Abstract
In the presence of a predator, foraging is a dangerous task. Social individuals can respond to risk by forming groups, benefiting from enhanced collective anti-predator behavior but suffering from increased conspicuousness to predators. Within groups, individuals exhibit variable foraging behavior. One important factor influencing risky foraging behaviour is current energetic state, and individuals must trade off food and safety by deciding when to leave a protected refuge in order to find food. We generated mixed groups of goldfish (Carassius auratus) containing equal numbers of underfed and well-fed individuals and examined individual refuge use and willingness to take risks venturing into risky foraging areas in the presence of an avian predator (little egret—Egretta garzetta). Underfed fish exhibited higher levels of risky behaviour by participating in more foraging outings and emerging from the refuge in frontal group positions, compared with well-fed individuals. As expected, underfed fish benefitted by consuming more food, but surprisingly did not experience higher rates of mortality. This may be due to the fact that the egret predator rarely captured the first fish to emerge from the refuge, preferentially attacked groups of three or more fish, and often captured fish in the chaotic period following a failed initial strike. We demonstrate how differences in energetic condition can influence risk-taking behaviours among social individuals that subsequently influence relative levels of foraging success and group fission–fusion dynamics. Moreover, our results illustrate the risk associated with foraging in larger groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foraging can be a dangerous task and, in order to optimize fitness, prey animals must attempt to enhance energetic gains while reducing the risk of injury or death from predation (McNamara and Houston 1992). Prey often respond to predation risk by forming social groups. Behavioral theory suggests that sociality evolves when the benefits of association with conspecific group members outweigh the costs (Krause and Ruxton 2002; Silk 2007). In the context of predator–prey interactions, individuals within social prey groups benefit from greater protection from predators via collective detection (Lima 1995) and the dilution effect (Foster and Treherne 1981). On the other hand, group formation can be costly for individuals as larger group sizes increase competition for resources (Hirsch 2007) and conspicuousness to predators (Krause and Godin 1995).
Within social groups, prey individuals exhibit variation in their behavioural responses to predation risk. One way that prey animals reduce predation risk is by increasing time spent in refuges (Lima 1998a), despite having to pay a missed opportunity cost of lost foraging time (Sih 1992, 1997). Thus, animals trade off food and safety and must constantly decide whether to stay in a safe, protected refuge or leave to forage in a more rewarding, but risky location (Lima and Dill 1990; Sih et al. 2003; Brown and Kotler 2004). Individuals that exhibit more risk-prone behaviour by choosing to forage more often in dangerous locations and position themselves at the front of the group leaving a refuge are known to benefit from increased energetic gains (e.g., Teichroeb et al. 2015), but also experience higher risk of predation (e.g., Bumann et al. 1997). Consequently, the decision of how often, and in what spatial group position, to leave a protected refuge can influence relative levels of individual foraging success and survival within social groups (Bateson 2002; Morrell and Romey 2008; Romey and Galbraith 2008).
The choices animals make concerning risky foraging behaviour are dependent on the fitness costs and benefits associated with leaving a refuge (Lima 1998b; Orrock et al. 2013). Individuals should only leave a safe refuge to forage when the cost of not consuming food outweighs the possible mortality cost of foraging in a risky environment (Godin 1997; Bateson 2002). The asset protection principle (Clark 1994) suggests that the benefits of risky behaviour are determined by an individual’s fitness potential, which can be influenced by its energetic and nutritional state. An animal that is well-fed and in good condition should take fewer risks in order to survive and protect its high fitness potential. Conversely, a hungry animal in poor condition has a lower fitness potential due to its current state, and such individuals must prioritize foraging efforts in order to survive and ultimately increase future reproductive potential (Bednekoff 1996). In this way, hungry foragers gain more from each additional food item acquired as the marginal fitness value of finding a food resource is higher in comparison with well-fed animals (Nonacs 2001). Thus, undernourished animals are expected to accept greater risks of predation in order to forage (e.g., Olsson et al. 2002; Kotler et al. 2004).
In fish shoals, when resources are limited, intraspecific exploitation competition can lead to differential foraging success among group members. Certain individuals are able to acquire sufficient resources, while others fail and remain food deficient (Hirsch 2007). Consequently, individual fish within social groups can greatly differ in nutritional condition (Major 1978; Krause 1993), and individual foraging behaviour can be influenced by hunger-state (e.g., Krause et al. 1999). Although there is evidence that individual fish with high energy deficits accept greater predation risks to obtain food (e.g., Gotceitas and Godin 1991; Krause et al. 1992), few studies have examined individual refuge use and risky foraging behaviour within mixed social groups containing members differing in hunger-state. Little is known about how differences in energetic state among social prey influence individual and group foraging behavior and refuge use under predation risk.
Here, we investigate the effects of hunger-state on individual risk-taking behaviour for goldfish (Carassius auratus auratus) experiencing predation risk from a live avian predator (little egret—Egretta garzetta). Specifically, we assess individual propensity to forage under predation risk and order of emergence from a safe refuge within mixed groups of underfed and well-fed fish. We predicted that (i) underfed individuals would more often forage in the risky environment and emerge from the protected refuge at the front of the group. Therefore, we predicted that (ii) underfed individuals would consume more food, but (iii) also be more likely to be captured by the predator.
Methods
Study species
We used common gold-coloured goldfish (C. auratus) as the foragers and little egrets (E. garzetta) as the predator. Goldfish are small domesticated cyprinids that school, forage as a group, and seek cover from predation (Pitcher and Magurran 1983). Goldfish are related to eastern Asian dark coloured carp species that dwell in streams, lakes, and ponds (Holopainen et al. 1997). Like other species that evolved in muddy water, C. auratus do not use vigilance as their primary anti-predator behaviour, and instead typically manage risk by utilizing time allocation and hiding in a refuge when a predator is present (Katz et al. 2013). Our goldfish were acquired in Northern Israel (Ma’agan Michael, Hof HaCarmel), where they are raised in large (4000–40,000 m2) outdoor stocking pools. Although goldfish are domesticated, they have changed little genetically from their wild carp ancestors, and therefore we can expect them to exhibit natural fish behaviour (Magurran 1984). Consequently, there is a growing body of literature that uses goldfish as a model fish organism for behavioral research (e.g., Weir and Grant 2004; Amano et al. 2005; Stenberg and Persson 2005; Dunlop et al. 2006; Ingrum et al. 2010; DeLong et al. 2017). Past experiments in our lab have demonstrated that goldfish naturally schooled, sought cover from predation, and generally re-emerged to forage in coordinated groups (e.g., Katz et al. 2013; Vijayan et al. 2018; Balaban-Feld et al. 2018).
The little egret (~400 g) is a small heron in the Ardeidae family. Little egrets are opportunistic hunters that ambush aquatic prey species in shallow water (Kushlan 1978). Our egrets were wild captured in Northern Israel (Kfar Ruppin, Beit She’an Valley), and released at the location of capture following the conclusion of the experiment.
Individual fish size, boldness, and feeding regimen
We collected groups of fish from the main 1880 l cylindrical holding tank (2.0 m diameter × 0.6 m height), and then randomly separated individuals into two treatment groups. For each set, we collected fish from the main tank using a net, and then selected individuals one by one and alternated which treatment group the fish would join. The two treatment groups were then held in matching 840 l rectangular holding tanks (1.2 × 1.0 × 0.7 m) that had identical environmental conditions. The water in the holding tanks was kept at a constant 25 °C, and the tanks were kept on a 12 h:12 h light–dark cycle. One week prior to the beginning of the experiment, individual fish were lightly anaesthetized using MS-222 and marked with a unique symbol using Biotouch micro-pigments to allow for individual identification as part of either the underfed or well-fed treatment groups (see Fig. S1). The following day, each individual fish was tested for boldness to enter a novel area. A rectangular tank (35 × 22 × 13 cm) that was split into two equal-sized sections by a dark plastic barrier was used as the boldness arena. Each fish was placed into one side of the arena and given 5 min to acclimate. After acclimation, a 6 cm wide gate was remotely raised. We recorded the length of time until the fish’s entire body crossed through the gate into the novel area. Trials were limited to 10 min; fish that did not cross through the gate in 10 min were given a score of 600 s. We calculated a boldness index for each fish as 1 − (latency to enter novel arena/600). Accordingly, bold fish scored close to 1, and the shiest fish that did not emerge in 10 min scored 0 (adapted from Brown et al. 2005). Individuals were tested once as fish modify their behaviour following exposure to a novel arena (White et al. 2013), and then returned to their respective holding tanks.
Next, a feeding regimen was used to generate distinct sets of well-fed and underfed fish. Following boldness testing, the two treatment groups of fish were fed different amounts of food for 6 days leading up to the experiment. Well-fed fish were provided ten (0.01 g) pellets/fish/day which met their daily energetic requirements. Underfed fish were provided only one pellet/fish/day. Following the six-day feeding regimen, each fish was measured from head-to-tail and weighed. All boldness testing and feeding occurred in 25 °C water.
Experimental design
Experiments were held in a specially designed outdoor cylindrical aviary (7 m diameter). The aviary contained three equally sized 1060 l pools (1.5 m diameter × 0.6 m depth) that were evenly spaced within the arena. In the centre of each pool, a 23.75 cm radius floating cover provided fish a safe refuge under which they could hide from the predator (Fig. S2). Plastic mesh placed around the refuge prevented the floating food pellets from entering under the cover, and the fish were forced to move into the surrounding open-water environment to forage (Fig. S1). Thus, the open-water microhabitat provided fish an opportunity to find food, but also left fish exposed to risk of attack by the predator. A plastic 1 cm mesh false bottom limited the fish to the upper 15 cm of the water and provided the egret with a surface on which to walk and hunt. Experiments were run during the day (09:00–13:00) so that natural sunlight could enter the aviary. All pools were kept at 25 °C.
For this experiment, we focused on one of the three pools that contained a mixed group of eight fish (four well-fed and four underfed). We limited access to the covered microhabitat by allowing fish to enter and exit the refuge through a 20 cm wide gate. An HD (Geovision model GV-EVD2100) underwater camera placed near the bottom of the focal pool allowed us to focus on the gate and assess the order in which individual fish exited the refuge to forage in the open-water environment (Fig. S1). Each group of eight fish was placed into the focal pool 12 h prior to testing to allow them to acclimate to their surroundings. The other two non-experimental pools contained an identical covered refuge and eight non-experimental fish, and provided the egret multiple hunting locations. It was important to encourage the egret to move away from the focal pool and return at a later time in order to provide the focal fish time to recover and behave naturally following periods of intense predation risk when the egret was at the experimental pool.
At the beginning of the experimental day, a single egret was released into the arena, and allowed free movement for 4 h. Throughout the trial, 60 floating food pellets were individually dropped onto the surface of the water at a constant rate using an overhead timed conveyor belt feeder. We tested N = 14 groups of fish, for a total of N = 112 individual fish (N = 56 well-fed and 56 underfed). Altogether, each set of fish underwent a 6-day feeding regimen, was then given 12 h to acclimate to the experimental arena, and then was used for a single experimental day. No fish were used twice. Egret hunting styles and levels of aggressiveness are highly variable. As such, a single egret was used as the predator throughout the experiment to ensure that the different groups of goldfish experienced the same predator hunting mode and overall level of risk.
Calculation of individual fish outing index
The experimental day began after the egret’s first visit to the focal pool to make sure we were assessing the behaviour of fish that were aware a predator was in the area. For each experimental day, we observed individual fish behaviour over 15 group foraging outings. A foraging outing began when the first fish crossed out of the refuge through the gate into the open risky habitat. The outing concluded when all participating fish returned to the covered refuge. To compare individual fish behaviour over 15 foraging outings, we developed an outing index to consider both the order of emergence into the risky habitat and the proportion of group outings participated in. For every outing, the first fish to leave the refuge received a score of 8, the second fish received a 7, and so on to the last fish to cross through the gate. Any fish that did not participate in the outing received a score of 0. At the end of the day, each fish received an outing index calculated as the average of the fish’s 15 outing scores (i.e., sum of outing scores/15). Thus, the highest possible outing index was 8, and the lowest possible score was 0.
Analysis of foraging success and mortality
To assess individual foraging success, we used two HD (Geovision model GV-EVD2100) cameras placed at the edge of the water surface to record which individual fish consumed fish pellets during each outing. At the end of the experimental day, we recorded which fish had survived, and all surviving fish were then weighed to determine weight change (%) as an additional indicator of foraging success. Using video analysis, we were able to observe each fish capture, record which treatment group the fish belonged to, and determine whether the fish was directly targeted in isolation by the egret upon emergence from the covered refuge, or if it was captured within a foraging group of three or more fish. Videos were recorded automatically and then viewed remotely at a later date. There was no observer inside of the aviary during the experiment.
Statistical analyses
To determine overall relationships between fish body length, boldness, outing index scores, pellets eaten, and weight change (%), regression analyses were run with group (N = 14) as a random effect. Log-likelihood ratio tests were used to determine the significance of each regression. When the response variable was boldness we used the coxme package from R (Therneau 2018) to perform a mixed-effects cox regression analysis with censored data reflecting the maximum time of 600 s (boldness = 0). In the case of count data (pellets eaten), we used a GLMM. All other analyses used the lme4 package (Bates et al. 2015) to construct general linear mixed models.
To examine how outing index scores influenced weight change (%) for both treatment groups, a linear mixed model was used with weight change (%) as the response variables, outing index and feeding treatment as fixed effects, and group as a random effect. For the relationship between outing index and pellets eaten, a Poisson GLMM was used with pellets eaten as the response variable, outing index and feeding treatment as fixed effects, and group as a random effect. We checked the residuals of the Poisson regression for overdispersion using the dispersion test in the R package DHARMa (Hartwig 2018).
We calculated mean outing participation, outing index, pellets eaten, and weight change (%) for the well-fed and underfed groups (N = 4 per treatment) on each day and ran two-tailed paired t tests to compare the two treatment groups. To compare mortality results between the treatment groups and between fish group sizes at the moment of capture (solitary fish versus group of fish) we ran Chi square goodness of fit analyses. All statistical tests were conducted using R (R Core Team 2016).
Data available on figshare: https://doi.org/10.6084/m9.figshare.6741371.
Results
Body length, (pre-test) boldness, outing index, and foraging success
Body length varied between 7.50 and 12.10 cm and was significantly correlated with body weight (r = 0.57, P < 0.001). We found no significant relationship between body length and boldness (Χ2(1) = 1.04, P = 0.37), body length and outing index score (Χ2(1) = 0.78, P = 0.78), or body length and either measure of foraging success (pellets eaten: Χ2(1) = 2.45, P = 0.12; weight change: Χ2(1) = 1.05, P = 0.31). Additionally, there was no significant effect of individual boldness on outing index (Χ2(1) = 0.46, P = 0.83), or foraging success (pellets eaten: Χ2(1) = 0.90, P = 0.34; weight change: Χ2(1) = 2.23, P = 0.14).
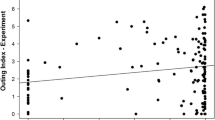
Overall, fish that scored higher outing index scores ate significantly more pellets and gained significantly more weight. More food pellets were eaten by fish with higher outing index scores (Χ2(1) = 138.23, P < 0.001) and by fish that were underfed (Χ2(1) = 2.69, P = 0.03); however, we did not find a significant interaction between feeding treatment and outing index (Χ2(1) = 2.04, P = 0.15), indicating that both underfed and well-fed fish groups exhibited a similar positive relationship between outing index and pellets eaten. We found no evidence of overdispersion in the residuals of the Poisson GLMM (DHARMa dispersion test: P = 0.50). Correspondingly, weight change (%) increased in fish with high outing index scores (Χ2(1) = 56.11, P < 0.001), and in fish that were underfed (Χ2(1) = 41.60, P < 0.001). Again, we did not find a significant interaction between feeding treatment and outing index (Χ2(1) = 1.61, P = 0.21), demonstrating that both underfed and well-fed fish exhibited a comparable positive relationship between outing index and weight change (%) (Fig. 1).
Relationship between Outing index scores on experimental days and weight change (%). Open circles and filled circles represent individual underfed and well-fed fish, respectively. Dashed and solid linear fit lines represent averages for N = 14 groups of underfed and well-fed fish, respectively. Data points above the grey dashed horizontal reference line indicate weight gain, and points below the reference line indicate weight loss
Foraging behaviour and success of underfed vs. well-fed fish
On average, underfed fish participated in significantly more group foraging outings (mean ± SE outing participation %: underfed 52 ± 5%; well-fed 34 ± 7%; paired t test: t13 = 5.92, P < 0.001) and accordingly scored significantly higher outing index scores compared with well-fed fish (mean ± SE outing index: underfed 2.71 ± 0.16; well-fed 1.53 ± 0.25; paired t test: t13 = 5.89, P < 0.001) (Fig. 2a). Furthermore, on 13 of the 14 experimental days, the top scoring fish was from the underfed treatment (Χ2(1) = 10.29, P = 0.001), and 24 of the 28 fish scoring the highest two outing index scores per day were from the underfed treatment (Χ2(1) = 14.29, P < 0.001).
Mean outing index scores (a), and weight change (%) (b) for underfed and well-fed fish. Each set of two points connected by a horizontal line represent data from the two treatment groups on a given day (N = 14). In b, data points above the grey dotted horizontal reference line indicate weight gain, and points below the reference line indicate weight loss
As a result, underfed fish consumed significantly more food pellets (mean ± SE pellets eaten: underfed 2.27 ± 0.27; well-fed 0.57 ± 0.12; paired t test: t13 = 5.81, P < 0.001), and the underfed fish gained significantly more weight compared with the well-fed fish that generally lost weight (mean ± SE weight gain (%): underfed 3.50 ± 0.73%; well-fed − 6.18 ± 1.14%; paired t test: t13 = 10.07, P < 0.001) (Fig. 2b).
Mortality of underfed vs. well-fed fish
The egret captured an equal number of underfed and well-fed fish (12 each). Overall, of the 24 total captures, the egret captured the first fish to emerge from the refuge ten times (6 underfed and 4 well-fed; Χ2(1) = 0.40, P = 0.53). The egret caught a significantly higher number of fish foraging in groups compared with single fish foraging in isolation (18 group captures and 6 single captures; Χ2(1) = 6.00, P = 0.01) (Fig. 3). Furthermore, the majority of the captures of fish within groups (13 of 18) transpired during the commotion resulting from the chaotic escape behaviour of goldfish individuals following a failed egret strike.
Discussion
We examined the behaviour of mixed groups of fish containing underfed and well-fed individuals that had the option to remain safe under a protective refuge or forage in the open while exposed to predation risk. We found that certain individuals more frequently took risks and left the safety of the refuge to find food in the dangerous open-water habitat. Although all fish in our study experienced the same level of risk, regardless of hunger-state, the underfed fish more frequently chose to leave the refuge to find food in the dangerous open-water habitat, and often left at the front of the group. In comparison, well-fed fish tended to participate in fewer outings, and emerged near the back of the group when they did venture out of the refuge. As a result, fish from the underfed treatment enjoyed greater relative foraging success compared with other well-fed group members. Furthermore, our study demonstrates how differences in nutritional condition among group members can impact group fission–fusion dynamics (Conradt and Roper 2000). Differences in hunger-state can generate variance in risk-taking behaviour among individuals that have the option to stay hidden in a refuge or forage in a risky location, ultimately resulting in smaller foraging groups.
Our study supports prior work in fishes that showed individuals in a poor energetic state are more likely to take risks and forage in the presence of a predator (e.g., Godin and Crossman 1994). Our results suggest that the fish in our underfed treatment that were provided with very little nourishment over the 6 days preceding the experiment desperately needed to find food to improve their poor energetic state in spite of greater mortality risks associated with foraging in open water. The opportunity cost of lost foraging time associated with hiding in the refuge was much higher for our undernourished fish, and this influenced the observed difference in risky behaviour between the underfed and well-fed treatment groups. Our finding that well-fed individuals with lower energetic needs were less likely to join a foraging group when there was the option to stay in a safe refuge supports prior work that indicated hungry fish spend more time foraging in risky open-water (Pettersson and Brönmark 1993). Initially, differences in time allocation strategies between individuals differing in hunger-state will lead to well-fed individuals reducing foraging time in risky environments and preferring safe environments that contain fewer resources, consequently decreasing energetic gains. Conversely, underfed fish are more willing to forage in risky environments, leading to enhanced energetic gains. Thus, over a longer time period, the differential response to the trade-off between food and safety would be expected to compress both well-fed and underfed individuals towards the mean energy state of the population (Brown 1999).
We predicted that individual fish that exhibited riskier behaviour would benefit from enhanced food consumption but would necessarily trade off foraging success with mortality risk (Brown and Kotler 2004). We were surprised to find that the more risk-prone underfed fish did not experience higher mortality risk, as an equal number of underfed and well-fed fish were captured by the egret predator. Our finding might be explained by the fact that bolder, more risk-seeking individuals have been shown to exhibit an enhanced ability to escape predators (Blake and Gabor 2014). However, we found no influence of individual boldness on risky behaviour in our experiment. Further, the mortality results were unexpected as the underfed fish in our experiment spent significantly more time in the open-water environment and tended to emerge at the front of the group, which we had predicted should have increased the probability they would be spotted and attacked by our ambush predator (Choi et al. 2008).
Upon video analysis of each instance in which a fish was captured by the egret, we found that the mortality risk associated with risky foraging behaviour was dependent on the circumstances surrounding a given attack. In the instances in which the egret successfully attacked the first fish to depart from the refuge, individual order of emergence within the foraging fish group determined survival. In such a situation, the egret killed more underfed fish (6 underfed versus 4 well-fed), and this was evidently due to the fact that underfed fish more often emerged at the front of the group. However, the egret most frequently targeted groups of three of more fish, and this may help explain why an equal number of underfed and well-fed fish were captured. Had the egret more often targeted and immediately captured the first fish to emerge from the refuge, significantly more underfed fish would have been captured. Furthermore, when attacking larger groups of fish, the egret often failed on its initial strike. In the ensuing chaotic seconds, the relative risk of mortality among well-fed and underfed group members was likely more random and equivalent.
Thus, while our mortality results demonstrate that it can be dangerous to be the first individual to emerge from the refuge (Bumann et al. 1997), the riskiest behavior in our experiment was to forage as part of a group of fish due to the preference of the predator to attack larger groups (Ioannou and Krause 2008). This result support prior work that has shown that predators preferentially attack larger shoal sizes (Krause and Godin 1995). The expected advantage of safety in numbers (i.e., the dilution effect—Foster and Treherne 1981) might have been negated as the predator most often attacked and successfully captured fish moving in groups containing multiple individuals, indicating that there was risk associated with foraging in large numbers. The risk of foraging in numbers does not implicitly favour either underfed or well-fed fish, but as the well-fed fish in our experiment participated in fewer outings and emerged near the back of foraging groups, they tended to spend more of their foraging time in larger groups. Therefore, as mentioned earlier, the underfed fish paid the cost of increased mortality from emerging first in order to gain access to food, while the well-fed fish tended to forage in larger groups that attracted more predator attacks.
Relative levels of refuge use and risky foraging behaviour were strongly influenced by hunger-state, but not by body size or boldness, suggesting that hunger-state has a stronger influence on foraging behaviour than individual differences in body size or personality. In contrast, previous work on fishes have demonstrated that large (e.g., Krause et al. 1998; Ward et al. 2002), and bold (e.g., Ward et al. 2004; Leblond and Reebs 2006; Balaban-Feld et al. 2018) individuals tend to occupy riskier frontal group positions. Moreover, Dowling and Godin (2002) showed that body length influenced time spent in a refuge while nutritional state did not. However, both the work of Dowling and Godin (2002) and our (pre-test) boldness assay examined the behaviour of solitary individual fish, while our experiment examined fish behaviour within a social group. Although examining individuals in an isolated setting can yield valuable information about underlying behavioural differences among individuals, it is also important to examine the behaviour of social animals in more realistic group settings. Behaviour recorded alone does not always coincide with behaviour tested in a group (e.g., Schuett and Dall 2009; McDonald et al. 2016), as social interactions and feedback among conspecifics can influence individual behaviour (Webster et al. 2007). As such, more work is needed to understand how risk-taking behaviours are modified by social interactions among conspecifics.
To conclude, individual fish in poor hunger-state took more risks to forage in a risky environment and benefitted by consuming more food but did not experience an increased cost of mortality compared with well-fed social group members. In terms of mortality risk, fish foraging in groups containing three or more fish were most often attacked and captured by the egret predator. Our study exemplifies how individual energetic state, social dynamics, trade-offs of food and safety, and predator behaviour can affect prey foraging behaviour. Future work is needed to better understand how differences in internal state among social prey, and the associated behavioural responses, influence individual and group risk-taking behaviours under predation risk.
References
Amano M, IiGo M, Yamamori K (2005) Effects of feeding time on approaching behavior to food odor in goldfish. Fish Sci 71:183–186
Balaban-Feld J, Mitchell WA, Kotler BP, Vijayan S, Elem LTT, Abramsky Z (2018) Influence of predation risk on individual spatial positioning and willingness to leave a safe refuge in a social benthic fish. Behav Ecol Sociobiol 72:87
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using {lme4}. J Stat Softw 67:1–48
Bateson M (2002) Recent advances in our understanding of risk-sensitive foraging preferences. Proc Nutr Soc 61:509–516
Bednekoff PA (1996) Risk-sensitive foraging, fitness, and life histories: where does reproduction fit into the big picture? Am Zool 36:471–483
Blake CA, Gabor CR (2014) Effect of prey personality depends on predator species. Behav Ecol 25:871–877
Brown JS (1999) Vigilance, patch use and habitat selection: foraging under predation risk. Evol Ecol Res 1:49–71
Brown JS, Kotler BP (2004) Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014
Brown C, Jones F, Braithwaite V (2005) In situ examination of boldness–shyness traits in the tropical poeciliid, Brachyraphis episcopi. Anim Behav 70:1003–1009
Bumann D, Krause J, Rubenstein D (1997) Mortality risk of spatial positions in animal groups: the danger of being in the front. Behaviour 134:1063–1076
Choi YS, Kwon IK, Yoo JC (2008) A study of feeding methods in five species of herons and egrets in Korea. J Ecol Environ 31:147–151
Clark CW (1994) Antipredator behavior and the asset-protection principle. Behav Ecol 5:159–170
Conradt L, Roper TJ (2000) Activity synchrony and social cohesion: a fission–fusion model. Proc R Soc B 267:2213–2218
DeLong CM, Barbato S, O’Leary T, Wilcox KT (2017) Small and large number discrimination in goldfish (Carassius auratus) with extensive training. Behav Process 141:172–183
Dowling LM, Godin JGJ (2002) Refuge use in a killifish: influence of body size and nutritional state. Can J Zool 80:782–788
Dunlop R, Millsopp S, Laming P (2006) Avoidance learning in goldfish (Carassius auratus) and trout (Oncorhynchus mykiss) and implications for pain perception. Appl Anim Behav Sci 97:255–271
Foster WA, Treherne JE (1981) Evidence for the dilution effect in the selfish herd from fish predation on a marine insect. Nature 293:466
Godin JGJ (1997) Evading predators. Behavioural ecology of teleost fishes. Oxford University Press, Oxford, pp 191–236
Godin JGJ, Crossman SL (1994) Hunger-dependent predator inspection and foraging behaviours in the three spine stickleback (Gasterosteus aculeatus) under predation risk. Behav Ecol Sociobiol 34:359–366
Gotceitas V, Godin JGJ (1991) Foraging under the risk of predation in juvenile Atlantic salmon (Salmo salar L.): effects of social status and hunger. Behav Ecol Sociobiol 29:255–261
Hartwig F (2018) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.2.0. https://CRAN.R-project.org/package-DHARMa. Accessed 27 Nov 2018
Hirsch BT (2007) Costs and benefits of within-group spatial position: a feeding competition model. Q Rev Biol 82:9–27
Holopainen IJ, Tonn WM, Paszkowski CA (1997) Tales of two fish: the dichotomous biology of crucian carp (Carassius carassius (L.)) in northern Europe. In: Annales Zoologici Fennici. Finnish Zoological and Botanical Publishing Board, Helsinki, pp 1–22
Ingrum J, Nordell SE, Dole J (2010) Effects of habitat complexity and group size on perceived predation risk in goldfish (Carassius auratus). Ethol Ecol Evol 22:119–132
Ioannou CC, Krause J (2008) Searching for prey: the effects of group size and number. Anim Behav 75:1383–1388
Katz MW, Abramsky Z, Kotler BP, Rosenzweig ML, Alteshtein O, Vasserman G (2013) Optimal foraging of little egrets and their prey in a foraging game in a patchy environment. Am Nat 181:381–395
Kotler BP, Brown JS, Bouskila A (2004) Apprehension and time allocation in gerbils: the effects of predatory risk and energetic state. Ecology 85:917–922
Krause J (1993) Positioning behaviour in fish shoals: a cost–benefit analysis. J Fish Biol 43:309–314
Krause J, Godin JGJ (1995) Predator preferences for attacking particular prey group sizes: consequences for predator hunting success and prey predation risk. Anim Behav 50:465–473
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Krause J, Bumann D, Todt D (1992) Relationship between the position preference and nutritional state of individuals in schools of juvenile roach (Rutilus rutilus). Behav Ecol Sociobiol 30:177–180
Krause J, Reeves P, Hoare D (1998) Positioning behaviour in roach shoals: the role of body length and nutritional state. Behaviour 135:1031–1039
Krause J, Loader SP, Kirkman E, Ruxton GD (1999) Refuge use by fish as a function of body weight changes. Acta Ethol 2:29–34
Kushlan JA (1978) Nonrigorous foraging by robbing egrets. Ecology 59:649–653
Leblond C, Reebs SG (2006) Individual leadership and boldness in shoals of golden shiners (Notemigonus crysoleucas). Behaviour 143:1263–1280
Lima SL (1995) Back to the basics of anti-predatory vigilance: the group-size effect. Anim Behav 49:11–20
Lima SL (1998a) Nonlethal effects in the ecology of predator–prey interactions. Bioscience 48:25–34
Lima SL (1998) Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. In: Advances in the study of behavior. Academic Press, New York, pp 215–290
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Magurran A (1984) Gregarious goldfish. New Sci 103:32–33
Major PF (1978) Predator–prey interactions in two schooling fishes, Caranx ignobilis and Stolephorus purpureus. Anim Behav 26:760–777
McDonald ND, Rands SA, Hill F, Elder C, Ioannou CC (2016) Consensus and experience trump leadership, suppressing individual personality during social foraging. Sci Adv 2:e1600892
McNamara JM, Houston AI (1992) Risk-sensitive foraging: a review of the theory. Bull Math Biol 54:355–378
Morrell LJ, Romey WL (2008) Optimal individual positions within animal groups. Behav Ecol 19:909–919
Nonacs P (2001) State dependent behavior and the marginal value theorem. Behav Ecol 12:71–83
Olsson O, Brown JS, Smith HG (2002) Long-and short-term state-dependent foraging under predation risk: an indication of habitat quality. Anim Behav 63:981–989
Orrock JL, Preisser EL, Grabowski JH, Trussell GC (2013) The cost of safety: refuges increase the impact of predation risk in aquatic systems. Ecology 94:573–579
Pettersson LB, Brönmark C (1993) Trading off safety against food: state dependent habitat choice and foraging in crucian carp. Oecologia 95:353–357
Pitcher TJ, Magurran AE (1983) Shoal size, patch profitability and information exchange in foraging goldfish. Anim Behav 31:546–555
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 27 Nov 2018
Romey WL, Galbraith E (2008) Optimal group positioning after a predator attack: the influence of speed, sex, and satiation within mobile whirligig swarms. Behav Ecol 19:338–343
Schuett W, Dall SR (2009) Sex differences, social context and personality in zebra finches, Taeniopygia guttata. Anim Behav 77:1041–1050
Sih A (1992) Prey uncertainty and the balancing of antipredator and feeding needs. Am Nat 139:1052–1069
Sih A (1997) To hide or not to hide? Refuge use in a fluctuating environment. Trends Ecol Evol 12:375–376
Sih A, Kats LB, Maurer EF (2003) Behavioural correlations across situations and the evolution of antipredator behaviour in a sunfish–salamander system. Anim Behav 65:29–44
Silk JB (2007) The adaptive value of sociality in mammalian groups. Proc R Soc B 362:539–559
Stenberg M, Persson A (2005) The effects of spatial food distribution and group size on foraging behaviour in a benthic fish. Behav Process 70:41–50
Teichroeb JA, White MM, Chapman CA (2015) Vervet (Chlorocebus pygerythrus) intragroup spatial positioning: dominants trade-off predation risk for increased food acquisition. Int J Primatol 36:154–176
Therneau TM (2018) coxme: mixed effects Cox models. R package version 2.2-10. https://CRAN.R-project.org/package=coxme. Accessed 27 Nov 2018
Vijayan S, Kotler BP, Elem LTT, Abramsky Z (2018) Effect of predation risk on microhabitat use by goldfish. Ethol Ecol Evol. https://doi.org/10.1080/03949370.2018.1477837
Ward AJ, Hoare DJ, Couzin ID, Broom M, Krause J (2002) The effects of parasitism and body length on positioning within wild fish shoals. J Anim Ecol 71:10–14
Ward AJ, Thomas P, Hart PJ, Krause J (2004) Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus). Behav Ecol Sociobiol 55:561–568
Webster MM, Ward AJW, Hart PJB (2007) Boldness is influenced by social context in three spine sticklebacks (Gasterosteus aculeatus). Behaviour 144:351–371
Weir LK, Grant JW (2004) The causes of resource monopolization: interaction between resource dispersion and mode of competition. Ethology 110:63–74
White JR, Meekan MG, McCormick MI, Ferrari MC (2013) A comparison of measures of boldness and their relationships to survival in young fish. PLoS ONE 8:e68900
Acknowledgements
This study was supported by Israel Science Foundation (Grant 05/14 to ZA). SV is grateful to the Azrieli Foundation for the award of a Postdoctoral Fellowship at Ben-Gurion University.
Author information
Authors and Affiliations
Contributions
JBF conceived the experiment and wrote the manuscript; WAM contributed to statistical analyses; BPK, SV, LTTE, and ZA contributed to experimental design, video analyses, and edited early drafts of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The experiments were conducted in full accordance with the animal care and ethical guidelines of Ben-Gurion University of the Negev, and the Abramsky lab was granted permission to use egrets and goldfish in this study by the committee for the ethical care and use of animals in experiments (authorization number: IL-37-07-2017).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Aaron J. Wirsing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Balaban-Feld, J., Mitchell, W.A., Kotler, B.P. et al. State-dependent foraging among social fish in a risky environment. Oecologia 190, 37–45 (2019). https://doi.org/10.1007/s00442-019-04395-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04395-z