Abstract
The high levels of biodiversity in the Amazon are maintained mostly due to its composition as a natural mosaic of different habitats, including both unflooded and flooded forests, campinaranas, and savannahs. Here, we compared multiple dimensions of α- and β- bat biodiversity between four natural Amazonian habitats (savannah, campinarana, forest patches, and continuous forest). In addition, we explored the extent to which bat communities in the different habitats are nested within one another, and compared the community-level functional uniqueness and community-weighted mean traits between habitats. Our results show that taxonomic, functional and phylogenetic α-diversity of bats is higher in continuous forest than in any of the other habitat types. The continuous forest also harbours more unique species, and indeed, the bat community assemblages in the less-complex habitats, including forest patches, campinarana and savannahs, are taxonomic, functional and phylogenetic sub-sets of the assemblage found in the continuous forest. By examining β-diversity partitions and species composition, we are able to shed light on the mechanisms behind the variation in diversity between the four habitat types, which reflect a process of environmental sorting along a habitat gradient going from a more complex to a less complex habitat. We conclude that nesting patterns along the mosaic of habitats are determined by differences in complexity between habitats and that taxonomic and functional uniqueness contribute to overall regional bat diversity and functionality. Ongoing human-induced disturbances of these habitats could provoke an unprecedented loss of bat diversity and functionality with negative consequences for biodiversity and ecosystem services.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Amazon is one of the most biodiverse places on the planet (Silva et al. 2005; Antonelli and Sanmartín 2011), owing in part to its composition as a natural mosaic of different habitats, including both unflooded (terra firme) and flooded (várzea) forests, white-sand ecosystems (such as campinaranas), and savannahs (Pires and Prance 1985; Prance 1996; Malhado et al. 2013; Adeney et al. 2016; Carvalho and Mustin 2017). The distribution and extent of, and threats to, these different habitat types vary significantly, with key threats largely linked to uncontrolled land-use change and occupation of the Amazon (but see Adeney et al. 2016; Carvalho and Mustin 2017; Matricardi et al. 2020). Understanding the ways in which species use these different habitats and how biodiversity is distributed among them is essential for a more effective conservation strategy.
The distribution of taxa between habitat types relies on the elucidation of key environmental filters. Studies carried out along different environmental gradients within natural Amazonian ecosystems have shown that functional traits help to determine the occurrence of species of butterflies and ants in different habitat types (Graça et al. 2017; Guilherme et al. 2019). In addition, taxonomic diversity of both vertebrates and invertebrates has been found to be higher in more complex Amazonian habitats, which display greater biomass and availability of food and shelters (Bobrowiec et al. 2014; Graça et al. 2017; Fraga et al. 2018; Naka et al. 2020). However, very few studies have used the taxonomic, functional and phylogenetic dimensions of diversity together to compare animal communities among natural habitats in neotropical ecosystems (see Moreno et al. 2018 for a review). Indices based on functional or phylogenetic trees can aid in our understanding of environmental filters that act on loss of functions or lineages in a community (Moreno et al. 2018; Aninta et al. 2019). Currently, we have robust tools to estimate the dimensions of α- and β-diversity, and nestedness (e.g., Melo et al. 2014; Moreno et al. 2018). Using these robust tools, we can both improve our understanding of how ecological and evolutionary processes act on communities, and better inform conservation actions for species and habitats.
Specifically for bats, the second most diverse order of Mammalia (Chiroptera) in the Neotropics, taxonomic diversity in the Amazon has also been shown to vary between natural habitat types (Bernard and Fenton 2007; Ramos Pereira et al. 2010; Bobrowiec et al. 2014; Carvalho et al. 2018, 2020), largely due to variation in rates of primary productivity, fruit yield and availability of other foods, such as arthropods and small vertebrates (Ramos Pereira et al. 2010; Bobrowiec et al. 2014). However, so far, few studies have explored the variation in the functional and phylogenetic dimensions of α- and β-diversity, or nestedness of tropical bat assemblages between different natural habitats (e.g., Carvalho et al. 2020). Most studies, including those that consider several locations throughout the Neotropics, have been conducted in areas that are already fragmented and degraded by anthropogenic actions. For example, there are studies considering the impacts of habitat change in anthropogenically modified landscapes on both taxonomic, functional and phylogenetic diversity together (Cisneros et al. 2015; Ramos Pereira et al. 2018; Carvalho et al. 2020) or separately (García-Morales et al. 2016; Farneda et al. 2018, 2020; Aninta et al. 2019), and their results show a decrease in taxonomic, functional and phylogenetic diversity as the intensity of habitat modification increases. Knowing how the different dimensions of bat diversity vary between different Amazonian natural ecosystems, according to their different environmental filters, will enable better management and conservation of the entire Amazon biome and its different species of bats.
Here, we assess taxonomic, functional and phylogenetic α- and β-diversity and nestedness of bat assemblages in four different Amazonian natural habitats, which form an environmental gradient from continuous forest through forest patches and campinarana, to savannah, a non-forest habitat. Owing to the more complex vegetation structure, we expect that taxonomic, functional and phylogenetic α-diversity will be highest in continuous forest, followed by forest patches and campinarana, and will be lowest in savannah, the least complex habitat type. Also based on the level of habitat complexity, we expect the two more complex habitat types, continuous forest and forest patches, will be more taxonomically, functionally and phylogenetically similar and thus β-diversity will be lower between these habitat types. Following the same logic, we also expect taxonomic, functional and phylogenetic β-diversity to be higher between continuous forest and both savannah and campinarana, which are less complex habitat types. Furthermore, we expect to find that these higher levels of taxonomic, functional and phylogenetic β-diversity between the most and least complex habitat types are driven mostly by differences in species richness (βrich) rather than by species turnover (βtur), due to nestedness of the bat assemblages of the less to more complex habitat types. In other words, we expect that the decrease in habitat complexity along the gradient from forests to forest patches to campinaranas to savannahs leads to a decrease in the availability and diversity of food and other resources, leading to loss of species and therefore declines in taxonomic, functional and phylogenetic diversity along the habitat-complexity gradient.
Materials and methods
We restricted our analyses to phyllostomids and the mormoopid Pteronotus sp., which can be adequately sampled using mist nets (Kalko et al. 1996). The packages used in all analyses were loaded using Program R (R Core Team 2020).
Study area
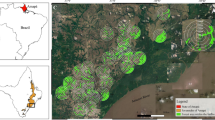
Fieldwork was conducted between 2008 and 2018 on the border between the states of Pará and Amapá, in the northeastern Amazon / Guiana Shield region in Brazil (geographical coordinates: 0° 16′ 23.19" N, 50° 54′ 19.74" W and 1° 32′ 23.05" S, 55° 12′ 29.15" W) (Fig. 1). The data were collected using two different sampling designs. The first sampling design consisted of four sites with five transects of 250 m (Fig. 1). The transects in each site followed the natural curve of the ground (same altitude), and were spaced at least 1 km apart (Fig. 1). The transects were distributed among areas of continuous forest (ten sites), campinarana (five), savannah (three) and forest patches within a savannah matrix (two). The second sampling design consisted of 20 sampling sites distributed in forest patches (10 sites) and savannah (10). Data were collected at a single sampling point located at least 50 m from the edge between the two habitat types (forest patches and savannah) (Fig. 1).
Map of the study area in the northern Brazil, with the states of Amapá and Pará highlighted (upper left corner). The largest map shows the five locations where bats were captured between 2008 and 2018. The smaller maps show the layout of the bat sampling sites; at sites A to D, sampling was carried out between 2008 and 2010 and at site E in 2018. In E, each forest sampling point was associated with a savannah sampling point (each one was located 50 m from the edge between the two environments)
Some of the continuous forest transects fell within a mosaic of secondary rainforest (aluvial and terra firme) crossed by streams (igarapés) (Carvalho et al. 2018). The continuous forest had an average vegetation height of 35 m, with trees up to 50 m high, and a closed canopy. The vegetation in the campinarana modules was characterized by trees up to 18 m in stature, and a closed canopy. The campinarana areas were patches of this type of habitat inserted in a continuous forest matrix (Fig. 1). The savannah modules were located on parkland Cerrado (Mustin et al. 2017), which is characterised by an open tree storey that does not surpass 3 metres in height, having a completely open canopy (Costa-Neto 2014). The forest patches, which were embedded in the savannah matrix, which borders the várzea and terra firme forest on both sides (Mustin et al. 2017; Fig. 1), had a closed canopy, and trees with an average height of 6 meters, with sparse trees reaching up to 25 m in stature. The sampled forest patches varied in size and shape, as well as in their edges, with the majority being longer (up to 3 km) than they were wide (minimum of 20 m in some parts of the patch). In summary, these habitats form a gradient in plant complexity and structure, ranging from tall and complex forested habitat (continuous forest), followed by shorter and less complex forest patches and campinaranas, to fully open habitat (savannah).
Bat sampling
In the first sampling design, we captured bats during three field seasons: (1) December 2008 to January 2009, (2) February to March 2010, and (3) June to July 2010. The bat sampling was carried out with 12 mist-nets (12 × 3 m; 14 mm mesh size) set in the understory. These nets were set up at dusk, around 18:00 h, and were kept open until 02:00 h, for 3 consecutive nights per site per season. We subsequently checked these data for a possible effect of “trap shyness” as a result of sampling on consecutive nights (see Marques et al. 2013), but found no qualitative difference in richness or diversity estimates and thus included the data from all 3 nights in subsequent analyses. Thus, 80 sampling nights were carried out in continuous forest, 40 in campinaranas, 24 in savannahs and 16 in forest patches (Supplementary Table 1).
In the second design, we captured bats between June and September 2018 using nine mist-nests (12 × 3 m; 14 mm mesh size) set in the understory along a ~ 110 m trail. The nets were set up at dusk, around 18:00 h, and were kept open until 00:00 h, for 2 nights per site per season. Thus, 40 sampling nights were carried out in savannah and 40 in forest patches. Total sampling effort for both sampling designs was calculated according to Straube and Bianconi (2002) and in total represented 708,480 m2 × h (savannah: 160,704 m2 × h; campinarana = 138,240 m2 × h; forest patches: 133,056 m2 × h; continuous forest: 276,480 m2 × h). Despite these data having been collected 8 years after the completion of data collection in the first sampling design, we do not expect any strong influence of habitat change or other disturbance as there have been no major changes in land use in the study region since 2009 (MapBiomas—https://plataforma.brasil.mapbiomas.org/), and the area has not experienced major forest fires or extreme seasonal events that could compromise our comparisons.
For both sampling designs, the bats captured in the nets were removed and placed in cotton bags for later identification. All individuals of the genus Platyrrhinus, except for P. brachycephalus, captured between 2008 and 2010 were considered to belong to a species complex of Platyrrhinus sp. as at the time genetic material was not available to separate them between the now known species P. fusciventris and P. incarum (Velazco et al. 2010). For the genus Pteronotus, we considered all individuals to be Pteronotus sp. because we cannot identify if the individuals are P. alinotus or P. rubiginosus, which has been recently recorded in this region (Thoisy et al. 2014; Pavan et al. 2018). Bats were sexed, weighed and identified in the field according to Lim and Engstrom (2001), Gardner (2008), Reis et al. (2017) and Lòpez-Baucells et al. (2018). Nomenclature of species follows Garbino et al. (2020). All applicable institutional and/or national guidelines for the care and use of animals were followed. In addition, the fieldwork, handling and processing of all captures at all study sites followed the guidelines of the American Society of Mammalogists (Sikes et al. 2011).
Species functional traits
To calculate functional α- and β-diversity, and functional nestedness, based on our own capture data and on published literature, we recorded five functional traits of Neotropical bats: body mass, trophic level, diet, vertical stratification, and wing morphology (Supplementary Table 2).
Body mass
Larger species tend to have greater energy requirements (Purvis et al. 2000) and thus higher occupancy in more complex habitats where food is more abundant and available (Castillo-Figueroa and Pérez-Torres 2021). Body mass was calculated as the average body mass of all adult individuals of each species captured in each habitat, excluding pregnant females. Logarithmic transformations were performed on body mass to normalize the values.
Diet and trophic levels
Habitat use by bats is influenced by their diet and trophic level, as these two functional traits are directly associated with the structure and complexity of the forest (Farneda et al. 2015). Information on diet was obtained from the Ecological Register database (ecoregister.org; on 15 January 2019), which includes a repository of published papers from around the world (Alroy 2017). We classified the species, according to their specific diet, as carnivores, frugivores, insectivores, nectarivores, omnivores or sanguinivores. In addition, bat species were classified into two broad trophic levels: animalivorous or phytophagous (Giannini and Kalko 2004).
Vertical stratification
There is a strong vertical stratification of bats in Neotropical forests, with some species occupying the upper forest strata (canopy) more than the lower strata (ground Marques et al. 2016; Kalko and Handley 2001; Ramos Pereira et al. 2010). The bat species were classified in terms of vertical stratification within the forest as either understory or canopy species (Bernard 2001; Kalko and Handley 2001; Ramos Pereira et al. 2010).
Wing morphology
Wing morphology includes both aspect ratio and relative wing loading, and these characteristics are important predictors of foraging habitat, dispersal ability and home range size (Norberg and Rayner 1987; Arita and Fenton 1997). For most species, we obtained data on wing morphology from Tavares (2013) and Marinello and Bernard (2014). When not available in the literature, we estimated the parameter based on body mass data from a closely related species and the formula indicated in Jovanovic and Levy (1997).
Phylogeny
Phylogenetic α- and β-diversity, and phylogenetic nestedness were estimated using a phylogenetic tree based on the phylogeny of Jones et al. (2002). Four species (Artibeus gnomus, Lichonycteris degener, Micronycteris microtis, and Tonatia maresi) are not present in this phylogeny and were substituted by their closest congener (Cisneros et al. 2015).
Data analysis
Taxonomic, functional and phylogenetic alpha diversity
We estimated α-diversity considering its three partitions—taxonomic, functional and phylogenetic. Taxonomic diversity (TD) was estimated using the Simpson's Index of Diversity (1-D), which was calculated using the R package ‘diverse’ (Guevara et al. 2017). Higher values for Simpson´s Index of Diversity (1-D) indicate more diverse communities and lower values indicate less diverse communities. Functional diversity (FD) was estimated using Rao's quadratic diversity index Q, which takes into account the differences (trait-based variance) between species pairs, weighted by relative abundance. Higher values of FD indicate that species are functionally different from each other, and lower values indicate that species are functionally equivalent (Botta-Dukát 2005). To better explore variation in FD, we also quantified community-level functional uniqueness (FU) of bat assemblages, that is, how important a species is in supporting a certain function. Functional uniqueness was estimated using the ratio between Q and D (FU = Q/D, see Ricotta et al. 2016), which considers all species maximally dissimilar (Botta-Dukát 2005), measuring how much functional diversity belongs to each taxonomic diversity unit. Higher values of FU indicate that all species are functionally dissimilar, and lower values indicate that all species are functionally identical (Ricotta et al. 2016). Both FD and FU were estimated using the function described in Ricotta et al. (2016) and the R package ‘FD’ (Laliberté et al. 2015). To assess how bat functional traits differ between the habitat types, we used the community-weighted mean trait values (CWM) (Lavorel et al. 2008). This method allows for assessment of the shifts in mean trait values weighted by relative species abundances, equalizing differences in capture effort between sites, areas or studies (see Lavorel et al. 2008 for more details). We calculated CWM traits using the “functcomp” function of the R package ‘FD’ (Laliberté and Legendre 2010). Finally, phylogenetic diversity (PD) was estimated using Faith's phylogenetic diversity (Faith 1992) in the R package ‘picante’ (Kembel et al. 2020).
Pairwise comparisons of taxonomic, functional and phylogenetic diversity, functional uniqueness and community-weighted mean trait values were made between the Amazonian habitats (continuous forest, forest patches, savannah and campinarana) using paired permutational tests with 9999 randomizations (Farneda et al. 2018).
Taxonomic, functional and phylogenetic beta diversity
To better understand the ecological processes underlying community structuring between the four Amazonian habitats, we examined the variation in taxonomic (TβD), functional (FβD) and phylogenetic (PβD) β-diversity, and each was measured and partitioned, through the Jaccard index, into their respective components of richness and turnover (Cardoso et al. 2015). As such, the relative contributions of species, functional and lineage replacement (TβDTur, FβDTur and PβDTur—species, functional and lineage turnover) and species, functional and lineage richness difference (TβDRich, FβDRich and PβDRich—due to loss or gain of bat species, functional traits and bat lineages) to TβD, FβD and PβD, respectively, were assessed.
To account for differences in capture effort between periods, the β-diversity components (i.e., βTur + βrich for each one of the partitions) were computed for each habitat type using rarefaction (1000 runs) to 676 individuals (corresponding to the total number of individuals sampled in campinarana, the habitat with the lowest number of captures). The analyses were conducted using the beta function of the R package ‘BAT’ (Cardoso et al. 2015).
Taxonomic, functional and phylogenetic nestedness
To estimate taxonomic, functional and phylogenetic nestedness, presence-absence matrices were first constructed where species were in the columns and habitat types were in the rows. Taxonomic nestedness was then estimated using the NODF index (Nestedness Metric Based on Overlap and Decreasing Fill). We chose to use NODF as it has been suggested that it exhibits more robust statistical properties than other indices, and because it is possible to quantify the degree to which each row of the matrix (in this case habitat type) is nested in each of the other rows (Almeida-Neto et al. 2008). NODF can assume values from 0 to 100, and higher values indicate more nested assemblages (Almeida-Neto et al. 2008). We evaluated the significance of the taxonomic nestedness using the fixed–fixed null model based on the “quasiswap” algorithm (Miklós and Podani 2004). Both NODF estimation and the significance test were conducted using the R package ‘vegan’ (Oksanen et al. 2007).
To estimate both functional (traitNODF) and phylogenetic (phyloNODF) nestedness, we used an extension of the NODF index called treeNODF index (Melo et al. 2014), and a functional and a phylogenetic tree, respectively. The treeNODF index calculates what proportion of the functional or lineage diversity that is present in more functionally or phylogenetically poor assemblages is also present in more functionally or phylogenetically rich assemblages (Melo et al. 2014; Almeida‐Gomes et al. 2019). The treeNODF can also assume values from 0 to 100, and this value is maximum when species composition in a community with low phylogenetic diversity is a perfect subset of those present in a community with high phylogenetic diversity (Melo et al. 2014). In addition, we also partitioned the traitNODF and phyloNODF into its two components: S.fraction and topoNODF. The S.fraction represents the degree to which habitats are or are not nested as a result of having assemblages composed of the same or different species, whereas topoNODF represents the degree to which habitats are nested or not as a result of functional or phylogenetic resemblance or dissimilarity, based on the functional and phylogenetic tree topologies (Melo et al. 2014). The treeNODF index was estimated using the R package ‘CommEcol’ (Melo 2019). The significance of the observed traitNODF and phyloNODF and their component values (S.fraction and topoNODE) was determined using a permutation null model (999 permutations).
Results
We captured 3686 bats representing 47 species (1263 bats and 39 species in continuous forest, 979 and 34 in savannah, 768 and 33 in forest patches, 676 and 24 in campinarana, respectively—Table S1). Four unique species, species that occurred only in one habitat, were recorded in continuous forest (Chrotopterus auritus, Lophostoma schulzi, Phyllostomus discolor, and Vampyrodes caraccioli), three in savannah (Chiroderma villosum, Lichonycteris degener, and Micronycteris minuta), and two in forest patches (Chiroderma trinitatum and Micronycteris hirsuta). No unique species were recorded in campinarana.
Taxonomic, functional and phylogenetic alpha diversity
Taxonomic, functional and phylogenetic diversity, and functional uniqueness, were significantly higher in continuous forest than in campinarana (p < 0.01; Fig. 2) and savannah (p < 0.01; Fig. 2). Functional and phylogenetic diversity was significantly higher in the continuous forest than in forest patches (p < 0.01; Fig. 2). However, there was no significant difference in taxonomic diversity or functional uniqueness between continuous forest and forest patches (p > 0.05; Fig. 2).
A Taxonomic, B functional and C phylogenetic diversity, and D functional uniqueness values that were estimated from bats sampled in four different Amazonian habitats in northern Brazilian Amazon. The vertical lines represent of 95% confidence intervals. CF continuous forest, CP campinarana, SV savannah, FP forest patches
There was no significant difference in taxonomic or phylogenetic diversity between forest patches and any other habitat type (p > 0.05; Fig. 2). However, forest patches were more functionally diverse and functionally unique than both campinarana (p < 0.05; Fig. 2) and savannah (p < 0.01; Fig. 2). There was no significant difference in taxonomic, functional or phylogenetic diversity, or functional uniqueness, between savannah and campinarana (p > 0.05; Fig. 2).
A number of traits helped to explain the higher functional diversity and functional uniqueness in the more complex (continuous forest and forest patches) than the less complex (campinarana and savannah) habitats. Firstly, nectarivorous species (e.g., Hsunycteris thomasi), and animalivorous species, represented by carnivores (e.g., Trachops cirrhosus) and insectivores (e.g., Gardnerycteris crenulatum and Lophostoma silvicola), were more closely associated with continuous forest and forest patches than with savannah and campinarana (Table 1; Fig. 3). Conversely, phytophagous bats, represented mainly by frugivorous species (e.g., Ametrida centurio, Artibeus lituratus, Artibeus concolor, Uroderma bilobatum, and Uroderma magnirostrum), were more associated with savannah and campinarana (Table 1; Fig. 3).
Community-weighted mean (CWM) trait values for all functional traits considered in present study, estimated from literature data and bats sampled in four different Amazonian habitats in northern Brazilian Amazon. Wing morph. Wing morphology. The vertical lines represent of 95% confidence intervals. CF continuous forest, CP campinarana, SV savannah, FP forest patches
Secondly, both wing traits (aspect and loading) increased sharply in savannah, being more than double compared with all three forest habitats (Table 1; Fig. 3), indicating that species with large and broad wings (e.g., Platyrrhinus brachycephalus and Uroderma magnirostrum) are more frequent in the savannah than in the other habitats. Body mass was also higher in the savannah than in continuous forest or campinarana (Table 1; Fig. 3), but was similarly high in the forest patches (Table 1; Fig. 3). However, body mass varied widely in savannah and forest patches indicating a wide range of species with different body sizes using these habitats.
Finally, canopy species were more frequently captured in the savannah than in any other environment, followed by campinarana, forest patches, and continuous forest (Table 1; Fig. 3), whereas understorey species were more frequently captured in continuous forest (Table 1; Fig. 3). However, both canopy and understorey species were frequently captured in the savannah, indicating a wide range of preferences related to vertical habitat.
Taxonomic, functional and phylogenetic beta diversity
Taxonomically (TβD), functionally (FβD) and phylogenetically (PβD), continuous forest and forest patches presented the most similar bat assemblages (Fig. 4; Supplementary Table 3). In contrast, taxonomic (FβD), functional and phylogenetic (PβD) beta-diversity was highest between continuous forest and campinarana, and intermediate between continuous forest and savannah. Taxonomic (TβD), functional and phylogenetic (PβD) β-diversity were lower between forest patches and savannah, than between forest patches and campinarana. Finally, the highest value for TβD, FβD and PβD showed that the bat assemblages between campinarana and savannah are less similar (Fig. 4; Supplementary Table 3).
Values of A taxonomic (TβD), B functional (FβD) and C phylogenetic (PβD) β-diversity (block dots) and percentage of each of the components (βTur and βRich—coloured bars) that make up the total beta diversity for the bat assembly sampled in four different Amazonian habitats in northern Brazilian Amazon. CF continuous forest, CP campinarana, SV savannah, FP forest patches
Turnover was more important than species richness difference in driving TβD between continuous forest and forest patches, continuous forest and savannah, and forest patches and savannah. Between campinarana and savannah, the turnover was slightly more important than species richness difference in driving TβD (Fig. 4; Supplementary Table 3). However, species richness difference was more important than turnover in driving TβD between continuous forest and campinarana. Whereas species richness and turnover contributed equally to TβD between forest patches and campinarana. Functional turnover and functional richness difference contributed equally to FβD between continuous forest and forest patches, whereas functional richness difference was more important than functional turnover in driving FβD between continuous forest and both campinarana, and savannah (Fig. 4; Supplementary Table 3). Functional turnover was the main factor responsible for FβD between forest patches and savannah, and campinarana and savannah, whereas functional richness difference and functional turnover made a similar contribution to FβD between forest patches and campinarana (Fig. 4; Supplementary Table 3). Lineage richness difference was more important than lineage turnover in driving PβD between continuous forest and forest patches, continuous forest and savannah, and continuous forest and campinarana, where lineage turnover made no contributions. Lineage turnover was more important than lineage richness difference in driving PβD between forest patches and savannah, between forest patches and campinarana, and between campinarana and savannah (Fig. 4; Supplementary Table 3).
Taxonomic, functional and phylogenetic nestedness
The site ordering hypothesis using presence-absence indicated that bat assemblages of the forest patches (NODF = 62.67, p = 0.04), campinarana (NODF = 63.52, p < 0.01) and savannah (NODF = 58.74, p < 0.01) are taxonomically nested within continuous forest (Fig. 5A–C). However, the analysis showed no evidence that the bat assemblages of the campinarana (NODF = 52.4, p = 0.54) and savannah (NODF = 47.8, p = 0.12) are taxonomically nested within forest patches (Fig. 5D and E), nor that the assemblage of the savannah (NODF = 48.8, p = 0.96) is taxonomically nested within that of the campinarana (Fig. 5F).
Taxonomic nestedness plot from six matrices of the four Amazonian habitats in the northern Brazil. A continuous forest versus forest patches; B continuous forest versus campinarana; C continuous forest versus savannah; D forest patches versus campinarana; E forest patches versus savannah; and F campinarana versus savannah. Habitats: CF continuous forest (dark green), FP forest patches (light green), C campinarana (yellow), and S savannah (brown). In all figures, the columns represent the species, and the lines represent the sampled sites. In each figure, the habitats (rows) would be perfectly nested if all interactions were above the “fill line” (black curved line)
Bat assemblages of all other habitats were found to be functionally and phylogenetically nested within the assemblage of the continuous forest (p < 0.05; Table 2). The bat assemblages of the campinarana and savannah were also found to be functionally and phylogenetically nested within the assemblage of the forest patches (p < 0.05; Table 2). Both functional and phylogenetic nesting partitions contributed to the nestedness in all comparisons, however, the observed traitNODF/phyloNODF value was mostly represented by the S. Fraction (species composition component) rather than by the topoNODF (dendrogram topology component—Table 2).
In summary, in relation to species composition (taxonomic nestedness), forest patches, campinarana and savannah are nested in the continuous forest. For functional traits (functional nestedness) and lineages (phylogenetic nestedness), less complex habitats (e.g., savannah) are subgroups of more complex habitats (e.g. forest patches), as we expected, and this nestedness is more driven by change in species composition (S.fraction) than functional or phylogenetic tree topology (topoNODF) between the habitats.
Discussion
Here we show that taxonomic, functional and phylogenetic diversity of bats is higher in continuous forest than in any of the other habitat types. The continuous forest also harbours more unique species, and indeed, the bat community assemblages in the less-complex habitats, including forest patches, campinarana and savannahs, are taxonomic, functional and phylogenetic sub-sets of the assemblage found in the continuous forest. The continuous forest is the most structurally complex habitat with more diurnal roosts and food availability (Meyer et al. 2016), which may help to explain these patterns. This result fits the pattern shown for other vertebrate and invertebrate groups across Amazonian habitats, including bats (Ramos Pereira et al. 2009; Bobrowiec et al. 2014; Graça et al. 2017; Carvalho et al. 2018, 2020; Fraga et al. 2018; Naka et al. 2020), and suggests that forest habitats maintain bat species diversity in the Amazon. Given the rapid expansion of crop and tree plantations, as well as deforestation linked to cattle production in the Amazon (Carvalho et al. 2019a), our results also have important implications for the conservation of bats and their habitats, beyond what they tell us about the ecological processes that structure bat assemblages.
The differences found between habitats in terms of taxonomic, functional and phylogenetic composition reflect a process of environmental sorting (Özkan et al. 2013), in accordance with species life history strategies, along a habitat gradient going from a more forested (i.e., continuous forest) to a non-forested habitat (i.e., savannah). Here we show that the continuous forest has more unique species, but also tends to harbour smaller and more specialised (nectarivorous and carnivorous) species than the other habitat types. The forest patch assemblage is however taxonomically, functionally and phylogenetically nested within the continuous forest assemblage and the two habitats tend to share more species which are dependent on forests (Klingbeil and Willig 2009; Meyer and Kalko 2008; Martins et al. 2017), being similar in terms of functional uniqueness (FU) and showing low taxonomic, functional and phylogenetic beta-diversity values. In both continuous forest and forest patches, there were relatively more animalivorous species, and particularly insectivorous species, than were found in the savannah, and also relatively more animalivorous species, including both insectivores and carnivores, than were found in the campinarana. This pattern is driven by the dependence of animalivorous species on forests with a more complex structure (Meyer and Kalko 2008; Klingbeil and Willig 2009; Martins et al. 2017; Farneda et al. 2018), and the fact that these species do not breed in open habitats or environments that have been deforested, and in general tend to avoid open habitats, as well as forest edges (Martins et al. 2017; Rocha et al. 2017). Indeed, although structurally different from one another, both savannahs and campinarana may have similarly low levels of diurnal shelter and food availability, particularly for animalivourous and nectarivorous bats, such that differences in floristic composition and vegetation structure compared to lowland forests in the Amazon (Veloso and Góes-Filho 1982) effectively act as environmental filters. Overall, the patterns described here likely reflect the higher number of micro-habitats in the more structurally complex continuous forest and forest patches, providing more resources for prey species of animalivorous species, and higher overall plant diversity providing resources for nectarivores.
Another important factor that helps to explain the gradients of diversity found here is the effect of the matrix in which different habitat patches are inserted. Our results support the idea that the diversity of bats in the campinarana and savannahs are determined by their insertion in a “matrix” of continuous forest, in the case of the former, and their adjacency to forest patches, in the case of the latter. The forest habitat around the campinarana is less hostile to bat species than the campinarana itself (as happens around planted forests—Pina et al. 2013) such that bats tend to use campinarana areas less than the continuous forest. On the other hand, the savannah is a matrix in which the forest patches are immersed (Mustin et al. 2017), and as such bats tend to cross it even though it is more hostile than the campinarana (e.g., open habitat with higher probability of predation—Lima and O'Keefe 2013), as there is seemingly little connectivity between the forest patches in this landscape of the Amazonian savannahs (Calle-Rendón et al. 2020). However, savannahs and campinaranas, as components of the overall landscape, may also play a role in food availability for bats, forming edges with otherwise continuous forest and forest patches, and promoting edge effects, thereby maintaining the populations of pioneer species (e.g. Cecropia spp., Vismia spp. and Piper spp.) which are important food resources for generalist frugivorous bats, such as Carollia perspicillata and Rhinophyla pumilio (Muylaert et al. 2016; Rocha et al. 2017). In addition, campinaranas are distinct in structure and species composition compared to other forest types in Amazonia, with almost a quarter of tree species found there being specialists (García-Villacorta et al. 2016), and tree species found uniquely in this habitat (e.g., Aldina heterophylla and several species of Protium, Licania, Clusia and Piper spp.) may be a complementary source of food and shelter for some species of bats. Taken together, these results suggest that the pattern of α- and β-diversity between the less to more complex habitats can be explained at least in part by the way in which each of them is represented in the landscape, and with a different landscape composition these patterns could be different. Thus, there is a need for further studies focusing on the transition environment between these Amazonian natural habitats, which should specifically seek to determine how landscape configuration and edge effects interact to contribute to the maintenance of bat diversity.
Exploring in more detail the patterns of diversity along the habitat gradient, we found that taxonomic and functional β-diversity (TβD and FβD) between paired habitat types were mostly driven by the replacement of some species/functional traits by others, with no change in the total number of species or functions. In contrast, phylogenetic β-diversity was mostly driven by the loss and gain of lineages rather than by the replacement between paired habitat types. These results are in line with previous bat studies in the Amazon (Bobrowiec et al. 2014; Farneda et al. 2018; Aninta et al. 2019) and the Atlantic Forest (Varzinczak et al. 2018). These β-diversity patterns are in part explained by the presence of generalist species that are more tolerant to environmental filters, such as Carollia perspicillata and Glossophaga soricina, in the less-complex habitats (Farneda et al. 2015; Rocha et al. 2017; Carvalho et al. 2019b). These species are opportunistic and tolerant, having the ability to adapt and take advantage of habitats that are less complex, due to human interventions (clearing Bobrowiec and Gribel 2010; Farneda et al. 2018), and, as our results have shown, naturally less-complex habitats. The key exception to the pattern is the difference in taxonomic and functional diversity between continuous forest and campinarana, which is largely driven by lower species and functional richness in the campinarana, suggesting that it is the least hospitable of the four habitat types for bats, with fewer species using the habitat, and consequent loss of functions. These results have important implications for conservation, as they suggest that forest habitats are crucial for the maintenance of more specialist species, and for lineage diversity, across the mosaic of Amazonian habitats.
Some of the other patterns found here, however, likely reflect an important methodological constraint that should be considered in future studies, rather than an ecological process. We found that, compared with the campinarana and the savannahs, the understorey of the continuous forest, and of forest patches, tends to harbour relatively fewer phytophagous species (particularly frugivores). This pattern is in part explained by behaviour of the frugivorous bats within the different habitat types, which use the canopy of the continuous forest and forest patches, and thus are not captured by the sampling method used here, but which fly lower in the savannah and campinarana and are thus more frequently captured. Indeed, this factor also helps to explain the observed difference in wing morphology between habitat types, with larger winged, heavier, phytophagous bats (Ramos Pereira et al. 2010; Marinello and Bernard 2014) being more commonly caught in the savannah. As such, although we found in our data that campinarana and savannah tend to have a predominance of frugivorous species, this result should be interpreted with caution, as this pattern likely does not reflect the availability of fruits and nectar as food in campinaranas and savannahs, but rather vertical stratification of habitat use in the continuous forest and forest patches. Indeed, these larger phytophagous bats use the canopy of the continuous forest and forest patches to reach and feed on canopy plant species. These plant species are rarely found in the savannah, and as such the frequent capture of the phytophagous bats in campinarana and savannah suggests that these species tend to use these habitats to cross between forest areas, rather than as habitat per se, as savannahs are very permeable to the movement of species of bats that can use open areas (Bernard and Fenton 2003; Loayza and Loiselle 2009). This movement pattern of bats between forest areas using an open matrix could also be considered in studies comparing the behaviour of bat species (e.g., habitat use or temporal activity) between forested and clearing areas or early growth vegetation. Thus, future studies should take into account sampling bats in the sub-canopy and canopy of forest habitats (e.g., continuous forest, forest patches and campinarana) to compare the bat communities with non-forest habitats (e.g., savannah), because species associated with the canopy in forest habitats, may fly closer to the ground in natural non-forest habitats (savannah). We also emphasize that here we are only discussing Phyllostomidae and that future studies with strictly insectivorous bats (e.g., Emballonuridae, Molossidae, and Vespertilionidae) may show other patterns.
The naturally eroded levels of functional and phylogenetic diversity in bat assemblages in campinaranas and savannahs, relative to forest and forest patches, make them more susceptible to environmental change. This is particularly troubling from a conservation perspective as campinaranas and Amazonian savannahs are suffering increasing anthropogenic pressures, such as clearing, replacement of native vegetation by commercial plantations and wildfire (Adeney et al. 2016; Carvalho and Mustin 2017; Mustin et al. 2017; Carvalho et al. 2019a), and this increases the likelihood of further functional and phylogenetic erosion of the bat assemblages, and consequent loss of ecosystems services such as seed dispersal and pollination. In addition, as this permeable matrix of campinarana and savannah has been modified in recent years (Adeney et al. 2016; Carvalho and Mustin 2017), studies should be carried out to assess the consequences of these modifications on bat use and movement. In the case of Amazonian savannahs, these issues are of greater concern, as the replacement or clearing of the savannah matrix may bring more serious consequences for the maintenance of bat populations in the forest patches, because the permeability of the matrix will change. Thus, bats will tend to no longer use the matrix, as other studies suggest for terrestrial mammals that occur in the Amazonian savannahs (Coelho et al. 2014), and there is already consensus for bats in sites with human-modified matrix in other South American biomes (Meyer et al. 2016; Farneda et al. 2020; Mendes and Srbek-Araújo 2020). Therefore, the conservation and maintenance of the environmental integrity of non-forest habitats throughout the Amazon is of fundamental importance for biodiversity, and for the maintenance of ecosystem services for the communities that live along this biome.
Our results show that the bat assemblages in the continuous forest are more taxonomically, functionally and phylogenetically diverse than those in natural forest patches, campinarana and savannahs in the Amazon. We conclude that this pattern is in part driven by a process of environmental sorting, reflecting the higher number of micro-habitats in the more structurally complex continuous forest and forest patches, and in part by the configuration of the landscape composition and the different roles different habitats play. However, we caution that further studies are required, focussing on landscape configuration and the edges between habitats, and how these factors interact to contribute to the maintenance of bat diversity. We also highlight an important methodological consideration common to ours and similar studies, whereby the use of mist-nets at ground level tends to suggest that frugivorous species are more common in the more open habitats than in the forest, where in reality they are using the sub-canopy and canopy of forest habitats. Finally, from a conservation perspective, we highlight two important key findings. Firstly, forest habitats are crucial for the maintenance of more specialist species, and for lineage diversity, across the mosaic of Amazonian habitats. Secondly, non-forest habitats make their own important contributions to taxonomic and functional diversity of bats in the Amazon, and these habitats are both vulnerable to environmental change, owing to naturally eroded levels of functional and phylogenetic diversity, and currently highly threatened by increasing anthropogenic pressures, such as clearing, replacement of native vegetation by commercial plantations and wildfire. Biodiversity conservation and the maintenance of ecosystem services in the Amazon therefore depends on both conservation of forest and non-forest habitats.
Change history
27 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00442-021-05018-2
References
Adeney JM, Christensen NL, Vicentini A, Cohn-Haft M (2016) White-sand ecosystems in Amazonia. Biotropica 48:7–23. https://doi.org/10.1111/btp.12293
Almeida-Gomes M, Vieira MV, Rocha CF, Melo AS (2019) Habitat amount drives the functional diversity and nestedness of anuran communities in an Atlantic Forest fragmented landscape. Biotropica 51:874–884. https://doi.org/10.1111/btp.12687
Almeida-Neto M, Guimaraes P, Guimaraes PR Jr, Loyola RD, Ulrich W (2008) A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117:1227–1239. https://doi.org/10.1111/j.0030-1299.2008.16644.x
Alroy J (2017) Effects of habitat disturbance on tropical forest biodiversity. Proc Natl Acad Sci 114:6056–6061. https://doi.org/10.1073/pnas.1611855114
Aninta SG, Rocha R, López-Baucells A, Meyer CF (2019) Erosion of phylogenetic diversity in Neotropical bat assemblages: findings from a whole-ecosystem fragmentation experiment. Biodivers Conserv 28:4047–4063. https://doi.org/10.1007/s10531-019-01864-y
Antonelli A, Sanmartín I (2011) Why are there so many plant species in the Neotropics? Taxon 60:403–414. https://doi.org/10.1002/tax.602010
Arita HT, Fenton MB (1997) Flight and echlocation in the ecology and evolution of bats. Trends Ecol Evol 12:53–58. https://doi.org/10.1016/S0169-5347(96)10058-6
Bernard E (2001) Vertical stratification of bat communities in primary forests of Central Amazon, Brazil. J Trop Ecol 17:115–126. https://doi.org/10.1017/S0266467401001079
Bernard E, Fenton MB (2003) Bat mobility and roosts in a fragmented landscape in central Amazonia, Brazil. Biotropica 35:262–277. https://doi.org/10.1111/j.1744-7429.2003.tb00285.x
Bernard E, Fenton M (2007) Bats in a fragmented landscape: Species composition, diversity and habitat interactions in savannas of Santarém, Central Amazonia, Brazil. Biol Conserv 134:332–343. https://doi.org/10.1016/j.biocon.2006.07.021
Bobrowiec PED, Gribel R (2010) Effects of different secondary vegetation types on bat community composition in Central Amazonia, Brazil. Anim Conserv 13:204–216. https://doi.org/10.1111/j.1469-1795.2009.00322.x
Bobrowiec PED, Rosa LS, Gazarini J, Haugaasen T (2014) Phyllostomid bat assemblage structure in Amazonian flooded and unflooded forests. Biotropica 46:312–321. https://doi.org/10.1111/btp.12102
Botta-Dukát Z (2005) Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J Veg Sci 16:533–540. https://doi.org/10.1111/j.1654-1103.2005.tb02393.x
Calle-Rendón BR, Toledo JJ, Mustin K, Hilário RR (2020) Drivers of primate richness and occurrence in a naturally patchy landscape in the Brazilian Amazon. Biodivers Conserv 29:3369–3391. https://doi.org/10.1007/s10531-020-02028-z
Cardoso P, Rigal F, Carvalho JC (2015) BAT–biodiversity assessment tools, an R package for the measurement and estimation of alpha and beta taxon, phylogenetic and functional diversity. Methods Ecol Evol 6:232–236. https://doi.org/10.1111/2041-210X.12310
Carvalho WD, Mustin K (2017) The little known, highly threatened and under-protected Amazonian Savannas. Nat Ecol Evol 1:0100. https://doi.org/10.1038/s41559-017-0100
Carvalho WD, Gomes LAC, Castro IJ, Martins AC, Esbérard CEL, Mustin K (2018) Beyond the Amazon forest: richness, abundance and flight height of bats in the understory of savannahs, campinaranas and terra firme forest. Acta Chiropt 20:407–419. https://doi.org/10.3161/15081109ACC2018.20.2.011
Carvalho WD, Mustin K, Hilário RR, Vasconcelos IM, Eilers V, Fearnside PM (2019a) Deforestation control in the Brazilian Amazon: a conservation struggle being lost as agreements and regulations are subverted and bypassed. Perspect Ecol Conser 17:122–130. https://doi.org/10.1016/j.pecon.2019.06.002
Carvalho WD, Martins MA, Esbérard CEL, Palmeirim JM (2019b) Traits that allow bats of tropical lowland origin to conquer mountains: Bat assemblages along elevational gradients in South American Atlantic Forests. J Biogeogr 46:316–331. https://doi.org/10.1111/jbi.13506
Carvalho WD, Meyer CFJ, Xavier BS, Mustin K, Castro IJ, Silvestre SM, Pathek DB, Capaverde UD Jr, Hilário R, Toledo JJ (2020) Consequences of replacing native savannahs with acacia plantations for the taxonomic, functional, and phylogenetic alfa- and beta-diversity of bats in the northern Brazilian Amazon. Front Ecol Evol 8:609214. https://doi.org/10.3389/fevo.2020.609214
Castillo-Figueroa D, Pérez-Torres J (2021) On the development of a trait-based approach for studying Neotropical bats. Pap Avulsos De Zool 61:20216124. https://doi.org/10.11606/1807-0205/2021.61.24
Cisneros LM, Fagan ME, Willig MR (2015) Effects of human-modified landscapes on taxonomic, functional and phylogenetic dimensions of bat biodiversity. Divers Distrib 21:523–533. https://doi.org/10.1111/ddi.12277
Coelho M, Juen L, Mendes-Oliveira AC (2014) The role of remnants of Amazon savanna for the conservation of Neotropical mammal communities in eucalyptus plantations. Biodivers Conserv 23:3171–3184. https://doi.org/10.1007/s10531-014-0772-9
Costa-Neto SV (2014) Fitofisionomia e florística de savanas do Amapá. PhD thesis, Universidade Federal Rural da Amazônia, Belém, Brazil
Faith DP (1992) Conservation evaluation and phylogenetic diversity. Biol Cons 61:1–10. https://doi.org/10.1016/0006-3207(92)91201-3
Farneda FZ, Rocha R, López-Baucells A, Groenenberg M, Silva I, Palmeirim JM, Bobrowiec PED, Meyer CFJ (2015) Trait-related responses to habitat fragmentation in Amazonian bats. J Appl Ecol 52:1381–1391. https://doi.org/10.1111/1365-2664.12490
Farneda FZ, Rocha R, López-Baucells A, Sampaio EM, Palmeirim JM, Bobrowiec PED, Grelle CEV, Meyer CFJ (2018) Functional recovery of Amazonian bat assemblages following secondary forest succession. Biol Conserv 218:192–199. https://doi.org/10.1016/j.biocon.2017.12.036
Farneda FZ, Meyer CFJ, Grelle CEV (2020) Effects of land-use change on functional and taxonomic diversity of Neotropical bats. Biotropica 52:120–128. https://doi.org/10.1111/btp.12736
Fraga R, Ferrão M, Stow AJ, Magnusson WE, Lima AP (2018) Different environmental gradients affect different measures of snake β-diversity in the Amazon rainforests. PeerJ 6:e5628. https://doi.org/10.7287/peerj.preprints.27146v1
Garbino GST, Gregorin R, Lima IP, Loureiro L, Moras LM, Moratelli R, Nogueira MR, Pavan AC, Tavares VC, Peracchi AL (2020) Updated checklist of Brazilian bats: versão 2020. Comitê da Lista de Morcegos do Brasil—CLMB. Sociedade Brasileira para o Estudo de Quirópteros (Sbeq). https://www.sbeq.net/lista-de-especies
García-Morales R, Moreno CE, Badano EI, Zuria I, Galindo-González J, Rojas-Martínez AE, Ávila-Gómez ES (2016) Deforestation impacts on bat functional diversity in Tropical landscapes. PLoS ONE 11:e0166765. https://doi.org/10.1371/journal.pone.0166765
García-Villacorta R, Dexter KG, Pennington T (2016) Amazonian white-sand forests show strong floristic links with surrounding oligotrophic habitats and the Guiana Shield. Biotropica 48:47–57. https://doi.org/10.1111/btp.12302
Gardner AL (2008) Mammals of South America, Volume 1, marsupials, xenarthrans, shrews, and bats. University of Chicago Press, Chicago
Giannini NP, Kalko EK (2004) Trophic structure in a large assemblage of phyllostomid bats in Panama. Oikos 105:209–220. https://doi.org/10.1111/j.0030-1299.2004.12690.x
Graça MB, Pequeno PA, Franklin E, Souza JL, Morais JW (2017) Taxonomic, functional, and phylogenetic perspectives on butterfly spatial assembly in northern Amazonia. Ecol Entomol 42:816–826. https://doi.org/10.1111/een.12454
Guevara MR, Hartmann D, Mendoza M (2017) diverse: an R Package to analyze diversity in complex systems. R J 8:60–78
Guilherme DR, Souza JLP, Franklin E, Pequeno PACL, Chagas AC, Baccaro FB (2019) Can environmental complexity predict functional trait composition of ground-dwelling ant assemblages? A test across the Amazon Basin. Acta Oecol 99:103434. https://doi.org/10.1016/j.actao.2019.05.004
Jones KE, Purvis A, Maclarnon ANN, Bininda-Emonds OR, Simmons NB (2002) A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biol Rev 77:223–259. https://doi.org/10.1017/s1464793101005899
Jovanovic BD, Levy PS (1997) A look at the rule of three. Am Stat 51:137–139. https://doi.org/10.2307/2685405
Kalko EK, Handley CO (2001) Neotropical bats in the canopy: diversity, community structure, and implications for conservation. Plant Ecol 153:319–333. https://doi.org/10.1023/A:1017590007861
Kalko EK, Handley CO, Handley D (1996) Organization, diversity, and long-term dynamics of a neotropical bat community-Chapter 16. In: Cody M, Smallwood J (eds) Long-term studies in vertebrate communities. Academic, Los angeles, pp 503–553
Kembel SW, Ackerly DD, Blomberg SP, Cornwell WK, Cowan PD, Helmus MR, Morlon H, Webb CO (2020) Package ‘picante’. URL https://cran.r-project.org/web/packages/picante/picante.pdf
Klingbeil BT, Willig MR (2009) Guild-specific responses of bats to landscape composition and configuration in fragmented Amazonian rainforest. J Appl Ecol 46:203–213. https://doi.org/10.1111/j.1365-2664.2008.01594.x
Laliberté E, Legendre P, Shipley B, Laliberté ME (2015) Package ‘FD’ – Measuring functional diversity from multiple traits, and other tools for functional ecology. URL https://cran.r-project.org/web/packages/FD/FD.pdf
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305. https://doi.org/10.1890/08-2244.1
Lavorel S, Grigulis K, McIntyre S, Williams NS, Garden D, Dorrough J, Berman S, Quétier F, Thébault A, Bonis A (2008) Assessing functional diversity in the field–methodology matters! Funct Ecol 22:134–147. https://doi.org/10.1111/j.1365-2435.2007.01339.x
Lim BK, Engstrom MD (2001) Species diversity of bats (Mammalia: Chiroptera) in Iwokrama Forest, Guyana, and the Guianan subregion: implications for conservation. Biodivers Conserv 10:613–657. https://doi.org/10.1023/A:1016660123189
Lima SL, O’Keefe M (2013) Do predators influence the behaviour of bats? Biol Rev 88:626–644. https://doi.org/10.1111/brv.12021
Loayza AP, Loiselle BA (2009) Composition and distribution of a bat assemblage during the dry season in a naturally fragmented landscape in Bolivia. J Mammal 90:732–742. https://doi.org/10.1644/08-MAMM-A-213R.1
López-Baucells A, Rocha R, Bobrowiec P, Bernard E, Palmeirim J, Meyer CFJ (2018) Field guide to the Bats of the Amazon. Pelagic Publishing, Exeter
Malhado A, Ladle R, Whittaker R, Neto A, Malhi Y, Ter Steege H (2013) The ecological biogeography of Amazonia. Front Biogeogr. https://doi.org/10.21425/F5FBG13314
Marinello M, Bernard E (2014) Wing morphology of Neotropical bats: a quantitative and qualitative analysis with implications for habitat use. Can J Zool 92:141–147. https://doi.org/10.1139/cjz-2013-0127
Marques JT, Ramos Pereira MJ, Marques TA, Santos CD, Santana J, Beja P, Palmeirim JM (2013) Optimizing sampling design to deal with mist-net avoidance in Amazonian birds and bats. PLoS ONE 8:e74505. https://doi.org/10.1371/journal.pone.0074505
Marques JT, Ramos Pereira MJ, Palmeirim JM (2016) Patterns in the use of rainforest vertical space by Neotropical aerial insectivorous bats: all the action is up in the canopy. Ecography 39:476–486. https://doi.org/10.1111/ecog.01453
Martins AC, Willig MR, Presley SJ, Marinho-Filho J (2017) Effects of forest height and vertical complexity on abundance and biodiversity of bats in Amazonia. For Ecol Manage 391:427–435. https://doi.org/10.1016/j.foreco.2017.02.039
Matricardi EA, Skole DL, Costa OB, Pedlowski MA, Samek JH, Miguel EP (2020) Long-term forest degradation surpasses deforestation in the Brazilian Amazon. Science 369:1378–1382. https://doi.org/10.1126/science.abb3021
Melo AS, Cianciaruso MV, Almeida-Neto M (2014) treeNODF: nestedness to phylogenetic, functional and other tree-based diversity metrics. Methods Ecol Evol 5:563–572. https://doi.org/10.1111/2041-210X.12185
Melo AS (2019) Package ‘CommEcol’. URL https://cran.r-project.org/web/packages/CommEcol/CommEcol.pdf
Mendes P, Srbek-Araújo AC (2020) Effects of land-use changes on Brazilian bats: a review of current knowledge. Mammal Rev (early View). https://doi.org/10.1111/mam.12227
Meyer CF, Kalko EK (2008) Assemblage-level responses of phyllostomid bats to tropical forest fragmentation: land-bridge islands as a model system. J Biogeogr 35:1711–1726. https://doi.org/10.1111/j.1365-2699.2008.01916.x
Meyer CF, Struebig MJ, Willig MR (2016) Responses of tropical bats to habitat fragmentation, logging, and deforestation. In: Voigt C, Kingston CC (eds) Bats in the anthropocene: conservation of bats in a changing world. Springer, Berlin, pp 63–103
Miklós I, Podani J (2004) Randomization of presence–absence matrices: comments and new algorithms. Ecology 85:86–92. https://doi.org/10.1890/03-0101
Moreno CE, Calderón-Patrón JM, Martín-Regalado N, Martínez-Falcón AP, Ortega-Martínez IJ, Rios-Díaz CL, Rosas F (2018) Measuring species diversity in the tropics: a review of methodological approaches and framework for future studies. Biotropica 50:929–941. https://doi.org/10.1111/btp.12607
Mustin K, Carvalho WD, Hilário RR, Costa-Neto SV, Silva CR, Vasconcelos IM, Castro IJ, Eilers V, Kauano EE, Mendes-Junior RNG, Funi C, Fearnside PM, Silva JMC, Euler A, Toledo JJ (2017) Biodiversity, threats and conservation challenges in the “Cerrado of Amapá”, an Amazonian savanna. Nat Conserv 22(107):127. https://doi.org/10.3897/natureconservation.22.13823
Muylaert RL, Stevens RD, Ribeiro MC (2016) Threshold effect of habitat loss on bat richness in cerrado-forest landscapes. Ecol Appl 26:1854–1867. https://doi.org/10.1890/15-1757.1
Naka LN, Laranjeiras TO, Lima GR, Plaskievicz A, Pinto F, Gonçalves-Souza T (2020) Climate as a major driver of avian diversity in riparian Amazon habitats along an environmental gradient. J Biogeogr 47:2328–2340. https://doi.org/10.1111/jbi.13957
Norberg UM, Rayner JM (1987) Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Phil Trans R Soc Lond B 316:335–427. https://doi.org/10.1098/rstb.1987.0030
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests M (2007) The vegan package. Commun Ecol Packag 10:631–637
Özkan K, Svenning JC, Jeppesen E (2013) Environmental species sorting dominates forest-bird community assembly across scales. J Anim Ecol 82:266–274. https://doi.org/10.1111/j.1365-2656.2012.02019.x
Pavan AC, Bobrowiec PED, Percequillo AR (2018) Geographic variation in a South American clade of mormoopid bats, Pteronotus (Phyllodia), with description of a new species. J Mammal 99:624–645. https://doi.org/10.1093/jmammal/gyy048
Pina S, Meyer C, Zortéa M (2013) A comparison of habitat use by bats in natural forest fragments and Eucalyptus plantations in Brazilian Savanna. Chirop Neotrop 19:14–30
Pires JM, Prance GT (1985) The vegetation types of the Brazilian Amazon. In: Prance GT, Lovejoy TE (eds) Key environments: Amazonia. Pergamon Press, Oxford, pp 109–145
Prance GT (1996) Islands in Amazonia. Philos Trans R Soc Lond B Biol Sci 351:823–833. https://doi.org/10.1098/rstb.1996.0077
Purvis A, Gittleman JL, Cowlishaw G, Mace GM (2000) Predicting extinction risk in declining species. Proc R Soc Lond B Biol Sci 267:1947–1952. https://doi.org/10.1098/rspb.2000.1234
R Core Team (2020) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria, R version 4.0.3, 2020. URL https://www.R-project.org
Ramos Pereira MJ, Marques JT, Santana J, Santos CD, Valsecchi J, De Queiroz HL, Beja P, Palmeirim JM (2009) Structuring of Amazonian bat assemblages: the roles of flooding patterns and floodwater nutrient load. J Anim Ecol 78:1163–1171. https://doi.org/10.1111/j.1365-2656.2009.01591.x
Ramos Pereira MJ, Marques JT, Palmeirim JM (2010) Vertical stratification of bat assemblages in flooded and unflooded Amazonian forests. Curr Zool 56:469–478. https://doi.org/10.1093/czoolo/56.4.469
Ramos Pereira MJ, Fonseca C, Aguiar LM (2018) Loss of multiple dimensions of bat diversity under land-use intensification in the Brazilian Cerrado. Hystrix 29:25–32. https://doi.org/10.4404/hystrix-00020-2017
Reis NR, Peracchi AL, Batista CB, Lima IP (2017) História Natural dos Morcegos Brasileiros: chave de identificação de espécies. Technical Books Livraria LTDA, Rio de Janeiro
Ricotta C, Bello F, Moretti M, Caccianiga M, Cerabolini BE, Pavoine S (2016) Measuring the functional redundancy of biological communities: a quantitative guide. Methods Ecol Evol 7:1386–1395. https://doi.org/10.1111/2041-210X.12604
Rocha R, López-Baucells A, Farneda FZ, Groenenberg M, Bobrowiec PE, Cabeza M, Palmeirim JM, Meyer CF (2017) Consequences of a large-scale fragmentation experiment for Neotropical bats: disentangling the relative importance of local and landscape-scale effects. Landsc Ecol 32:31–45. https://doi.org/10.1007/s10980-016-0425-3
Sikes RS, Gannon WL, Care A, Mammalogists UCotASo (2011) Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 92:235–253. https://doi.org/10.1644/10-MAMM-F-355.1
Silva JMC, Rylands AB, Fonseca GAB (2005) The fate of the Amazonian areas of endemism. Conserv Biol 19:689–694. https://doi.org/10.1111/j.1523-1739.2005.00705.x
Straube FC, Bianconi GV (2002) Sobre a grandeza e a unidade utilizada para estimar esforço de captura com utilização de redes-de-neblina. Chirop Neotrop 8:150–152
Tavares VC (2013) Phyllostomid bat wings from Atlantic Forest bat ensembles: an ecomorphological study. Chirop Neotrop 19:57–70
Thoisy BD, Pavan AC, Delaval M, Lavergne A, Luglia T, Pineau K, Ruedi M, Rufray V, Catzeflis F (2014) Cryptic diversity in common mustached bats Pteronotus cf. parnellii (Mormoopidae) in French Guiana and Brazilian Amapá. Acta Chiropt 16:1–13. https://doi.org/10.3161/150811014X683228
Varzinczak LH, Moura MO, Lima CS, Passos FC (2018) How do bat, rodent, and marsupial communities respond to spatial and environmental gradients? Insights from a deconstruction of mammal beta diversity from the Atlantic Forest of South America. Oecologia 189:851–861. https://doi.org/10.1007/s00442-018-4288-y
Velazco PM, Gardner AL, Patterson BD (2010) Systematics of the Platyrrhinus helleri species complex (Chiroptera: Phyllostomidae), with descriptions of two new species. Zool J Linn Soc 159:785–812. https://doi.org/10.1111/j.1096-3642.2009.00610.x
Veloso HP, Góes-Filho L (1982) Fitogeografia brasileira: classificação fisionômico-ecológica da vegetação neotropical. Ministério de Minas e Energia/Boletim Técnico Projeto RADAMBRASIL, Salvador, Bahia, Brazil
Acknowledgements
William Douglas Carvalho and Fábio Zanella Farneda are supported by a post-doctoral scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES PNPD and PrInt—Finance Code 001, respectively). Two companies, Ecology and Environment do Brasil and the Group Isolux, funded our study. We thank our field assistants Domingos Ramos, Valdomiro, Tonivelton and Edilson Luis (Bocão), Eddeivid Reis and Mariana Chandaliê for help in the collection of field data. The fieldwork was authorized by the appropriate Brazilian authority, namely the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA; Collection licenses n° 144/2008 and nº 256/2009).
Author information
Authors and Affiliations
Contributions
WDC originally formulated the idea; WDC, KM, IJC, ACM, BSX and JDM conducted fieldwork; WDC and FZF performed statistical analyses and WDC and KM wrote the drafts of the main manuscript and the online resources. All authors contributed critically to the manuscript and gave final approval for publication.
Corresponding author
Additional information
Communicated by Thomas Lilley.
The original online version of this article was revised: The co-author name Renato R. Hiláro is corrected as Renato R. Hilário.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carvalho, W.D., Mustin, K., Farneda, F.Z. et al. Taxonomic, functional and phylogenetic bat diversity decrease from more to less complex natural habitats in the Amazon. Oecologia 197, 223–239 (2021). https://doi.org/10.1007/s00442-021-05009-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-021-05009-3