Abstract
Land-use intensification (LUI) and biological invasions are two of the most important global change pressures driving biodiversity loss. However, their combined impacts on biological communities have been seldom explored, which may result in misleading ecological assessments or mitigation actions. Based on an extensive field survey of 445 paired invaded and control plots of coastal vegetation in SW Spain, we explored the joint effects of LUI (agricultural and urban intensification) and invasion on the taxonomic and functional richness, mean plant height and leaf area of native plants. Our survey covered five invasive species with contrasting functional similarity and competitive ability in relation to the native community. We modeled the response of native communities for the overall and invader-specific datasets, and determined if invader-native functional differences could influence the combined impacts of LUI and invasion. Overall, we found that urban intensification reduced taxonomic richness more strongly at invaded plots (synergistic interactive effects). In contrast, functional richness loss caused by urban intensification was less pronounced at invaded plots (antagonistic interactive effects). Overall models showed also that urban intensification led to reduced mean leaf area, while agriculture was linked to higher mean plant height. When exploring invader-specific models, we observed that the combined effects of agricultural and urban intensification with invasion were heterogeneous. At invaded plots, invader-native functional differences accounted for part of this variability. Our findings demonstrate the importance of considering the interactive effects of global change pressures for a better assessment and management of ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Land-use intensification (LUI) and biological invasions are two of the most important global change pressures driving biodiversity loss (Sala 2000; Butchart et al. 2010). A recent global assessment examining the effects of LUI on ecosystems shows a net loss of local richness across taxonomic groups, ecosystems and regions (Newbold et al. 2015). Moreover, LUI can also reduce the functional diversity of biological communities (Laliberté et al. 2010; Pakeman 2011; Allan et al. 2015; Gutiérrez-Cánovas et al. 2015). At the local scale, biological invasions also tend to reduce the diversity and abundance of native species (Vilà et al. 2011, 2015; Pyšek et al. 2012), as well as their functional and phylogenetic diversity (Hejda and de Bello 2013; Jucker et al. 2013; Castro-Díez et al. 2016). While many studies have explored how the invader similarity to the local native community determines invasion success (e.g., Carboni et al. 2013; Castro-Díez et al. 2014), it is still unclear how functional similarity and competitive ability influence the invader impact on native communities (Gallien and Carboni 2017) and their interaction with other pressures (Gooden and French 2014).
Despite the fact that invasions are often closely linked to LUI (MacDougall and Turkington 2005; Vilà and Ibáñez 2011; Jauni et al. 2015; González-Moreno et al. 2017), so far, the impacts of these two pressures on community structure have been considered in isolation, which precludes from determining their combined effects and the most appropriate management practices (Didham et al. 2007; Côté et al. 2016; Feld et al. 2016). If LUI interacts with invasions, its impact on invaded sites may either be stronger (synergistic effects) or weaker (antagonistic effects) compared to non-invaded sites. Therefore, understanding how the combined effects of LUI and invasion affect multiple functional traits and their diversity in the native community is key to predicting which species could cope with multiple anthropic pressures (Hooper et al. 2005; Laliberté et al. 2010), identifying the most important impacts on ecosystem functioning (Suding et al. 2008; Gross et al. 2017) and improving mitigation and management strategies to preserve biodiversity (Didham et al. 2007).
Habitat filtering and coexistence theories offer non-exclusive mechanisms that can explain native species’ tolerance to LUI and their response to invasion (Chesson 2000; Weiher et al. 2011). The habitat filtering hypothesis postulates that, under the intense abiotic stress produced by LUI, species showing non-suitable traits will be extirpated from the community (Weiher et al. 2011), leading to communities with functionally similar species and reduced diversity (i.e., trait clustering; Flynn et al. 2009; Laliberté et al. 2010; Gutiérrez-Cánovas et al. 2015). On the other hand, invaders could extirpate not only functionally distant but also similar species, depending on the strength of competitive ability differences (hierarchical functional trait differences) and niche similarity (non-directional functional trait differences) to the native community (Mayfield and Levine 2010; Kraft et al. 2015). When invaders possess certain trait values providing a better ability to compete, and reflecting fitness differences across species (e.g., plant height), inferior competitors (e.g., smaller plants) can be excluded (Cahill et al. 2008; Van Kleunen et al. 2010; Lai et al. 2015). When invader and native species have equivalent competitive abilities, invader species will compete more intensively with those native species showing a similar niche (functional similarity), some of them being excluded (Swenson et al. 2006; Cavender-Bares et al. 2009). In both cases, native and invader species can co-exist via niche complementarity if they are sufficiently different and use distinct resources (functionally distant) (Tilman et al. 1997; Mayfield and Levine 2010).
Here, using an extensive survey of coastal vegetation, we explored the combined effects of LUI (i.e., agricultural and urban intensification) and invasion by exotic plants on native plant communities. Our hypothesis is that both the impacts of invader presence and its interaction with LUI will depend on their functional similarity and competitive ability in comparison to native plant communities. To assess this hypothesis, we firstly characterized the distribution of plant species along the main axes of trait variation and the functional similarity between native and invasive plants at regional and local levels. Secondly, we modeled how native community metrics (i.e., taxonomic richness, functional richness, community mean plant height and leaf area) responded to agricultural and urban intensification, invader presence, and their interactions. Third, to assess whether overall patterns for native community metrics depend or not on invader identity, we ran separate models for each invader dataset. Finally, we analysed if invader functional similarity (i.e., overall functional trait distance) and competitive ability differences (i.e., height difference) to the native species influence the combined effects of agricultural and urban intensification, and invasion on native community metrics.
Materials and methods
Study area and invasive species
A floristic survey was conducted along a 125-km strip of the Atlantic coast in South-western Spain. This area spans a gradient of LUI, including natural protected areas (mainly, coastal dune and wetland habitats), urban areas (i.e., mostly summer residential areas) and crops. The climate of the study region is typically Mediterranean, with warm and dry summers and mild winters (mean annual temperature: 17.6 °C; mean annual precipitation: 535 mm). The most common invasive species were Arctotheca calendula (L.), Arundo donax (L.), Carpobrotus spp. N. E. Br., Conyza bonariensis (L.) Cronq. and Opuntia dillenii (Ker-Gawler) Haw, all of which are considered invasive in Spain (Sanz-Elorza et al. 2004).
The South African A. calendula (Asteraceae) is a small, annual forb widely distributed in coastal habitats of Mediterranean climate. It was introduced to Europe in the eighteenth century as an ornamental plant, and shows preferences for areas affected by agriculture or urbanization (Sanz-Elorza et al. 2004). Its invasive potential is linked to a high reproductive capacity, high seed dispersal, and tolerance to stress (Brundu et al. 2015). A. donax (Gramineae) is a tall perennial gramminoid native to India and South East Asia. It was introduced to Europe about 400 years ago, and now it is globally present in sand dunes, wetlands, riparian zones and disturbed areas of temperate, subtropical and tropical climates (Sanz-Elorza et al. 2004), as a result of its rapid growth rates, clonal reproduction by rhizomes, and its capacity to thrive under variable soil conditions (Quinn and Holt 2008). The South African succulent perennial clonal Carpobrotus acinaciformis (L.) Bolus and C. edulis (L.) N.E. Br. (Aizoaceae) were introduced as ornamentals and for erosion prevention. Currently, their hybrids are widely naturalized on coastal rocks, cliffs and sand dunes (Suehs et al. 2004; Traveset et al. 2008a). Its invasive capacity is associated with a prolific mat-forming clonal growth, and high plasticity to cope with different light intensities (Traveset et al. 2008b). Here, we considered them together as Carpobrotus spp. group, given they are ecologically and functionally similar species, and difficult to distinguish in the field in the absence of inflorescences. The American C. bonariensis (Asteraceae) is an 80-cm-tall, annual forb, accidentally introduced to Europe in the eighteenth century. This species tends to invade degraded, ruderal habitats (Sanz-Elorza et al. 2004), thanks to its high reproduction capacity and early seed germination (Thebaud and Abbott 1995). Finally, O. dillenii (Cactaceae) is an American 3-m-tall succulent shrub, which was introduced to Europe around the sixteenth century for ornamental and green fence purposes (Sanz-Elorza et al. 2004). Its invasive capacity is linked to high vegetative and sexual reproductive capacities, and high tolerance to drought conditions (Padrón et al. 2011).
Floristic survey
In spring of 2010 and 2011, we performed a vegetation survey by walking along the coastal vegetation at a distance of c.a. 50 m from the shoreline to avoid the influence of the highest tides. The vegetation surveyed is composed by sparse foredune vegetation (Valdés et al. 1987). This vegetation is subjected to salt spray, episodic over-wash, highly permeable substrate, low field capacity, high temperatures, drought, high winds, and substrate mobility (García-Mora et al. 1999). When the strip of coastal vegetation was wide enough, a parallel walk was also placed inwards at approximate 50 m from the previous one but within the same vegetation type. Although we intended to survey the entire coast, due to accessibility problems (e.g., military areas) or lack of coastal vegetation in urbanized coastal areas, we finally sampled 70 km out of the 125-km total coastal line. The occurrence of the five invasive plants along each transect was noted and the coordinates recorded. For each occurrence, we set up a 10 × 10 m plot including only one invasive species (i.e., we avoided plots with two or more co-occurring invaders; hereafter, invaded plot). We did not survey plots totally dominated by the invader. The average cover of each invader species in a subsample of plots was 23% for A. calendula, 21% for A. donax, 32% for Carpobrotus spp., 14% for C. bonariensis and 28% for O. dillenii. Another plot containing only native species (i.e., control plot) was chosen in close vicinity (< 20 m) to ensure that each pair of plots was subjected to similar land-use and environmental conditions (Vilà et al. 2006). Our survey resulted in a total of 445 paired invaded and control plots. We identified all plant species in each plot, which resulted in a total of 127 native species, being Ammophila arenaria (Gramineae), Lotus creticus (Fabaceae), Malcomia littorea (Brassicaceae) and Rumex tingitanus (Polygonaceae) the most common taxa.
Land-use intensity assessment
For each pair of plots, land-use intensity was assessed using the 2007 land-cover maps for Andalusia based on ortho-rectified aerial photographs, which were the closest to the sampling data (Moreira et al. 2010; scale 1:25,000). For each pair of plots, we estimated urban and agricultural land-use percentages in a 500-m radius buffer area around the central point of the plots, excluding the area occupied by sea (i.e., we removed sea cover in the buffers and calculated the percentage of inland land-uses to have comparable values). This radius buffer was selected based on previous research in the same study area that identified the scale at which the effects of land-use intensity on plant communities were more conspicuous (González-Moreno et al. 2017). As a measure of natural landscape variability, we also estimated the percentage of dune and wetland habitats within the same buffer area.
Our dataset contained 445 pairs of invaded and non-invaded plots, which resulted from combining 99 pairs for A. calendula dataset (urban plus agricultural land-use: 0.0–61.2%), 130 pairs for A. donax dataset (0.0–50.7%), 76 pairs for Carpobrotus spp. dataset (0.0–56.4%), 99 pairs for C. bonariensis dataset (0.0–44.2%) and 41 pairs for O. dillenii dataset (0.0–61.2%). Fifty plots were used more than once as controls for different invaded plots, which overall resulted in 840 plots (445 invaded plus 395 control plots).
Species traits
To characterize functional similarity and competitive ability differences between invader and native species, we compiled information on six traits (plant height, leaf area, life form, life span, N-fixing capacity and photosynthetic pathway) for the 132 studied plant taxa, i.e., 127 natives plus the five invasive species (Devictor et al. 2010; Mayfield and Levine 2010). Trait values were obtained from regional or national botanical guides (Valdés et al. 1987; Sanz-Elorza et al. 2004; Castroviejo 2012) and expert knowledge. Although the invader-native functional similarity and competitive capacity differences is likely to change over the LUI gradients (Maire et al. 2012; Kraft et al. 2015; Lai et al. 2015), our static average trait data are unable to capture such intra-specific variation.
Plant height (range 8–3000 cm) was estimated as the mean of the maximum and minimum values found in the literature. Plant height is a surrogate of several key aspects of plant biology and ecology, which are related to critical trade-offs for growth, reproduction, energy allocation and competitive strategies (Pérez-Harguindeguy et al. 2013). Larger plants have a higher capacity to intercept light, disperse diaspores, build deep root systems or cope with mechanical stress; whilst small plants need less space and resources to thrive and can be present at high densities (Brown et al. 2004). We used plant height as a proxy of plant fitness and competitive ability, in a way that larger plants will be superior competitors as a result of their enhanced capacity to acquire soil resources (water, nutrients) and occupy space (Gaudet and Keddy 1988; Violle et al. 2009; Maire et al. 2012; Younginger et al. 2017). Leaf area was estimated as the surface of the most similar geometrical form (e.g., oval, cylinder, triangle, among others), using mean leaf width and length as input data (average from minimum and maximum literature values, range 0.02–5222.90 cm2). For compound leaves, we multiplied the leaflet area by the total number of leaflets. Leaf area plays an important role in energy acquisition and water balance. Larger leaves can capture more energy at the cost of evaporating more water through stomata (Wright et al. 2004, 2005). Life form (forb, gramminoid, liana, shrub, sub-shrub, tree) is a proxy of multiple life-histories and physiological aspects, which combines plant size with other aspects as resistance forms of protection to disturbance and stress, lateral and vertical span or lignin content, all of which represent different strategies to grow and cope with disturbance (Pérez-Harguindeguy et al. 2013). Life span (annual, perennial or variable) reflects the plant allocation pattern between reproduction, growth and defence (Herms and Mattson 1992), and determines plant resilience to disturbance. For example, annual plants recover their populations faster after disturbance as a result of an earlier and profuse reproduction, whilst perennial plants have higher ability to cope with adverse conditions, thanks to a higher investment in support and storage. The capacity to fix atmospheric nitrogen (coded as a binary trait, no/yes) provides an advantage for plants to colonize nitrogen-poor soils, increasing soil fertility when tissues of these plants die. Finally, photosynthetic pathways (C3, C4, CAM) represent different plant strategies to fix carbon during photosynthesis, involving different rates of water loss for the plant (Pérez-Harguindeguy et al. 2013). The majority of the plants considered show a C3 strategy to fix carbon, which involves great water loss under intense heat. However, C4 and CAM plants have developed more efficient strategies to fix carbon, which reduce water loss, providing a potential advantage in hot and arid environments.
Trait similarity between native and invasive species
To characterize functional similarity between native and invasive species, we built a species × traits matrix, including both native and invasive species (n = 132 species). First, using Gower’s index (Gower 1971), we computed a matrix containing the pair-wise functional dissimilarity across species based on the six traits considered here to capture the multidimensional nature of species niches (hereafter, Gower trait matrix). Gower’s index can derive species dissimilarities from both quantitative and qualitative traits, allowing for missing values. Second, based on this Gower trait matrix, we built a functional space through a Principal Coordinate Analysis (PCoA). This analysis reduced all traits to a few main axes (or coordinates), retaining a high proportion of cross-species variance, and representing invasive and native species in the space defined by these main axes (Villéger et al. 2008). Third, to identify which traits were correlated with each axis, we used Pearson correlation coefficients (r) for continuous traits and ANOVAs for binomial and categorical traits (i.e., species coordinates as response variable and trait categories as predictors). We considered that a trait was associated with an axis when \( r\, \ge \,\left| {0.50} \right| \). We ranked ANOVAs according to their r2 values to identify the categorical traits most associated with each axis. To select the number of relevant functional axes, we assessed the minimum number of dimensions (from two to ten) that provided a good representation of the original Gower trait matrix (Maire et al. 2015). We kept three dimensions (mean squared deviance, mSD = 0.019) because functional spaces of higher dimensions did not substantially improve the representation accuracy of the original Gower trait matrix (e.g., 10 dimensions yielded a mSD = 0.013). These three axes were the most explanatory and represented 70.0% of the original traits variation.
To have a general view of invader functional similarity to the native species at the regional level, we quantified the functional distance between each invader and the average trait values of all the studied native species in the species pool (n = 127). To do this, we estimated the Euclidean distance between the position of each invader in the functional space and the native centroid, which was calculated as the average of the 127 native species coordinates for each functional axis in the 3D space.
Additionally, to explore if invader functional differences to native communities can modify LUI effects at invaded plots, we quantified invader functional distance (measure of functional similarity) and height difference (measure of competitive ability) in comparison to the native species present in the paired plots (Hejda et al. 2009; Mayfield and Levine 2010; Lai et al. 2015). We quantified invader functional distance as the Euclidean distance between the invader and the centroid of the native species present in the paired control plot. We calculated invader-native community height differences as a measure of the potential difference in competitive ability, given that plant height can capture differences in fitness across species (Gaudet and Keddy 1988; Violle et al. 2009; Maire et al. 2012; Younginger et al. 2017). For each invaded plot, we quantified log-transformed absolute height differences between each invader and the mean value of the native community from the paired control plot. Log-absolute differences in plant height were multiplied by − 1 when the invader height was smaller than the mean native community value.
Plant community structure and functional trait metrics
For each plot, we counted the number of native species as a measure of taxonomic richness. The functional richness of each plot, which represents the trait range variation for the native community (Villéger et al. 2008), was estimated as the ratio (from zero to one) between the functional space volume of the convex hulls enclosing all the native species in the plot and that encompassing all the native and invasive species present in the study area (Villéger et al. 2008). This index could not be calculated for plots with less than four species (i.e., a minimum of four points is required to build a volume). Finally, using only native species, we estimated the mean plant height and mean leaf area for each plot to assess how native plant traits change with LUI and invasion (Suding et al. 2008).
Statistical analysis
To reduce distribution skewness, we applied a log-transformation to functional richness, mean plant height, mean leaf area and the percentage of dune habitat, and a squared-root transformation to invader functional distance. All quantitative predictors were standardized to mean = 0 and SD = 1.
To explore the combined effects of LUI and invasion on native taxonomic richness, functional richness, community mean plant height and mean leaf area for the overall and for each invader dataset, we used linear mixed-effect models (LMM) with a Gaussian error distribution. For the overall dataset (n = 840), we explored the combined effects of agricultural and urban intensification, invader presence, and their interaction terms. Interactions with agriculture were not considered in subsequent models to simplify models and because main overall analyses suggested minor importance (see Table 1). To see how far overall patterns depend on invader identity, we ran separate models for each invader dataset using agricultural and urban intensity, invader presence, and urban × invader presence interaction term as predictors. Overall and invader-specific models included control-invaded plot pair as random factor to account for repeated measures in the same location. Overall models also included invader species identity as random factor to control for invader-specific effects.
We focused on invaded plots (n = 445) to determine if invader-native functional differences could influence the combined impacts of LUI and invasion on taxonomic richness, functional richness, mean plant height and mean leaf area. For this analysis, we ran linear regression models (LM) including the following fixed factors: agricultural and urban intensity, invader-native functional distance, invader-native height difference and the interactions between urban intensity × invader-native functional distance and urban intensity × invader-native height difference. These models included also invader identity as fixed covariate to control for invader-specific effects.
All these models included the percentage of dune and wetland habitats as fixed covariates to account for landscape heterogeneity of the native community across the study area. For each model, we also checked the spatial autocorrelation structure of the models’ residuals using Moran’s Index (Moran’s I) based on each site’s coordinates. When the Moran’s I values were higher than I > |0.50| (i.e., invader-specific models), we added a residual spatial autocorrelation covariate (RAC) as predictor to capture the spatial effects non-considered by the fixed factors (Crase et al. 2012). This RAC term considers the correlation between the residuals at a given plot and those from its neighboring locations. Before running the models, we checked pairwise Pearson correlation coefficients (r) among fixed factors to control for collinearity. When |r| ≥ 0.7 between two predictors, we removed the predictor with a mean lower correlation with the response variable.
To quantify predictor’s standardized effect sizes (SES) and significance of LMM and LM, we adopted a multi-model inference approach (Grueber et al. 2011), using the MuMIn R package (Bartoń 2016). This statistical technique ranks all the models generated using all the possible combination of predictors using AIC. Then, a set of top models is selected to produce an average model only if the model ranking first is ambiguously supported (model weight < 0.90). We chose top models differing in no more than four AIC units (delta ≤ 4) from the model ranked first (minimum AIC). We adopted a natural average method to conduct the model averaging, which consists in averaging predictors only over models in which the predictor appears, and weighting predictor’s SES by the summed weights of these models (Burnham and Anderson 2002). All final models were validated by visually checking the distribution of residuals for normality and homoscedasticity (Zuur et al. 2009). For each LMM model, two measures of goodness-of-fit were estimated (Nakagawa and Schielzeth 2013) using the MuMIn package (Bartoń 2016): marginal goodness-of-fit \( \left( {r^{ 2}_{\text{m}} } \right) \) indicates the variance explained just by the fixed factors, while conditional goodness-of-fit \( \left( {r^{ 2}_{\text{c}} } \right) \) shows the variance accounted for by both fixed and random terms.
Given that taxonomic and functional richness can be correlated by chance (selection probability effect; e.g., Huston 1997), we performed null models to confirm whether the observed functional patterns were not simply a consequence of the underlying taxonomic variation (Stevens et al. 2003; Gutiérrez-Cánovas et al. 2015; Bruno et al. 2016). Thus, we produced a distribution of 999 null coefficient values for each empirical model term (fixed factors) following this procedure: (1) randomly re-assigning trait combinations to each species on the trait table, (2) re-estimating functional richness for each plot and (3) re-examining its relationship using global LMM models including all fixed factors, instead of the multi-model inference approach. We used global models because averaged null models could not include some of the focal fixed factors, which would preclude their comparison against observed parameters. Given that trait randomisation was performed on the trait table, the overall species frequency and the species richness for each plot were kept unmodified in the null models. Depending on the sign of the observed model parameter, we examined whether the empirical model parameters were significantly lower (negative model parameters) or greater (positive model parameters) than the null model distributions using an exact one-tailed test at α = 0.05 (i.e., occurrence of values greater or lower than the null model parameters/null model runs + 1). Model parameters that did not significantly differ from the null distribution indicated that changes in functional richness are indistinguishable from taxonomic richness variation.
We could expect that agricultural and urban intensification will reduce functional richness more than expected by chance (Online Resource 1; i.e., trait clustering due to abiotic filtering), while invader presence could have a more complex response (Online Resource 1; i.e., trait clustering when the invader displaces inferior species with different trait values; invader causes trait overdispersion as a way to avoid competition and allow co-existence via niche complementarity) (Mayfield and Levine 2010; Kraft et al. 2015). Departures from null expectations of the interaction parameters (urban intensity × invader presence) would imply different trait responses depending on the signs of the observed individual and interactive model parameters (Online Resource 1). If observed model parameters are all negative, LUI and invader presence would result in an enhanced trait clustering. When LUI and invader presence have a negative effect on functional richness, but interaction has a positive sign that departs from null expectations, the interactive effect produces a mitigated trait clustering. Finally, when agriculture/urban intensity and invader presence have different signs and the interaction term departs from null expectations, both trait clustering and overdispersion occur depending on invader functional distance to control plots.
All the analyses were conducted using the R statistical software (R Development Core 2019). Code and functions to derive the biodiversity metrics and run the statistical analysis are available (Online Resource 2).
Results
Invasive and native species position along functional axes
Details of the functional space axes are available in Online Resource 3. At the species pool level, the median of the functional distance of all native species to the centroid of the functional space was 0.295 (first quartile = 0.250, third quartile = 0.402). Invasive species showed contrasting distances to the centroid of the native community (Online Resource 4 and 5). C. bonariensis and A. calendula were the most similar species to the native species pool (functionally similar invaders, hereafter), with functional distances to the native centroid lower than the native median (0.249 and 0.232, respectively). By contrast, A. donax (0.378), Carpobrotus spp. (0.575) and O. dillenii (0.594) were more distant to the native species pool median (functionally distant invaders, hereafter). The main differences between the native species and functionally distant invaders were found for functional axis 1 (plant height, life span, N-fixation and life form) and axis 3 (photosynthetic pathway). On average, the three functional distant invaders O. dillenii, A. donax and Carpobrotus spp. were taller than the native species mean and, additionally, Carpobrotus spp. and O. dillenii were the only plants showing CAM photosynthetic pathway in our study. At the plot level, we found a similar pattern, except for A. donax, that fell within the group of similar invaders: C. bonariensis (median = 0.135), A. calendula (0.145), A. donax (0.146); the most distant invaders were O. dillenii (0.177) and Carpobrotus spp. (0.189).
Overall combined effects of LUI and invasion
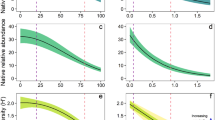
Urban intensification and invader presence had interactive effects on taxonomic and functional richness (Table 1, Online Resource 6). Specifically, urban intensification caused a greater taxonomic richness loss in invaded plots relative to control plots (synergistic response), while functional richness loss was greater in control plots relative to invaded plots (antagonistic response). Besides their interactive effects with invasion, urban intensification reduced mean leaf area, while agricultural intensification increased mean plant height (Table 1, Fig. 1a–d). Invader presence did not show any significant effect on mean plant height or leaf area, nor any main effect on richness and functional richness. These overall patterns were generally weak as reflected by the limited portion of explained variance (3–13%). Null models revealed that urban intensification caused more functional richness loss than expected by chance, reflecting non-random trait clustering (Online resource 8). Null model analyses also confirmed that invader presence tended to mitigate functional richness loss caused by LUI more than expected by chance (mitigation effect).
The combined effects of land-use intensification (LUI) and invasion on a taxonomic richness, b functional richness, c mean plant height and d mean leaf area for the overall dataset (n = 840) in invaded and non-invaded plots (light orange dots and light blue triangles, respectively). Solid blue lines represent the fitted values at control plots, and orange solid lines represent fitted values at invaded plots. Color version of this figure is available online
Combined effects of LUI and invasion for each invader dataset
When analysed independently, our five datasets showed that the effects of LUI and invader presence depended on invader identity (Table 2, Fig. 2, Online resource 7), being generally stronger than those observed in the overall models, as reflected by the higher proportions of explained variance (11–66%).
The interactive effects of urban intensification and invasion on functional richness of aCarpobrotus spp. and bO. dillenii and c mean plant height of Carpobrotus spp. in invaded and non-invaded plots (light orange dots and light blue triangles, respectively). Solid blue lines represent the fitted values at control plots, and orange solid lines represent fitted values at invaded plots. Color version of this figure is available online
Interactive effects occurred only in two invader datasets (Carpobrotus spp. and O. dillenii datasets). In response to combined urban intensification and invader presence (Fig. 2), these interactions reflected antagonistic responses of functional richness (both datasets) and a synergistic plant height decline (Carpobrotus spp. dataset). Urban intensification tended to reduce taxonomic and functional richness, and mean plant height and leaf area, but some exceptions occurred (O. dillenii dataset). Besides, individual impacts caused by invader presence were heterogeneous and depended on invader identity: A. calendula presence was associated with lower mean plant height and leaf area. A. donax invasion was linked to reduced taxonomic richness, functional richness and mean leaf area. Plots invaded by Carpobrotus spp. showed higher mean leaf area. C. bonariensis presence was linked to higher taxonomic richness. Null model results generally showed that urban intensity (Carpobrotus spp., C. bonariensis and O. dillenii datasets) and invader presence (A. donax dataset) caused more functional richness loss than expected by chance (Online resource 8).
Overall combined effects of LUI and invader functional differences to the native communities
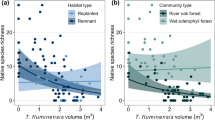
Urban intensification and invader functional distance had a synergistic interactive effect on taxonomic richness (Table 3, Fig. 3a, Online resource 9). Thus, urban intensification reduced taxonomic richness more strongly at plots invaded by functionally distant plants in comparison to paired native communities. Mean plant height declined with greater functional distance and height difference between invader and native community, and increased with urban intensification (Fig. 3c). Functional richness and mean leaf area did not show any significant association with agriculture, urban intensification or invader functional differences to native communities (Fig. 3b, d).
The combined effects of LUI and invasion on a taxonomic richness, b functional richness, c mean plant height and d mean leaf area for the overall dataset at invaded plots (n = 445). Solid blue lines represent the fitted values where invaders showed either low functional distance (low dist.) or low height difference (low height dif.) to native communities. Orange solid lines represent fitted values where invader showed either large functional distance (large dist.) or large height difference (large height dif.) to native communities. Color version of this figure is available online
Discussion
Our results showed that LUI and biological invasion have variable and invader-specific effects on plant communities, including both additive and interactive effects. We also demonstrated that such impacts can cause non-random changes in functional richness, mostly arising from trait clustering. Furthermore, we observed that the combined effects of LUI and invasion can vary considerably across invader datasets, but interactive effects were more common for functionally distant invaders with greater competitive traits (plant height) relative to the native species pool. At invaded plots, both urban intensity and functional differences between invaders and native communities were important to explain changes in taxonomic richness and mean plant height. Overall, these results highlight the need of considering the combined effects of global change pressures for a better assessment of their impacts (Didham et al. 2007; Côté et al. 2016; Feld et al. 2016).
In our study, trends in response to urban intensification showed a reduction of taxonomic and functional richness in the overall, Carpobrotus spp. and C. bonariensis datasets, which seems to be linked to abiotic filtering, as found in previous research (Laliberté et al. 2010; Pakeman 2011; Allan et al. 2015; Gutiérrez-Cánovas et al. 2015). Our data also showed that LUI can reduce mean plant height and leaf area, particularly as a result of urban intensification. Urban intensification caused a greater impact than agriculture, probably, because it was the most pervasive anthropogenic land-use in the study area. These impacts may emerge from changes in landscape fragmentation, local disturbance and soil degradation (Lee et al. 2006; González-Moreno et al. 2013), which could have been also facilitated by past land-use legacies in the study area (González-Moreno et al. 2017).
Biological invasion caused variable effects, which added to or modified urban intensification effects, and were partly linked to the functional similarity and competitive ability differences between invaders and native community (Hejda et al. 2009; Lai et al. 2015). The invaders A. calendula and C. bonariensis, which are annual, C3, forbs with similar overall and competitive traits (plant height) relative to the native species pool, caused very different effects, which in any case interacted with LUI. Despite their similarity, A. calendula caused negative impacts on mean plant height and leaf area, likely through competitive exclusion (Lai et al. 2015), while C. bonariensis seems to coexist with native species without producing negative impacts, as previously reported (Prieur-Richard et al. 2002). The resource-acquisitive strategy of A. calendula based on greater leaf area and root system and, perhaps, its high toxicity to mammals (Brundu et al. 2015; de la Riva et al. 2019) might have resulted in a greater ability to compete with native species and avoid grazing. In contrast, the reduced impact of C. bonariensis could be linked to a more ruderal invasive strategy, which involves a greater ability to colonize empty sites but not to displace pre-exiting native plants (Thebaud and Abbott 1995). A. donax, which was the invader with the greatest height and leaf area, produced negative effects on native communities, which did not interact with LUI and caused a reduction in taxonomic and functional richness, and mean leaf area of native communities. A. donax can produce strong reductions of plant and invertebrate diversity, such as those observed in invaded riparian zones, where it forms large stands favored by humid soils (Maceda-Veiga et al. 2016). Surprisingly, the invasion by Carpobrotus spp. did not cause great negative changes in native taxonomic or functional richness, as opposed to the results previously reported in coastal systems (Vilà et al. 2006; Jucker et al. 2013; Castro-Díez et al. 2016). O. dillenii did not caused strong impacts on native communities, potentially due to a nursery effect either from grazing protection (Opuntia spp. has spines) or as a result of habitat amelioration, i.e. increased soil humidity or wind shelter thanks to canopy protection (Vilà and Gimeno 2003; Padrón et al. 2011). Interestingly, Carpobrotus spp. and O. dillenii, the most functionally distant invaders with a great competitive ability compared to the native pool of species, were involved in various interactive effects. As occurred in the overall models, interactions of urban intensification with functionally distant invaders tended to attenuate functional richness loss due to urban intensification, although mean plant height declined with LUI more strongly at plots invaded by Carpobrotus spp. A potential explanation could be the replacement of LUI-sensitive species by other taxa showing higher tolerance (Laliberté et al. 2010), which could have mitigated functional richness loss at invaded plots.
In combination, empirical and null models performed with different datasets (overall, invader-specific, overall invaded) revealed that taxonomic and functional richness showed different responses to LUI at control and invaded plots. Thus, while urban intensification reduced both taxonomic and functional richness through non-random trait clustering for the full dataset (Laliberté et al. 2010; Pakeman 2011), our results suggest more complex patterns at invaded plots. In this case, taxonomic richness declined more strongly in the presence of functionally distant invaders. Besides, mean plant height was reduced by a non-interacting combination of urban intensification, increased invader functional distance and competitive ability difference to native communities. Recent application of the co-existence theory to invasion ecology suggests that the competitive exclusion of native species in response to invasion occurs when competitive ability overwhelms functional similarity; while, invaders and native species with very different traits can coexist (Chesson 2000; Mayfield and Levine 2010; Lai et al. 2015). In this sense, our results for mean plant height seem to match this framework, where smaller and functionally distant native plants in comparison to the invader seem to occur at invaded plots. However, our patterns for taxonomic richness did not fit well these predictions, particularly regarding the greater reduction in taxonomic richness caused by functionally distant invaders to the native community and not by those with higher height differences. There are some potential explanations for this disagreement, which require further research to be tested and confirmed. For example, some plant species can be extirpated due to a lower fitness caused by modifications in ecosystem functioning (e.g., pollination, seed dispersal) or properties (e.g., soil nutrients or humidity) mediated by the altered trait composition caused by the functionally distant invader (Vilà et al. 2011; Albrecht et al. 2014). In addition, indirect competitive exclusion meditated by native species can occur if some of them become more competitive and dominant in response to the new conditions created by the presence of the functionally distant invader and high LUI (White et al. 2006; Didham et al. 2007; Lai et al. 2015).
Despite the fact that coastal vegetation is spatially variable, and that the lack of abundance data and information on fine soil properties could have limited our results, we provide novel evidence on the combined effects of LUI and invasion at the local scale, for which empirical evidence was still strikingly scarce (Didham et al. 2007). Our results suggest that the interaction between LUI and functionally distant invaders with higher competitive capacities may result in interactive effects, which may exacerbate LUI impacts. Although the few studies performed so far found additive effects of LUI and invasion on native plant communities (Gooden and French 2014), we should be cautious when suggesting management measures to mitigate LUI and invasion, since the competitive ability of some invaders can increase with soil nutrient content (Lai et al. 2015). We should acknowledge that our inference regarding the ecological mechanisms driving the observed community changes is limited because of the use of observational data and average trait data from literature, but this could be improved and addressed in future research. Previous works found that the combined impacts of LUI and invasion can also alter ecosystem functions, such as pollination, where they result in additive (Grass et al. 2013) and interactive effects (Bartomeus et al. 2010). Overall, all the reported changes in plant diversity and trait composition as a result of the combined effects of LUI and biological invasion are likely to affect food webs and ecosystem functions delivered by coastal plants and soils (Mokany et al. 2008; Allan et al. 2015; Gross et al. 2017), requiring the attention of managers and future research on global change ecology.
This study represents a first step in addressing the impact of multiple global change pressures on plant communities. Taken together, our findings demonstrate the importance of considering the interactive impacts of global change pressures at the local scale. We showed that the co-occurrence of LUI and biological invasion can cause variable impacts, which include the reduction of taxonomic and functional richness, and shifts in community mean traits through additive and interactive effects. The use of large databases including different types of invaders along with their functional traits provides a useful strategy to explore the response of communities to multiple global change pressures and to provide guidance for a better ecosystem management.
References
Albrecht M, Padrón B, Bartomeus I, Traveset A (2014) Consequences of plant invasions on compartmentalization and species’ roles in plant-pollinator networks. Proc R Soc B Biol Sci 281:20140773. https://doi.org/10.1098/rspb.2014.0773
Allan E, Manning P, Alt F, Binkenstein J, Blaser S, Blüthgen N, Böhm S, Grassein F, Hölzel N, Klaus VH, Kleinebecker T, Morris EK, Oelmann Y, Prati D, Renner SC, Rillig MC, Schaefer M, Schloter M, Schmitt B, Schöning I, Schrumpf M, Solly E, Sorkau E, Steckel J, Steffen-Dewenter I, Stempfhuber B, Tschapka M, Weiner CN, Weisser WW, Werner M, Westphal C, Wilcke W, Fischer M (2015) Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol Lett 18:834–843. https://doi.org/10.1111/ele.12469
Bartomeus I, Vilà M, Steffan-Dewenter I (2010) Combined effects of Impatiens glandulifera invasion and landscape structure on native plant pollination. J Ecol 98:440–450. https://doi.org/10.1111/j.1365-2745.2009.01629.x
Bartoń K (2016) MuMIn: multi-model inference. R package version 1.15.6. https://cran.r-project.org/web/packages/MuMIn/index.html
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. https://doi.org/10.1890/03-9000
Brundu G, Lozano V, Manca M, Celesti-Grapow L, Sulas L (2015) Arctotheca calendula (L.) Levyns: an emerging invasive species in Italy. Plant Biosyst 149:954–957. https://doi.org/10.1080/11263504.2015.1125963
Bruno D, Gutiérrez-Cánovas C, Sánchez-Fernández D, Velasco J, Nilsson C (2016) Impacts of environmental filters on functional redundancy in riparian vegetation. J Appl Ecol 53:846–855. https://doi.org/10.1111/1365-2664.12619
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Butchart SHM, Walpole M, Collen B, van Strien A, Scharlemann JPW, Almond REA, Baillie JEM, Bomhard B, Brown C, Bruno J, Carpenter KE, Carr GM, Chanson J, Chenery AM, Csirke J, Davidson NC, Dentener F, Foster M, Galli A, Galloway JN, Genovesi P, Gregory RD, Hockings M, Kapos V, Lamarque J-F, Leverington F, Loh J, McGeoch MA, McRae L, Minasyan A, Morcillo MH, Oldfield TEE, Pauly D, Quader S, Revenga C, Sauer JR, Skolnik B, Spear D, Stanwell-Smith D, Stuart SN, Symes A, Tierney M, Tyrrell TD, Vie J-C, Watson R (2010) Global biodiversity: indicators of recent declines. Science 328(80):1164–1168. https://doi.org/10.1126/science.1187512
Cahill JF, Kembel SW, Lamb EG, Keddy PA (2008) Does phylogenetic relatedness influence the strength of competition among vascular plants? Perspect Plant Ecol Evol Syst 10:41–50. https://doi.org/10.1016/j.ppees.2007.10.001
Carboni M, Münkemüller T, Gallien L, Lavergne S, Acosta A, Thuiller W (2013) Darwin’s naturalization hypothesis: scale matters in coastal plant communities. Ecography (Cop) 36:560–568. https://doi.org/10.1111/j.1600-0587.2012.07479.x
Castro-Díez P, Godoy O, Alonso A, Gallardo A, Saldaña A (2014) What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecol Lett 17:1–12
Castro-Díez P, Pauchard A, Traveset A, Vilà M (2016) Linking the impacts of plant invasion on community functional structure and ecosystem properties. J Veg Sci 27:1233–1242. https://doi.org/10.1111/jvs.12429
Castroviejo S (2012) Flora ibérica 1-8, 10-15, 17-18, 21. Real Jardín Botánico. CSIC, Madrid
Cavender-Bares J, Kozak KH, Fine PVA, Kembel SW (2009) The merging of community ecology and phylogentic biology. Ecol Lett 12:693–715. https://doi.org/10.1111/j.1461-0248.2009.01314.x
Chesson P (2000) Mechanisms of Maintenance of Species Diversity. Annu Rev Ecol Syst 31:343–366. https://doi.org/10.1146/annurev.ecolsys.31.1.343
Côté IM, Darling ES, Brown CJ (2016) Interactions among ecosystem stressors and their importance in conservation. Proc R Soc B Biol Sci 283:20152592. https://doi.org/10.1098/rspb.2015.2592
Crase B, Liedloff AC, Wintle BA (2012) A new method for dealing with residual spatial autocorrelation in species distribution models. Ecography (Cop) 35:379–388. https://doi.org/10.1111/j.1600-0587.2011.07138.x
de la Riva EG, Godoy O, Castro-Díez P, Gutiérrez-Cánovas C, Vilà M (2019) Functional and phylogenetic consequences of plant invasion for coastal native communities. J Veg Sci 30:510–520. https://doi.org/10.1111/jvs.12748
Devictor V, Clavel J, Julliard R, Lavergne S, Mouillot D, Thuiller W, Venail P, Villéger S, Mouquet N (2010) Defining and measuring ecological specialization. J Appl Ecol 47:15–25. https://doi.org/10.1111/j.1365-2664.2009.01744.x
Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM (2007) Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol Evol 22:489–496
Feld CK, Segurado P, Gutiérrez-Cánovas C (2016) Analysing the impact of multiple stressors in aquatic biomonitoring data: A ‘cookbook’ with applications in R. Sci Total Environ 573:1320–1339. https://doi.org/10.1016/j.scitotenv.2016.06.243
Flynn DFB, Gogol-Prokurat M, Nogeire T, Molinari N, Richers BT, Lin BB, Simpson N, Mayfield MM, DeClerck F (2009) Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett 12:22–33. https://doi.org/10.1111/j.1461-0248.2008.01255.x
Gallien L, Carboni M (2017) The community ecology of invasive species: where are we and what’s next? Ecography (Cop). https://doi.org/10.1111/ecog.02446
García-Mora MR, Gallego-Fernández JB, García-Novo F (1999) Plant functional types in coastal foredunes in relation to environmental stress and disturbance. J Veg Sci 10:27–34. https://doi.org/10.2307/3237157
Gaudet CL, Keddy PA (1988) A comparative approach to predicting competitive ability from plant traits. Nature 334:242–243. https://doi.org/10.1038/334242a0
González-Moreno P, Pino J, Carreras D, Basnou C, Fernández-Rebollar I, Vilà M (2013) Quantifying the landscape influence on plant invasions in Mediterranean coastal habitats. Landsc Ecol 28:891–903. https://doi.org/10.1007/s10980-013-9857-1
González-Moreno P, Pino J, Cózar A, García-de-Lomas J, Vilà M (2017) The effects of landscape history and time-lags on plant invasion in Mediterranean coastal habitats. Biol Invasions 19:549–561. https://doi.org/10.1007/s10530-016-1314-z
Gooden B, French K (2014) Non-interactive effects of plant invasion and landscape modification on native communities. Divers Distrib 20:626–639. https://doi.org/10.1111/ddi.12178
Gower JC (1971) A general coefficient of similarity and some of its properties. Biometrics 27:857–871. https://doi.org/10.2307/2528823
Grass I, Berens DG, Peter F, Farwig N (2013) Additive effects of exotic plant abundance and land-use intensity on plant-pollinator interactions. Oecologia 173:913–923. https://doi.org/10.1007/s00442-013-2688-6
Gross N, Le Bagousse-Pinguet Y, Liancourt P, Berdugo M, Gotelli NJ, Maestre FT (2017) Functional trait diversity maximizes ecosystem multifunctionality. Nat Ecol Evol 1:0132. https://doi.org/10.1038/s41559-017-0132
Grueber CE, Nakagawa S, Laws RJ, Jamieson IG (2011) Multimodel inference in ecology and evolution: challenges and solutions. J Evol Biol 24:699–711. https://doi.org/10.1111/j.1420-9101.2010.02210.x
Gutiérrez-Cánovas C, Sánchez-Fernández D, Velasco J, Millán A, Bonada N (2015) Similarity in the difference: changes in community functional features along natural and anthropogenic stress gradients. Ecology 96:2458–2466. https://doi.org/10.1890/14-1447.1
Hejda M, de Bello F (2013) Impact of plant invasions on functional diversity in the vegetation of Central Europe. J Veg Sci 24:890–897. https://doi.org/10.1111/jvs.12026
Hejda M, Pyšek P, Jarošík V (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97:393–403. https://doi.org/10.1111/j.1365-2745.2009.01480.x
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335. https://doi.org/10.1086/417659
Hooper DU, Chapin FS III, Ewel JJ (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35. https://doi.org/10.1890/04-0922
Huston MA (1997) Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia 110:449–460. https://doi.org/10.1007/s004420050180
Jauni M, Gripenberg S, Ramula S (2015) Non-native plant species benefit from disturbance: a meta-analysis. Oikos 124:122–129. https://doi.org/10.1111/oik.01416
Jucker T, Carboni M, Acosta ATR (2013) Going beyond taxonomic diversity: deconstructing biodiversity patterns reveals the true cost of iceplant invasion. Divers Distrib 19:1566–1577. https://doi.org/10.1111/ddi.12124
Kraft NJB, Adler PB, Godoy O, James EC, Fuller S, Levine JM (2015) Community assembly, coexistence and the environmental filtering metaphor. Funct Ecol 29:592–599. https://doi.org/10.1111/1365-2435.12345
Lai HR, Mayfield MM, Gay-des-combes JM, Spiegelberger T, Dwyer JM (2015) Distinct invasion strategies operating within a natural annual plant system. Ecol Lett 18:336–346. https://doi.org/10.1111/ele.12414
Laliberté E, Wells JA, Declerck F, Metcalfe DJ, Catterall CP, Queiroz C, Aubin I, Bonser SP, Ding Y, Fraterrigo JM, McNamara S, Morgan JW, Merlos DS, Vesk PA, Mayfield MM (2010) Land-use intensification reduces functional redundancy and response diversity in plant communities. Ecol Lett 13:76–86. https://doi.org/10.1111/j.1461-0248.2009.01403.x
Lee SY, Dunn RJK, Young RA, Connolly RM, Dale PER, Dehayr R, Lemckert J, McKinnon S, Powell B, Teasdale PR, Welsh DT (2006) Impact of urbanization on coastal wetland structure and function. Austral Ecol 31:149–163. https://doi.org/10.1111/j.1442-9993.2006.01581.x
MacDougall AS, Turkington R (2005) Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86:42–55. https://doi.org/10.1890/04-0669
Maceda-Veiga A, Basas H, Lanzaco G, Sala M, de Sostoa A, Serra A (2016) Impacts of the invader giant reed (Arundo donax) on riparian habitats and ground arthropod communities. Biol Invasions 18:731–749. https://doi.org/10.1007/s10530-015-1044-7
Maire V, Gross N, Börger L, Proulx R, Wirth C, da Pontes LS, Soussana JF, Louault F (2012) Habitat filtering and niche differentiation jointly explain species relative abundance within grassland communities along fertility and disturbance gradients. New Phytol 196:497–509. https://doi.org/10.1111/j.1469-8137.2012.04287.x
Maire E, Grenouillet G, Brosse S, Villéger S (2015) How many dimensions are needed to accurately assess functional diversity? A pragmatic approach for assessing the quality of functional spaces. Glob Ecol Biogeogr 24:728–740. https://doi.org/10.1111/geb.12299
Mayfield MM, Levine JM (2010) Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol Lett. https://doi.org/10.1111/j.1461-0248.2010.01509.x
Mokany K, Ash J, Roxburgh S (2008) Functional identity is more important than diversity in influencing ecosystem processes in a temperate native grassland. J Ecol 96:884–893. https://doi.org/10.1111/j.1365-2745.2008.01395.x
Moreira JM, Gámez MF, Frieyro E (2010) Mapa de usos y coberturas vegetales del suelo de Andalucía. Junta de Andalucía, Sevilla, Spain. http://www.juntadeandalucia.es/medioambiente/site/rediam/menuitem.aedc2250f6db83cf8ca78ca731525ea0/?vgnextoid=1474d61d8470f210VgnVCM2000000624e50aRCRD
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Newbold T, Hudson LN, Hill SL, Contu S, Lysenko I, Senior RA, Börger L, Bennett DJ, Choimes A, Collen B, Day J, De Palma A, Dıáz S, Echeverria-Londoño S, Edgar MJ, Feldman A, Garon M, Harrison MLK, Alhusseini T, Ingram DJ, Itescu Y, Kattge J, Kemp V, Kirkpatrick L, Kleyer M, Laginha Pinto Correia D, Martin CD, Meiri S, Novosolov M, Pan Y, Phillips HRP, Purves DW, Robinson A, Simpson J, Tuck SL, Weiher E, White HJ, Ewers RM, Mace GM, Scharlemann JP, Purvis A (2015) Global effects of land use on local terrestrial biodiversity. Nature 520:45–50. https://doi.org/10.1038/nature14324
Padrón B, Nogales M, Traveset A, Vilà M, Martínez-Abraín A, Padilla DP, Marrero P (2011) Integration of invasive Opuntia spp. by native and alien seed dispersers in the Mediterranean area and the Canary Islands. Biol Invasions 13:831–844. https://doi.org/10.1007/s10530-010-9872-y
Pakeman RJ (2011) Functional diversity indices reveal the impacts of land use intensification on plant community assembly. J Ecol 99:1143–1151. https://doi.org/10.1111/j.1365-2745.2011.01853.x
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, De Vos AC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, Ter Steege H, Van Der Heijden MGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234. https://doi.org/10.1071/BT12225
Prieur-Richard AH, Lavorel S, Linhart YB, Dos Santos A (2002) Plant diversity, herbivory and resistance of a plant community to invasion in Mediterranean annual communities. Oecologia 130:96–104. https://doi.org/10.1007/s004420100774
Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Chang Biol 18:1725–1737. https://doi.org/10.1111/j.1365-2486.2011.02636.x
Quinn LD, Holt JS (2008) Ecological correlates of invasion by Arundo donax in three southern California riparian habitats. Biol Invasions 10:591–601. https://doi.org/10.1007/s10530-007-9155-4
R Development Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Sala OE (2000) Global biodiversity scenarios for the year 2100. Science 287(80):1770–1774. https://doi.org/10.1126/science.287.5459.1770
Sanz-Elorza M, Sobrino E, Dana ED (2004) Atlas de las plantas alóctonas invasoras en España. Ministerio de Medio Ambiente, Madrid
Stevens RD, Cox SB, Strauss RE, Willig MR (2003) Patterns of functional diversity across an extensive environmental gradient: vertebrate consumers, hidden treatments and latitudinal trends. Ecol Lett 6:1099–1108. https://doi.org/10.1046/j.1461-0248.2003.00541.x
Suding KN, Lavorel S, Chapin FS, Cornelissen JHC, Díaz S, Garnier E, Goldberg D, Hooper DU, Jackson ST, Navas ML (2008) Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob Chang Biol 14:1125–1140. https://doi.org/10.1111/j.1365-2486.2008.01557.x
Suehs CM, Affre L, Médail F (2004) Invasion dynamics of two alien Carpobrotus (Aizoaceae) taxa on a Mediterranean island: I. Genetic diversity and introgression. Heredity (Edinb) 92:31–40. https://doi.org/10.1038/sj.hdy.6800374
Swenson NG, Enquist BJ, Pither J, Thompson J, Zimmerman JK (2006) The problem and promise of scale dependency in community phylogenetics. Ecology 87:2418–2424. https://doi.org/10.1890/0012-9658(2006)87[2418:TPAPOS]2.0.CO;2
Thebaud C, Abbott RJ (1995) Characterization of invasive Conyza species (Asteraceae) in Europe: quantitative trait and isozyme analysis. Am J Bot 82:360–368. https://doi.org/10.2307/2445581
Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E (1997) The influence of functional diversity and composition on ecosystem processes. Science 277(80):1300–1302. https://doi.org/10.1126/science.277.5330.1300
Traveset A, Brundu G, Carta L, Mprezetou I, Lambdon P, Manca M, Médail F, Moragues E, Rodríguez-Pérez J, Siamantziouras ASD, Suehs CM, Troumbis AY, Vilà M, Hulme PE (2008a) Consistent performance of invasive plant species within and among islands of the Mediterranean basin. Biol Invasions 10:847–858. https://doi.org/10.1007/s10530-008-9245-y
Traveset A, Moragues E, Vallardes F (2008b) Spreading of the invasive Carpobrotus aff. acinaciformis in Mediterranean ecosystems: the advantage of performing in different light environments. Appl Veg Sci 11:45–54. https://doi.org/10.3170/2007-7-18303
Valdés B, Talavera S, Fernández-Galiano E (1987) Flora vascular de Andalucía Occidental. Ketres, Barcelona (Spain)
Van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245. https://doi.org/10.1111/j.1461-0248.2009.01418.x
Vilà M, Gimeno I (2003) Seed predation of two alien Opuntia species invading Mediterranean communities. Plant Ecol 167:1–8. https://doi.org/10.1023/A:1023956108731
Vilà M, Ibáñez I (2011) Plant invasions in the landscape. Landsc Ecol 26:461–472. https://doi.org/10.1007/s10980-011-9585-3
Vilà M, Tessier M, Suehs CM, Brundu G, Carta L, Galanidis A, Lambdon P, Manca M, Médail F, Moragues E, Traveset A, Troumbis AY, Hulme PE (2006) Local and regional assessments of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. J Biogeogr 33:853–861. https://doi.org/10.1111/j.1365-2699.2005.01430.x
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Vilà M, Rohr RP, Espinar JL, Hulme PE, Pergl J, Le Roux JJ, Schaffner U, Pyšek P (2015) Explaining the variation in impacts of non-native plants on local-scale species richness: the role of phylogenetic relatedness. Glob Ecol Biogeogr 24:139–146. https://doi.org/10.1111/geb.12249
Villéger S, Mason NWH, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301. https://doi.org/10.1890/07-1206.1
Violle C, Garnier E, Lecoeur J, Roumet C, Podeur C, Blanchard A, Navas ML (2009) Competition, traits and resource depletion in plant communities. Oecologia 160:747–755. https://doi.org/10.1007/s00442-009-1333-x
Weiher E, Freund D, Bunton T, Stefanski A, Lee T, Bentivenga S (2011) Advances, challenges and a developing synthesis of ecological community assembly theory. Philos Trans R Soc B Biol Sci 366:2403–2413. https://doi.org/10.1098/rstb.2011.0056
White EM, Wilson JC, Clarke AR (2006) Biotic indirect effects: a neglected concept in invasion biology. Divers Distrib 12:443–455
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Hikosaka K, Lamont BB, Lee W, Oleksyn J, Osada N, Poorter H, Villar R, Warton DI, Westoby M, Garnier E (2005) Assessing the generality of leaf trait of global relationships. New Phytol 166:485–496. https://doi.org/10.1111/j.1469-8137.2005.01349.x
Younginger BS, Sirová D, Cruzan MB, Ballhorn DJ (2017) Is biomass a reliable estimate of plant fitness? Appl Plant Sci 5:1600094. https://doi.org/10.3732/apps.1600094
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM, Corporation Ebooks (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank E. Manzano and J. L. Espinar for their contribution in the field survey. We are grateful to K. Gross, Y.-L. Feng and the three anonymous reviewers for their helpful comments to previous versions of the manuscript. This study was supported by the project IMPLANTIN (CGL2015-65346-R) of the Spanish Ministerio de Economía y Competitividad (MINECO) and POII10˗0179˗4700 of the Junta de Comunidades de Castilla˗La Mancha (Spain). CG-C is supported by a “Juan de la Cierva-Formación” research contract (MINECO, FJCI-2015-25785) and by the European Regional Development Fund (COMPETE2020 and PT2020) and the Portuguese Foundation for Science and Technology (FCT), through the Centre of Molecular and Environmental Biology (CBMA) strategic program UID/BIA/04050/2019 (POCI-01-0145-FEDER-007569) and the STREAMECO project (Biodiversity and ecosystem functioning under climate change: from the gene to the stream, PTDC/CTA-AMB/31245/2017). DS-F is supported by a post-doctoral contract funded by Universidad de Castilla-La Mancha and the European Social Fund (ESF). PGM was supported by CABI Development Fund, with contributions from Department for International Development (DFID, UK), Australian Center for International Agricultural Research (ACIAR, Australia) and Directorate for International Cooperation (DGIS, Netherlands), and by a “Juan de la Cierva-Incorporación” contract (MINECO, IJCI-2017-31733). MV acknowledges support through the Junta de Andalucía projects RNM-4031 (Spain) and the Spanish Severo Ochoa Program for Centers of Excellence in R+D+I (SEV-2012-0262).
Author information
Authors and Affiliations
Contributions
CG-C, DS-F, PG-M, PC-D and MV conceived the ideas and designed methodology; PG-M and MV collected the data; CG-C, DS-F and EM-N compiled and codified trait data with critical inputs from the rest of authors; CG-C analysed the data with assistance of PG-M; CG-C led the writing of the manuscript. All authors contributed critically to data interpretation and manuscript reviewing and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Yu-Long Feng.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gutiérrez-Cánovas, C., Sánchez-Fernández, D., González-Moreno, P. et al. Combined effects of land-use intensification and plant invasion on native communities. Oecologia 192, 823–836 (2020). https://doi.org/10.1007/s00442-020-04603-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04603-1