Abstract
Despite being central concepts for life history theory, little is known about how reproductive effort and costs vary with individual age once plants have started to reproduce. We conducted a 5-year field study and estimated age-dependent reproductive effort for both sexes in the extraordinarily long-lived dioecious plant Borderea pyrenaica. We also evaluated costs of reproduction on vital rates for male and female plants, both by examining effects of differences in individual reproductive effort under natural conditions, and by conducting a flower removal experiment, aimed at decreasing reproductive effort. Reproductive effort was fairly constant and independent of age for males, which may reflect a strategy of adjusting overall reproductive output by spreading reproduction over the life course. Females had a higher total effort, which first increased and then decreased with age. The latter may be a response to an increasing reproductive value—an inverse of a terminal investment—or a sign of reproductive senescence due to an age-related physiological decline. Seed production was lower in plants with higher previous reproductive effort and this effect increased with age. We found no evidence for costs of reproduction on other vital rates for either sex. Experimental flower removal only resulted in progressively more negative effects on flower production in older male plants, whereas female vital rates were unaffected. Overall, this study demonstrates that not only sex, but also age influences resource allocation trade-offs and, thus, plant life history evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproduction is necessary for persistence of all species. Investing resources into producing offspring has short- and long-term consequences, and the optimal investment strategies depend on the abiotic and biotic environment. As a result, there is a substantial variation of reproductive strategies in terms of when to start, how often to reproduce, and how much to invest. Reproductive effort, the proportion of an organism’s resources allocated to reproduction (Samson and Werk 1986), may be expected to change over the life span of individuals as an evolutionary response to changes in reproductive value [the expected average contribution of individuals of a given age to future population growth rate (Caswell 2001)]. However, although some studies have documented increases in reproductive effort with age for young plants (Lacey 1986; Ehlers and Olesen 2004), little is known about how reproductive effort changes with age in polycarpic plants that have started to reproduce. In species where survival decreases with advancing age (actuarial senescence), such as birds and mammals, future reproduction events become progressively less certain, and a progressive increase in reproductive effort could be expected to maximize fitness (Williams 1966; Pianka and Parker 1975). In particular, a large investment into reproduction very late in life has been called ‘terminal investment’ (Williams 1966) and seems to be common in animals (Galimberti et al. 2007; Descamps et al. 2007, but see Clutton-Brock 1984; Velando et al. 2006). Alternatively, reproductive senescence may accompany actuarial senescence, that is, reproductive effort may decline throughout an organism’s life span due to physiological decline (Thomas 2013).
Plants show a wide variation in life span (Ehrlén and Lehtilä 2002), but few studies have investigated late-life changes in reproductive effort. Koenig and colleagues (2017) found no evidence for terminal investment, in terms of that trees of eight Quercus species in California that died during the study had invested more into reproduction compared to surviving trees. In most investigated species, reproductive investment has been found to be relatively constant over age for established plants of similar size (Dahlgren and Roach 2017). However, we are not aware of studies explicitly investigating how reproductive effort and reproductive value change with age. For some plants, such as the extremely long-lived species Pinus longaeva and Borderea pyrenaica, increases in reproductive value due to increases in survival may occur (Hiebert and Hamrick 1983; García et al. 2011). In these species, where future reproduction becomes more certain with age, we may expect a decrease in reproductive effort as plants become older.
Likewise, reproductive “costs” (lowered future reproduction, growth and survival; Stearns 1992; Roff 1992) may change over life spans. The concept of resource allocation trade-offs is central to life-history theory and reproductive costs have been shown in many taxa, including long-lived plants (Obeso 2002; Sletvold and Ågren 2015). Changes in the cost of reproduction over age could occur if individuals either senesce or become physiologically more robust as they age. However, little is known about how effects of reproduction on future performance change with age.
In dioecious organisms, reproductive effort and costs usually differ between sexes, and female plants often appear to invest more into reproduction than males (Meagher and Antonovics 1982a). A lower reproductive effort in males than in females would imply that male plants have more resources to invest in growth and survival (Stearns 1992). However, female plants may compensate for the higher reproductive investment by reproducing less frequently, delaying the onset of reproduction, and increasing their reproductive investment from low levels in young plants. If male reproduction is less costly, we may thus expect earlier reproduction and overall flatter age trajectories of reproductive effort than in females.
In this study, we used observational demographic data recorded over 5 years and field experiments to examine age- and sex-dependent reproductive effort and costs of reproduction in the long-lived dioecious herb Borderea pyrenaica. Age determination in our study species is possible because of a morphological characteristic of the tubers, offering an unusual opportunity to explore how reproductive parameters change over the life course of individuals. Our data set included almost seven hundred individuals of ages up to 260 years in an ecologically stressful but stable alpine scree environment in the central Pyrenees. Previous studies did not find evidence of demographic or physiological senescence in this plant, but instead showed that reproductive value increased with age for both sexes (García et al. 2011; Morales et al. 2013) and that males tend to flower more frequently than females (García and Antor 1995b). Here, we tested the following three hypotheses: (a) reproductive effort is similar in males and females over their life courses, because the higher investment per reproductive event in females is balanced by a lower frequency of reproductive events; (b) reproductive effort decreases with age in both sexes because the reproductive value increases, but the decrease in males is less pronounced because they have a lower overall effort; (c) reproductive costs are age dependent and decrease with age after that individuals have attained their maximum size and have more resources to invest into reproduction, again the decrease being less pronounced in male plants.
Materials and methods
Study system
Borderea pyrenaica (Dioscoreaceae) is a small relict species endemic to limestone screes in the Central Pyrenees, usually at altitudes higher than 1800 m a.s.l. Pollinators include ants, flies and lady beetles, fruit set is high and there is no evidence for pollen limitation (García et al. 1995). By the latter half of May, each plant has grown one short aboveground stem, flowering begins in late June and fruit dispersal in early September. At the end of the growing season, this stem dies back, leaving behind a scar on the tuber (García and Antor 1995a). Counting the number of scars gives an age estimate. Sex of reproductive individuals is usually easily identifiable throughout the growing season as flowers remain on the plant. Males start reproducing at younger ages (10–20 years) than females (15–35 years), have a larger number of flowers, and flower more frequently (almost every year) than females (García and Antor 1995b).
Data collection and preparation
Data were collected yearly from 1995 to 1999 in the Pineta valley (42°41′N, 0°06′E; 2000 m a.s.l.), located in the central Pyrenees. The density of B. pyrenaica in this population varies considerably, reaching up to a few hundred plants per square metre (García et al. 2011). Individuals were carefully mapped to be relocated in successive years (aboveground parts wilt in fall), and for each plant and year we recorded state (dead or alive), sex (vegetative, male or female), number of leaves, length of the longest leaf, number of flowers in males, and the number of fruits and seeds within each fruit in females. Aerial vegetative biomass (“size” hereafter) was estimated for all plants as log(number of leaves × leaf length2). Tubers were collected the final year of the study and were weighed and aged. Due to a severe drought that made male flowers die back early in the season and counts unreliable, the number of flowers in males was not recorded in 1999. Age estimates for individuals of unknown age but known size were imputed for both sexes based on generalised additive models (GAM) of age on size (García et al. 2011).
The analyses were based on two demographic data sets: observational data on 518 individuals during the period 1995–1999 which was analysed previously to investigate changes in flowering probability, flower number, seed number and reproductive value with age (García et al. 2011), and experimental data on 181 reproductive individuals of both sexes, whose reproductive effort was manipulated by flower removal both in 1996 and 1997. Experimental plants were assigned to one of two treatments: (1) control (no treatment) and (2) flower removal (in both years for individuals that flowered the second year, otherwise only in the first year). We conducted the experiment because we expected costs of reproduction to potentially be obscured by positive correlations between reproduction and plant condition.
To convert our measures of size and reproductive output into dry weights (g), we also collected aerial parts of 32 female and 32 male reproductive plants, counted the number of fruits and male flowers, estimated size as defined above, and dried and weighed reproductive and vegetative parts separately. The dry weights were then regressed on corresponding counts of fruits or male flowers, or size, and the regression coefficients were used to translate field recordings of number of fruits, male flowers and size into corresponding dry weights (Online Resource 1).
Age trajectories of reproductive investment and effort
Reproductive investment (weight of reproductive parts) and reproductive effort (proportion of aerial biomass allocated to flowers and fruits for males and females) were regressed on age using generalised additive models, which allow flexible nonlinear patterns reflecting potential non-monotonic changes in reproductive effort and investment with age. Each individual plant was assigned one value of reproductive investment or effort, being the sum of values for each year. We did not include tuber biomass in these calculations, as this was only measured once, at the end of the study. However, aerial and tuber biomass were tightly correlated (Online Resource 2), and our estimates of aerial reproductive investment and effort should, therefore, be proportional to values based on plant total biomass. We applied the default thin-plate smoothing splines in the “gam” function of the package “mgcv” for the statistical software R. Initially, models including all individuals were fitted, but since sex and the interaction term of sex and age had statistically significant effects on trajectories of flower number, seed number and size (p < 0.05), separate models were fitted for the two sexes.
Effects of flower removal
Generalised linear models (GLMs) were used to examine age-specific effects of flower removal for males and females separately. Treatment, individual age, and the interaction between treatment and age were used as predictor variables. Size in the previous year was also included as a covariate. We tested effects on five response variables: tuber biomass in the final year of the study, and size and fecundity in 1998 and 1999. Size was set to zero for plants that were dormant in 1 year and for 14 plants that did not emerge in the last year. Death is a very rare event in this species. Thus, survival was not modelled, and the potential effects of treatment on dormancy and survival are included in the analyses of size. Models of tuber biomass and size were fitted as ordinary linear regression models, in GLMs with “identity” link functions and Gaussian error distributions. Fecundity was first analysed as total seed number and male flower number in all individuals (reproducing or not), in models with log link functions and quasi-Poisson error distributions (accounting for overdispersion relative to the Poisson distribution). Female fruit number and seed number were strongly correlated (Online Resource 3). Effects on fecundity were also examined for separate components: flowering probability, flower and fruit number in flowering individuals, and seed number in female plants that produced fruits. Both methods yielded similar results, and only the models of seed number and male flower number for all individuals are presented. Wald tests were used to calculate p values.
Observational study of costs of reproduction
Data from the observational study were used also to examine the cost of reproduction, in terms of relationships between past reproduction and current vital rates. The response variables were the same as in the analyses of the experimental flower removal study and were analysed using the same types of regression models. All individuals that flowered at least once during the study were included in analyses. The total numbers of seeds produced per female (Online Resource 4) and flowers produced per male (Online Resource 5) in 1995, 1996 and 1997 were regarded as measures of reproductive investment and used as predictor variables, together with age and size the previous year.
Results
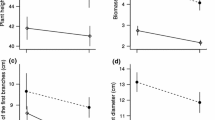
Generalised linear models indicated that for all ages, females invested more biomass into reproduction per year than males, also when accounting for the lower frequency of reproductive events in females (Fig. 1a, b). The pattern was similar for reproductive effort (Fig. 1c, d).
Reproductive investment measured as (ln) weight of reproductive structures (g) and reproductive effort (proportion of plant biomass invested in reproductive elements) over individual age for a, c female and b, d male individuals of Borderea pyrenaica, respectively. Each point represents an individual plant’s summed reproductive investment or effort over the entire observation period. Continuous lines represent spline fits of GAM models and dashed lines represent the 95% confidence intervals
There were also sex differences in the age trajectories of reproductive investment and effort. In male plants, there was a continuous increase in reproductive investment with age. In contrast, in females there was an early increase in reproductive investment, followed by a decline starting at ages of about 70 years, when individuals attained their maximum size. The divergence of age trajectories between sexes was slightly more pronounced for reproductive effort (Fig. 1c, d). In males, there was no apparent increase with age in reproductive effort, whereas reproductive effort decreased in females at ages above 70 years. The decreases in female reproductive investment and effort above 70 years were statistically significant (Online Resource 6).
Experimental flower removal did not affect size or seed production in female plants in the short term (Table 1). In males, flower removal reduced size in 1998, but not in 1999. There was also an interactive effect of age and treatment on male flower number: flower removal resulting in lower flower number in plants older than 40 years (median and mean ages were 65 and 80.5, respectively), and effects of treatment being larger in older plants (note that effects in Table 1 are the effects of not receiving the flower removal treatment, i.e. of an increased reproductive investment, and thus corresponding to effects of increased reproductive investment in Table 2).
The results of the observational study suggest an age-dependent cost of reproduction for female plants: fecundity (in 1999 but not 1998) was lower in plants with higher previous reproductive effort and this negative effect of previous reproduction increased with age (Table 2; note that model predictions based on both main effects and the interaction term are that, except for plants with combinations of low previous reproduction and low age, effects of increasing previous reproduction are negative). For other response variables, our analyses suggested overall positive effects of having a higher previous reproduction in both sexes (Table 2). Female plants with higher previous reproductive investment had higher aerial biomass in 1998 and tuber biomass in 1999. However, older females with higher previous reproductive effort also tended to have a lower aerial biomass in 1999 and this effect became stronger with increasing age (p = 0.095). Male plants with higher previous reproductive investment had a higher tuber biomass and subsequent flower number, with a tendency (p = 0.07) of increasingly positive relationship between previous reproductive effort and flower number with age.
Discussion
Our results show that reproductive effort and costs of reproduction are age dependent in the long-lived Borderea pyrenaica and that age trajectories differ between male and female plants. Males had an overall lower reproductive effort than females and were hardly affected by plant age, while female reproductive effort first slightly increased and then decreased with age. Also costs of reproduction were age dependent in females. Late-life age dependence of reproductive effort has been shown previously for animals (e.g. Ericsson et al. 2001), but not in plants, and we are not aware of any previous study with plants suggesting age-dependent reproductive costs.
In Borderea pyrenaica, the greater reproductive effort per reproductive event in females than in males is not fully compensated for by a lower frequency of female reproductive events. Sex differences in reproductive effort may reflect differing strategies of resource allocation. In polygynous animals, males appear to invest more resources into reproduction than females through high costs of traits favouring competition for mates (Promislow et al. 1992; Clutton-Brock and Isvaran 2007). Male plants do not compete in other ways than by increasing pollen (flower) production, and females often invest more resources than males into reproductive tissue (Meagher and Antonovics 1982b). The higher female reproductive effort in B. pyrenaica is somewhat surprising given the lack of differences in growth or survival between sexes (García et al. 2011). It is possible that our measure of reproductive effort is not fully adequate, since it is only based on biomass and does not account for nutrient concentrations. Moreover, quantifying reproductive effort is difficult in our study species because both male inflorescences and female fruits are photosynthetically active. However, we would not expect higher nutrient concentrations in male tissues than in females, and despite that fruits might contribute to energy acquisition during ripening, resources invested in fruits are eventually lost at dispersal whereas nutrients may be resorbed from male inflorescences after the growing season. As a result, we expect the identified difference in reproductive effort to be conservative.
Also the shape of age trajectories of reproductive effort differed between sexes. One explanation of the observed differences in both overall effort and the shape of trajectories between sexes may be that a lower effort, per flower and also over their life course, allows males to spread their reproduction more evenly over their life spans than females. That females show lower reproductive effort early in life may be because the high minimum cost of reproduction, in terms of producing one fruit, makes it optimal for females to stay non-reproductive for a longer period than males. Our observation of a lower effort with advancing age in older female plants may be explained by the fact that their reproductive value seems to increase with age due to increasing survival (García et al. 2011). Under these circumstances, it should not be beneficial for individuals to invest more resources into reproduction as they age if such investments affect survival negatively, because future reproduction becomes gradually more certain (Williams 1966). The observed tendency of decreasing reproductive effort with age could also simply be a consequence of physiological decline (Thomas 2013), even though no evidence of such declines has been detected previously in this species (Morales et al. 2013).
Our results regarding reproductive costs, in terms of effects of previous reproduction on vital rates, may at first glance seem contradictory. In our experimental study, we found no effect of flower removal on females and a negative effect on male plants. Small effects of a 2-year treatment for plants that can live longer than two centuries and have a prominent storage organ (tuber) are not surprising (Ehrlén and van Groenendael 2001; Obeso 2002; and see also Aragon et al. (2009) for effects on a short-lived plant). However, we still expect to detect age-dependent costs if the effect of age is strong. For male plants, it appears that negative effects of removing photosynthetic tissue associated with inflorescences outweighed any positive effects of reduced reproductive investment on future vital rates. It is possible that the treatment had negative effects also in females, which cancelled out positive effects of reduced reproductive effort. However, in several other studies flower removal has led to increases in future reproduction or in other vital rates (Hartemink et al. 2004). Analysing the observational data, we found some positive relationships between high reproductive effort and future vital rates, which are likely a result of reproductive effort being positively correlated with plant condition (Obeso 2002). However, we also found that older female plants produced more seeds after having a lower previous reproductive effort, indicating a cost of reproduction, and that this effect increased with plant age. The fact that we did find evidence for an age-dependent cost of reproduction, despite the short study period in relation to the maximum life span of this plant, suggests to us that costs may be substantial. Taken together, and also considering the observed differences in reproductive investment and effort over the plants’ life course, these results may illustrate causes of differences in life history strategies among sexes. Males maintain a fairly constant reproductive effort as they age, but at the cost of becoming frailer at higher ages, as indicated by the larger negative effect of the treatment in older males. Females, on the other hand, having higher costs per reproductive event than males, and a larger reproductive effort that first increases (young females) but then decreases (old females) seem to adjust their fecundity to their resource state.
In conclusion, the age dependence of reproductive effort and reproductive costs, and the differences between sexes shown in this study, suggests that age and sex shape plant life history strategies. Borderea pyrenaica shows an increasing survival and reproductive value with advancing age, and no evidence for an increase of investment into reproduction at high ages, but instead a slight decline in female reproductive effort after reproductive maturity. This pattern may either be a response to an increasing reproductive value, or the consequence of physiological decline. More demographic studies considering plant ageing are needed to understand plant life history diversification and how this is affected by resource allocation trade-offs.
References
Aragón CF, Méndez M, Escudero A (2009) Survival costs of reproduction in a short-lived perennial plant: live hard, die young. Am J Bot 96(5):904–911. https://doi.org/10.3732/ajb.0800223
Caswell H (2001) Matrix population models, 2nd edn. Sinauer Associates, Sunderland
Clutton-Brock TH (1984) Reproductive effort and terminal investment in iteroparous animals. Am Nat 123(2):212–229. https://doi.org/10.1086/284198
Clutton-Brock TH, Isvaran K (2007) Sex differences in ageing in natural populations of vertebrates. Proc R Soc B Biol 274(1629):3097–3104. https://doi.org/10.1098/rspb.2007.1138
Dahlgren JP, Roach DA (2017) Demographic senescence in herbaceous plants. In: Shefferson R, Jones OR, Salguero-Gómez R (eds) The evolution of senescence in the tree of life. Cambridge University Press, Cambridge, pp 303–319. https://doi.org/10.1017/9781139939867.015
Descamps S, Boutin S, Berteaux D, Gaillard JM (2007) Female red squirrels fit Williams’ hypothesis of increasing reproductive effort with increasing age. J Anim Ecol 76(6):1192–1201. https://doi.org/10.1111/j.1365-2656.2007.01301.x
Ehlers BK, Olesen JM (2004) Flower production in relation to individual plant age and leaf production among different patches of Corydalis intermedia. Plant Ecol 174:71–78. https://doi.org/10.1023/B:VEGE.0000046060.77491.b9
Ehrlén J, Lehtilä K (2002) How perennial are perennial plants? Oikos 98:308–322. https://doi.org/10.1034/j.1600-0706.2002.980212.x
Ehrlén J, van Groenendael J (2001) Storage and the delayed costs of reproduction in the understorey perennial Lathyrus vernus. J Ecol 89(2):237–246. https://doi.org/10.1046/j.1365-2745.2001.00546.x
Ericsson G, Wallin K, Ball JP, Broberg M (2001) Age-related reproductive effort and senescence in free-rangin moose, Alces alces. Ecology 82(6):1613–1620. https://doi.org/10.1890/0012-9658
Galimberti F, Sanvito S, Braschi C, Boitani L (2007) The cost of success: reproductive effort in male southern elephant seals (Mirounga leonina). Behav Ecol Sociobiol 62(2):159–171. https://doi.org/10.1007/s00265-007-0450-y
García MB, Antor RJ (1995a) Age and size structure in populations of a long-lived dioecious geophyte: Borderea pyrenaica (Dioscoreaceae). Int J Plant Sci 156(2):236–243. https://doi.org/10.1086/297246
García MB, Antor RJ (1995b) Sex ratio and sexual dimorphism in the dioecious Borderea pyrenaica (Dioscoreaceae). Oecologia 101(1):59–67. https://doi.org/10.1007/BF00328901
García MB, Antor RJ, Espadaler X (1995) Ant pollination of the palaeoendemic dioecious Borderea pyrenaica (Dioscoreaceae). Plant Syst Evol 198(1–2):17–27. https://doi.org/10.1007/BF00985105
García MB, Dahlgren JP, Ehrlén J (2011) No evidence of senescence in a 300-year-old mountain herb. J Ecol 99(6):1424–1430. https://doi.org/10.1111/j.1365-2745.2011.01871.x
Hartemink N, Jongejans E, de Kroon H (2004) Flexible life history responses to flower and rosette bud removal in three perennial herbs. Oikos 105:159–167. https://doi.org/10.1111/j.0030-1299.2004.12784.x
Hiebert RD, Hamrick JL (1983) Patterns and levels of genetic variation in Great Basin Bristlecone Pine, Pinus longaeva. Evolution 37(2):302–310. https://doi.org/10.1111/j.1558-5646.1983.tb05540.x
Koenig WD, Knops JMH, Carmen WJ, Pesendorfer MB (2017) Testing the terminal investment hypothesis in California oaks. Am Nat 189(5):564–569. https://doi.org/10.1086/691161
Lacey EP (1986) Onset of reproduction in plants: size versus age-dependency. Trends Ecol Evol 1(3):72–75. https://doi.org/10.1016/0169-5347(86)90021-2
Meagher TR, Antonovics JJ (1982a) Life history variation in dioecious plant populations: a case study of Chamaelirium luteum. In: Dingle H, Hegmann JP (eds) Evolution and genetics of life histories. Springer, Berlin, pp 139–154. https://doi.org/10.1007/978-1-4684-6270-8_9
Meagher TR, Antonovics JJ (1982b) The population biology of Chamaelirium luteum, a dioecious member of the lily family. Ecology 63(6):1690–1700. https://doi.org/10.2307/1940111
Morales M, Oñate M, García MB, Munné-Bosch S (2013) Photo-oxidative stress markers reveal absence of physiological deterioration with ageing in Borderea pyrenaica, an extraordinarily long-lived herb. J Ecol 101(3):555–565. https://doi.org/10.1111/1365-2745.12080
Obeso JR (2002) The costs of reproduction in plants. New Phytol 155(139):321–348. https://doi.org/10.1046/j.1469-8137.2002.00477.x
Pianka ER, Parker WS (1975) Age-specific reproductive tactics. Am Nat 109(968):453–464. https://doi.org/10.1086/283013
Promislow DEL, Montgomerie R, Martin TE (1992) Mortality cost of sexual dimorphism in birds. Proc R Soc B Biol 250(1328):143–150. https://doi.org/10.1098/rspb.1992.0142
Roff DA (1992) The evolution of life histories. Chapman and Hall, New York
Samson DA, Werk KS (1986) Size-dependent effects in the analysis of reproductive effort in plants. Am Nat 127(5):667–680. https://doi.org/10.1086/284512
Sletvold N, Ågren J (2015) Nonlinear costs of reproduction in a long-lived plant. J Ecol 103(5):1205–1213. https://doi.org/10.1111/1365-2745.12430
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Thomas H (2013) Senescence, ageing and death of the whole plant. New Phytol 197:696–711. https://doi.org/10.1111/nph.12047
Velando A, Drummond H, Torres R (2006) Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proc R Soc B Biol 273(1593):1443–1448. https://doi.org/10.1098/rspb.2006.3480
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100(916):687–690. https://doi.org/10.1086/282461
Acknowledgements
We are grateful to Owen Jones for comments on a previous version of the manuscript and would like to give particular thanks to the Regional Government of Aragón for permission to work with a plant of “special interest”. DAS and JPD acknowledge funding from the Max Planck Society. MBG acknowledges PERDIVER project (BBVA Foundation) and JPD acknowledges the Independent Research Fund Denmark (DFF) for funding during the analysis and writing stages.
Author information
Authors and Affiliations
Contributions
MBG and JE designed the experiments. MBG carried out the field work. All authors conceived the specific study questions. DAS and JPD conducted the statistical analyses. DAS wrote the first draft of the manuscript and all other authors contributed substantially with revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Monica Geber.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sherman, D.A., Dahlgren, J.P., Ehrlén, J. et al. Sex and the cost of reproduction through the life course of an extremely long-lived herb. Oecologia 191, 369–375 (2019). https://doi.org/10.1007/s00442-019-04491-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04491-0