Abstract
Nitrogen deposition in the northeastern US changed N availability in the latter part of the twentieth century, with potential legacy effects. However, long-term N cycle measurements are scarce. N isotopes in tree rings have been used as an indicator of N availability through time, but there is little verification of whether species differ in the strength of this signal. Using long-term records at the Fernow Experimental Forest in West Virginia, we examined the relationship between soil conditions, including net nitrification rates, and wood δ15N in 2014, and tested the strength of correlation between tree ring δ15N of four species and stream water NO3− loss from 1971 to 2000. Higher soil NO3− was weakly associated with higher wood δ15N across species, and higher soil net nitrification rates were associated with higher δ15N for Quercus rubra only. The δ15N of Liriodendron tulipifera and Q. rubra, but neither Fagus grandifolia nor Prunus serotina, was correlated with stream water NO3−. L. tulipifera tree ring δ15N had a stronger association with stream water NO3− than Q. rubra. Overall, we found only limited evidence of a relationship between soil N cycling and tree ring δ15N, with a strong correlation between the wood δ15N and NO3− leaching loss through time for one of four species. Tree species differ in their ability to preserve legacies of N cycling in tree ring δ15N, and given the weak relationships between contemporary wood δ15N and soil N cycle measurements, caution is warranted when using wood δ15N to infer changes in the N cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forests in the northeastern United States experienced high deposition of reactive nitrogen (N) over the last half-century (Galloway et al. 2004). Since primary production in these ecosystems is often N-limited, the retention of N in forests is typically high and supply is low relative to demand (a “closed” N cycle). However, high inputs of N from atmospheric deposition can alter N transformations within the system and reduce forest watershed N retention. For example, elevated N deposition can cause high rates of N mineralization and nitrification, and elevated losses of mobile nitrate (NO3−) in stream water (Peterjohn et al. 1996; Aber et al. 1998; Lovett and Goodale 2011), with possible long-term impacts including the loss of important base cations and changes in understory and overstory species composition (Edwards and Helvey 1991; Gilliam et al. 1996, 2016; May et al. 2005). Changes in species composition, caused by climate change or forest pests, can also alter N availability and promote N leaching losses (Lovett et al. 2002; Peterjohn et al. 2015; Crowley and Lovett 2017), and watershed N losses shift throughout the course of succession (Vitousek and Reiners 1975; Lovett et al. 2018). However, few long-term records of changes in N cycling exist, complicating rigorous assessment of the legacy effects of N deposition and other long-term changes in N retention. Sediment cores are an effective proxy of catchment N cycling over extended time scales (McLauchlan et al. 2007), but lack the potential for fine temporal or spatial resolution in forests affected by N deposition. However, the δ15N signature of plant tissue could potentially serve as such an indicator of soil N cycling.
Plant tissue δ15N should reflect changes in the local soil N cycle (Robinson 2001; Pardo et al. 2006; Pardo and Nadelhoffer 2012; Falxa-Raymond et al. 2012). In the absence of fractionation during N uptake, N assimilated by plants and incorporated into tissue should have a similar isotopic signature as the soil N pool from which it was acquired. In the N cycle of a temperate forest, the primary fractionating step in well-drained soils with little denitrification is microbial nitrification, a process that preferentially uses the lighter 14N isotope, and that results in a 15N-enriched NH4+ substrate pool and a 15N-depleted NO3− product pool (Handley and Raven 1992). As an anion, NO3− is readily leached into stream water, while NH4+ remains associated with cation exchange sites in the soil. So, when N deposition causes an increase in nitrification, more 14NO3− is leached from the plant available N pool, resulting in a shift towards higher 15N abundance under more “open” N cycling (Martinelli et al. 1999; Pardo and Nadelhoffer 2012). Although plants acquire N as both NH4+ and NO3−, over time, the loss of 15N-depleted NO3− increases the overall δ15N of N retained in the system. One complication is the potential for fractionation upon the uptake of N, particularly in species associated with ectomycorrhizal fungi (ECM) (Hobbie and Högberg 2012), which would decouple the δ15N signatures of plant tissue and available soil N. However, ECM colonization and abundance typically declines in areas of high N deposition (Kjøller et al. 2012; Bahr et al. 2013), reducing the effect of ECM on plant δ15N Central Appalachian forests. So, if the δ15N signature of plant tissue reflects the plant available N pool, it would shift accordingly when the supply of N into the system exceeds N demand and could be used as a proxy for reduced N retention in the absence of direct measurements.
Since central Appalachian temperate broadleaf forests historically received high anthropogenic N deposition, which is now declining, the δ15N signature of tree rings may be particularly useful as a long-term indicator of changes in N cycling in these ecosystems. Annual preservation of tissue in tree rings potentially make them a powerful tool for studying temporal changes in the N cycle (Gerhart and McLauchlan 2014). Past research using tree ring δ15N to detect distinct isotope signatures from N pollution from fossil fuel burning (Saurer et al. 2004; Bukata and Kyser 2007; Savard et al. 2009) and track N cycle perturbations after known disturbances and fertilizer additions provide support for the signal preservation in wood N isotopes (Elhani et al. 2003, 2005; Hart and Classen 2003; Burnham et al. 2016). Generally, the direct influence of point source pollution tends to drive tree ring δ15N signatures towards the signature of the source, which is typically high δ15N for fossil-fuel pollution sources (Elliott et al. 2007). In addition, elevated long-term deposition may cause a change in soil nitrification, N isotopic fractionation, and mycorrhizal function, increasing the δ15N of plant available N over time (Högberg and Johannisson 1993; Bukata and Kyser 2007; Högberg et al. 2014). As a result, the record of δ15N in tree rings has been used to infer changes in ambient N availability. For example, declines in tree ring δ15N have been detected in North America (Poulson et al. 1995; Härdtle et al. 2013; Kranabetter et al. 2013), and are often attributed to lower N availability and reduced ecosystem N losses (McLauchlan et al. 2007, 2017; McLauchlan and Craine 2012; Elmore et al. 2016). Thus, tree ring δ15N could be used as an indicator of past N cycle perturbation leading to low N retention in Central Appalachian watersheds and a measure of changes in the cycling of N as anthropogenic deposition declines.
Previous research suggests that higher foliar δ15N is associated with elevated nitrification and soil N availability in tropical (Martinelli et al. 1999) and temperate forests (Garten 1993; Falxa-Raymond et al. 2012). However, species may differ in the degree to which tree ring δ15N would reflect soil processes. The form of N taken up can differ between species (Templer and Dawson 2004), and fractionation by soil N transformation processes changes the δ15N signature of different N pools. Also, there is evidence of fractionation against 15N during the transfer of N compounds to plants by ectomycorrhizal (EM) fungi (Hobbie and Högberg 2012; Kranabetter et al. 2013), decoupling the δ15N of EM species’ tree rings from that of the soil when trees are well colonized with their EM symbionts. Arbuscular mycorrhizal (AM) species are often associated with greater rates of nitrification relative to EM trees (Phillips et al. 2013; Lin et al. 2017), further distinguishing their potential tree ring δ15N trends. Furthermore, differences in wood anatomy and function could lead to varying N retranslocation (Del Arco et al. 1991), lateral mobility of N within bole wood (Elhani et al. 2003; Tomlinson et al. 2014; Goodale 2017), and internal cycling of N within active woody tissue (El Zein et al. 2011) between species. So, it is important to determine how well soil N processes and availability relate to wood δ15N of different species and mycorrhizal types, and to use existing records of N retention and loss to validate tree ring δ15N as a proxy of changes in N cycling.
Final considerations that could impact the tree ring δ15N record are any temporal changes in the δ15N signature of N deposition due to different emission sources (Elliott et al. 2007). For example, the δ15N of deposition tends to be higher during the dormant season as a result of the greater influence of coal-fired power generation (Elliott et al. 2007; Rose et al. 2015). If the δ15N signature from power generation is typically high (Heaton 1990), then vegetation downwind from power plants might have tree rings enriched in δ15N regardless of the ecosystem’s inherent rates of nitrification and N retention. A reduction in the amount of power plant-derived deposition would reduce its influence on the δ15N of the system and the temporal trend in tree ring δ15N. Thus, it is currently far from established that tree ring δ15N effectively records and preserves the N status of ecosystems through time.
Given the uncertainty in how well tree ring δ15N of different species reflects soil N processes and availability and how closely tree ring δ15N relates to watershed N retention and loss through time, we investigated how nitrification and other soil factors influenced wood δ15N of two arbuscular mycorrhizal (AM) and two ectomycorrhizal (EM) tree species within a reference watershed (WS 4) at the Fernow Experimental Forest (FEF) that exhibits very low N retention. We also determined if tree ring δ15N reflected temporal changes in stream water NO3− discharge. Differences in the δ15N of tree rings should reflect the microbially mediated processes of nitrification and mycorrhizal N contribution. As such, we expected to find a positive relationship between wood δ15N and both nitrification and soil NO3−, with AM trees having stronger relationships due to the lower N contribution, and thus reduced fractionation, by their mycorrhizal symbionts. This watershed has one of the longest continuous stream water NO3− records in the eastern United States, and exhibited a 145% increase in stream water NO3−, which has been attributed to long-term N deposition and N saturation (Peterjohn et al. 1996). There is a positive correlation between nitrification, soil NO3−, and stream water NO3− concentration through time at the FEF (Gilliam and Adams 2011), and so the increase in WS 4 stream water NO3− was likely caused by temporal trends towards greater soil nitrification and reduced N retention. Thus, we expected tree ring δ15N to be positively related to stream water NO3− concentration through time. Finally, we expected a stronger relationship between tree ring δ15N and stream water NO3− in portions of the watershed with higher nitrification and extractable NO3− pools.
Methods
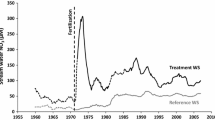
The FEF is a US Forest Service research site located in Tucker County, WV, USA (39.054839°N, 79.688580°W). Watershed 4 (WS 4, 38.7 ha) (Fig. 1) is a long-term reference watershed, last commercially logged circa 1910 and allowed to naturally regrow since that time. The predominant soil is a Calvin channery silt loam (loamy-skeletal, mixed, mesic Typic Dystrochrept), elevation ranges from approximately 760–840 m, and the slope averages 16% at a southeasterly aspect (Kochenderfer 2006). Nearly continuous hydrologic and biogeochemical measurements in the watershed include stream flow and precipitation since 1951 and 1952, stream chemistry including N export since 1970, and bulk N deposition (wet + particulate) since 1983 (Fig. 2). Annually, FEF WS 4 receives an average of ~ 145 cm of rainfall. The forest is a stand of mixed hardwood species consisting of Quercus rubra (32.3% of basal area), Acer saccharum (13.1%), Acer rubrum (11.2%), Prunus serotina (9.4%), Liriodendron tulipifera (7.9%), Quercus prinus (5.9%), and Fagus grandifolia (3.4%). Around 1980, stream water NO3− concentration increased ~ 145% (Fig. 3a), contributing to a 435% increase in watershed NO3−–N discharge from 1975 to 1984. Bulk N deposition data (wet + particulate N), which extend back to 1983 and can be estimated via relationship to wet deposition data back to 1979 (NADP 2016), reveal a long-term decline in deposition (Fig. 2). In contrast, after the increase in N output circa 1980, discharge remained elevated through ~ 2010. The elevated stream water NO3− and decrease in N deposition resulted in a shift in N retention in WS 4 from ~ 80% of annual N inputs in 1979–1980 to less than 30% since the early 1990s, and it exhibited the lowest total N retention of measured watersheds in the Northeastern United States over this time frame (Campbell et al. 2004). Although no records of the δ15N signature of N deposition exist here, if a reduction in isotopically enriched power plant-derived N (Heaton 1990) caused the long-term decline in N deposition, then it is possible that the δ15N of deposited N declined as well.
Stream water NO3− concentration (a) and tree ring δ15N signature (b–e) since 1971. The temporal trend in stream water NO3− is visualized using a 3-year running average of the monthly, flow-weighted stream water NO3− concentration. The average tree ring δ15N (n = 7 for each species) is shown for each 3-year time segment, 1971–2000. Note the difference in y-axis scale for L. tulipifera
During July 2014, we collected increment cores from four major tree species within FEF WS 4 (seven individual trees per species): Q. rubra, P. serotina, L. tulipifera, and F. grandifolia (Fig. 1). These represent both arbuscular mycorrhizal (P. serotina and L. tulipifera) and ectomycorrhizal (Q. rubra and F. grandifolia) species, and Burnham et al. (2017) found that they take up both NO3− and NH4+ in approximately equal proportion. To ensure that the tree ring record extended back to the commencement of stream water NO3− measurements (1970), we chose mature, canopy trees of each species > 40 cm in diameter at breast height (DBH), except for one somewhat smaller F. grandifolia tree (32 cm DBH). Since each species is not evenly distributed across the watershed, we opportunistically selected trees that were near (within ~ 25 m) trees from the other species being examined, when possible, to minimize species differences in δ15N due to their spatial location and elevation (Garten 1993). Due to the relative scarcity of canopy beech trees, two cored trees were spatially separated from the other species (Fig. 1). We collected two increment cores from each tree, one from either side of the tree parallel to the contour of the land, using 5-mm diameter increment borers (Mora of Sweden, Mora, Sweden). Increment corers were rinsed with deionized water between trees, and the cores were air dried prior to processing for ring width and wood δ15N. One core from each tree was mounted and sanded to better visualize the annual growth rings. The ring widths were measured and cores were cross-dated, using the dplR package for R to assess cross-dating accuracy (Bunn 2010). The second core from each tree was used for δ15N analysis. Due to the lack of an established, consistent labile-N extraction method (Gerhart and McLauchlan 2014), we analyzed raw, non-extracted wood. The most recently formed wood, from the 2014 tree ring, was separated from each core to compare its δ15N signal with contemporary N cycle measurements in nearby soil. To analyze the temporal relationship between tree ring δ15N and stream water NO3− concentration, each core was cut into 3-year segments from 1971 to 2000, which included the ~ 1980 increase in stream NO3− and the subsequent discharge dynamics through the year 2000. Each ring from 2014, and the 1971–2000 ring segments, were ground into a fine powder using a dental amalgamator (Henry Schein, Inc., Melville, NY), and 8–10 mg of tissue from each were wrapped in tin capsules. The δ15N of each segment was measured via isotope ratio gas chromatography–mass spectrometry by the Central Appalachians Stable Isotope Facility at the University of Maryland Center for Environmental Science Appalachian Laboratory (Frostburg, MD). The mobility of non-structural N compounds within the tree spurred efforts to extract labile N prior to isotopic analysis (Gerhart and McLauchlan 2014), but these have met mixed results, with some reduction of δ15N variability (Elhani et al. 2003), but little change in temporal trends (Elhani et al. 2003; Caceres et al. 2011; Bunn et al. 2017). As such, we did not perform any labile N extraction.
To determine if measures of soil N status are related to tree ring δ15N, we collected mineral soils from under each cored tree in July of 2014. The area of the vertically projected canopy under each tree was divided into four quadrants, and two soil cores were extracted from each quadrant, within ~ 3 m of the trunk, using a 2.2-cm inner diameter soil-recovery probe (AMS, Inc., American Falls, ID, USA). The top 5 cm of mineral soil from the soil cores were pooled into one sample for each tree. Prior to sieving, the soils were weighed to calculate their total dry mass (using the gravimetric water content) and bulk density. The soils were then sieved to pass through a 5.6-mm (#3.5) mesh testing sieve, and a subsample used for gravimetric determination of moisture content, in which 5–6 g of soil were weighed before and after drying for 48 h at 65 °C.
We measured net mineralization, ammonification, and nitrification potentials in the collected soils using a lab incubation. One ~ 10-g subsample of soil was incubated in the dark at room temperature for 5 days to account for any collection disturbance effect on N transformation rates, and a second subsample was incubated for an additional 28 days to measure the rate of NH4+ and NO3− production in the soil. After incubation, both subsamples were extracted in 1 M KCl by gently shaking for 30 min, filtering through a polyethersulfone filter with 0.45 μM pore size (Supor membrane, Pall Life Sciences, Ann Arbor, MI, USA), and storing at − 20 °C until NO3− and NH4+ analyses were performed using a Lachat QuikChem 8500 Series 2 Auto-analyzer (QuikChem Methods 12-107-04-1-B and 12-107-06-2-A).
Statistical analyses
We used multiple linear regressions to test if current inorganic soil N pools or soil N transformation rates could predict non-standardized tree ring δ15N of the most recent wood (2014). We ran a regression with the δ15N of the 2014 tree ring of each species as the dependent variable and independent variables of soil potential net mineralization, potential net nitrification, the percent of mineralized N nitrified to NO3− (percent nitrification), the pool of extractable NH4+, and the pool of extractable NO3−. Due to the nature of the predictor variables examined, we assessed multicollinearity using variance inflation factors (VIF values). To adhere to the assumption of independence of predictors, as best as possible, we excluded those variables from the regression model that had VIF values of > 5. In our final models, the VIF values were all < 1.5.
Raw, unstandardized tree ring δ15N data are supplied as an online resource (ESM 1) and are available in the National Centers for Environmental Information tree ring paleoclimate data repository. Differences in N content and raw δ15N between species and mycorrhizal types were assessed via 2-way ANOVA. To reduce the between-tree variability, and focus on the long-term temporal trend in tree ring δ15N, we standardized the isotope values of the 3-year time segments by subtracting the within-tree mean wood δ15N (Gerhart and McLauchlan 2014; Burnham et al. 2016). The seven replicate trees for each species were then averaged within each 3-year time segment. We used a Pearson correlation analysis to measure the strength and significance (α = 0.05) of the relationship between both the basal area-weighted average of δ15N across species and average tree ring δ15N within each species, and the annual average of monthly flow-weighted stream water NO3− concentrations through time. To determine if the strength of the relationship between tree ring δ15N and stream water NO3− concentration depended on tree species, we tested this interaction via ANCOVA with tree ring δ15N as the response, species as a categorical factor, and stream water NO3− concentration as a covariate. In all analyses, we originally included a categorical watershed position variable to account for the loose clustering of sampled trees (Fig. 1), and a covariate of elevation, but these never had significant effects and were not included in the final results. All statistical analyses were completed using Minitab 17 statistical software (Minitab, Inc., State College, PA, USA) and the R statistical environment (R Core Team 2018).
Results
The average net mineralization and nitrification potentials under the tree canopies were 1.02 μg N g−1 dry soil day−1 and 1.01 μg N g−1 dry soil day−1, respectively, and they did not significantly differ between species (Table 1). The net nitrification potential was 96.7% of the net mineralization potential (i.e. “relative nitrification”), and this, as well as extractable soil NO3− levels, also did not differ between species. However, the extractable NH4+ pool was different between species (F = 6.45, P = 0.002), with more extractable NH4+ present in soils under P. serotina and Q. rubra trees (6.1 and 5.2 μg N g−1 dry soil, respectively) than under L. tulipifera (2.0 μg N g−1 dry soil). Soils under F. grandifolia had intermediate levels of extractable NH4 (2.9 μg N g−1 dry soil) (Table 1).
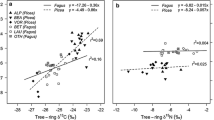
We did not find any concurrent changes in BAI across species, and within-species mean inter-series ring-width correlations were relatively low (Q. rubra =0.09, L. tulipifera = 0.16, P. serotina = 0.13, F. grandifolia = − 0.05). This is likely because our site is a relatively undisturbed, mesic forest and not a sensitive site typical of dendrochronological studies that investigate strong growth responses to changes in climate or disturbance. The mean wood N content differed between species (F = 28.9, P < 0.01), with P. serotina having lower mean N content from 1971 to 2000 (0.07% ± 0.01%) than the other species (0.10% ± 0.01%). However, in 2014, the species did not differ in N content. The mean raw, non-standardized δ15N of 2014 wood was 1.44‰, and did not differ between species (F = 0.04, P = 0.96) nor by mycorrhizal type (F = 0.17, P = 0.69). However, there was an 8.6‰ range of 2014 tree ring δ15N values, from − 3.04 to 5.56‰ (Table ESM1), and the overall mean raw δ15N value, across all species, increased from − 0.13 to 1.44‰ from 1971 to 2014. Across all species examined, higher soil NO3− was weakly positively associated with 2014 tree ring δ15N (R2 = 18.3%, F = 6.27, P = 0.022), and there was a trend towards this effect depending on species (F = 2.50, P = 0.092), with 2014 tree ring δ15N of Q. rubra, but not other species, increasing with higher soil NO3− (P = 0.029) (Fig. 4). For L. tulipifera trees, extractable soil NH4+ was negatively associated with the 2014 tree ring δ15N (R2 = 59.9%, F = 7.45, P = 0.041, data not shown). For Q. rubra, the 2014 tree ring δ15N was positively associated with net nitrification potential (R2 = 79.7%, F = 19.6, P = 0.007) (Fig. 4). There were no soil N pool or transformation rate variables that were significantly associated with the 2014 tree ring δ15N of P. serotina or F. grandifolia.
Relationship between non-standardized 2014 tree ring δ15N, 2014 soil KCl-extractable NO3− (a), and net nitrification potential (b). Only significant (P < 0.05) trend lines are shown: all species (solid line) and Q. rubra (dashed line) for soil NO3−, Q. rubra only (dashed line) for net nitrification potential
The monthly flow-weighted mean stream water NO3− concentration in FEF WS 4 increased from ~ 12 μM in the early 1970s to ~ 50 μM in the early 1980s (Fig. 3a). Despite long-term declines in N deposition (Fig. 2), stream water NO3− concentration and N discharge remained constant from the mid-1980s through 2000 (Fig. 3a), only starting to decline around 2009 (Fig. 2). Across species, the basal area-weighted tree ring δ15N was positively correlated with stream water NO3− concentration from 1971 to 2000 (r = 0.88, P < 0.001). However, this was driven by two of the four species. Within tree species, the average Q. rubra (r = 0.81, P = 0.004) and L. tulipifera (r = 0.91, P < 0.001) standardized tree ring δ15N were positively correlated with stream water NO3− concentration from 1971 to 2000, but significant correlations were not found for P. serotina (P = 0.46) or F. grandifolia (P = 0.44). As such, the relationship between stream water NO3− and tree ring δ15N differed between species (F = 27.3, P < 0.001). The temporal dynamics found in stream water NO3− concentrations (1971–2000) were well characterized by the temporal changes in the standardized tree ring δ15N of L. tulipifera (Fig. 3b). Over the ~ 10-year period from 1977 to 1979 until 1986–1988, L. tulipifera standardized tree ring δ15N increased from − 2.2 to 1.2‰ (+ 3.4‰). While the temporal pattern found in the δ15N record of Q. rubra tree rings captured the overall trend in stream water NO3− concentrations (Fig. 3c), it was less sensitive to the abruptness of change than the record contained in the rings of L. tulipifera. The standardized tree ring δ15N of Q. rubra increased from − 0.39 to 0.06‰ (+ 0.45‰) from 1971–1973 through 1980–1982, and later increased to 0.23‰ in 1989–1991 and 0.31‰ in 1998–2000.
Discussion
Across the four species examined, we found only mixed evidence that nitrification and soil NO3− affect wood δ15N. Although soil NO3− was related to wood δ15N across species, it explained only a small portion (R2 = 18%) of the variability in tree ring δ15N signatures. The strength of this relationship differed between species, with higher nitrification and soil NO3− only increasing tree ring δ15N of Q. rubra, and L. tulipifera 2014 tree ring δ15N only responding (negatively) to soil NH4+. These effects lend only marginal support for using tree ring δ15N as an indicator of soil N availability, and only for one species, Q. rubra. In the FEF, nitrification is relatively high across the watersheds (Gilliam et al. 2018), particularly mid-season (Gilliam et al. 2001). These site characteristics and our mid-season sampling may have reduced likelihood of detecting a strong relationship between soil N cycling and the δ15N of contemporary tree rings of different species. In addition, remobilized N contributes substantially to current-year leaf and root growth (Millard and Grelet 2010; Bazot et al. 2016). If internal non-structural N stores contribute to new wood N, then this would decouple wood δ15N from current-year soil N cycle processes. It is also likely that internal cycling of N within woody tissue, which has been measured in Quercus and Fagus species (El Zein et al. 2011), is partly responsible for this lack of relationship. The large range and high variability of δ15N values in newly formed wood (8.6‰) greatly complicated detecting any species differences in effects of soil N cycling on isotope values. Although we found a large range of wood δ15N values, previous studies report ranges of 4–5‰ (Savard et al. 2009; Reimchen and Arbellay 2018) and often reduce between-tree variability by standardizing δ15N values within tree core to isolate temporal trends across a site or region (Savard et al. 2009; McLauchlan and Craine 2012; McLauchlan et al. 2017).
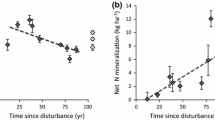
Although our results do not support the use of wood δ15N as an indicator of soil N cycling, other studies have reported positive relationships between soil N and tree ring δ15N. Kranabetter and Meeds (2017) and Kranabetter et al. (2013) found that Douglas fir wood δ15N was positively associated with N availability and site index. The forest used in these studies was a low N coniferous forest with lower N process rates, whereas we measured high rates of percent nitrification (Table 1), typical across the FEF (Gilliam et al. 2018), and we sampled mid-season when nitrification rates are highest in this forest (Gilliam et al. 2001). Furthermore, Kranabetter et al. (2013) show that the difference between soil and wood δ15N (termed Δ15N) is negatively associated with soil N supply, suggesting that higher N availability may have caused lower transfer of N from EM fungi to the plant because fractionation during N transfer leads to high Δ15N values (Kranabetter et al. 2013). If the positive association between soil N availability and Douglas fir wood δ15N was driven by reductions in mycorrhizal colonization, then such an association would not be detectable in higher N systems already characterized by low transfer of N to plants from mycorrhizae, such as the FEF and Catskill Mountains (Templer et al. 2007).
The inconsistent relationship between wood δ15N and soil N cycling makes it difficult to support the use of tree ring δ15N records as a proxy for N retention and loss through time in Central Appalachia. However, our study differs from prior research in that we also used stream water NO3− concentration as an independent, long-term index of N retention. In our study, the tree ring δ15N records for L. tulipifera and Q. rubra were positively correlated with direct measurements of stream water NO3− concentration in FEF WS 4, and the similarity between the tree ring δ15N record for L. tulipifera and the temporal pattern in stream water NO3− concentration was particularly striking. The 3‰ increase in δ15N coincided with a 38 μM increase (317%) in stream water NO3− that has been attributed to N saturation under long-term anthropogenic deposition (Peterjohn et al. 1996). The heartwood–sapwood transition for this species did not occur until the rings formed in the late-1980s, and so did not contribute to the prior change in δ15N. Although the tree ring δ15N of Q. rubra reflected the overall trend in stream NO3− concentrations, it appears to be less sensitive to shorter term dynamics. Therefore, our results suggest that wood δ15N is a poor indicator of contemporary nitrification rates, but tree ring δ15N in some species preserves long-term changes in the δ15N of the plant available pool under elevated NO3− leaching.
It is surprising that tree ring δ15N of two species appears to relate to N retention and loss through time, but not to current-year N cycle dynamics in a temperate forest with relatively high N availability. As an explanation for this phenomenon, we suggest that changes in tree ring δ15N through time are controlled most by shifts in mycorrhizal N contribution. Since mycorrhizal transfer of N fractionates against 15N, high rates of colonization and mycorrhizal N contribution would reduce plant tissue δ15N relative to the soil (Hobbie and Ouimette 2009; Hobbie and Högberg 2012). We then hypothesize that as N availability increased in the FEF under high N deposition, reductions in mycorrhizal colonization and N transfer increased plant tissue δ15N towards that of the soil, which is typically higher than plant tissue (Templer et al. 2007). However, the extent to which AM fungi contribute to tree N nutrition and the fractionation during their possible N transfer is uncertain (Hobbie and Ouimette 2009). Thus, this may be a particularly important consideration for the use of δ15N in tree rings of EM species, whereas fractionation by nitrifying soil microbes may be a stronger influence of tree ring δ15N of AM species. Further measurements of soil and plant δ15N and mycorrhizal colonization across a gradient of N availability for these EM and AM species are needed to discern the mechanisms behind changes in tree ring δ15N.
While Q. rubra tree ring δ15N was positively correlated with stream water NO3−, it appeared to better capture the long-term trend in stream NO3− concentration (Fig. 3), rather than the shorter term dynamics. Though no measurements of mycorrhizal colonization exist at this site, it is possible that N deposition-induced reductions in EM colonization causing lower fungal transfer to Q. rubra trees were gradual, since Quercus species are typically associated with lower N cycling rates (Templer et al. 2007; Piatek et al. 2009; Peterjohn et al. 2015). Also, there is evidence of the movement of N compounds between rings after ring formation in Q. rubra (Burnham et al. 2016) and in other species (Elhani et al. 2003; Hart and Classen 2003). Inter-annual mobility of N would smooth out the preserved signal of any response of tree ring δ15N to a perturbation in the N cycle and result in the observed response of Q. rubra to long-term N cycle changes in WS 4. Interestingly, Q. rubra was the only species that had a relationship between soil nitrification and contemporary tree ring δ15N. This suggests that inter-annual mobility of N compounds within this species is partial and/or takes place in subsequent years after wood formation. Finally, a long-term increase in Acer saccharum at the FEF (Schuler and Gillespie 2000) might also contribute to gradual changes in N availability and nitrification (Lovett et al. 2002, 2004; Phillips et al. 2013), in turn affecting the δ15N signature of co-occurring species such as Q. rubra. Although Q. rubra does not capture short-term dynamics as precisely as L. tulipifera in this study, it appears to more consistently respond to both short-term perturbations in the N cycle (Burnham et al. 2016) and the long-term changes in N availability.
It was also notable that two species, P. serotina and F. grandifolia, showed no relationship between tree ring δ15N and stream water NO3− in WS 4 or between any soil N pools or transformation rates and δ15N of newly formed woody tissue. It is unclear why P. serotina tree ring δ15N did not respond to changes in watershed N cycling, particularly since urea fertilization increased P. serotina tree ring δ15N in a nearby watershed (Burnham et al. 2016). However, this species had lower mean wood percent N from 1971 to 2000 than the other species, but equal in contemporary 2014 wood. This could indicate higher mobility of N compounds within this species, obscuring any temporal δ15N trend. For F. grandifolia, Elhani et al. (2003) used 15N-enriched label addition to show that N can be mobile within the woody tissue by detecting δ15N enrichment in wood formed prior to 15N treatment. This species may also be an ineffective recorder of the N saturation signal, because it can be more responsive to surrounding stand dynamics and changes in the light environment than patterns of long-term N deposition. Indeed, we observed that individual F. grandifolia trees would often exhibit short-term increases in ring width that did not coincide with other F. grandifolia trees in the same watershed, which caused a poor mean ring-width inter-series correlation for this species (− 0.05 vs. 0.13 for all other species). This pattern we attribute to the fact that, in WS 4, F. grandifolia is common in the sub-canopy, where it grows slowly until the canopy is disturbed. This is consistent with the observation that the annual coefficient of variation in F. grandifolia BAI (85% across all years) was much higher than the values for the other species (all ~ 50% or lower). Further evidence was also seen in the concurrent growth response of four F. grandifolia trees in a nearby watershed when it was harvested in the late 1950s (Burnham et al. 2016). As a result, we suspect that the short-term increases in F. grandifolia N demand during periods of rapid growth generally reduced leaching in the surrounding soil and possibly increased mycorrhizal colonization and N transfer, hindering this species’ ability to integrate long-term changes in N supply and demand within WS 4.
Since power plant N pollution is typically enriched in δ15N in this region (Elliott et al. 2007), the reduction in deposition since the early 1980s (Fig. 2) suggests a reduction in δ15N of N input into the watershed. Studies that use tree ring isotopes to detect changes in pollution rely on pollution signatures that are distinct from the background δ15N of the ecosystem and N input rates that are high relative to internal N cycling (Gerhart and McLauchlan 2014). Though long-term data on the δ15N signal of precipitation do not exist here, Rose et al. (2015) found that within-year precipitation δ15N values are highly variable at this site, ranging from − 6.0 to + 5.1‰, with an annual average of − 0.1‰. Additionally, inputs of N via deposition, ~ 15 kg N ha−1 year−1 at their highest, are low compared to internal rates of N cycling, 114 kg N ha−1 year−1 for nitrification (Gilliam et al. 2001). If the δ15N of deposition exerted strong control over tree ring δ15N, we would expect a decline in the wood δ15N through time and similar δ15N values across trees that reflect the signature of deposition. However, we detected an overall increase in tree ring δ15N through time, and high variability in raw δ15N (Table ESM1). Thus, we do not believe that the δ15N signal of deposited N greatly impacted the δ15N record in tree rings at this site.
Although tree ring δ15N has been used as an indicator of changes in N availability and retention through time (Craine et al. 2009; Hietz et al. 2011; Elmore et al. 2016; McLauchlan et al. 2017), there is little verification using independent measurements of N availability and leaching loss. In our study, contemporary measurements of N cycle processes and N availability were not related to δ15N of newly formed wood. This finding contributes to a small body of evidence that wood δ15N is, at best, an inconsistent indicator of local N cycling (Templer et al. 2007; Kranabetter et al. 2013; Tomlinson et al. 2016; Kranabetter and Meeds 2017). However, we were able to detect a meaningful temporal signal of N availability, based on stream water NO3− losses, in two of the four species we examined. At our site, these mixed results suggest that past changes in mycorrhizal colonization and N transfer caused by increasing N availability under long-term N deposition exerted significant control over tree ring and plant δ15N, at least for EM species, and this mechanism should be further explored across a broader range of N availability. To further our understanding of temporal changes in plant δ15N, studies should also focus on determining the dominant forms of N in wood and how they are formed and cycled within the plant, and any if isotopic fractionation occurs during these processes. Overall, our results warrant caution in interpreting tree ring δ15N trends until more is known about the mechanisms that govern wood δ15N variation.
References
Aber J, McDowell W, Nadelhoffer K et al (1998) Nitrogen saturation in temperate forest ecosystems. Bioscience 48:921–934
Bahr A, Ellström M, Akselsson C et al (2013) Growth of ectomycorrhizal fungal mycelium along a Norway spruce forest nitrogen deposition gradient and its effect on nitrogen leakage. Soil Biol Biochem 59:38–48. https://doi.org/10.1016/j.soilbio.2013.01.004
Bazot S, Fresneau C, Damesin C, Barthes L (2016) Contribution of previous year’s leaf N and soil N uptake to current year’s leaf growth in sessile oak. Biogeosciences 13:3475–3484. https://doi.org/10.5194/bg-13-3475-2016
Bukata AR, Kyser TK (2007) Carbon and nitrogen isotope variations in tree-rings as records of perturbations in regional carbon and nitrogen cycles. Environ Sci Technol 41:1331–1338
Bunn AG (2010) Statistical and visual crossdating in R using the dplR library. Dendrochronologia 28:251–258. https://doi.org/10.1016/j.dendro.2009.12.001
Bunn AG, Helfield JM, Gerdts JR et al (2017) A solvent-based extraction fails to remove mobile nitrogen from western redcedar (Thuja plicata). Dendrochronologia 44:19–21. https://doi.org/10.1016/j.dendro.2017.03.001
Burnham MB, McNeil BE, Adams MB, Peterjohn WT (2016) The response of tree ring δ15N to whole-watershed urea fertilization at the Fernow Experimental Forest, WV. Biogeochemistry 130:133–145. https://doi.org/10.1007/s10533-016-0248-y
Burnham MB, Cumming JR, Adams MB, Peterjohn WT (2017) Soluble soil aluminum alters the relative uptake of mineral nitrogen forms by six mature temperate broadleaf tree species: possible implications for watershed nitrate retention. Oecologia. https://doi.org/10.1007/s00442-017-3955-8
Caceres MLL, Mizota C, Yamanaka T, Nobori Y (2011) Effects of pre-treatment on the nitrogen isotope composition of Japanese black pine (Pinus thunbergii) tree-rings as affected by high N input. Rapid Commun Mass Spectrom 25:3298–3302. https://doi.org/10.1002/rcm.5227
Campbell JL, Hornbeck JW, Mitchell MJ et al (2004) Input-output budgets of inorganic nitrogen for 24 forest watersheds in the northeastern United States: a review. Water Air Soil Pollut 151:373–396
Crowley KF, Lovett GM (2017) Effects of nitrogen deposition on nitrate leaching from forests of the northeastern United States will change with tree species composition. Can J For Res 47:997–1009. https://doi.org/10.1139/cjfr-2016-0529
Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TE, Hobbie EA, Kahmen A, Mack MC, McLauchlan KK, Michelsen A, Nardoto GB, Pardo LH, Peñuelas J, Reich PB, Schuur EAG, Stock WD, Templer PH, Virginia RA, Welker JM, Wright IJ (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183(4):980–992
Del Arco JM, Escudero A, Garrido MV (1991) Effects of site characteristics on nitrogen retranslocation from senescing leaves. Ecology 72:701–708. https://doi.org/10.2307/2937209
Edwards P, Helvey J (1991) Long-term ionic increases from a Central Appalachian forested watershed. J Environ Qual 20:250–255
El Zein R, Maillard P, Bréda N et al (2011) Seasonal changes of C and N non-structural compounds in the stem sapwood of adult sessile oak and beech trees. Tree Physiol 31:843–854. https://doi.org/10.1093/treephys/tpr074
Elhani S, Lema B, Zeller B et al (2003) Inter-annual mobility of nitrogen between beech rings: a labelling experiment. Ann For Sci 60:503–508. https://doi.org/10.1051/forest
Elhani S, Guehl J-M, Nys C et al (2005) Impact of fertilization on tree-ring δ15N and δ13C in beech stands: a retrospective analysis. Tree Physiol 25:1437–1446
Elliott EM, Kendall C, Wankel SD et al (2007) Nitrogen isotopes as indicators of NOx source contributions to atmospheric nitrate deposition across the midwestern and northeastern United States. Environ Sci Technol 41:7661–7667
Elmore AJ, Nelson DM, Craine JM (2016) Earlier springs are causing reduced nitrogen availability in North American eastern deciduous forests. Nat Plants 2:16133. https://doi.org/10.1038/nplants.2016.133
Falxa-Raymond N, Patterson AE, Schuster WSF, Griffin KL (2012) Oak loss increases foliar nitrogen, δ15N and growth rates of Betula lenta in a northern temperate deciduous forest. Tree Physiol 32:1092–1101. https://doi.org/10.1093/treephys/tps068
Galloway J, Dentener F, Capone D et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Garten C (1993) Variation in foliar 15N abundance and the availability of soil nitrogen on Walker Branch Watershed. Ecology 74:2098–2113
Gerhart LM, McLauchlan KK (2014) Reconstructing terrestrial nutrient cycling using stable nitrogen isotopes in wood. Biogeochemistry 120:1–21. https://doi.org/10.1007/s10533-014-9988-8
Gilliam FS, Adams MB (2011) Effects of nitrogen on temporal and spatial patterns of nitrate in streams and soil solution of a central hardwood forest. ISRN Ecol 2011:1–9
Gilliam FS, Adams MB, Yurish BM (1996) Ecosystem nutrient responses to chronic nitrogen inputs at Fernow Experimental Forest, West Virginia. Can J For Res 26:196–205
Gilliam FS, Yurish BM, Adams MB (2001) Temporal and spatial variation of nitrogen transformations in nitrogen-saturated soils of a central Appalachian hardwood forest. Can J For Res 31:1768–1785. https://doi.org/10.1139/cjfr-31-10-1768
Gilliam FS, Welch NT, Phillips AH et al (2016) Twenty-five-year response of the herbaceous layer of a temperate hardwood forest to elevated nitrogen deposition. Ecosphere 7:e01250. https://doi.org/10.1002/ecs2.1250
Gilliam FS, Walter CA, Adams MB, Peterjohn WT (2018) Nitrogen (N) dynamics in the mineral soil of a Central Appalachian hardwood forest during a quarter century of whole-watershed N additions. Ecosystems 21:1489–1504. https://doi.org/10.1007/s10021-018-0234-4
Goodale CL (2017) Multiyear fate of a 15N tracer in a mixed deciduous forest: retention, redistribution, and differences by mycorrhizal association. Glob Change Biol 23:867–880. https://doi.org/10.1111/gcb.13483
Handley LL, Raven JA (1992) The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant Cell Environ 15:965–985. https://doi.org/10.1111/j.1365-3040.1992.tb01650.x
Härdtle W, Niemeyer T, Assmann T et al (2013) Long-term trends in tree-ring width and isotope signatures (δ13C, δ15N) of Fagus sylvatica L. on soils with contrasting water supply. Ecosystems 16:1413–1428. https://doi.org/10.1007/s10021-013-9692-x
Hart SC, Classen AT (2003) Potential for assessing long-term dynamics in soil nitrogen availability from variations in δ15N of tree rings. Isotopes Environ Health Stud 39:15–28. https://doi.org/10.1080/1025601031000102206
Heaton THE (1990) 15N/14N ratios of NOx from vehicle engines and coal-fired power stations. Tellus 42:304–307. https://doi.org/10.3402/tellusb.v42i3.15223
Hietz P, Turner BL, Wanek W, Richter A, Nock CA, Wright SJ (2011) Long-term change in the nitrogen cycle of tropical forests. Science 334(6056):664–666
Hobbie EA, Högberg P (2012) Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol 196:367–382. https://doi.org/10.1111/j.1469-8137.2012.04300.x
Hobbie EA, Ouimette AP (2009) Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 95:355–371. https://doi.org/10.1007/s10533-009-9328-6
Högberg P, Johannisson C (1993) 15N abundance of forests is correlated with losses of nitrogen. Plant Soil 157:147–150
Högberg P, Johannisson C, Högberg MN (2014) Is the high 15N natural abundance of trees in N-loaded forests caused by an internal ecosystem N isotope redistribution or a change in the ecosystem N isotope mass balance? Biogeochemistry 117:351–358. https://doi.org/10.1007/s10533-013-9873-x
Kjøller R, Nilsson L, Hansen K et al (2012) Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytol 194:278–286
Kochenderfer JN (2006) Fernow and the Appalachian Hardwood Region. In: Adams M, DeWalle D, Hom J (eds) The Fernow Watershed acidification study. Springer, Dordrecht, pp 17–39
Kranabetter JM, Meeds JA (2017) Tree ring δ15N as validation of space-for-time substitution in disturbance studies of forest nitrogen status. Biogeochemistry 134:201–215. https://doi.org/10.1007/s10533-017-0355-4
Kranabetter JM, Saunders S, MacKinnon JA et al (2013) An assessment of contemporary and historic nitrogen availability in contrasting coastal Douglas-fir forests through δ15N of tree rings. Ecosystems 116:111–122. https://doi.org/10.1007/s10021-012-9598-z
Lin G, Mccormack ML, Ma C, Guo D (2017) Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol 213:1440–1451. https://doi.org/10.1111/nph.14206
Lovett GM, Goodale CL (2011) A new conceptual model of nitrogen saturation based on experimental nitrogen addition to an oak forest. Ecosystems 14:615–631. https://doi.org/10.1007/s10021-011-9432-z
Lovett GM, Weathers KC, Arthur MA (2002) Control of nitrogen loss from forested watersheds by soil carbon:nitrogen ratio and tree species composition. Ecosystems 5:712–718. https://doi.org/10.1007/s10021-002-0153-1
Lovett GM, Weathers KC, Arthur MA, Schultz JC (2004) Nitrogen cycling in a northern hardwood forest: do species matter? Biogeochemistry 67:289–308. https://doi.org/10.1023/B:BIOG.0000015786.65466.f5
Lovett GM, Goodale CL, Ollinger SV et al (2018) Nutrient retention during ecosystem succession: a revised conceptual model. Front Ecol Environ 16:1–7. https://doi.org/10.1002/fee.1949
Martinelli LA, Piccolo MC, Townsend AR et al (1999) Nitrogen stable isotopic composition of leaves and soil: tropical versus temperate forests. Biogeochemistry 46:45–65
May JD, Burdette SB, Gilliam FS, Adams MB (2005) Interspecific divergence in foliar nutrient dynamics and stem growth in a temperate forest in response to chronic nitrogen inputs. Can J For Res 35:1023–1030. https://doi.org/10.1139/x05-036
McLauchlan KK, Craine JM (2012) Species-specific trajectories of nitrogen isotopes in Indiana hardwood forests, USA. Biogeosciences 9:867–874. https://doi.org/10.5194/bg-9-867-2012
McLauchlan KK, Craine JM, Oswald WW et al (2007) Changes in nitrogen cycling during the past century in a northern hardwood forest. Proc Natl Acad Sci USA 104:7466–7470. https://doi.org/10.1073/pnas.0701779104
McLauchlan KK, Gerhart LM, Battles JJ et al (2017) Centennial-scale reductions in nitrogen availability in temperate forests of the United States. Sci Rep 7:7856. https://doi.org/10.1038/s41598-017-08170-z
Millard P, Grelet GA (2010) Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiol 30:1083–1095. https://doi.org/10.1093/treephys/tpq042
National Atmospheric Deposition Program (NADP) (2016) NADP Program Office, Wisconsin State Laboratory of Hygiene, 465 Henry Mall, Madison, WI 53706
Pardo LH, Nadelhoffer KJ (2012) Using nitrogen isotope ratios to assess terrestrial ecosystems at regional and global scales. In: West JB, Bowen GJ, Dawson TE, Tu KP (eds) Isoscapes. Springer, Dordrecht, pp 221–249
Pardo LH, Templer PH, Goodale CL et al (2006) Regional assessment of N saturation using foliar and root δ15N. Biogeochemistry 80:143–171. https://doi.org/10.1007/s10533-006-9015-9
Peterjohn WT, Adams MB, Gilliam FS (1996) Symptoms of nitrogen saturation in two central Appalachian hardwood forest ecosystems. Biogeochemistry 35:507–522
Peterjohn WT, Harlacher MA, Christ MJ, Adams MB (2015) Testing associations between tree species and nitrate availability: do consistent patterns exist across spatial scales? For Ecol Manag 358:335–343. https://doi.org/10.1016/j.foreco.2015.09.018
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol 199:41–51. https://doi.org/10.1111/nph.12221
Piatek KB, Munasinghe P, Peterjohn WT et al (2009) Oak contribution to litter nutrient dynamics in an Appalachian forest receiving elevated nitrogen and dolomite. Can J For Res 39:936–944. https://doi.org/10.1139/X09-028
Poulson SR, Chamberlain CP, Friedland AJ (1995) Nitrogen isotope variation of tree rings as a potential indicator of environmental change. Chem Geol 125:307–315. https://doi.org/10.1016/0009-2541(95)00097-6
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reimchen TE, Arbellay E (2018) Intra-annual variability in isotopic and total nitrogen in tree rings of old growth Sitka spruce from coastal British Columbia. Botany 96:851–857
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
Rose LA, Elliott EM, Adams MB (2015) Triple nitrate Isotopes indicate differing nitrate source contributions to streams across a nitrogen saturation gradient. Ecosystems 18:1209–1223. https://doi.org/10.1007/s10021-015-9891-8
Saurer M, Cherubini P, Ammann M et al (2004) First detection of nitrogen from NOx in tree rings: a 15 N/14 N study near a motorway. Atmos Environ 38:2779–2787. https://doi.org/10.1016/j.atmosenv.2004.02.037
Savard MM, Bégin C, Smirnoff A et al (2009) Tree-ring nitrogen isotopes reflect anthropogenic NOx emissions and climatic effects. Environ Sci Technol 43:604–609
Schuler T, Gillespie A (2000) Temporal patterns of woody species diversity in a central Appalachian forest from 1856 to 1997. J Torrey Bot Soc 127:149–161
Templer PH, Dawson TE (2004) Nitrogen uptake by four tree species of the Catskill Mountains, New York: implications for forest N dynamics. Plant Soil 262:251–261. https://doi.org/10.1023/B:PLSO.0000037047.16616.98
Templer PH, Arthur MA, Lovett GM, Weathers KC (2007) Plant and soil natural abundance δ15N: indicators of relative rates of nitrogen cycling in temperate forest ecosystems. Oecologia 153:399–406. https://doi.org/10.1007/s00442-007-0746-7
Tomlinson G, Siegwolf R, Buchmann N et al (2014) The mobility of nitrogen across tree-rings of Norway spruce (Picea abies L.) and the effect of extraction method on tree-ring δ15N and δ13C values. Rapid Commun Mass Spectrom 28:1–7. https://doi.org/10.1002/rcm.6897
Tomlinson G, Buchmann N, Siegwolf R et al (2016) Can tree-ring δ15N be used as a proxy for foliar δ15N in European beech and Norway spruce? Trees Struct Funct 30:627–638. https://doi.org/10.1007/s00468-015-1305-1
Vitousek PM, Reiners WA (1975) Ecosystem succession and nutrient retention: a hypothesis. Bioscience 25:376–381
Acknowledgements
The authors thank Chris Walter, Rachel Arrick, Jessica Graham, Hoff Lindberg, Hannah Hedrick, and Leah Baldinger for their field and laboratory assistance on this study. We also acknowledge the USDA Forest Service staff at the Fernow Experimental Forest for long-term management of the site and support of this project. This work was supported by the Long-Term Research in Environmental Biology (LTREB) program at the National Science Foundation (Grant nos. DEB-0417678 and DEB-1019522) and the WVU Department of Biology and Eberly College of Arts and Sciences.
Author information
Authors and Affiliations
Contributions
MBB and WTP conceived the idea. MBA maintained and processed long-term N data, and MBB and WTP performed field core and soil collection and laboratory analyses. MBA provided editorial advice, and MBB and WTP analyzed the data and wrote the manuscript.
Corresponding author
Additional information
Communicated by Richard P. Phillips.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Burnham, M.B., Adams, M.B. & Peterjohn, W.T. Assessing tree ring δ15N of four temperate deciduous species as an indicator of N availability using independent long-term records at the Fernow Experimental Forest, WV. Oecologia 191, 971–981 (2019). https://doi.org/10.1007/s00442-019-04528-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04528-4