Abstract
Habitat loss is currently a major threat to biodiversity, affecting species interactions, such as plant–pollinator interactions. This is particularly important in self-incompatible plants relying on pollinators to reproduce and sustain their populations. Here, we evaluated how habitat loss affects the pollination system, plant individual–pollinator species interaction network, and plant reproductive fitness of the self-incompatible Jasione maritima var. sabularia, a threatened taxon from dune systems. This plant is a pollinator generalist, visited by 108 species from distinct taxonomic groups. Results suggest that increasing habitat loss led to a significant decline in pollinator richness, increased pollen limitation, and a decrease in reproductive fitness of J. maritima var. sabularia. Visitation rate per individual did not significantly change with available area, indicating that the quality of pollen differed across populations. The topology of the network between J. maritima var. sabularia individuals and its pollinator species did not change, which may be attributed to the stability in the core of pollinator species. This suggests that the lower fitness of plants with increasing habitat degradation may be explained not only by the lower richness of peripheral pollinators but also by the genetic structure of the plant populations, as there is a possible higher transference of less quality pollen by pollinators, ultimately compromising the persistence of plant populations. Our study highlights the need of future studies to integrate the fine details provided by individual-level networks, which will increase our understanding of the pattern of species interactions and its consequences for the fitness of threatened plant populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and fragmentation of natural habitats are a major threat to biodiversity worldwide, currently being the primary cause of species extinction (Pimm and Raven 2000; IPBES 2018). It leads to changes in land cover composition and configuration, and to a gradual degradation of habitat quality (Fischer and Lindenmayer 2007; Hanski 2011; Hadley and Betts 2012). Landscape changes affect plant and animal populations alike, reducing their sizes and/or increasing their isolation and, consequently, increasing inbreeding depression and the risk of extinction (Kearns et al. 1998; Vanbergen 2014). Habitat transformation jeopardizes not only biodiversity itself but also disrupts interactions between species on which ecosystem functioning depends (Haddad et al. 2015). Among these interactions are mutualisms between flowering plants and their pollinators, which play a critical role in shaping much of Earth’s biodiversity and complexity (Bascompte 2009). Pollination is crucial for the sexual reproduction of plants (more than 87.5% of flowering plants require, to some degree, animals for pollination; Ollerton et al. 2011), as well as for the maintenance of insect populations relying on wild plants nectar and/or pollen as food resources (e.g.,Carvell et al. 2006; Müller et al. 2006; Baude et al. 2016). Habitat degradation may disrupt pollination functioning through the reduction of plant and pollinator diversity in a given area (positive species-area relationship theory; MacArthur and Wilson 1967) and by changing pollinator’s foraging behavior (Hadley and Betts 2012; Blaauw and Isaacs 2014; IPBES 2016).
Pollinator decline at local, regional and global scales is currently threatening the persistence of wild plant populations that rely on animal vectors for pollination (Kearns et al. 1998; Ollerton 2017), and among its causes is anthropogenic-driven habitat disturbance (Kearns et al. 1998; Potts et al. 2010; IPBES 2016). The combination of multiple stressors increases the overall pressure on living organisms and triggers complex negative feedback loops between animals and the plants they pollinate (Hadley and Betts 2012). Habitat loss and fragmentation may influence pollination directly through changes in plant and pollinator densities, and indirectly through changes in pollinator foraging behavior. First, the reduction of available habitat, and subsequent loss of nesting, oviposition and foraging sites, directly results in a decline of diversity and abundance of pollinators at local scales (Potts et al. 2010; Winfree et al. 2011). In addition, with the decline in plant density and diversity, food resources for pollinators become scarcer and scattered. These changes in food resources alter pollinator’s movements and foraging strategies, resulting in changes in visitation patterns as well as flight distances. Consequently, this also changes the costs to meet pollinator’s energetic needs (Wilcock and Neiland 2002; Xiao et al. 2016), ultimately impacting pollinator communities in the landscape (Tewksbury et al. 2002; Kremen et al. 2007).
Declines in pollinators will inevitably decrease the pollination service to wild plant populations and increase pollen limitation (Burd 1994; Potts et al. 2010). In addition, habitat degradation also results in impoverished plant populations and lower genetic diversity of conspecific plants, impacting the availability and quality of mating partners (Hadley and Betts 2012; Xiao et al. 2016). Consequently, there is a decrease in seed quantity and quality, plant reproductive output, and ultimately leading to the demographic collapse of plant populations (Aizen et al. 2002; Wilcock and Neiland 2002). Nevertheless, flowering plants present a wide array of reproductive strategies and the dependence on the pollination mutualism is conditioned by the plant’s breeding system (Bond 1994; Richards 1997). Self-incompatible plants are highly dependent on the availability of pollen vectors and mating partners since they can only use outcross pollen to produce seeds (Aizen et al. 2002; Hiscock and McInnis 2003). Therefore, self-incompatible plants pollinated by biotic vectors are expected to be highly vulnerable to habitat degradation, with extra concerns in the case of endemic, threatened and/or rare plant species (Bond 1994; Aguilar et al. 2006).
Over the last decade, there has been an increase in the number of studies that analyzed biological interactions using a network approach (Ings et al. 2009; Heleno et al. 2014). Ecological networks present a framework to simultaneously explore the role of species and their interactions, while simultaneously exploring emergent community properties (Bascompte and Jordano 2007; Heleno et al. 2014). Moreover, a network approach has proved to be a powerful tool to foresee the indirect effects of biodiversity loss (Tylianakis and Morris 2017), which may trigger extinction cascades and threaten the persistence of natural ecosystems (Kaiser-Bunbury et al. 2010; Rumeu et al. 2017). To date, however, most ecological network studies focused on interactions between species (Heleno et al. 2014; Timóteo et al. 2018). Although the potential of habitat loss to disrupt ecological interactions and to alter network structure at the community level is well documented (e.g., Grass et al. 2018; Heleno et al. 2020; Udy et al. 2020), to date its effect at the individual level, and the consequences for the functioning and stability of populations is still largely overlooked (but see Dáttilo et al. 2015). Recent studies have highlighted the importance of understanding interaction patterns at different levels of organization because network patterns may be scale-dependent (reviewed in Guimarães 2020). This includes downscaling ecological networks to the individual level (Dupont et al. 2011; Gómez et al. 2011; Gómez and Perfectti 2012; Tur et al. 2014; Valverde et al. 2016), because in the end it is the pattern of interactions between individuals that will dictate the dynamics and persistence of each population (Dupont et al. 2014; Tur et al. 2014; Arroyo-Correa et al. 2021). In the case of pollination networks specifically, downscaling networks to the individual plant level may be viewed as a map of pollen flow (Fortuna et al. 2008), revealing the dynamics of a population and how interaction patterns will impact plant fitness.
Here, we investigated the vulnerability to habitat loss of an insect-pollinated plant species endemic to dune habitat. Coastal sand dunes are characterized by an environmental gradient that determines a characteristic coast-to-inland plant community zonation (Acosta et al. 2007) and harbor a structurally and floristically distinct community of native plants. The environmental isolation of the dune system has been associated with speciation processes, resulting in a high proportion of endemic plant species (Neto et al. 2007). Coastal sand dunes are considered one of the most vulnerable and disturbed landscapes in Europe, and the Portuguese coastal dunes are no exception, being strongly subjected to natural and anthropogenic pressures (Marchante 2007; Martínez et al. 2008; Fantinato 2019). In Portugal, coastal zones are the most densely populated areas of the country, with more than 75% of the population living on the coast (DGA 2000; Calvão et al. 2013). Moreover, the increasing touristic activity and associated pressures (e.g., infrastructures, dune trampling, removal and substitution of native by alien species, and agricultural tillage) (Marchante 2007; Calvão et al. 2013) have contributed to decrease the heterogeneity of this landscape. These activities lead to a significant reduction in dune area, and consequently, of the availability of suitable habitat for native dune vegetation (Curr et al. 2000; Calvão et al. 2013), which may jeopardize the persistence of insect-dependent plants and its pollinators (e.g., Traveset et al. 2018).
In this study, we used the threatened Jasione maritima var. sabularia (Cout.) Sales & Hedge (Campanulaceae) as a study system to understand how habitat loss may affect the topology of the network between individuals of J. maritima var. sabularia and its pollinator species, as well as the plant fitness. Jasione maritima is a self-incompatible plant (Siopa et al. 2020; Castro et al. unpublished data) from the west coast of the Iberian Peninsula dune systems (Sales and Hedge 2001a; ICNF 2002), relying on pollinators for successful reproduction. The dependence on flower visitors suggests that this plant could be particularly vulnerable to the loss of dune habitats, not only through the obvious lack of habitat but also by the reduction of flower visitors and, consequently, of its reproductive fitness. Moreover, downscaling networks from species to individuals may allow new insights into how habitat loss affects the pattern of interactions at the individual plant level and how this translates into changes in the fitness of populations (Gómez and Perfectti 2012).
In this context, we studied the pollination ecology of the threatened J. maritima var. sabularia at 15 localities along the Portuguese coast, that differ in the availability of suitable area for J. maritima var. sabularia. First, we evaluated how habitat loss (represented as the percentage of available area for the focal plant species) affects the populations of J. maritima var. sabularia and its pollinators. We hypothesize that J. maritima var. sabularia density and the richness of its pollinator community will increase with increasing available area and, consequently, the number of visits per plant will also increase. Second, we assessed whether habitat loss reduces the fitness of J. maritima var. sabularia due to limited pollination services. For this, we quantified plant reproductive fitness and pollen limitation through a hand-pollination experiment at each population. We expect that pollen limitation will decrease, and J. maritima var. sabularia fitness will increase with increasing available area. For each locality, we built plant individual–pollinator species networks to assess whether individual-level network structure changes with the available area. We expect that networks will become more generalized as the available area increases, with individual plants receiving visits from more partners. Since Jasione maritima var. sabularia was the only resource flowering by the end of spring, these networks represent a proxy for pollinator foraging patterns in each locality. This allowed us to explore whether and how the pattern of interactions at the plant individual level differs with available area, and how it will affect the fitness of its individuals and ultimately of its populations. Finally, we investigated the network structural role of pollinator species, based on pollinators connectivity, along the gradient of available area. We expect that the pollinator community will be composed by a stable core of reliable pollinators along the gradient of available area (Dáttilo et al. 2013).
The results provide significant insights to our understanding of the dynamics of the interaction between insect-pollinated plants and the community of its pollinators across environments with different degradation levels.
Materials and methods
Study plant
Jasione maritima var. sabularia (Cout.) Sales & Hedge (Campanulaceae) is an endemic taxon from the west coast of the Iberian Peninsula (Sales and Hedge 2001a, b), with a distribution confined to the coastal sand dunes, mainly to the interdunal space and grey dunes among open vegetation (ICNF 2002). This taxon has received several taxonomic treatments; however, its recognition as a distinct taxon is supported by morphological traits (indumentum and leaf shape; Sales and Hedge 2001a, b) and by molecular analyses (Pérez-Espona et al. 2005). It is classified by the IUCN as Threatened due to the current destruction of the dune systems of the Iberian Peninsula (Bilz 2011). In Portugal, it occurs between Caminha and Aveiro, with an estimated area of occupancy of 1300 km2 (Commission of the European Communities 2009).
Jasione maritima is a perennial plant flowering from June to July. Controlled self-pollinations reveal that this species is self-incompatible (seed set, mean ± SE (n = 8): self-pollinations 8.8 ± 5.9; outcross pollinations 241.6 ± 68.7) and failed to produce seed under pollinator exclusion (Castro et al. unpublished data), similar to its sister species (J. montana, Parnell 1982, 1987; J. maritima subsp. maritima, Siopa et al. 2020). Consequently, this plant relies on pollinators for successful reproduction. Individual flowers are protandrous and open acropetally, with inflorescences lasting several days depending on their size, and have 33 ± 2 ovules (mean ± SE, n = 10; Castro et al. unpublished data). Nectar is secreted from the top of the ovary in small quantities, accessible to a large variety of insects, and pollen is actively collected as a reward by several bee species (author’s field observations). In addition, it exhibits secondary pollen presentation on a distal cylindrical stylar brush of small hairs (Faegri and Van Der Pijl 1979; Yeo 1993), and pollen removal by flower visitors triggers the female phase.
Study site and experimental design
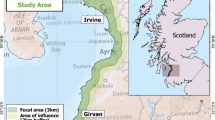
The present study was carried out in the northern Portuguese coastal sand dunes, between Silvalde (40 59′9.93″N, 8°38′43.99″W) and Torreira Sul (40°44′27.55″N, 8°43′6.02″W), stretching 29 km along the north-west coast of Portugal. The study area is characterized by a low sandy shore and is considered one of the most vulnerable habitats along the Portuguese coastline (Martins et al. 2012). This dune system has been greatly impacted by human activity, from infrastructures construction directly on the dunes, to the removal of natural vegetation, usually starting in the grey dune and continuing further inland, with conversion of land to agriculture and other uses (Martins et al. 2013).
The study area is located in the transition between the Eurosiberian and the Mediterranean biogeographic regions (Costa et al. 1998), which influences the type of vegetation present in the dune system (Martins et al. 2013). The native vegetation of the interdunal space and the grey dunes, the main habitat of J. maritima var. sabularia, is characterized by low plant cover with herbaceous species [e.g., Malcomia littorea (L.) R.Br., Helichrysum italicum subsp. picardi (Boiss. & Reut.) Franco, Silene L. species, Linaria Mill. species, Anagallis monelli L.] and a few shrubs [e.g., Corema album (L.) D. Don and Artemisia campestris subsp. maritima Arcang.]. Moreover, the dune system is affected by an extensive proliferation of invasive species, mainly Acacia longifolia (Andrews) Willd. and Carpobrotus edulis (L.) N.E. Br., considered the most impactful threats to the Portuguese native flora (Marchante 2007). The native vegetation flowers earlier in the spring, whereas invasive species flower in winter and early spring, and thus flowering periods did not overlap with J. maritima var. sabularia blooming.
Characterization of habitat quality and J. maritima var. sabularia density
Along the study area, 29 1-ha quadrangular plots parallel to the sea, and 1 km apart, were established to prospect for J. maritima var. sabularia. Each plot was characterized according to: (1) geographical coordinates; (2) presence/absence of Jasione maritima, and (3) type of habitat anthropogenic-driven disturbance (urbanization and altered dune vegetation, such as presence of invasive species or grasses) (Supplementary material; Table S1). Jasione maritima var. sabularia was only present in 15 of the 29 sites, further selected for this study.
Habitat loss was characterized using the percentage of available habitat for the focal plant, being proportionally inverse to available area. In each 1-ha plot, the percentage of available area for J. maritima var. sabularia growth was quantified with the aid of satellite images from Google Earth v7.3.2, and later validated in the field (Supplementary material; Fig. S1). The available area for J. maritima var. sabularia was defined as the dune area with native dune vegetation (Table S1). Thus, the area occupied by urbanization and altered vegetation due to invasive plant expansion or other vegetation (e.g., grasslands) changes due to anthropogenic activities (both forming dense stands) was calculated using the Earth Point tool for Google Earth (http://www.earthpoint.us/) and subtracted from the total plot area. Both the changes in vegetation considered here and the urbanization impede completely the growth of J. maritima var. sabularia and other native dune vegetation. Bare sand paths present along each plot were not considered unavailable area because we frequently observe this taxon thriving in these paths.
The density of J. maritima var. sabularia in each 1-ha plot was estimated in April of 2018, along four transects perpendicular to the sea and 25 m apart. Along each transect, a 1 m2 quadrat was placed at every 15 m and the number of reproductive individuals of J. maritima var. sabularia was counted (Table S1). A total of 28 quadrats per plot were analyzed (seven quadrats per transect). Thus, density of J. maritima var. sabularia is defined as the number of individuals of J. maritima per square meter in each 1-ha plot.
Flower visitor assemblage
The assemblage of floral visitors of J. maritima var. sabularia was characterized by direct observation during the flowering peak of 2017 (from June to July), on sunny and low to moderate windy days. In each of the 15 sites, observations were made in 10 patches of 2.25 m2 (1.5 m × 1.5 m), randomly distributed in areas where the studied plant grows. The observer was positioned at approximately 1 m from the patch, with small range binoculars, being able to monitor all floral visitors without interfering with the foraging activity of visitors. Visits were recorded in 15 min census for one day (from 0900 to 1800 h, GMT), or in two consecutive days when the lack of sunny conditions due to fog limited the number of censuses in the first day, totalling 384 censuses and 96 h of observations. In each patch, the following parameters were registered: (1) number of J. maritima var. sabularia individuals; (2) number of open inflorescences per individual plant; (3) identity and number of each flower visitor that interacted with the reproductive organs of J. maritima var. sabularia; (4) number and sequence of inflorescences visited by each flower visitor. One specimen of each insect type was collected for further identification to the lowest taxonomic level possible (hereafter called species for simplicity). Visitation rate per plant was calculated as the number of inflorescences of each plant individual visited per minute (Castro-Urgal et al. 2012).
Reproductive fitness and pollen limitation
To determine the effect of habitat loss on the reproductive fitness of J. maritima var. sabularia and on pollen limitation, the following treatments were applied at each site, during the flowering period of 2017: (1) open pollination (control), i.e., flowers without treatment, left open for natural levels of pollination; (2) supplementary pollination, i.e., flowers left open to natural levels of pollination and additionally supplemented with fresh pollen from five different genotypes of J. maritima var. sabularia. For this, at each site, 30 individuals were arbitrarily selected to receive both treatments, with one inflorescence marked to receive natural levels of pollination, while another received pollen supplementation. These plants were permanently labeled. Inflorescences with the exterior row of receptive flowers (visible by the lack of pollen in the stylar brush and by the bilobed stigmatic surface) were selected for pollen supplementation. Only this row was considered in the treatment due to time constraints to perform pollinations in all the study sites. Pollen supplementation was made by gently rubbing the selected inflorescence with inflorescences from five different genotypes. When mature, but prior to dehiscence, infructescences were collected to estimate the number of fruits and seeds. In the laboratory, fruit set (percentage of flowers that developed into fruits) and seed set (number of viable seeds per fruit) were quantified for each individual and treatment. To calculate the reproductive fitness of each individual of J. maritima var. sabularia, seed set was multiplied by fruit set. Only the exterior row of fruits of the infructescences from both the control and supplement treatments was used to estimate reproductive fitness to avoid differential resource allocation within the inflorescence. Although this fitness variable might not account for differences in internal rows, considering external rows in both treatments enabled us to obtain comparable results for the purpose of our objectives (i.e., calculate pollen limitation values). Pollen limitation (PL) was estimated for each individual based on the reproductive fitness according to Larson and Barrett (2000) as: PL = 1 − C/S, where C is the reproductive fitness of the control treatment and S is the reproductive fitness of the pollen supplementation treatment. Positive values resulting from higher reproductive fitness in pollen supplemented than in control treatment indicate the existence of pollen limitation in natural populations, while zero or negative values indicate no pollen limitation.
Network analysis
Quantitative plant individual-floral visitor species interaction matrices were built for each of the 15 sites, pooling the observations from the ten monitored plots. Each Jasione maritima individual and each floral visitor species observed in the day of pollinator monitoring were considered as a node in the visitation networks. These networks represent the potential mating events between individuals of J. maritima in a given population that may result from visiting insect species. Link weight was quantified as frequency of interaction between floral visitors and plants and calculated as the number of insect visits per minute (Castro-Urgal et al. 2012; Traveset et al. 2018).
To compare the structure of the individual-based plant–pollinator networks along our gradient of increasing available area, the following network-level descriptors were calculated: (1) weighted connectance, the linkage density divided by the number of species in the network, and reflecting the fraction of realized interactions (Tylianakis et al. 2007); (2) interaction evenness, based on Shannon diversity, reflects the uniformity of the interactions between species at network level (Bersier et al. 2002) often negatively associated with habitat disturbance (Tylianakis et al. 2007); (3) network specialization (H2′), a measure of the selectivity of interaction partners across the network, derived from Shannon entropy, and is a robust metric not affected by network size and sampling effect (Blüthgen et al. 2006); (4) generality, the mean number of preys per predator; in the present work interpreted as the mean number of plant individuals per pollinator (Bersier et al. 2002; Tylianakis et al. 2007); (5) vulnerability, the number of predators per prey, in the present work interpreted as the mean number of pollinators per plant individual (Bersier et al. 2002; Tylianakis et al. 2007); (6) pollinator robustness, a metric quantifying how much the pollinator community can withstand the random loss of plant individuals; and (7) plant robustness, quantifying how much the plant population can withstand the random loss of floral visitor species (Memmott et al. 2004). Moreover, two node-level descriptors for plants and for pollinators were calculated: (1) normalized degree for plants and for pollinators, number of pollinator species visiting each plant individual and the number of plant individuals visited by each pollinator species, respectively, divided by the number of possible interaction partners (Martín González et al. 2010); (2) species strength for plants and for pollinators is the sum of dependencies, i.e., is a measure of plant individual’s importance for the pollinator community and a measure of pollinator species’ importance for plant population, respectively (Bascompte et al. 2006). Since in our networks plant nodes are individuals rather than species, we will refer to individual plant strength from now on.
Many network descriptors can be affected by differences in sampling effort and network size (Fründ et al. 2016). To overcome this potential issue, network-level descriptors were corrected with a null model, which allows reliable comparisons between the networks (Vázquez and Aizen 2003; Costa et al. 2016). Due to the presence of decimal values in the matrix and to allow the use of a quantitative null model, networks were standardized by dividing its link weight by the lowest non-zero link weight in the matrix and rounded to the nearest integer (Timóteo et al. 2018). Afterwards, we generated 1000 randomizations of the original networks and, for each network descriptor, we calculated its z-score (z = [observed − null mean]/null standard deviation) (Sebastián-González et al. 2015; Dalsgaard et al. 2017), which were used in subsequent analysis. The randomized networks were obtained using the Patefield’s null model (Patefield 1981), which fixes network size (species richness) and marginal sums (total interaction frequency of each species). Network and species level descriptors were calculated using the bipartite R package (Dormann et al. 2008, 2009).
Finally, pollinators were classified for they role in each network as core or peripherals, according to the number of links of each species, i.e., number of individuals of J. maritima they visited (Dáttilo et al. 2013, 2015). Following Dátillo et al. 2013, this classification is based on standardizing the number of links of each pollinator species through: Gc = (ki − kmean)/σk, where ki is the number of links of the species in a network, kmean is mean number of links of all pollinator species in a network, and σk is the standard deviation of the number of links of a species in a network. Pollinator species with Gc > 1 have higher number of links than other pollinator species, thus structural core species of the pollination network, whereas those with Gc < 1 have lower number of links than the other species, being structural peripheral species in.
Statistical analysis
All statistical analyses were performed in R version 3.5.0 (R Core Team 2018). To evaluate the level of sampling completeness of pollinator species, we calculated species accumulation curves for each study site [vegan package (Oksanen et al. 2017)]. We estimated the minimum asymptotic richness of pollinators using the non-parametric estimator Chao 2 (Chao 1987), which is known to be robust for reduced sample sizes, and more reliable than other estimators (Walther and Moore 2005). The percentage of sampling completeness was calculated as the observed number of species divided by the estimated number of species (Costa et al. 2016).
To assess the effect of the available area on the number of open inflorescences per patch of pollination observation, number of flowers per inflorescence, number of inflorescences per plant individual, reproductive fitness, pollinator normalized degree and pollinator species strength we resorted to Generalized Linear Mixed Models [GLMM, lme4 (Bates et al. 2014) and lmerTest (Kuznetsova et al. 2017) packages]. To explore the effect of available area on J. maritima var. sabularia density, visitation rate per plant, pollen limitation, plant normalized degree and plant strength, we used Robust Linear Mixed Models [RLMM, robustlmm package (Koller 2016)]. Latitude was included as a random factor to control for the potential variability associated with the geographic location of the sampling locations. In the analyses regarding pollinator node-level metrics, we also included pollinator species as a random factor to account for differences in species composition between locations and to avoid pseudo-replication. Moreover, for response variables based on pollination observations, we included observation patch as a nested factor within each latitude, i.e., within location, to account for the nested structure of the experimental design (patches of pollination observation within location).
For each network-level metric [weighted connectance, network specialization (H2′), vulnerability and generality, interaction evenness, pollinator and plant robustness], we have only obtained one value per location, and latitude could not be entered as a random factor. Thus, we used Generalized Linear Models (GLM) and latitude was included as a covariate instead.
The models for the number of open inflorescences, number of flowers per inflorescence, number of inflorescences per plant individual, and pollinator species richness were fitted with Poisson errors distribution, with a square-root link function for the first model, an identity link function for the next two models, and a log link function for the last model. The model for reproductive fitness was fitted with Gaussian errors distribution and a square-root link function. The models for network specialization H2′ and pollinator normalized degree and species strength were fitted with Gamma errors distribution, with an inverse link function for the first model, and a log link function for the latter two models. The models for the remaining variables were fitted with Gaussian errors distribution and an identity link function.
For all GLMMs, RLMMs and GLMs mentioned before, J. maritima var. sabularia density (number of individuals of J. maritima per square meter) was also included as a covariate to account the effect of J. maritima density (the density of J. maritima in 1-ha plot), to test if the results are independent of population density.
The effect of pollen supplementation on reproductive fitness for all populations combined was analyzed using a GLMM, including pollination treatment as fixed factor, latitude and individual as random factors, fitted with Gaussian errors distribution and an identity link function. To assess the effect of pollen supplementation for each locality, differences were also investigated for each population using linear models (LMs).
To test if the community of pollinators changed across the gradient of available area, we used a Permutational Multivariate Analysis of Variance (PERMANOVA) (Anderson 2001), based on a Bray–Curtis’ distances with 9999 permutations, with function adonis () from the R package vegan (Oksanen et al. 2017). Besides available area, Gc classification was included to test for differences between the composition of the core and peripheral communities. Finally, we tested whether each of those subcommunities changed across the gradient of available area, and whether the number of core pollinator and peripheral species was affected by the available area, using a GLM fitted with Poisson errors distribution with a log link, including density of J. maritima to control. Core species are those that contribute most to pollination with visits to several J. maritima individuals, and likely the most effective pollinators.
For all the LM, GLM and GLMM analyses, residuals were plotted and analyzed for departures from normality and homoscedasticity, and response variables were transformed to ensure the best fit to the assumptions of standard regression. When the assumptions for linear models were not met, Robust Linear Mixed Models were used as they down-weight influential points on the general trend of the data and provide better estimates of the regression parameters and their standard errors (Koller 2016). Given that likelihood ratio tests are not available for robust models, significance of the explanatory variables was calculated using Satterthwaite approximations of degrees of freedom (Luke 2017; Geniole et al. 2019). The details and significance of all models are presented in Tables S2 to S9.
Regarding visitation per plant and network analyses, two extreme values related to the frequency of interaction of ant species during two observation periods were tested as outliers (once they corresponded to observations performed in monitoring patches located above ant colonies) and removed to meet the assumptions of normality and homoscedasticity.
Pearson correlation analysis was performed to evaluate the correlation between the following variables: J. maritima var. sabularia density, pollinator richness, reproductive fitness and pollen limitation.
Results
Effect of available area on Jasione maritima populations
The density of J. maritima var. sabularia varied between 0.04 and 8.61 plants per square meter (Table S1), but we did not find a significant effect of available area on J. maritima var. sabularia density (t = 1.862, 13 df, P = 0.085; Fig. 1a and Table S2). Nevertheless, data suggest a slight trend for higher J. maritima var. sabularia density with increasing area.
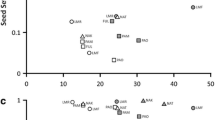
Effect of the available area on (a) Jasione maritima var. sabularia density (number of individuals per square meter), (b) number of open inflorescences of J. maritima var. sabularia per monitored patch, (c) pollinator species richness (number of floral visitor’s taxa), (d) visitation rate per plant (number of inflorescences of each plant individual visited per minute), (e) reproductive fitness (number of seeds per flower), and (f) pollen limitation (PL) across the increasing gradient of available area. Values were back-transformed. Solid lines represent the predicted relationships from the respective model and shaded areas correspond to 95% confidence interval. Significance values for the effect of area on each variable are given at the upper left corner
The number of open inflorescences of J. maritima var. sabularia per patch significantly increased with available area (χ2 = 6.548, 1 df, P = 0.011), ranging between 13.8 and 59.9 inflorescences per patch (Fig. 1b; Table S2). Available area did not significantly affect the number of flowers per inflorescence (χ2 = 0.015, 1 df, P = 0.902; Table S2 and Table S3), or the number of inflorescences per individual plant (χ2 = 0.315, 1 df, P = 0.574; Table S2 and Table S3).
In addition, J. maritima var. sabularia density had no effect on any response variable (P > 0.101 for all response variables; Table S2, Table S4–Table S8) analyzed in the following sections of the results, suggesting that the results are independent of population density.
Effect of available area on pollinator assemblage and visitation rate per plant
A total of 1258 insects, belonging to 108 morphospecies, were observed visiting the flowers of J. maritima var. sabularia. Floral visitors included ants, wasps and bees [Hymenoptera (38.9%), 42 morphospecies]; flies [Diptera (39.8%), 43 morphospecies]; beetles [Coleoptera (4.6%), 5 morphospecies]; and butterflies [Lepidotera (16.7%), 18 morphospecies] (Fig. 2; Table S9 for a detailed list of species). Overall, 3044 interactions between J. maritima var. sabularia and its visitors were recorded across the 15 sites. Most of the interactions were with Hymenoptera (1687 interactions—55.4%), followed by Diptera (918 interactions—30.2%), Coleoptera (353 interactions—11.6%) and Lepidoptera (86 interactions—2.8%). Sampling completeness ranged between 32.6% and 89.6% (Table S1). Nevertheless, in most sites (11 out of 15 studied), we achieved a moderate to high level (64.0% or above) of sampling completeness.
Examples of pollinators of Jasione maritima var. sabularia: (a) Stizus ruficornis, (b) Podalonia hirsute, (c) Apis mellifera, (d) Megachile morphospecies 1, (e) Lasioglossum morphospecies 1, (f) Paragus morphospecies 1, (g) Eristalis tenax, (h) Sarcophaga morphospecies, (i) Sphaerophoria sp., (j) Syritta pipiens, (k) Paracorymbia stragulata, (l) Oedemera flavipes, (m) Pyronia cecilia, (n) Leptotes pirithous, and (o) Pyropteron hispanica
Pollinator species richness ranged from 10 to 25 species, whereas the abundance of pollinators ranged between 33 and 176 individuals (Table S1). Available area for J. maritima var. sabularia had a significant positive effect on pollinator richness (χ2 = 7.677, 1 df, P = 0.006; Fig. 1c and Table S4), but not on the visitation rate per plant (t = − 0.142, 12 df, P = 0.889; Fig. 1d and Table S5).
Effect of available area on reproductive fitness
Regarding reproductive fitness, available area showed a significant effect on the number of seeds J. maritima var. sabularia produced (χ2 = 6.579, 1 df, P = 0.010; Fig. 1e and Table S5 and Table S11).
At the regional level, strong significant differences were detected between open pollinated and pollen supplemented flowers (χ2 = 208.65, 1df, P < 0.001; Table S8), with supplemented flowers reaching a higher reproductive fitness, i.e., producing more seeds per flower than open pollinated flowers (mean ± SE; 18.95 ± 0.42 vs 12.21 ± 0.44, respectively, Table S11). At the population level, in 13 of the 15 sites studied, supplemented flowers had a significantly higher reproductive fitness than open pollinated flowers (Fig. S2, Table S9 and Table S11). In the remaining two populations, supplemented flowers had a greater reproductive fitness than open pollinated ones, but no significant differences were observed between the two pollination treatments (Fig. S2 and Table S10).
Pollen limitation index increased as available area for J. maritima decreased (t = − 2.197, df = 10.66, P = 0.051; Table S5; Fig. 1f).
Correlation between plant and pollinator variables
Pollinator richness was significantly correlated with reproductive fitness of J. maritima var. sabularia (r = 0.5204, P = 0.047). None of the other correlations between the studied variables was significant (Table 1).
Effect of available area on network structure and node-level descriptors
Contrary to our expectations, available area did not have a significant effect on any network-level descriptor (P > 0.260 for all network-level metrics; Table S6; Table S12 and Table S13). Regarding node-level descriptors, available area had a significant negative effect on both pollinator normalized degree (χ2 = 5.187, 1 df, P = 0.023) and plant normalized degree (t = − 3.382, 10.60 df, P = 0.006), a marginally significant positive effect on pollinator species strength (χ2 = 3.454, 1 df, P = 0.063), and no significant effect on individual plant strength (t = − 1.562, 12.60 df, P = 0.143; Table S7, Table S14 and Table S15).
Globally, pollinator community composition did not change across the gradient (PERMANOVA: Pseudo-F = 1.070, R2 = 0.006, P = 0.298), and no differences were detected between the composition of the community of core and peripheral pollinators (PERMANOVA: Pseudo-F = 1.067, R2 = 0.007, P = 0.300). Moreover, when looking into each of these communities of pollinators, neither the core nor the peripheral community changed with available area (PERMOVA: core—Pseudo-F = 0.933, R2 = 0.025, P = 0.646; peripheral—Pseudo-F = 1.096, R2 = 0.008, P = 0.244). However, while the number of core species did not change across the gradient (χ2 = 0.011, 1 df, P = 0.916; Table S4), the number of peripheral species increase with available area (χ2 = 8.842, 1 df, P = 0.003; Fig. 1i, Table S4).
Discussion
Jasione maritima var. sabularia is a pollinator generalist, with its flowers being visited by 108 species, mostly Hymenoptera and Diptera. This threatened endemic taxon is self-incompatible and, thus, it highly relies on pollinators for its successful reproduction. Here, we observed a decline in pollinator richness and pollination service (indicated by increased pollen limitation levels), resulting in a reduction in reproductive fitness as available habitat for J. maritima decreases (i.e., with increasing habitat loss). Consequently, the ongoing expansion of urbanization and of invasive plant species in Portuguese dune systems can lead to the decline of the pollinators of J. maritima var. sabularia and compromise the persistence of its populations. In addition, the vulnerability to the loss of pollinators due to habitat loss observed here could be extended to other insect-pollinated plants growing in disturbed habitats.
Species sensitivity to habitat degradation depends on the combination of species traits, such as, for example, in plants, the competitive and dispersal abilities, reproductive potential across generations, iteroparity or clonal reproduction (Henle et al. 2004). However, habitat area is fundamental to determine the persistence of plant and pollinator populations, since smaller areas will commonly contain fewer species and individuals (MacArthur and Wilson 1967; Bender et al. 1998; Hagen et al. 2012). Indeed, several authors (e.g.,Aizen and Feinsinger 1994; Blaauw and Isaacs 2014; Jauker et al. 2019) have demonstrated decreases in pollinator species richness due to smaller areas generated by habitat loss and fragmentation. Larger habitat areas are structurally more heterogeneous and usually support larger communities of pollinators by presenting a higher availability and quality of nesting opportunities (Potts et al. 2005; Hopfenmüller et al. 2014), as well as higher diversity and abundance of food resources (Blaauw and Isaacs 2014; Hopfenmüller et al. 2014). Although the increase in the density of J. maritima var. sabularia with available area was not statistically significant, larger areas presented significantly higher number of open inflorescences, which may affect attractiveness to pollinators. This result supports the consensus that smaller populations are less attractive or less detectable, which is a fundamental factor in the diversity of pollinators and also the patterns of pollen flow (Sih and Baltus 1987). Importantly, in the populations studied in this work, at the time of its peak flowering (late spring to early of summer), J. maritima var. sabularia was nearly the only food resource for the pollinators. Indeed, and except for a very few late flowering individuals of other native plants in a few localities, there were no other co-flowering plants. This lack of alternative food resources highlights the critical importance of J. maritima var. sabularia as a key resource for the maintenance of the pollinator’s community in these dune systems.
The present study shows that habitat loss influences the reproductive fitness of J. maritima var. sabularia, as the decrease in available area was associated to lower number of seeds per flower. The reduction in the reproductive fitness of J. maritima var. sabularia with habitat loss is consistent with other studies that document decreases in reproductive fitness of self-incompatible plants due to habitat degradation (Steffan-Dewenter and Tscharntke 1999; Moody-weis and Heywood 2001; Aguilar et al. 2006). A review by Ghazoul (2005) showed that, in 12 out of 16 studies, small populations of self-incompatible plants had lower fitness than large ones. Although seed and fruit set can be influenced by other ecological and genetic factors (Caruso et al. 2005; Campbell and Husband 2007), pollen limitation is a key factor for reproductive fitness (Ashman et al. 2004) and has been pointed out as one of the main causes for the reduction of plant fitness in fragmented habitats (Aguilar et al. 2006). Our pollen supplementation experiment showed that J. maritima var. sabularia plants were strongly pollen limited at the regional level, with plants supplemented with outcross pollen presenting higher fitness when compared to control plants. However, pollen limitation can vary temporally and spatially within the same species (e.g.,Knight 2003; Castro et al. 2008) and two of the populations from habitats with larger available area were not pollen limited. Moreover, habitats with larger available area presented lower pollen limitation when compared to habitats with smaller areas. Pollen limitation is a consequence of the reduction, in quantity and quality, of the pollen deposited on stigmas (Wilcock and Neiland 2002). Successful pollination depends on factors related with pollinator traits, such as pollinator diversity, abundance and efficiency and pollinator foraging strategies, as well as plant traits, such as breeding system and population genetic composition (Ghazoul 2005; Castro et al. 2009; Hadley and Betts 2012). Thus, one may hypothesize that increased levels of pollen limitation with increased habitat loss may result from (1) changes in pollinator communities and plant–pollinator interaction networks, and/or (2) genetic dilapidation in this self-incompatible plant populations.
Changes in pollinator communities and plant–pollinator networks may affect pollen transfer between plant individuals and, consequently, impact its reproductive fitness. However, contrary to our expectations, the overall topology of the individual-based plant–pollinator networks did not change along the gradient of available area, with no changes detected for any network-level descriptor. Available area did not significantly affect the diversity or distribution of interactions across networks, as no trend was found for weighted connectance, interaction evenness, network specialization, vulnerability and generality. Furthermore, available area did not affect pollinator and plant robustness, whereby the structure of the individual networks was robust to loss of available area. Nonetheless, we found an effect of the gradient of available area on the number of interaction partners (normalized degree) for both pollinators and plants. In smaller areas, pollinators tended to visit more plant individuals, whereas each plant individual was also visited by a greater number of pollinator species. Moreover, the core pollinators did not change across the entire gradient of available area, being reliable deliverers of the pollination service. Core pollinators contribute with most interactions to networks, visiting more individuals of J. maritima var. sabularia and being likely the most effective pollinators and the reason why we did not detect changes in network topology (Dáttilo et al. 2015). To date, very little information exists about the effect of habitat degradation on individual-based networks, but the pattern we found here is consistent with a previous study from tropical rainforests showing that reliable core pollinators helped to stabilize network topology (Dáttilo et al. 2015). However, we also observed that along the gradient of available area the number of peripheral pollinator species increased, leading to a higher richness of pollen vectors. For plants depending on pollinators for seed production, visits from a more diverse pollinator community, with distinct foraging patterns, has been associated with better reproductive success and fitness (Gómez and Perfectti 2012; Tur et al. 2013). A diverse pollinator community introduces functional redundancy and weakens mutual dependencies, strengthening community resilience and robustness to local species loss (Tylianakis et al. 2007; Kaiser-bunbury et al. 2017). In this study system, the higher reproductive fitness along the gradient of available area may be partially explained by the cumulative positive effect of peripheral pollinator species, whose richness increases along the gradient. Successful pollination in a population is determined not only by individual pollinator abundance or pollinator richness, but also by pollinator efficiencies, and because pollinator efficiencies are variable, the relation between those factors and reproductive success might not be straightforward.
The fact that visitation rate per individual plant did not change across the gradient of available area also suggests that the quality of pollen may differ across populations, raising the hypothesis of genetic dilapidation of J. maritima var. sabularia populations with habitat loss. Smaller areas tend to hold smaller populations of J. maritima var. sabularia and plant individuals are probably genetically more related, which may lead to increase inbreeding levels and reproductive failures, thus facing higher fitness costs (Fischer and Lindenmayer 2007; Sletvold et al. 2012). This is likely associated with transference of lower quality pollen (incompatible or genetically related pollen in self-incompatible species) by pollinators that can result in stigmatic clogging and a consequent decrease in reproductive success (Ehlers 1999; Waites and Ågren 2004). Larger areas are likely to have higher genetic diversity with most of the pollen received by individual plants being compatible, thus increasing the reproductive fitness in such populations. Future studies accounting for the genetic structure and inbreeding levels may provide further insights into the processes involved in the dynamics of plant populations under disturbance. Given that the pollinator community is more homogeneous in smaller areas, J. maritima var. sabularia could still be at risk of a higher reproductive failure if it relies heavily on a guild of pollinators that responds similarly to a future anthropogenic effect (Kremen et al. 2007). Anthropogenic actions and ecological processes acting together can result in a selective decline of important species of the system, leading to detrimental effects on the plant population, and ultimately to the irreversible collapse of the plant–pollinator network (Kaiser-Bunbury et al. 2010).
Conclusions
This is one of the first studies investigating the effect of habitat loss on the reproductive fitness of the threatened J. maritima var. sabularia, and how the pattern of interactions of its individuals with the pollinator community is affected. Here, we show that the loss of suitable habitat within the dune system is a main factor leading to a decrease in pollination services, and consequently in the reproductive fitness of J. maritima var. sabularia. This self-incompatible species highly relies on the pollinator community for sexual reproduction, and although the structure of its network of interaction did not change, future disturbances impacting its core pollinators may further compromise the persistence of viable populations. The sensitivity of plant–pollination interactions across disturbance gradients may constitute a major threat to animal-pollinated plant populations growing in habitats highly susceptible to disturbance. This study provides information on the pollination ecology of this endemic plant, which can be a first step to devise a strategy for the implementation of conservation measures.
Our results provide a novel insight to the study of individual-based networks in degraded environments, highlighting the need to develop more comprehensive experimental designs that include for example the evaluation of the genetic structure of the populations, to better understand how environmental gradients may affect the structure of mutualistic networks and how it may translate into the fitness of its individual constituents and of the overall population.
Data availability
Data supporting the results will be made publicly available upon acceptance.
References
Acosta A, De PV, Beach WP, Pillar VDP, Blasi C (2007) Coastal vegetation zonation and dune morphology in some Mediterranean ecosystems. J Coast Res 23:1518–1524. https://doi.org/10.2112/05-0589.1
Aguilar R, Ashworth L, Galetto L, Aizen M (2006) Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecol Lett 9:968–980. https://doi.org/10.1111/j.1461-0248.2006.00927.x
Aizen M, Feinsinger P (1994) Habitat fragmentation, native insect pollinators, and feral honey bees in Argentine “Chaco Serrano.” Ecol Appl 4:378–392. https://doi.org/10.2307/1941941
Aizen MA, Ashworth L, Galetto L (2002) Reproductive success in fragmented habitats: do compatibility systems and pollination specialization matter? J Veg Sci 13:885–892. https://doi.org/10.1111/j.1654-1103.2002.tb02118.x
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Arroyo-Correa B, Bartomeus I, Jordano P (2021) Individual-based plant-pollinator networks are structured by phenotypic and microsite plant traits. J Ecol 00:1–13. https://doi.org/10.1111/1365-2745.13694
Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell DR et al (2004) Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421. https://doi.org/10.1890/03-8024
Bascompte J (2009) Mutualistic networks. Front Ecol Environ 7:429–436. https://doi.org/10.1890/080026
Bascompte J, Jordano P (2007) Plant-animal mutualistic networks: the architecture of biodiversity. Annu Rev Ecol Evol Syst 38:567–593. https://doi.org/10.1146/annurev.ecolsys.38.091206.095818
Bascompte J, Jordano P, Olesen JM (2006) Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312:431–433. https://doi.org/10.1126/science.1123412
Bates D, Mächler M, Bolker BM, Walker SC (2014) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Baude M, Kunin WE, Boatman ND, Conyers S, Davies N, Gillespie MA et al (2016) Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 530:85–88. https://doi.org/10.1038/nature16532
Bender DJ, Contreras TA, Fahrig L (1998) Habitat loss and population decline: a meta-analysis of the patch size effect. Ecology 79:517–533. https://doi.org/10.1890/0012-9658(1998)079[0517:HLAPDA]2.0.CO;2
Bersier L-F, Banasek-Richter C, Cattin MF (2002) Quantitative descriptors of food-web matrices. Ecology 83:2394–2407. https://doi.org/10.2307/3071801
Bilz M (2011) Jasione lusitanica. In: IUCN Red List Threat. Species. https://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T161853A5504370.en. Accessed 28 Nov 2019
Blaauw BR, Isaacs R (2014) Larger patches of diverse floral resources increase insect pollinator density, diversity, and their pollination of native wildflowers. Basic Appl Ecol 15:701–711. https://doi.org/10.1016/j.baae.2014.10.001
Blüthgen N, Menzel F, Blüthgen N (2006) Measuring specialization in species interaction networks. BMC Ecol 6:1–12. https://doi.org/10.1186/1472-6785-6-9
Bond WJ (1994) Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Philos Trans R Soc B 344:83–90. https://doi.org/10.1098/rstb.1994.0055
Burd M (1994) Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot Rev 60:83–139. https://doi.org/10.1007/BF02856594
Calvão T, Pessoa MF, Lidon FC (2013) Impact of human activities on coastal vegetation—a review. Emirates J Food Agric 25:926–944. https://doi.org/10.9755/ejfa.v25i12.16730
Campbell LG, Husband BC (2007) Small populations are mate-poor but pollinator-rich in a rare, self-incompatible plant, Hymenoxys herbacea (Asteraceae). New Phytol 174:915–925. https://doi.org/10.1111/j.1469-8137.2007.02045.x
Caruso CM, Remington DLD, Ostergren KE (2005) Variation in resource limitation of plant reproduction influences natural selection on floral traits of Asclepias syriaca. Oecologia 146:68–76. https://doi.org/10.1007/s00442-005-0183-4
Carvell C, Roy DB, Smart SM, Pywell RF, Preston CD, Goulson D (2006) Declines in forage availability for bumblebees at a national scale. Biol Conserv 132:481–489. https://doi.org/10.1016/j.biocon.2006.05.008
Castro S, Silveira P, Navarro L (2008) How flower biology and breeding system affect the reproductive success of the narrow endemic Polygala vayredae Costa (Polygalaceae). Bot J Linn Soc 157:67–81. https://doi.org/10.1111/j.1095-8339.2008.00784.x
Castro S, Luis S, Navarro L (2009) Floral traits variation, legitimate pollination, and nectar robbing in Polygala vayredae (Polygalaceae). Ecol Re 24:47–55. https://doi.org/10.1007/s11284-008-0481-5
Castro-Urgal R, Tur C, Albrecht M, Traveset A (2012) How different link weights affect the structure of quantitative flower-visitation networks. Basic Appl Ecol 13:500–508. https://doi.org/10.1016/j.baae.2012.08.002
Chao A (1987) Estimating the population size for capture-recapture data society, international biometric with unequal catchability. Biometrics 43:783–791. https://doi.org/10.2307/2531532
Commission of the European Communities (2009) Composite Report on the Conservation Status of Habitat Types and Species as requiered under Article 17 of the Habitats Directive. Brussels
Costa JC, Aguiar C, Capelo JH, Lousã M, Neto C (1998) Biogeografia De Portugal Continental. Quercetea 1:5–56
Costa JM, da Silva LP, Ramos JA, Heleno RH (2016) Sampling completeness in seed dispersal networks: when enough is enough. Basic Appl Ecol 17:155–164. https://doi.org/10.1016/j.baae.2015.09.008
Curr RH, Koh A, Edwards E, Williams AT, Davies P (2000) Assessing anthropogenic impact on Mediterranean sand dunes from aerial digital photography. J Coast Conserv 6:15–22. https://doi.org/10.1007/BF02730463
Dalsgaard B, Schleuning M, Maruyama PK, Dehling DM, Sonne J, Vizentin-Bugoni J et al (2017) Opposed latitudinal patterns of network-derived and dietary specialization in avian plant–frugivore interaction systems. Ecography 40:1395–1401. https://doi.org/10.1111/ecog.02604
Dáttilo W, Guimarães PR, Izzo TJ (2013) Spatial structure of ant-plant mutualistic networks. Oikos 122:1643–1648. https://doi.org/10.1111/j.1600-0706.2013.00562.x
Dáttilo W, Aguirre A, Quesada M, Dirzo R (2015) Tropical forest fragmentation affects floral visitors but not the structure of individual-based palm-pollinator networks. PLoS ONE 10:1–15. https://doi.org/10.1371/journal.pone.0121275
DGA (2000) Relatório do estado do ambiente 1999
Dormann CF, Gruber B, Fründ J (2008) Introducing the bipartite package: analysing ecological networks. R News 8:8–11
Dormann CF, Frund J, Bluthgen N, Gruber B (2009) Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol J 2:7–24. https://doi.org/10.2174/1874213000902010007
Dupont YL, Trøjelsgaard K, Olesen JM (2011) Scaling down from species to individuals: a flower-visitation network between individual honeybees and thistle plants. Oikos 120:170–177. https://doi.org/10.1111/j.1600-0706.2010.18699.x
Dupont YL, Trøjelsgaard K, Hagen M, Henriksen MV, Olesen JM, Pedersen NM et al (2014) Spatial structure of an individual-based plant-pollinator network. Oikos 123:1301–1310. https://doi.org/10.1111/oik.01426
Ehlers BK (1999) Variation in fruit set within and among natural populations of the self-incompatible herb Centaurea scabiosa (Asteraceae). Nord J Bot 19:653–664. https://doi.org/10.1111/j.1756-1051.1999.tb00675.x
Faegri K, Van Der Pijl L (1979) Principles of pollination ecology, 3rd edn. Pergamon, Oxford
Fantinato E (2019) The impact of (mass) tourism on coastal dune pollination networks. Biol Conserv 236:70–78. https://doi.org/10.1016/j.biocon.2019.05.037
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 16:265–280. https://doi.org/10.1111/j.1466-8238.2006.00287.x
Fortuna MA, García C, Guimarães PRJ, Bascompte J (2008) Spatial mating networks in insect-pollinated plants. Ecol Lett 11:490–498. https://doi.org/10.1111/j.1461-0248.2008.01167.x
Fründ J, Mccann KS, Williams NM (2016) Sampling bias is a challenge for quantifying specialization and network structure: lessons from a quantitative niche model. Oikos 125:502–513. https://doi.org/10.1111/oik.02256
Geniole SN, Proietti V, Bird BM, Ortiz TL, Bonin PL, Goldfarb B et al (2019) Testosterone reduces the threat premium in competitive resource division. Proc R Soc B 286:20190720. https://doi.org/10.1098/rspb.2019.0720
Ghazoul J (2005) Pollen and seed dispersal among dispersed plants. Biol Rev 80:413–443. https://doi.org/10.1017/S1464793105006731
Gómez JM, Perfectti F (2012) Fitness consequences of centrality in mutualistic individual-based networks. Proc R Soc B Biol Sci 279:1754–1760. https://doi.org/10.1098/rspb.2011.2244
Gómez JM, Perfectti F, Jordano P (2011) The functional consequences of mutualistic network architecture. PLoS ONE 6:e16143. https://doi.org/10.1371/Citation
Grass I, Jauker B, Steffan-Dewenter I, Tscharntke T, Jauker F (2018) Past and potential future effects of habitat fragmentation on structure and stability of plant–pollinator and host–parasitoid networks. Nat Ecol Evol 2:1408–1417. https://doi.org/10.1038/s41559-018-0631-2
Guimarães PR (2020) The structure of ecological networks across levels of organization. Annu Rev Ecol Evol Syst 51:433–460. https://doi.org/10.1146/annurev-ecolsys-012220-120819
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:1–10. https://doi.org/10.1126/sciadv.1500052
Hadley AS, Betts MG (2012) The effects of landscape fragmentation on pollination dynamics: absence of evidence not evidence of absence. Biol Rev 87:526–544. https://doi.org/10.1111/j.1469-185X.2011.00205.x
Hagen M, Kissling WD, Rasmussen C, De Aguiar MA, Brown LE, Carstensen DW et al (2012) Biodiversity, species interactions and ecological networks in a fragmented world. Adv Ecol Res 46:89–210. https://doi.org/10.1016/B978-0-12-396992-7.00002-2
Hanski I (2011) Habitat loss, the dynamics of biodiversity, and a perspective on conservation. Ambio 40:248–255. https://doi.org/10.1007/s13280-011-0147-3
Heleno R, Garcia C, Jordano P, Traveset A, Gómez JM, Blüthgen N et al (2014) Ecological networks: delving into the architecture of biodiversity. Biol Lett 10:20131000
Heleno RH, Ripple WJ, Traveset A (2020) Scientists ’ warning on endangered food webs. Web Ecol 20:1–10. https://doi.org/10.5194/we-20-1-2020
Henle K, Davies KF, Kleyer M, Margules C, Settele J (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13:207–251. https://doi.org/10.1023/B:BIOC.0000004319.91643.9e
Hiscock SJ, McInnis SM (2003) The diversity of self-incompatibility systems in flowering plants. Plant Biol 5:23–32. https://doi.org/10.1055/s-2003-37981
Hopfenmüller S, Steffan-Dewenter I, Holzschuh A (2014) Trait-specific responses of wild bee communities to landscape composition, configuration and local factors. PLoS ONE 9:e104439. https://doi.org/10.1371/journal.pone.0104439
ICNF (2002) Fichas de caracterização e gestão das espécies constantes no Anexo II da Directiva Habitats-Flora. http://www2.icnf.pt/portal/pn/biodiversidade/rn2000/resource/doc/rn-plan-set/flora/jas-lusit. Accessed 28 Nov 2019
Ings TC, Montoya JM, Bascompte J, Blüthgen N, Brown L, Dormann CF et al (2009) Ecological networks—beyond food webs. J Anim Ecol 78:253–269. https://doi.org/10.1111/j.1365-2656.2008.01460.x
IPBES (2016) The assessment report on pollinators, pollination and food production of the intergovernmental science-policy platform on biodiversity and ecosystem services
IPBES (2018) The IPBES assessment report on land degradation and restoration
Jauker F, Jauker B, Grass I, Steffan-Dewenter I, Wolters V (2019) Partitioning wild bee and hoverfly contributions to plant-pollinator network structure in fragmented habitats. Ecology 100:e02569. https://doi.org/10.1002/ecy.2569
Kaiser-Bunbury CN, Muff S, Memmott J, Müller CB, Caflisch A (2010) The robustness of pollination networks to the loss of species and interactions: a quantitative approach incorporating pollinator behaviour. Ecol Lett 13:442–452. https://doi.org/10.1111/j.1461-0248.2009.01437.x
Kaiser-Bunbury CN, Mougal J, Whittington AE, Valentin T, Gabriel R, Olesen JM et al (2017) Ecosystem restoration strengthens pollination network resilience and function. Nature 542:223–227. https://doi.org/10.1038/nature21071
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualisms: the conservation of plant-pollinator interactions. Annu Rev Ecol Syst 29:83–112. https://doi.org/10.1146/annurev.ecolsys.29.1.83
Knight TM (2003) Floral density, pollen limitation, and reproductive success in Trillium grandiflorum. Oecologia 137:557–563. https://doi.org/10.1007/s00442-003-1371-8
Koller M (2016) robustlmm : an R package for Robust estimation of linear mixed-effects models. J Stat Softw 75:1–24. https://doi.org/10.18637/jss.v075.i06
Kremen C, Williams NM, Aizen MA, Gemmill-Herren B, LeBuhn G, Minckley R et al (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10:299–314. https://doi.org/10.1111/j.1461-0248.2007.01018.x
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects Models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Larson BMH, Barrett SCH (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linn Soc 69:503–520. https://doi.org/10.1006/bij1.1999.0372
Luke SG (2017) Evaluating significance in linear mixed-effects models in R. Behav Res Methods 49:1494–1502. https://doi.org/10.3758/s13428-016-0809-y
MacArthur RH, Wilson EO (1967) The theory of island biogeography, 1st edn. Princeton University Press, Princepton
Marchante E (2007) Invasion of Portuguese coastal dunes by Acacia longifolia: impacts on soil ecology. Master thesis. Department of Life Sciences. University of Coimbra. Coimbra
Martín González AM, Dalsgaard B, Olesen JM (2010) Centrality measures and the importance of generalist species in pollination networks. Ecol Complex 7:36–43. https://doi.org/10.1016/j.ecocom.2009.03.008
Martínez ML, Maun MA, Psuty NP (2008) The fragility and conservation of the World’s Coastal Dunes: geomorphological, ecological and socioeconomic perspectives. Coastal dunes—ecology and conservation. Springer-Verlag, Berlin, pp 355–369
Martins VN, Pires R, Cabral P (2012) Modelling of coastal vulnerability in the stretch between the beaches of Porto de Mós and Falésia, Algarve (Portugal). J Coast Conserv 16:503–510. https://doi.org/10.1007/s11852-012-0191-6
Martins MC, Neto CS, Costa JC (2013) The meaning of mainland Portugal beaches and dunes’ psammophilic plant communities: a contribution to tourism management and nature conservation. J Coast Conserv 17:279–299. https://doi.org/10.1007/s11852-013-0232-9
Memmott J, Waser NM, Price MV (2004) Tolerance of pollination networks to species extinctions. Proc R Soc B Biol Sci 271:2605–2611. https://doi.org/10.1098/rspb.2004.2909
Moody-weis JM, Heywood JS (2001) Pollination limitation to reproductive success in the Missouri Evening Primrose, Oenothera macrocarpa (Onagraceae). Am J Bot 88:1615–1622. https://doi.org/10.2307/3558406
Müller A, Diener S, Schnyder S, Stutz K, Sedivy C, Dorn S (2006) Quantitative pollen requirements of solitary bees: implications for bee conservation and the evolution of bee-flower relationships. Biol Conserv 130:604–615. https://doi.org/10.1016/j.biocon.2006.01.023
Neto C, Costa JC, Honrado J, Capelo J (2007) Phytosociologic associations and Natura 2000 habitats of Portuguese coastal sand dunes. Fitosociologia 44:29–35
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB et al. (2017) Vegan: community ecology package. R Package version 2.4–2
Ollerton J (2017) Pollinator diversity: distribution, ecological function, and conservation. Annu Rev Ecol Evol Syst 48:353–376. https://doi.org/10.1146/annurev-ecolsys-110316-022919
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326. https://doi.org/10.1111/j.1600-0706.2010.18644.x
Parnell J (1982) Some observations on the breeding biology of Jasione montana. J Life Sci 4:1–7
Parnell J (1987) Variation in Jasione montana L. (Campanulaceae) and related species in Europe and North Africa. Watsonia 16:249–267
Patefield AWM (1981) Algorithm AS 159: an efficient method of generating random R × C tables with given row and column totals algorithm random R x C tables with an efficient method of generating given row and column totals. J R Stat Soc Ser C 30:91–97
Pérez-Espona S, Sales F, Hedge I, Möller M (2005) Phylogeny and species relationships in Jasione (Campanulaceae) with emphasis on the “Montana-complex.” Edinburgh J Bot 62:29–51. https://doi.org/10.1017/S0960428606000047
Pimm SL, Raven P (2000) Extinction by numbers. Nature 403:843–845. https://doi.org/10.1038/35002708
Potts SG, Vulliamy B, Roberts S, O’Toole C, Dafni A, Ne’eman G et al (2005) Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecol Entomol 30:78–85. https://doi.org/10.1139/b99-015
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
R Core Team (2018) R: A language and environment for statistical computing
Richards AJ (1997) Plant breeding systems, Second. Garland Science
Rumeu B, Devoto M, Traveset A, Olesen JM, Vargas P, Nogales M et al (2017) Predicting the consequences of disperser extinction: richness matters the most when abundance is low. Funct Ecol 31:1910–1920. https://doi.org/10.1111/1365-2435.12897
Sales F, Hedge IC (2001a) Jasione L. In: Flora iberica: plantas vasculares de la Península Ibérica e Islas Baleares. Real Jardín Botánico, Consejo Superior de Investigaciones Científicas 153–170
Sales F, Hedge IC (2001b) Nomenclature and typification of western european Jasione (Campanulaceae). In: Anales del Jardín Botánico de Madrid. pp 163–172
Sebastián-González E, Dalsgaard B, Sandel B, Guimarães PR (2015) Macroecological trends in nestedness and modularity of seed-dispersal networks: human impact matters. Glob Ecol Biogeogr 24:293–303. https://doi.org/10.1111/geb.12270
Sih A, Baltus M-S (1987) Patch size, pollinator behavior, and pollinator limitation in catnip. Ecology 68:1679–1690. https://doi.org/10.2307/1939860
Siopa C, Dias MC, Castro M, Loureiro J, Castro S (2020) Is selfing a reproductive assurance promoting polyploid establishment ? Reduced fitness, leaky self-incompatibility and lower inbreeding depression in neotetraploids. Am J Bot 107:526–538. https://doi.org/10.1002/ajb2.1441
Sletvold N, Grindeland JM, Zu P, Agren J (2012) Strong inbreeding depression and local outbreeding depression in the rewarding orchid Gymnadenia conopsea. Conserv Genet 13:1305–1315. https://doi.org/10.1007/s10592-012-0373-7
Steffan-Dewenter I, Tscharntke T (1999) Effects of habitat isolation on pollinator communities and seed set. Oecologia 121:432–440. https://doi.org/10.1007/s004420050949
Tewksbury JJ, Levey DJ, Haddad NM, Sargent S, Orrock JL, Weldon A et al (2002) Corridors affect plants, animals, and their interactions in fragmented landscapes. Proc Natl Acad Sci U S A 99:12923–12926. https://doi.org/10.1073/pnas.202242699
Timóteo S, Correia M, Rodríguez-Echeverría S, Freitas H, Heleno R (2018) Multilayer networks reveal the spatial structure of seed-dispersal interactions across the Great Rift landscapes. Nat Commun 9:1–11. https://doi.org/10.1038/s41467-017-02658-y
Traveset A, Castro-Urgal R, Rotllàn-Puig X, Lázaro A (2018) Effects of habitat loss on the plant–flower visitor network structure of a dune community. Oikos 127:45–55. https://doi.org/10.1111/oik.04154
Tur C, Castro-Urgal R, Traveset A (2013) Linking plant specialization to dependence in interactions for seed set in pollination networks. PLoS ONE 8:e78294. https://doi.org/10.1371/journal.pone.0078294
Tur C, Vigalondo B, Trøjelsgaard K, Olesen JM, Traveset A (2014) Downscaling pollen-transport networks to the level of individuals. J Anim Ecol 83:306–317. https://doi.org/10.1111/1365-2656.12130
Tylianakis JM, Morris RJ (2017) Ecological networks across environmental gradients. Annu Rev Ecol Evol Syst 48:25–48. https://doi.org/10.1146/annurev-ecolsys-110316-022821
Tylianakis JM, Tscharntke T, Lewis OT (2007) Habitat modification alters the structure of tropical host-parasitoid food webs. Nature 445:202–205. https://doi.org/10.1038/nature05429
Udy K, Hannah R, Scherber C, Tscharntke T (2020) Plant-pollinator interactions along an urbanization gradient from cities and villages to farmland landscapes. Ecosphere 11:e03020. https://doi.org/10.1002/ecs2.3020
Valverde J, Maria G, Perfectti F (2016) The temporal dimension in individual-based plant pollination networks. Oikos 125:468–479. https://doi.org/10.1111/oik.02661
Vanbergen AJ (2014) Landscape alteration and habitat modification: impacts on plant-pollinator systems. Curr Opin Insect Sci 5:44–49. https://doi.org/10.1016/j.cois.2014.09.004
Vázquez DP, Aizen MA (2003) Null model analyses of specialization in plant-pollinator interactions. Ecology 84:2493–2501. https://doi.org/10.1890/02-0587
Waites AR, Ågren J (2004) Pollinator visitation, stigmatic pollen loads and among-population variation in seed set in Lythrum salicaria. J Ecol 92:512–526. https://doi.org/10.1111/j.0022-0477.2004.00893.x
Walther BA, Moore JL (2005) The concepts of bias, precision and accuracy, and their use in testing the performance of species richness estimators, with a literature review of estimator performance. Ecography 28:815–829. https://doi.org/10.1111/j.2005.0906-7590.04112.x
Wilcock C, Neiland R (2002) Pollination failure in plants: why it happens and when it matters. Trends Plant Sci 7:270–277. https://doi.org/10.1016/S1360-1385(02)02258-6
Winfree R, Bartomeus I, Cariveau DP (2011) Native pollinators in anthropogenic habitats. Annu Rev Ecol Evol Syst 42:1–22. https://doi.org/10.1146/annurev-ecolsys-102710-145042
Xiao Y, Li X, Cao Y, Dong M (2016) The diverse effects of habitat fragmentation on plant-pollinator interactions. Plant Ecol 217:857–868. https://doi.org/10.1007/s11258-016-0608-7
Yeo PF (1993) Secondary pollen presentation: form, function and evolution. Springer-Verlag, New York
Acknowledgements
The authors are very grateful to Catarina Siopa, Mariana Castro and Helena Castro for their valuable help in the field, and to Hugo Gaspar for assistance with insect identification. The authors are thankful to ICNF—Instituto da Conservação da Natureza e das Florestas for providing authorizations to collect samples and undertake scientific research. The authors are also thankful to Dra. Monica Geber and two anonymous reviewers for all the helpful comments and suggestions that enabled to significantly improve the manuscript.
Funding
Project RENATURE—“Programa Operacional Regional do Centro 2014-2020 (Centro2020)—CENTRO-01–0145-FEDER-000007”. Project UID/BIA/04004/2020; ST was funded by the Portuguese Foundation for Science and Technology (FCT) contract CEECIND/00135/2017. SC was financed by CULTIVAR project (CENTRO-01-0145-FEDER-000020), co-financed by the Regional Operational Programme Centro 2020, Portugal 2020 and European Union, through European Fund for Regional Development (ERDF).
Author information
Authors and Affiliations
Contributions
SC, ST and JL conceived and designed the experiment. SM conducted field and laboratory work with the assistance of the remaining authors. SM, ST and SC analyzed the data and SM and ST developed the plant–pollinator networks. SM wrote the manuscript with the contributions of all the other authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Permits to collect samples and undertake scientific research were obtained from ICNF—Instituto da Conservação da Natureza e das Florestas, Reference 866/2018.
Additional information
Communicated by Monica Geber.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mendes, S.B., Timóteo, S., Loureiro, J. et al. The impact of habitat loss on pollination services for a threatened dune endemic plant. Oecologia 198, 279–293 (2022). https://doi.org/10.1007/s00442-021-05070-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-021-05070-y