Abstract
The resilience of an ecological unit encompasses resistance during adverse conditions and the capacity to recover. We adopted a ‘resistance-recovery’ framework to experimentally partition the resilience of a foundation species (the seagrass Cymodocea nodosa). The shoot abundances of nine seagrass meadows were followed before, during and after simulated light reduction conditions. We determined the significance of ecological, environmental and genetic drivers on seagrass resistance (% of shoots retained during the light deprivation treatments) and recovery (duration from the end of the perturbed state back to initial conditions). To identify whether seagrass recovery was linearly related to prior resistance, we then established the connection between trajectories of resistance and recovery. Finally, we assessed whether recovery patterns were affected by biological drivers (production of sexual products—seeds—and asexual propagation) at the meadow-scale. Resistance to shading significantly increased with the genetic diversity of the meadow and seagrass recovery was conditioned by initial resistance during shading. A threshold in resistance (here, at a ca. 70% of shoot abundances retained during the light deprivation treatments) denoted a critical point that considerably delays seagrass recovery if overpassed. Seed densities, but not rhizome elongation rates, were higher in meadows that exhibited large resistance and quick recovery, which correlated positively with meadow genetic diversity. Our results highlight the critical role of resistance to a disturbance for persistence of a marine foundation species. Estimation of critical trade-offs between seagrass resistance and recovery is a promising field of research to better manage impacts on seagrass meadows.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resilience is the self-organization capacity of a system to maintain its identity and function after a perturbation occurs (Holling 1973; Gunderson et al. 2010). Resilience encompasses both resistance, as the initial ability to persist during adverse conditions, and subsequent recovery, once these unfavourable conditions cease (Hodgson et al. 2015; Nimmo et al. 2015; Ingrisch and Bahn 2018; Falk et al. 2019). This ‘resistance–recovery’ (also known as the ‘resistance–resilience’) framework is an idea that is not linked to a particular biological level; ecosystems, communities, populations, or even individuals can be measured in terms of their resistance and recovery to perturbations (Nimmo et al. 2015; Falk et al. 2019). The rate at which the perturbed biological/ecological unit recovers is known as the ‘elasticity’, while the duration back to the (initial) state is the ‘return time’ (Hodgson et al. 2015). For a particular perturbation, one system/ecological unit is more resilient because it recovers with high ‘elasticity’, i.e., a low ‘return time’, while another is more resilient because it is more ‘resistant’, i.e., the system does not get severely ‘displaced’ from the initial state. This methodological approach, therefore, assesses whether resilience is majorly achieved via resistance or recovery (Falk et al. 2019). The ‘resistance–recovery’ framework proposes direct measures of resistance and recovery that are simple and interpretable measures of change to assess ecological resilience (Hodgson et al. 2015; Nimmo et al. 2015; Ingrisch and Bahn 2018).
Identifying ecological and environmental drivers of both resistance and recovery offers relevant insights to guide conservation and management policies (Holling 1973; Hoover et al. 2014; Connell and Ghedini 2015). Understanding resilience of ‘foundation species’ is particularly relevant, because of the global environmental crisis that affects the health of these key species, including kelps, seagrasses, corals and mangroves, in the marine realm (Orth et al. 2006; Waycott et al. 2009; Nyström et al. 2012; Bulleri et al. 2018). Marine ‘foundation species’ can influence the ability of stress-sensitive species to exhibit plastic responses (Bulleri et al. 2018) and their loss or degradation has cascading effects on species that depend on them (Orth et al. 2006; Connell and Ghedini 2015). In some cases, drivers influencing resistance and recovery are similar, because they arise from similar mechanisms; for example, a large soil microbial biomass favours resistance and recovery of soils to perturbations (Orwin and Wardle 2004). In other occasions, however, there is no link between determinants of resistance and recovery, so mechanisms that underpin each stage can vary, and/or operate at different spatio-temporal scales. In turn, resistance to and recovery from perturbation can jointly, and/or independently, influence resilience (Ingrisch and Bahn 2018). Importantly, given that recovery is the ‘bounce back’, it is partially determined by how much change was initially experienced (i.e., resistance) (Nimmo et al. 2015). The extent to which resistance affects subsequent recovery of ecological units, i.e., the connection between measures of resistance and recovery, remains largely unknown for many ecological systems (Falk et al. 2019). Furthermore, potential non-linear relationships, including critical thresholds, need to be identified (Gunderson et al. 2010; Nyström et al. 2012; Nimmo et al. 2015; Connell and Ghedini 2015; Boschetti et al. 2019).
In ecological systems, simultaneous measurements of both resistance and recovery are sparse (Ingrisch and Bahn 2018), despite their potential to shed light on potential trade-offs/feedbacks between ‘resistance’ (= change in state) and ‘recovery’ (= elasticity) (Hoover et al. 2014; Hodgson et al. 2015; Connell et al. 2016). Several difficulties are obvious. First, variables to describe ecosystem (or population) structure and functions are sometimes hard to select. Moreover, studies should collect data over large temporal scales, including periods before (baseline) and under perturbations, as well as during subsequent recovery until new equilibrium points are reached (Hodgson et al. 2015; Nimmo et al. 2015). Experimentally, manipulations to recreate varying regimes in the intensity and frequency of natural perturbations are complicated, particularly in the context of including adequate combinations, and replication, of processes underpinning both resistance and recovery, e.g., environmental gradients, varying levels of genetic diversity, etc. (Selwood et al. 2015; Wernberg et al. 2018; Falk et al. 2019).
In nearshore waters of temperate and tropical latitudes, seagrasses are ‘foundation species’ providing many goods and services to humans (Hemminga and Duarte 2000; York et al. 2017). However, the position of seagrasses in shallow waters exposes these plants, and the meadows they create, to numerous anthropogenic and natural disturbances. Seagrass losses have been described worldwide (Orth et al. 2006; Waycott et al. 2009), in many cases linked to perturbations that decrease the amount of light reaching seagrass above-ground tissues (Ralph et al. 2007). Seagrass resilience includes both resistance and recovery from perturbations (O’Brien et al. 2017; Barry et al. 2018), which have been classified as species (or genus)-specific. Some seagrasses are more resistant than others, while other species recover faster after perturbations (Hemminga and Duarte 2000; Erftemeijer et al. 2006; Kilminster et al. 2015; Roca et al. 2016; O’Brien et al. 2017; York et al. 2017). Local-scale conditions, as well as the genetic structure at the meadow scale, may considerably affect patterns of seagrass resilience (Procaccini et al. 2007). For example, many genotypes (genets) represent an optimal scenario for adaptation to, and recovery from, perturbations (Hughes and Stachowicz 2004; Procaccini et al. 2007; Salo et al. 2015; Evans et al. 2017), even though low genetic diversity meadows, chronically stressed, can exhibit large resilience (Connolly et al. 2018). Seagrasses can be ideal case-study candidates to partition the resilience of ‘foundation species’, as population responses can be empirically tracked before, during and after perturbations with minimum manipulation, using simple population descriptors, in particular seagrass shoot abundances (Hughes and Stachowicz 2004; Roca et al. 2016; Tuya et al. 2019). This is particularly the case of opportunistic seagrass species (sensu Kilminster et al. 2015; i.e., ‘fast-growing’ species), because it is logistically feasible to track the duration of resistance and recovery phases (i.e., a few years). The clonal structure of seagrasses implies the integration of genotypic (the number of genets per area) and genetic diversity (e.g., heterozygosity indices) attributes, as drivers of resilience (Procaccini et al. 2007; Massa et al. 2013), in addition to potential environmental influences mediating seagrass responses (O’Brien et al. 2017; Connolly et al. 2018).
In this study, we adopted a ‘resistance-recovery’ framework to experimentally partition seagrass resilience according to its initial resistance (decrease in shoot abundances during perturbations, relative to controls) and further recovery (increase in shoot abundances after perturbations, until reaching controls), following episodes of local light deprivation. Specifically, the shoot abundances of nine seagrass meadows of Cymodocea nodosa were followed before, during and after simulated light reduction episodes, to address resistance and recovery trajectories. We selected three meadows, encompassing a range of ecological and environmental conditions, at each of three regions across the Atlanto-Mediterranean province. We initially determined the significance of ecological, environmental and genetic drivers on seagrass resistance and recovery patterns. To identify whether seagrass recovery is linearly, or not, related to prior resistance, we then established the connection between trajectories of resistance and recovery of seagrass shoot abundances. Finally, we assessed whether recovery patterns were affected by biological drivers (production of sexual products -seeds—and asexual propagation) at the meadow-scale.
Materials and methods
Case-study species and experimental design
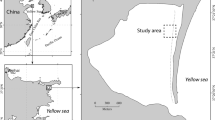
Cymodocea nodosa (Ucria) Ascherson is a seagrass distributed across the whole Mediterranean basin and the adjoining Atlantic coasts, including Madeira and the Canary Islands (Alberto et al. 2006; Tuya et al. 2014). Meadows from the Canary Islands are genetically isolated from the Iberian and Mediterranean populations (Alberto et al. 2008). An experiment was carried out at three regions, including: Southeast Iberia (Alicante) and the Balearic Sea (Mallorca Island), within the Western Mediterranean eco-region, and Gran Canaria Island within the Macaronesian eco-region, in the eastern Atlantic. At each region, we selected three meadows (Table 1, Fig. 1a). To encompass intra-regional (local) variation of seagrass genotypic/genetic diversity, we selected the meadows to encompass a gradient of genotypic/genetic diversity within each region. This strategy accounted for the variable genetic histories of each region, but also incorporating local environmental variation. A preliminary study supplied information on the genetic attributes of a set of meadows per region (Tuya et al. 2019) (Appendix 1 in ESM). The genetic assessment was performed via nine microsatellite markers: Cy1, Cy18, Cy3, Cy4, Cy16, Cn4-19, Cn4-6, Cn2-38 and Cn2-14 (Alberto et al. 2003; Ruggiero et al. 2004). Sampling, laboratory procedures and overall genetic methods, followed Alberto et al. (2003, 2006). At each of the nine meadows, we assessed seagrass shoot abundances, through n = 10 haphazardly allocated quadrats (20 × 20 cm), in November 2016 and February 2017; this provided baseline shoot abundance information previous to the simulation of experimental perturbations (which was started in May 2017; see below for specific details). All sampling was carried out within a window of 10 days for all meadows.
a Location of the study region in the eastern Atlantic and Western Mediterranean, including location of seagrass meadows in Gran Canaria Island (1, 2 and 3, Canary Islands), Alicante (4,5 and 6, Southeast Iberia) and Mallorca Island (7, 8 and 9, Balearic Islands). Light experimental treatments included b high and c moderate light reduction plots, as well as d procedural controls. Shading resulted in e severely reduced seagrass canopy within some plots
Light reduction manipulations were applied in 15 (1 × 1 m) plots established at each meadow. For each plot, a shade cloth was tethered to four metal bars inserted into the bottom. Three treatments were established: ‘high shading’ (Fig. 1b), ‘moderate shading’ (Fig. 1c) and ‘no shading’ (procedural control with a plastic 4 cm2 pore-sized mesh, Fig. 1d). Five plots per treatment were then set up per meadow. A one-week preliminary study indicated that we were able to create a decline of between 1 and 2 orders of magnitude in total light intensity within ‘moderate’ and ‘high’ shading plots, relative to the procedural control (ca. 83% and 98% in mean light reduction, respectively, Tuya et al. 2019). Cloths, at ca. 1 m above the bottom, were replaced every three to four weeks, for a total duration of 13–14 weeks, between May and September 2017. This period encompasses the season of maximum seagrass growth of C. nodosa (Tuya et al. 2006). Light loggers (Hobo Pendant) uninterruptedly recorded total light intensity above seagrass canopies underneath shading cloths within each of three plots, one per treatment, at each of the nine meadows, as well as before and after the light perturbation (Appendices 2, 3 and 4 in ESM). During the ‘resistance’ phase, we counted the number of shoots in each plot, every three to four weeks, by deploying n = 4 (10 × 10 cm) quadrats, at least 10 cm away from each plot edges; this provided an estimate of shoots abundance per plot (values published in Tuya et al. 2019). At the end of this perturbation stage, we removed all shade cloths (Fig. 1e). We then re-visited all plots every three months and, again, counted seagrass shoots in each plot by deploying n = 4 haphazardly allocated quadrats. This temporal monitoring provided data (mean shoot abundances per plot and treatments) on the ‘recovery’ phase of the experiment. We ended up the experiment in December 2018, when recovery was obvious at all meadows.
Measures of resistance and recovery
In this study, we followed ecological change through the abundances of seagrass shoots (= the number of seagrass shoots per m2). Shoot density is a robust descriptor, which does not involve destructive sampling in experimental plots and approximates seagrass above-ground biomass and restoration success (Hughes and Stachowicz 2004; Massa et al. 2013). As a result, we plotted mean shoot abundances (per treatment) through time, i.e., before, during, and after the light perturbation, to graphically infer patterns of resistance and recovery for each of the nine meadows. Resistance was then estimated, for each meadow, as the mean proportion (%) of shoot abundances retained during the light deprivation episodes, i.e., the mean percent change between the shading plots and controls at the end of the press (light deprivation) perturbation. Our approach differs from Nimmo et al. (2015) in the way resistance was calculated, because values at the end of the perturbation state were expressed relative to controls, and not relative to initial conditions. We also used a repeated measures design to analyse our data. This was necessary because the seagrass naturally fluctuates through seasonal scales (Reyes et al 1995; Tuya et al. 2006). Recovery was calculated, for each meadow, as the duration (months) from the end of perturbed state back to the unperturbed (initial) state (‘return time’, sensu Hodgson et al. 2015). In practice, such a time represents the convergence between perturbed and non-perturbed (control) plots, following the seasonal (natural) variation of the seagrass. For each meadow, the first post-perturbation time, in which there was no significant differences in mean shoot abundances between perturbed and control plots, was then obtained (this was verified through 1-way repeated measures ANOVAs); means at the plot level were used as replicates. These metrics were calculated for each of the nine meadows and for the high and moderate shading treatments, respectively.
Role of ecological, environmental and genetic drivers in resistance and recovery trajectories
To partition the relative effects of local ecological (depth, mean meadow shoot density before the perturbation, and meadow area), environmental (mean daily maximum light intensity and mean SST during the study, Table 1) and genetic attributes (the number of genotypes per sampled ramets, R, also known as the clonal richness, and the observed heterozygosity, Hobs, Appendix 1 in ESM) on both resistance and recovery patterns, Generalized Linear Models (GLMs) were applied by means of the ‘R Commander’ library (Fox 2005) in the R3.6.1 statistical package. We firstly visualized and tested for correlations (Spearman) between each pair of ecological, environmental (Table 1) and genetic attributes (Appendix 1 in ESM) through the ‘corrplot’ R library (Wei and Simko 2017). This was necessary to limit the inclusion of over-correlated predictor variables (R2 > 0.6, Harrison et al. 2018, Appendix 5 in ESM) in the subsequent modelization ('model selection') of both resistance and recovery trajectories. When two predictive variables were correlated, we selected that one with a larger biological significance (Bolker 2008). For example, the depth and mean SST of meadows were strongly correlated with their genetic (Hobs) and genotypic diversity (R), respectively, so both environmental factors were initially not included. Initially, we were more interested in addressing the effect of integrated descriptors that operate at large temporal scales and reveal the clonal structure of the meadows (genetic descriptors), rather than descriptors that operate at small scales (SST), as drivers of resilience (Procaccini et al. 2007; Massa et al. 2013). Models were separately fitted for each light deprivation event, i.e., high and moderate shading. Rather than applying statistical procedures for each plot, we focussed on the meadow scale, as ecological, environmental and genetic descriptors operate at this scale. Resistance (here, the mean proportion of shoot abundances retained during the light deprivation events) at the start of the recovery phase was additionally considered, for each meadow, to assess ecological influences on recovery patterns. A ‘Gaussian’ family of errors, with a ‘log’ link function, was selected in the case of resistance patterns, whereas a ‘Poisson’ family of errors, with a ‘log’ link function, was selected to analyse recovery trends. In all cases, we checked the assumptions of linearity and normality of errors through visual inspection of residuals and Q–Q plots (Harrison et al. 2018). To select the best predictors, a ‘stepwise’ model selection procedure, with a ‘forward/backward’ direction was implemented; the AICc (the Akaike Information Criteria corrected to small sample sizes) provided a principle to select the most parsimonious models (Bolker 2008). For all models, ‘Variance Inflation Factors’ (VIF) routines (Harrison et al. 2018) assessed correlation between selected predictor variables. To validate our model selection, we used the 'MuMIn' R library (Bartoń 2016), a flexible package for conducting model selection and model averaging with a variety of candidate GLMs. Model averaging is a way to incorporate model selection uncertainty; the parameter estimates for each candidate model are weighted using their corresponding model weights and summed. For both resistance and recovery patterns, we fitted several candidate GLMs, which contained all combinations between those most parsimonious variables previously selected by the ‘stepwise’ procedure. Models were then ranked by their AICc and importance weights for individual predictor variables calculated.
Connecting recovery and resistance trajectories
Bivariate resilience plots, where resistance (the proportion of shoot abundances retained during the light deprivation events) and recovery (1/‘return time’) are plotted together, were obtained (Hodgson et al. 2015); in our case-study, bivariate measures for each of the nine meadows for each of the two experimental shading treatments. To model meadow recovery as a function of prior resistance, we fitted non-linear sigmoidal (4-parameters) regression curves to the measures of recovery as a function of initial resistance of the nine meadows. Models were fitted separately for the high and moderate shading events, by means of the ‘drc’ R library (Ritz et al. 2015).
Intensity of seagrass sexual and asexual processes
To shed light on the role of sexual and asexual mechanisms driving seagrass recovery (Kendrick et al. 2012; Paulo et al. 2019), we estimated production of seeds and rhizome elongation rates per meadow. Sexual products (seeds) were counted from corers (n = 50, 10 cm of inner diameter) haphazardly allocated in each meadow in October 2017, i.e., 6 months after the main flowering season of the species in Mediterranean (Terrados 1993) and Atlantic waters (Reyes et al. 1995). The density of seeds was expressed per m2. To estimate elongation rates of plagiotropic rhizomes, apical shoots were tagged (April 2017) with cable-ties, which were retrieved after 6 months to encompass the period of seagrass vegetative growth (Terrados et al. 1997); final rhizome elongation rates (in cm) were expressed per month. Linear simple regressions, also implemented in R3.6.1, were used to test whether the density of seeds and mean meadow rhizome elongation rates were significantly predicted by the genetic diversity (observed heterozygosity, Hobs) of meadows; we expected this because meadow genetic diversity has been previously shown to promote seagrass recovery (Massa et al. 2013).
Results
Description of environmental context and genotypic/genetic diversity of meadows
Meadows from Mallorca were at shallower waters (2–3 m depth) than those from Alicante (9–12 m depth) and Gran Canaria (5–10 m depth) (Table 1). However, meadows from Mallorca were under less mean available light regimes relative to those from Alicante and Gran Canaria (Table 1). Within each region, meadows varied in extension (area) in an order of magnitude, with meadows from Alicante being larger than those from Mallorca and Gran Canaria (Table 1). Initial shoot densities at each meadow also varied within and between regions (Table 1). Overall, the genotypic (clonal richness, R) genetic and diversity, in terms of allelic richness (Â38) and heterozygosity (Hobs and Hexp) of seagrass meadows from Gran Canaria were lower than those from the Mediterranean meadows (Appendix 1 in ESM).
Trajectories of seagrass resilience
Patterns of seagrass meadow resistance and recovery notably differed between the three regions. Visually, resilience of seagrass meadows from Gran Canaria (Fig. 2) was low relative to meadows from the Mediterranean, including both Alicante (Fig. 3) and Mallorca (Fig. 4). These patterns were statistically demonstrated with the results from the model selection procedures for both shading experiments. Initial resistance to shading of both intensities (high and moderate) significantly increased with the genetic diversity (Hobs) of the meadow (Table 2). In addition, our model selection suggested that clonal richness (R, Table 2) may be a parsimonious predictor to explain meadow resistance to shading of both intensities.
Changes in seagrass shoot density before, during and after the light deprivation events at meadows from Gran Canaria: a Gando, b Castillo and c Arinaga. The vertical lines indicate the start and the end of the light deprivation treatment. Error bars are + SE of means (N = 4). The grey and black arrows denote the ‘return time’ for the moderate and high shading plots, respectively (the first post-perturbation time, in which there was no differences in shoot abundances between perturbed and control plots). Date format is day/month/year
Changes in seagrass shoot density before, during and after the different light deprivation events at meadows from Alicante: a Tabarca, b Albufereta and c San Juan. The vertical line indicates the start and the end of the light deprivation treatment. Error bars are + SE of means (N = 4). The grey and black arrows denote the ‘return time’ for the moderate and high shading plots, respectively (the first post-perturbation time, in which there was no differences in shoot abundances between perturbed and control plots). Date format is day/month/year
Changes in seagrass shoot density before, during and after the light deprivation events at meadows from Mallorca: a Formentor, b Aucanada, and c Es Barcarés. The vertical line indicates the start and the end of the light deprivation treatment. Error bars are + SE of means (N = 4). The grey and black arrows denote the ‘return time’ for the moderate and high shading plots, respectively (the first post-perturbation time, in which there was no differences in shoot abundances between perturbed and control plots). Date format is day/month/year
Subsequent seagrass meadow recovery, measured as 1/return time, was significantly facilitated by initial resistance to shading of both intensities (Table 3, Fig. 5). The sigmoidal fitting underpins a non-linear relationship between seagrass recovery and resistance (Fig. 5). A visual threshold in resistance, at a ca. 70% of shoot abundance retained during the light deprivation treatments, was detected, which denotes a critical point that notably delays seagrass recovery if exceeded.
Bivariate resilience plots, where meadow recovery (‘1/return time’) is expressed as a non-linear function of prior resistance (the proportion of shoot abundances retained during the light deprivation treatments). Fitted sigmoidal curves are plotted for a high and b moderate shading treatments. Values (with statistical significance) of the 4-parameters (‘b’, ‘c’, ‘d’ and ‘e’, where ‘d’ denotes the asymptote) of sigmoidal adjustments are also included
Intensity of seagrass sexual and asexual processes
Seed densities were higher at meadows of high resistance and quick recovery (Appendix 6 in ESM). In contrast, mean meadow rhizome elongation rates were independent of trajectories of resistance and recovery (Appendix 7 in ESM). The mean density of seeds per meadow (Table 1) was significantly (P-value = 0.01) predicted by the genetic diversity (Hobs) of the meadow; meadows of high genetic diversity showed larger seed densities relative to meadows of low genetic diversity (Fig. 6a). On the other hand, mean meadow elongation rates of apical shoots (Table 1) were not predicted (P-values > 0.2) by seagrass meadow genetic diversity (Fig. 6b).
Discussion
Seagrass degradation and subsequent recovery depends on the relative timescales of resistance and recovery, according to varying intensity, duration and frequency of perturbations (O’Brien et al. 2018). Our experimental approach was based on simulated perturbations of the same intensity, extent and duration over seagrass canopies at meadows under different ecological and environmental contexts. By embracing a ‘resistance-recovery’ strategy, this study has provided evidence that the temporal scale of seagrass recovery after a perturbation is determined by initial resistance during the perturbation phase. Although it could be initially criticized that we did not measure resilience of particular ecosystem functions (sensu Olivier et al. 2015), we followed a state variable, i.e., shoot abundances, which underpins several ecosystem functions of seagrass meadows, e.g., supply of primary production (Roca et al. 2016) and provision of habitat for associated biota (Gartner et al. 2013).
For studies adopting a 'bivariate resilience' approach, Nimo et al. (2015) established several types of relationships between measures of resistance and recovery, one of them being the existence of a positive connection between resistance and recovery, which is what we detected in our study. Specifically, our results demonstrate that a large initial resistance, i.e., a small loss of plant shoots, facilitated a quick recovery, i.e., a short return time to initial conditions. A priori, this might suggest that mechanisms limiting deleterious change (i.e., providing resistance) and those processes facilitating recovery from perturbation (here, shading) would be analogous (Nimo et al. 2015). Initially, mechanisms conferring seagrass resistance to light limitation majorly include physiological processes at the shoot-scale, such as increased efficiency of radiation capture, consumption of stored carbon reserves, and decrease in growth rates and carbon loss (Ralph et al. 2007; O’Brien et al. 2018). In our case-study, moreover, translocation of resources between adjacent shoots is also plausible, because we maintained the belowground clonal integration of seagrass shoots within plots with those outside plots (Tuya et al. 2013a).
Mechanisms mainly contributing to seagrass recovery include vegetative rhizome elongation by the formation of new shoots from apical shoot meristems, re-growth from live vertical meristems, as well as the development of seedlings via seeds derived from sexual reproduction events (Hemminga and Duarte 2000; Kendrick et al. 2012; El-Hacen et al. 2018); these mechanisms mostly operate at the meadow (population) patch scale. Because the timeframes of recovery were conditioned to initial resistance at the meadow-scale, the relative contribution of these processes (sexual versus asexual propagation) to recovery most likely change. Previously, asexual recolonization has been identified as the prevalent mechanism of recovery when experimental plots are slightly altered, or the size of the perturbed patch is small; a priori this should be the main mechanism contributing to recovery of our disturbed plots. In contrast, sexual reproduction (i.e., appearance of seedlings from seeds) tends to be more relevant when plots are severely affected, and/or the size of the disturbed area is much greater than that area potentially colonized through vegetative growth (El-Hacen et al. 2018; O’Brien et al. 2018). The tempo of both types of mechanisms is very different. At small-scales, regeneration through vegetative (asexual) propagation is quick, while at landscape-scales regeneration under this mechanism is considerably delayed (O’Brien et al. 2018). In turn, sexual reproduction becomes important for seagrass recovery from disturbances at large temporal scales (Paulo et al. 2019).
Initial seagrass resistance during adverse conditions involves some degree of physiological adaptation to perturbations (Hemminga and Duarte 2000; O’Brien et al. 2018). At the meadow-scale, our study demonstrated that the genetic diversity (here measured through the Hobs, observed heterozygosity) of the meadow notably associates to initial resistance. Importantly, however, genetic diversity was correlated to meadow genotypic diversity (clonal richness, R, Appendix 5 in ESM), as also shown in other seagrass species (e.g., Posidonia oceanica; Jahnke et al. 2015), so both mechanisms covary and cannot be disentangled. In turn, our model selection procedure also pointed out that clonal richness (R) could also be a relevant driver of meadow resistance to shading. In other seagrass field experiments, it was unrealistic to dissociate the effect of allelic and genotypic diversity, so each one could reflect the other and be an equivalent proxy for the resistance or resilience of seagrass populations (Massa et al. 2013). In any case, at first, a large and more diverse number of genotypes (seagrass clones) per area represent an optimal scenario for initial acclimation and adaptation to perturbations, including shading episodes (Hughes and Stachowicz 2004; Procaccini et al. 2007; Jahnke et al. 2015; Salo et al. 2015; Evans et al. 2017). Moreover, it could be criticized that certain confounding factors may concurrently drive resilience patters. For example, it is true that sea water temperatures seem to be confounded with genetic patterns. However, mean sea water temperatures during the study were very similar among meadows (Table 1; mean temperatures at Alicante and Mallorca were around ca. 19 °C, while at Gran Canaria mean temperatures were around ca. 20 °C). As a result, it is unlikely that water temperatures drive varying resilience patters among meadows.
In our case-study, different ecological/environmental contexts, and population histories between the three regions help to understand differences in resistance according to genetic diversity (Tuya et al. 2019). Notably, our results also demonstrated that seagrass recovery was positively correlated with genetic diversity, because seed production was significantly correlated with meadow genetic diversity. A correlation between heterozygosity and sexual reproduction has also been found for other seagrasses, indicative of sexual success (Jahnke et al. 2015; Ruiz et al. 2018; Paulo et al. 2019). It was remarkable that the energetic costs of intense sexual reproduction (flowering and fruiting) in these meadows does not seem to compromise their asexual propagation, because rhizome elongation rates were similar between meadows. Because asexual propagation was similar between meadows, this is a mechanism promoting recovery irrespective of the meadow levels of genotypic/genetic diversity and ecological/environmental settings of the meadows.
In our study, we experimentally simulated 1 m2 plot disturbances, as a compromise between experimental feasibility and scenarios of light reductions. Of course, a large shading event (for example, river runoff, port construction) could severely affect most of the area covered by the seagrass, therefore decreasing the ‘buffering’ effect provided by seagrass clonal integration from adjacent shoots (Tuya et al. 2013b). Hence, our experimental approach may have underestimated resistance and recovery rates. Understanding resilience of ‘foundation species’ implies, among other things, describing and identifying thresholds and the non-linear dynamics of ecological units (Nyström et al. 2012; Boschetti et al. 2019). In particular, seagrass resistance and recovery are influenced by complex feedbacks (Maxwell et al. 2016). In our study, a threshold in resistance, at a ca. 70% of shoot abundances retained during shading, was evident. Despite this threshold is conditioned to the scale of our experimental plots, and the fact that light-induced perturbations can operate at larger scales, it does suggest the existence of critical points in terms of resistance, which would greatly delay seagrass recovery if overpassed. Such knowledge has practical significance, contributing to more effective management of seagrass meadows. Specifically, managers focussing on the conservation of seagrass meadows should aim at unravelling such critical thresholds for their target seagrass species and the range of impacts that might affect their health. This study has demonstrated that, for our case-study seagrass, conservation strategies majorly focussing on resistance (e.g., through controlling levels of impacts) may be more important than strategies focussing on facilitating recovery, for example via transplants of vegetative fragments or seedlings produced in vitro (see Bulleri et al. 2018 for a series of possible management action on ‘foundation species’). Moreover, in practice, it is almost virtually impossible to assess if, in general, seagrass recovery is delayed because environmental conditions prolong seagrass absence, or simply because of a lack of source material for replenishment (O’Brien et al. 2018). Several processes can, moreover, interrupt seagrass recovery, because of certain feedbacks preventing recolonization (Maxwell et al. 2016; Nyström et al. 2012). In brief, keeping high resistance against perturbations is the best way to assure resilience and persistence of seagrass meadows. Estimation of critical trade-offs between seagrass resistance and recovery is a promising field of research that will help to better manage seagrass meadows.
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alberto F, Correia L, Billot C, Duarte CM, Serrao E (2003) Isolation and characterization of microsatellite markers for the seagrass Cymodocea nodosa. Mol Ecol Notes 3:397–399
Alberto F, Arnaud-Haond S, Duarte CM, Serrao EA (2006) Genetic diversity of a clonal angiosperm near its range limit: the case of Cymodocea nodosa at the Canary Islands. Mar Ecol Prog Ser 309:117–129
Alberto F, Massa S, Manent P, Diaz-Almela E, Arnaud-Haond S, Duarte CM, Serrao EA (2008) Genetic differentiation and secondary contact zone in the seagrass Cymodocea nodosa across the Mediterranean-Atlantic transition region. J Biogeogr 35:1279–1294
Barry SC, Jacoby CA, Frazer TK (2018) Resilience to shading influenced by differential allocation of biomass in Thalassia testudinum. Limnol Oceanogr 63:1817–1831
Bartoń K (2016) MuMIn: multi-model inference. R Package Version 1(15):6
Bolker BM (2008) Ecological models and data in R. Princeton University Press Princeton, USA
Boschetti F, Prunera K, Vanderklift MA, Thomson DP, Babcock RC, Doropoulos C, Cresswell A, Lozano-Montes H (2019) Information-theoretic measures of ecosystem change, sustainability, and resilience. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsz105
Bulleri F, Eriksson BK, Queiros A, Airoldi L, Arenas F, Arvanitidis C, Bouma TJ, Crowe TP, Davoult D, Guizien K, Ivesa L, Jenkins SR, Michalet R, Olabarria C, Procaccini G, Serrao EA, Wahl M, Benedetti-Cecchi L (2018) Harnessing positive species interactions as a tool against climate-driven loss of coastal biodiversity. PLoS Biol 16(9):2006852
Connell SD, Ghedini G (2015) Resisting regime-shifts: the stabilising effect of compensatory processes. Trends Ecol Evol 30:513–515
Connell SD, Nimmo SD, Ghedini G, Mac Nally R, Bennett AF (2016) Ecological resistance—why mechanisms matter: a reply to Sundstrom et al. Trends Ecol Evol 31:413–414
Connolly RM, Smith TM, Maxwell PS, Olds AD, Macreadie PI, Sherman CDH (2018) Highly disturbed populations of seagrass show increased resilience but lower genotypic diversity. Front Plant Sci 9:894
El-Hacen EH, Bouma TJ, Fivash GS, Sall AA, Piersma T, Olff H, Govers LL (2018) Evidence for ‘critical slowing down’ in seagrass: a stress gradient experiment at the southern limit of its range. Sci Rep 8:17263
Erftemeijer PL, Lewis R III, Roy R (2006) Environmental impacts of dredging on seagrasses: a review. Mar Pollut Bull 52:1553–1572
Evans SM, Sinclair EA, Poore AGB, Bain KF, Vergés A (2017) Genotypic diversity and short-term response to shading stress in a threatened seagrass: does low diversity mean low resilience? Front Plant Sci 8:1417
Falk DA, Watts AC, Thode AE (2019) Scaling ecological resilience. Front Ecol Evol 7:275
Fox J (2005) The R Commander: a basic statistics graphical user interface to R. J Stat Softw 14:1–42
Gartner A, Tuya F, Lavery PS, McMahon K (2013) Habitat preferences of macroinvertebrate fauna among seagrasses with varying structural forms. J Exp Mar Biol Ecol 439:143–151
Gunderson LH, Allen CR, Holling CS (2010) Foundations of ecological resilience. Island Press, Washington
Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, Robinson BS, Hodgson DJ, Inger R (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. Peer J 6:e4794
Hemminga MA, Duarte CM (2000) Seagrass ecology. Cambridge University Press, Cambridge
Hodgson D, McDonald JL, Hosken DJ (2015) What do you mean, ‘resilient’? Trends Ecol Evol 30:503–506
Holling CS (1973) Resilience and stability of ecological systems. Annu Rev Ecol Syst 1:1–23
Hoover DL, Knapp AK, Smith MD (2014) Resistance and resilience of a grassland ecosystem to climate extremes. Ecology 95:2646–2656
Hughes AR, Stachowicz JJ (2004) Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc Natl Acad Sci USA 101:8998–9002
Ingrisch J, Bahn M (2018) Towards a comparable quantification of resilience. Trends Ecol Evol 33:251–259
Jahnke M, Pagès JF, Alcoverro T, Lavery PS, McMahon KM, Procaccini G (2015) Should we sync? Seascape-level genetic and ecological factors determine seagrass flowering patterns. J Ecol 103:1464–1474
Kendrick G, Waycott M, Carruthers TJB, Cambridge ML, Hovey R, Krauss SL, Lavery PS, Les DH, Lowe RJ, Mascaró O, Ooi JLS, Orth RJ, Rivers DO, Ruiz-Montoya L, Sinclair EA, Statton J, Kornelis J, Verduin JJ (2012) The central role of dispersal in the maintenance and persistence of seagrass populations. Bioscience 62:56–65
Kilminster K, McMahon K, Waycott M, Kendrick GA, Scanes P, McKenzie L, O’Brien KR, Lyons M, Ferguson A, Maxwell P, Glasby T, Udy J (2015) Unravelling complexity in seagrass systems for management: Australia as a microcosm. Sci Total Environ 534:97–109
Massa SI, Paulino CM, Serrão EA, Duarte CM, Arnaud-Haond S (2013) Entangled effects of allelic and clonal (genotypic) richness in the resistance and resilience of experimental populations of the seagrass Zostera noltii to diatom invasion. BMC Ecol 13:39
Maxwell P, Eklof J, van Katwijk MM, O’Brien KR, de la Torre-Castro M, Boström C, Bouma TJ, Krause-Jensen D, Unsworth RKF, van Tussenbroek BI (2016) The fundamental role of ecological feedback mechanisms in seagrass ecosystems—a review. Biol Rev 92:1521–1538
Nimmo DG, Mac Nally R, Cunningham SC, Haslem A, Bennet AF (2015) Vive la résistance: reviving resistance for 21st century conservation. Trends Ecol Evol 30:516–523
Nyström M, Norström AV, Blenckner T, de la Torre-Castro M, Eklöf JS, Folke C, Österblom H, Steneck RS, Thyresson M, Troell M (2012) Confronting feedbacks of degraded marine ecosystems. Ecosystems 15:695–710
O’Brien KR, Waycott M, Maxwell P, Kendrick GA, Udy JW, Ferguson AJP, Kilminster K, Scanes P, McKenzie LJ, McMahon K, Adams MP, Samper-Villarreal J, Collier C, Lyons M, Munby PJ, Radke L, Christianen MJA, William C, Dennison WC (2018) Seagrass ecosystem trajectory depends on the relative timescales of resistance, recovery and disturbance. Mar Poll Bull 134:166–176
Oliver TH, Heard MS, Isaac NJB, Roy DB, Procter D, Eigenbrod F, Freckleton R, Hector A, Orme CDL, Petchey OL, Proença V, Raffaelli D, Blake Suttle K, Mace GM, Martín-López B, Woodcock BA, Bullock JM (2016) A synthesis is emerging between biodiversity–ecosystem function and ecological resilience research: reply to Mori. Trends Ecol Evol 31:89–92
Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S, Short FT, Waycott M, Williams SL (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996
Orwin KH, Wardle DA (2004) New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biol Biochem 36:1907–1912
Paulo D, Diekmann O, Alexandre A, Alberto F, Serrão A (2019) Sexual reproduction vs. clonal propagation in the recovery of a seagrass meadow after an extreme weather event. Sci Mar 83:357–363
Procaccini G, Olsen J, Reusch TBH (2007) Contribution of genetics and genomics to seagrass biology and conservation. J Exp Mar Biol Ecol 350:234–259
Ralph PJ, Durako MJ, Enriquez S, Collier CJ, Doblin MA (2007) Impact of light limitation on seagrasses. J Exp Mar Biol Ecol 350:176–193
Reyes J, Sansón M, Afonso-Carrillo J (1995) Distribution and reproductive phenology of the seagrass Cymodocea nodosa (Ucria) Ascherson in the Canary Islands. Aquat Bot 50:171–180
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS ONE 10:e0146021
Roca G, Alcoverro T, Krause-Jensen D, Balsby TJS, van Katwijk MM, Marbà N, Santos R, Arthur R, Mascaró O, Fernández-Torquemada Y, Perez M, Duarte CM, Romero J (2016) Response of seagrass indicators to shifts in environmental stressors: a global review and management synthesis. Ecol Indic 63:310–323
Ruggiero MV, Reusch TBH, Procaccini G (2005) Local genetic structure in a clonal dioecious angiosperm. Mol Ecol 14:957–967
Ruiz JM, Marín-Guirao L, García-Muñoz R, Ramos-Segura A, Bernardeau-Esteller J, Pérez M, Sanmartí N, Ontoria Y, Romero J, Arthur R, Alcoverro T, Procaccini G (2018) Experimental evidence of warming-induced flowering in the Mediterranean seagrass Posidonia oceanica. Mar Poll Bull 134(49):54
Salo T, Reusch TBH, Boström C (2015) Genotype-specific responses to light stress in eelgrass Zostera marina, a marine foundation plant. Mar Ecol Prog Ser 519:129–140
Selwood KE, Thomson JR, Clarke RH, McGeoch MA, Mac Nally R (2015) Resistance and resilience of terrestrial birds in drying climates: do floodplains provide drought refugia? Global Ecol Biogeogr 24:838–848
Terrados J (1993) Sexual reproduction and seed banks of Cymodocea nodosa (Ucria) Ascherson meadows on the southeast Mediterranean coast of Spain. Aquat Bot 46:293–299
Terrados J, Duarte CM, Kenworthy WJ (1997) Is the apical growth of Cymodocea nodosa dependent on clonal integration? Mar Ecol Prog Ser 148:263–268
Tuya F, Martín JA, Luque A (2006) Seasonal cycle of a Cymodocea nodosa (Ucria) Ascherson seagrass meadow and associated ichthyofauna in Playa Dorada (Lanzarote Canary Islands eastern Atlantic). J Cienc Mar 32:695–704
Tuya F, Viera-Rodríguez MA, Guedes R, Espino F, Haroun R, Terrados J (2013a) Seagrass responses to nutrient enrichment depend on clonal integration, but not flow-on effects on associated biota. Mar Ecol Prog Ser 490:23–35
Tuya F, Espino F, Terrados J (2013b) Preservation of seagrass clonal integration buffers against burial stress. J Exp Mar Biol Ecol 439:42–46
Tuya F, Ribeiro-Leite L, Arto-Cuesta N, Coca J, Haroun R, Espino F (2014) Decadal changes in the structure of Cymodocea nodosa seagrass meadows: natural vs. human influences. Estuar Coast Shelf Sci 137:41–49
Tuya F, Fernández-Torquemada Y, Zarcero J, del Pilar RY, Csenteri I, Espino F, Manent P, Curbelo L, Antich A, de la Ossa JA, Royo L, Castejón I, Procaccini G, Terrados J, Tomas F (2019) Biogeography modulates seagrass resistance to small-scale perturbations. J Ecol 107:1263–1275
Waycott M, Duarte C, Carruthers TRO, Dennison W, Olyarnik S, Fourqurean J, Heck K, Hughes AR, Kendrick GA, Kenworthy W, Short FT, Williams SL (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA 106:12377–12381
Wei T, Simko V (2017) R package “corrplot”: visualization of a Correlation Matrix (Version 0.84)
Wernberg T, Coleman MA, Bennett S, Thomsen MS, Tuya F, Kelaher BP (2018) Genetic diversity and kelp forest vulnerability to climatic stress. Sci Rep 8:1851
York PH, Smith TM, Coles RG, McKenna SA, Connolly RM, Irving AD, Jackson EL, McMahon K, Runcie JW, Sherman CDH, Sullivan BK, Trevathan-Tackett SM, Brodersen KE, Carter AB, Ewers CJ, Lavery PS, Roelfsema CM, Sinclair EA, Strydom S, Tanner JE, van Dijk KJ, Warry FY, Waycott M, Whitehead S (2017) Identifying knowledge gaps in seagrass research and management: an Australian perspective. Mar Environ Res 127:163–172
Acknowledgements
We acknowledge Paula Bonet, Nestor Bosch, Luis M. Ferrero-Vicente, Andrea García, Tony Sánchez, and José L. Sánchez-Lizaso for their help during fieldwork.
Funding
This work was funded by a project (RESIGRASS, CGL2014-58829) supported by the Secretaría de Estado de Investigación, Desarrollo e Innovación (MINECO, Government of Spain).
Author information
Authors and Affiliations
Contributions
F Tuya, F Tomas, YFT and JT conceived the ideas and designed the experiments. F Tuya, YFT, YPR, FE, PM, LC, FOT, JADO, LR, LA, IC, JMC, AMR, GP, CM, JT and F Tomas performed the experiments. F Tuya analysed the data. F Tuya wrote the manuscript; other authors provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Ethics approval was not required for this study according to local legislation.
Additional information
Communicated by James Fourqurean.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tuya, F., Fernández-Torquemada, Y., del Pilar-Ruso, Y. et al. Partitioning resilience of a marine foundation species into resistance and recovery trajectories. Oecologia 196, 515–527 (2021). https://doi.org/10.1007/s00442-021-04945-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-021-04945-4