Abstract
Space and environment are fundamental in influencing the community structure. However, their relative influences vary according to species’ biological characteristics. Here we test whether differences in life-history traits mainly linked to dispersal abilities influence bat, rodent and marsupial beta diversity along spatial and environmental gradients. We expect bat beta diversity to be weakly related with space in comparison to dispersal-limited rodents and marsupials. Using data from communities distributed along the Atlantic Forest of South America, we calculated the total beta diversity and its turnover and nestedness components for each group. We estimated the strength of correlation of beta diversity and its components along spatial and environmental gradients, comparing their importance within and between groups. Space had the higher influence on rodent and marsupial beta diversity. For bats, both gradients influenced similarly their community composition. Between taxa, the influence of these gradients did not differ for rodents and marsupials, while bats presented a stronger relationship with environment compared to non-volant small mammals. Also, all groups presented a similar influence of the spatial gradients on their community structure, despite their differences in dispersal abilities. Our results suggest that differences in biological characteristics partially influence the community structure of these mammals, with their responses along space likely reflecting similar biogeographical dynamics affecting their distribution. Overall, our results improve the understanding of the processes structuring these communities, highlighting the benefits of comparative analyses within a beta diversity perspective to better understand the influence of multiple processes on the community assembly along geographical gradients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beta diversity corresponds to one of the most classic concepts in ecology. Dating back to the late 1960s, it is one of the components of Whittaker’s triad of diversity, providing a link between local (alpha) and regional (gamma) diversities (Whittaker 1960; Anderson et al. 2011). Beta diversity can be defined as either variation, in which interest lies on the overall variation in community composition among communities, or a directional change in community composition, in which pairwise comparisons take place allowing to examine how community composition is linked to a gradient of interest, such as geographical or temporal (Anderson et al. 2011).
Of increased interest has been the recognition that beta diversity can arise by two complementary processes: species turnover and nestedness (Baselga 2010, 2012). Turnover occurs when species are replaced along a gradient without changes in the number of species between communities, while nestedness occurs when species are lost or gained, promoting changes in species richness between sites and reflecting a nested pattern with species-poor sites as subsets of the richest ones (Baselga 2010, 2012). The partitioning of beta diversity into these two components has provided new insights and advanced the understanding of the mechanisms and processes underlying the factors structuring communities (Baselga et al. 2012; Dobrovolski et al. 2012; Hill et al. 2017; Soininen et al. 2017).
Considering the main factors driving beta diversity along geographical gradients, the influence of the spatial distance and environmental differences between sites has been pervasive, dominating much of the ecological literature. These gradients highlight the importance of species’ life-history traits that confer them different dispersal abilities and niche breadth on the factors underlying their community structure (Nekola and White 1999; Tuomisto et al. 2003; Cottenie 2005; Soininen et al. 2007; Buckley and Jetz 2008; Qian and Ricklefs 2012; Hill et al. 2017; Gómez-Rodríguez and Baselga 2018). Also, species with contrasting life histories tend to show differences in their response along these gradients. For example, the beta diversity of communities of dispersal-limited species tends to be influenced to a higher degree by spatial dynamics in comparison to better dispersers (Nekola and White 1999; Qian 2009; De Bie et al. 2012; Qian and Ricklefs 2012). Conversely, species with larger niche width can establish and persist in different environments, which reduce the effects of environmental differences on their community structure in comparison to species with narrower environmental niches (Buckley and Jetz 2008). Comparative analyses among groups with distinct biological characteristics in a beta-diversity framework are thus not only essential to understand how community composition changes along geographical gradients, but they have also allowed for different hypotheses to be tested to untangle the drivers of community assembly (Nekola and White 1999; Tuomisto et al. 2003; Qian 2009; Qian and Ricklefs 2012; Hill et al. 2017; Gómez-Rodríguez and Baselga 2018).
Because of their biological differences, bats, rodents, and marsupials tend to respond differently to the ecological mechanisms structuring their communities (Lyons and Willig 1997; Arita and Rodríguez 2004). Bat species have flight adaptations, which allow them to disperse over different distances (Norberg and Rayner 1987; Esbérard et al. 2017). This is the main reason they are expected to be better dispersers than rodents and marsupials (Arita and Rodríguez 2004). Flight adaptations thus reduce the effects of spatial dynamics on bat distribution, making them more prone to be influenced by environmental variations between sites, mainly in terms of climate and vegetation structure (Stevens 2004; López-González et al. 2015; but also see Varzinczak et al. 2018). Opposite patterns are expected for rodents and marsupials, since their dispersal limitation tends to overshadow the influence of the environment, translating into a strong influence of spatial dynamics in their community structure (Arita and Rodríguez 2004; Dambros et al. 2015). However, no study has addressed formally this hypothesis, which could be essential to broaden the understandings on the mechanisms structuring mammal communities along geographical gradients.
In this study, we compare beta diversities for communities of bats, rodents, and marsupials from the highly diverse Atlantic Forest of South America. We first determined the relative importance of the turnover and nestedness in driving beta diversity for each group. Then, we performed a within- and between-taxa comparison addressing two main hypotheses, considering the factors underlying variations in their community composition (fully described in Table 1). Based on the broad ecological literature covering the relationship of beta diversity along space and environment, the core of our expectations is to find differences in the drivers of beta diversity and its components, both within and between these mammals. We expect communities of rodents and marsupials to show similar structures along environmental and spatial gradients compared to bats. Also, given their dispersal limitations, we predict that the beta diversity of rodents and marsupials should be higher with the space, but not for flying bats. Similarly, between groups, we expect the beta diversity of rodents and marsupials to be more influenced by space in comparison to bats, for which we expect a predominance of environment on their community structure.
Methods
Background: an overview of the Atlantic Forest
The Atlantic Forest is a region of high importance for biodiversity, since it is the second largest tropical forest in South America, extending from Northeastern Brazil to the inner-south of the continent in Paraguay and Argentina (Oliveira-Filho and Fontes 2000). It encompasses a broad latitudinal extent, including tropical and subtropical regions and a wide range of altitudinal gradients, allowing for several climatic regimes that provide a diverse set of environmental features throughout its extension (Oliveira-Filho and Fontes 2000; Ribeiro et al. 2009). These conditions produced a highly complex and heterogeneous forest, with high levels of biological diversity (Ribeiro et al. 2009). Further, the presence of several endemic and threatened species, together with a long history of anthropogenic activities that reduced most of its original land-cover, makes the Atlantic Forest one of the main hotspots for biodiversity conservation worldwide (Myers et al. 2000).
Data on Atlantic Forest mammal assemblages
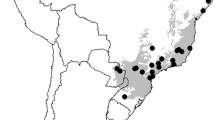
We used compositional data for Atlantic Forest mammal assemblages obtained from the Atlantic bats (Muylaert et al. 2017) for bats and Atlantic small-mammal (Bovendorp et al. 2017) for rodents and marsupials. Briefly, these are comprehensive datasets representing the largest effort to date to assemble information on the distribution and community composition of several taxa and sites along the whole extension of the Atlantic Forest. All taxonomies were reviewed by experts to make these data as accurate as possible. Further details on the methodology employed to assemble these datasets are fully described therein. Because data from different sources are subject to differences in sampling effort, we controlled for this shortcoming by selecting only communities for which sampling effort information was made available (see below). Overall, our final database included 174 bat, 293 rodent, and 231 marsupial communities throughout the Atlantic Forest (Fig. 1). Codes for all communities are provided in the Appendix S1 and can be easily retrieved from the original databases. The number of species encompassed is representative: 99 for bats (85% of all bat species recorded in the Atlantic Forest), 78 for rodents (80%), and 21 for marsupials (95%). For each taxon, we transformed data into binary matrices representing the species incidence in each community.
Distribution of a bat, b rodent, and c marsupial communities along the Atlantic Forest (green area) used to test patterns of mammal beta diversity along spatial and environmental gradients. Points falling out of the green area represent sites located in enclaves of the Atlantic Forest in South America
Beta-diversity metrics: total beta diversity and its components
By means of a pairwise approach, we calculated in R (R Core Team 2016) with the “betapart” package (Baselga and Orme 2012) the variation in community composition between sites for each taxon using the Sørensen index of similarity:
in which a is the number of shared species between two communities, and b and c are the number of exclusive species in each community. This index expresses the relative similarity between two communities in terms of their taxonomic composition, and therefore, its complement represents the variation in community composition for a given pair of sites.
We used the additive partitioning of beta diversity into its turnover (βsim) and nestedness (βnes) components to estimate the importance of species turnover and loss, respectively, in generating the beta diversity for each community pair (Baselga 2010, 2012). The βsor index ranges from 0 to 1, and the sum of βsim and βnes equals βsor for each community pair. Thus, if the main driver of the taxonomic variation between communities is the species replacement, then the turnover component (βsim) is higher and corresponds to the largest fraction of the βsor. On the other hand, if communities differ in their composition because species-poor sites are subsets of the richest ones, then the nestedness component (βnes) accounts for most of βsor. Although other approaches for decoupling beta diversity into different components are available (reviewed in Legendre 2014; Baselga and Leprieur 2015), the interpretation of turnover and nestedness components from Baselga (2010, 2012) is straightforward, and these two indexes have been shown to be truly independent from each other (Baselga 2010; Baselga and Leprieur 2015). This approach yielded for each taxon three pairwise matrices representing the importance of each beta-diversity component in driving taxonomic differentiation between sites.
Spatial and environmental differences between sites
From the geographic coordinates of each site, we calculated a pairwise matrix of geographic distance (spatial distances) in kilometers between communities using the “fossil” package (Vavrek 2015) in R. We characterized the environment of each community with variables representing:
-
1.
climatic gradients: mean annual temperature, temperature seasonality, mean annual precipitation, and precipitation seasonality, all from the WorldClim database (Hijmans et al. 2005);
-
2.
vegetation structure: canopy height (Simard et al. 2011);
-
3.
topography: altitude (USGS 2003);
-
4.
energy: potential evapotranspiration and actual evapotranspiration (Trabucco and Zomer 2009) and actual evapotranspiration (Trabucco and Zomer 2010). The potential evapotranspiration is a measure of the energetic balance at a given site independent of water availability, while the actual evapotranspiration is the real evaporation in a region and thus depends on the potential evapotranspiration and water availability (Currie 1991).
All variables represent different hypotheses by which species distributions and community diversity in a broad macro-ecological context can be influenced by environment (as reviewed in Currie 1991; Field et al. 2009; Stein et al. 2014). Moreover, such environmental parameters are also thought to exert influence on the diversity patterns for the groups we are studying (Stevens 2004; Stevens and Tello 2012; Dambros et al. 2015; López-González et al. 2015). To circumvent the multicollinearity between environmental variables, we summarized environmental data in a principal component analysis (PCA) with the “vegan” package (Oksanen et al. 2017) in R on their standardized (mean = 0, SD = 1) values. Then, we selected the PCA axes that accounted for most of the variation in environmental data using the broken stick criteria (Legendre and Legendre 2012). Scores of communities along these axes were then used to calculate a matrix of pairwise Euclidean distances separately for each taxon analyzed. Differences in these scores represent how much each community pair is similar or different in relation to their environmental features.
Estimating the influence of space and environment on beta diversity
For each group, we calculated the influence of space and environment on each beta-diversity component (βsor, βsim, and βnes) using partial Mantel’s correlation tests. The strength of the correlation was determined with the Mantel’s statistics (r) (Legendre and Legendre 2012). High absolute values of r indicate a stronger correlation of beta diversity with the gradient analyzed. Mantel tests were performed with the “ecodist” package (Goslee and Urban 2007) in R. In all analyses, we controlled the differences in sampling effort between communities (Appendix S2) using a pairwise matrix of Euclidean differences in sampling effort as a covariate. Also, we assessed the significance of each partial Mantel’s r using 10.000 permutations.
Comparing the drivers of beta diversity for bats, rodents, and marsupials
To test our initial hypotheses, we performed within- (H1; Table 1) and between-group (H2; Table 1) comparisons. For H1, we tested for differences in each group, considering the effects of space and environment on its community structure. We combined the values of r from the Mantel tests for each component of beta diversity into a new parameter, hereafter called rdiff. It is simply the differences from the observed value for the original correlation coefficient of beta diversity between the geographic distance and environmental differences between sites:
The observed difference was then compared with a null model in which we permutated the rows in the original matrices 1000 times and recalculated a null distribution of rdiff in relation to space and environment for each taxon. With these permutations, the relationship between communities with their environmental parameters and spatial locations was broken, generating a null distribution of sites along environment and space. Then, we compared the observed values with those obtained in the permutations, calculating the probability of obtaining by chance values of rdiff higher than or equal to the observed. It worth note that while partial Mantel tests provide probabilities for each correlation coefficient, it has limitations in comparing two or more coefficients, an issue solved using the differences in the strength of correlation and the permutations. Under the null hypothesis of no difference between spatial distance and environment in shaping beta diversity for each group, rdiff should be random in comparison to our null distribution. We adopted 0.05 as the cut-off level.
For our H2, in which we tested whether there are differences between groups in the processes structuring their communities, we used a similar approach compared to our H1 test. For this case, however, we were interested in the differences between taxa, and thus we calculated the observed differences for the parameter between groups and considering each gradient. Thus, for a given pair of taxa:
We then compared these results with a null distribution of these differences in a similar way as in H1, calculating the probability of obtaining by chance a difference higher than or equal to the one we observed for each group. Under the null hypothesis of no difference between taxa along a gradient (whether spatial or environmental), the observed differences of each parameter will be random in comparison to our null distribution. Because testing H2 involves three paired comparisons between groups (bats × rodents, bats × marsupials, and rodents × marsupials), we used a Bonferroni correction for the significance level, adopting a cut-off of 0.0166 (0.05/3). All null models and comparisons were performed in R.
Results
Overall patterns of beta diversity for bats, rodents, and marsupials
Communities of bats, rodents, and marsupials present similar patterns of beta diversity in the Atlantic Forest (Fig. 2). Considering all pairwise comparisons between communities, their beta diversity (βsor) is relatively high, although for bats the variation in community composition is more moderated (mean βsor-bats = 0.64 ± 0.18) compared to rodents (mean βsor-rodents = 0.83 ± 0.19) and marsupials (mean βsor-marsupials = 0.82 ± 0.24). We found that for all groups the species turnover (βsim) is the main responsible for differences in taxonomic composition between communities, again with moderated results for bats (mean βsim-bats = 0.45 ± 0.24) in comparison to rodents (mean βsim-rodents = 0.74 ± 0.30) and marsupials (mean βsim-marsupials = 0.73 ± 0.36) (Fig. 2). The contributions of nestedness were higher for bats (mean βnes-bats = 0.18 ± 0.17) than for rodents (mean βnes-rodents = 0.09 ± 0.15) and marsupials (mean βnes-marsupials = 0.08 ± 0.17) (Fig. 2).
Influence of space and environment on the community structure of bats, rodents and marsupials
For all groups, we found that βsor and βsim are positively associated with variable strength to the spatial distance and environmental differences between sites (Table 2; Figs. 3, 4). Conversely, βnes is mainly negatively and weakly associated to space and environment. Except for the βnes of rodents and marsupials along environmental gradients, all correlation coefficients are significant.
For bats, we found that all components of beta diversity did not show differences in the strength of correlation with the spatial distance and environmental dissimilarities between sites (Table 2). Such a result is the opposite of what we predicted initially, given bats’ flight capabilities (P1; Table 1). For rodents and marsupials, however, our results indicate a stronger correlation between all beta-diversity components and spatial distance than with environment (Table 2), which is in line with our initial predictions, considering their reduced capacity for dispersal (P1; Table 1).
Comparing between groups, for rdiff, bats presented a stronger relationship of βsor and βsim with environmental gradients compared to rodents and marsupials, and for βnes along environment in comparison to rodents (Table 3). All other comparisons were non-significant. Overall, this indicates that changes in community composition of all three taxa have the same trend with the geographic distance. Furthermore, in all comparisons of rdiff, rodents and marsupials did not present differences, supporting our initial predictions considering the similarities in biological traits between these mammals in comparison to bats. Therefore, we found only partial support for our hypothesis H2 and our prediction P2, mainly considering the comparison between non-volant small mammals (Table 1).
Discussion
Understanding the influence of spatial and environmental gradients on beta diversity has been essential to improve the knowledge about the mechanisms structuring communities. For mammals, however, there is a lack of comparative studies applying a community ecology approach in a broad macroecological context to test the influence of these gradients on their beta diversity patterns. Here we used a comprehensive database for communities of bats, rodents and marsupials from the Atlantic Forests of South America to ask whether differences in biological characteristics, mainly in terms of dispersal abilities, translate into different processes structuring their communities in this region. Besides revealing that the beta diversity for these groups is governed by a similar process of species turnover between communities, our results suggest only partial support for our initial hypotheses on the differential influence of spatial and environmental dynamics on the structure of these mammals’ assemblages.
A dominance of space over environment in driving rodent and marsupial community compositions can be expected based on their low vagility. In agreement with this prediction, previous studies showed that the beta diversity of these groups in the Atlantic Forest was explained mainly in terms of the spatial distance between sites (Dambros et al. 2015). Furthermore, besides their limited dispersal, such spatial influence probably reflects biogeographical processes linked to past vicariance events that caused the fauna of these non-volant small mammals to be distributed in several centers of endemism along the Atlantic Forest, with high degree of community compartmentation in relation to their species composition (Costa et al. 2000; de la Sancha et al. 2014). Overall, these factors cause a common spatial structure in non-volant small mammal community composition (de la Sancha et al. 2014; Dambros et al. 2015), as we observed in this study. Bats, however, did not show differences in the strength of correlation of their beta diversity between space and environment, against our initial predictions of an increased effect of environment in comparison to space on bat diversity (Stevens 2013; López-González et al. 2015). These results thus indicate the importance of niche dynamics due to bats’ environmental requirements coupled with biogeographical mechanisms such as dispersal limitation or biogeographical barriers that causes a spatial pattern in the community assembly of volant mammals, as previously observed at regional (Stevens et al. 2007) and continental scales (Varzinczak et al. 2018).
Although makes sense to initially expect the beta diversity of rodents and marsupials to be influenced mostly by the spatial configuration of sites owing to their low dispersal, which was supported by our results, non-volant small mammals are not homogeneous in dispersal abilities, and some species can be influenced to a higher degree by environmental dynamics compared to space (Stevens and Tello 2012). Additionally, Maestri and Patterson (2016) found differences between caviomorph and sigmodontine rodents considering the importance of environmental and topographic variables influencing their diversity in the Neotropics. Analogously, Neotropical bat species are influenced in different ways by the environment. For example, phyllostomid bats have been shown to be highly dependent on tropical climatic conditions and vegetation structure because of their metabolic constraints and plant-based diet for most species, respectively (Stevens 2004, 2013). Nonetheless, at larger scales (e.g., Neotropics). phyllostomid beta diversity was better predicted by spatial rather than environmental or historical dynamics (Varzinczak et al. 2018). On the other hand, insectivorous vespertilionid bats are better adapted to cope with the temperate climates found at extra-tropical areas (Stevens 2004). Such differences and their influence constitute a likely explanation of why our results for bats departed from our initial expectations. Altogether, these factors highlight that differences within each group can complicate attempts to make a single prediction for the effect of space and environment on beta-diversity patterns. Accordingly, the effect of the phylogenetic scale (sensu Graham et al. 2018) chosen to conduct analysis constitutes an important question that can be further addressed from a beta diversity perspective, since it could influence the inferences drawn from the multiple processes underlying the community structure within the major clades as the ones studied here.
Differences in the factors structuring communities are commonly found when comparing groups with distinct dispersal abilities and niche width, and comparative analysis have uncovered interesting patterns that agree with theoretical predictions based on life-history traits and their influence on beta-diversity patterns. For example, amphibians have a higher turnover rate in community composition along spatial and environmental gradients compared to birds because of their low dispersal abilities and high sensitivity to changes in environmental conditions (Buckley and Jetz 2008). For plants, taxa with high-vagile propagules have beta diversity weakly related to the spatial distance in comparison to dispersal-limited ones (Tuomisto et al. 2003; Qian 2009). Here, comparisons between rodents and marsupials supported our hypotheses in all scenarios and for all components of beta diversity, highlighting that their biological similarities mainly considering their dispersal limitations in fact translate into similar responses underlying their community assembly along geographical gradients. Both groups did not show differences in the strength of the processes related to variations in the taxonomic composition of their communities. Nonetheless, rodents and marsupials exhibit differences in basic niche attributes (e.g., resources and habitat use) at local scales, which favor their coexistence by reducing competitive pressures (Galetti et al. 2016). When comparing bats with non-volant small mammals, however, we found that the community composition of bats is more sensitive to environmental changes at this large scale in comparison to rodents and marsupials (Table 3). Also, although differences in life-history traits linked to dispersal seem to be reasonable to expect differences in the effects of space and environment among groups, we did not observe differences between bats and non-volant small mammals for the influence of the space on their community dynamics. This is surprisingly by at least two reasons. First, it is counter intuitive and goes against the classical ecological literature considering that better dispersers tend to present a reduced effect of space in comparison to better dispersers (e.g., Nekola and White 1999; Soininen et al. 2007; Qian 2009, De Bie et al. 2012). Second, the abilities of bats to fly are a distinguishing characteristic in comparison to rodents and marsupials. Therefore, besides demonstrating a similar spatial trend in their community composition along the Atlantic Forest, these comparisons shed light on the possibility that historical contingencies due to similar biogeographical dynamics and common barriers to dispersal are influencing spatially the composition of their communities in this region, regardless of their marked differences in dispersal abilities. In fact, there are evidences that several mammal species present common patterns of distribution that have been influenced by similar processes influencing the complex geological history of the Atlantic Forest (Costa and Leite 2012).
When considering βnes, we did not find strong support for our hypotheses. In all cases, the correlation of βnes with the gradients studied here was weak in comparison to the ones found for its βsor and βsim counterparts (Figs. 3, 4; Table 3). It is likely that additional processes other than spatial and environmental ones might be responsible for a nested pattern between communities. For example, the climatic history following the Last Glacial Maximum (21,000 years before the present) caused alterations in species’ geographical ranges, being as important as space and environment in driving nestedness among vertebrate assemblages (Baselga et al. 2012; Dobrovolski et al. 2012). Also, Dobrovolski et al. (2012) showed that groups with different niche widths and dispersal abilities responded differently to these dynamics. Our focus here was mainly to compare the effects of spatial distance and current environmental conditions on these mammals, so we did not address such potential role of history in shaping their community structure. Nonetheless, these results sum up with previous ones highlighting the importance of separating beta diversity into its different components to understand how communities are structured along different gradients (Hill et al. 2017; Soininen et al. 2017).
References
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye B, Freestone AL, Sanders N, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG (2011) Navigating the multiple meanings of beta diversity: a roadmap for the practicing ecologist. Ecol Lett 14:19–28. https://doi.org/10.1111/j.1461-0248.2010.01552.x
Arita HT, Rodríguez P (2004) Local-regional relationships and the geographical distribution of species. Glob Ecol Biogeogr 13:15–21. https://doi.org/10.1111/j.1466-882X.2004.00067.x
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Baselga A (2012) The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob Ecol Biogeogr 21:1223–1232. https://doi.org/10.1111/j.1466-8238.2011.00756.x
Baselga A, Leprieur F (2015) Comparing methods to separate components of beta diversity. Methods Ecol Evol 6:1069–1079. https://doi.org/10.1111/2041-210X.12388
Baselga A, Orme CDL (2012) Betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812. https://doi.org/10.1111/j.2041-210X.2012.00224.x
Baselga A, Gómez-Rodríguez C, Lobo JM (2012) Historical legacies in world amphibian diversity revealed by the turnover and nestedness components of beta diversity. PLoS One. https://doi.org/10.1371/journal.pone.0032341
Bovendorp RS, Villar N, de Abreu-Junior EF, Bello C, Regolin AL, Percequillo AR, Galetti M (2017) Atlantic small-mammal: a dataset of communities of rodents and marsupials of the Atlantic Forests of South America. Ecology. https://doi.org/10.1002/ecy.1893
Buckley LB, Jetz W (2008) Linking global turnover of species and environments. Proc Natl Acad Sci USA 105:17836–17841. https://doi.org/10.1073/pnas.0803524105
Costa LP, Leite YLR (2012) Historical fragmentation shaping vertebrate diversification in the Atlantic Forest biodiversity hotspot. In: Patterson B, Costa LP (eds) Bones, clones and biomes: the history and geography of recent Neotropical mammals. The University of Chicago Press, Chicago, pp 283–306
Costa LP, Leite YLR, Fonseca GA, Fonseca MT (2000) Biogeography of South American Forest mammals: endemism and diversity in the Atlantic Forest. Biotropica 32:872–881. https://doi.org/10.1111/j.1744-7429.2000.tb00625.x
Cottenie K (2005) Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett 8:1175–1182. https://doi.org/10.1111/j.1461-0248.2005.00820.x
Currie DJ (1991) Energy and large-scale patterns of animal- and plant-species richness. Am Nat 137:27–49
Dambros CS, Cáceres NC, Magnus L, Gotelli NJ (2015) Effects of neutrality, geometric constraints, climate, and habitat quality on species richness and composition of Atlantic Forest small-mammals. Glob Ecol Biogeogr 24:1084–1093. https://doi.org/10.1111/geb.12330
De Bie T, De Meester L, Brendonck L, Martens K, Goddwweis B, Ercken D, Hampel H, Denys L, Vanhecke L, Van der Gucht K, Van Wichelen J, Vyverman V, Declerck S (2012) Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecol Lett 15:740–747. https://doi.org/10.1111/j.1461-0248.2012.01794.x
de la Sancha NU, Higgins CL, Presley SJ, Strauss RE (2014) Metacommunity structure in a highly fragmented forest: has deforestation in the Atlantic Forest altered historic biogeographic patterns? Divers Distrib 20:1058–1070. https://doi.org/10.1111/ddi.12210
Dobrovolski R, Melo AS, Cassemiro FAS, Diniz-Filho JAF (2012) Climatic history and dispersal ability explain the relative importance of turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 21:191–197. https://doi.org/10.1111/j.1466-8238.2011.00671.x
Esbérard CEL, Godoy MSM, Renovato L, Carvalho WD (2017) Novel long-distance movements by Neotropical bats (Mammalia: Chiroptera: Phyllostomidae) evidenced by recaptures in Southeastern Brazil. Stud Neotropical Fauna Environ 52:75–80. https://doi.org/10.1080/01650521.2016.1273751
Field R, Hawkins BA, Cornell HV, Currie DJ, Diniz-Filho JAF, Guégan JF, Kaufman DM, Kerr J, Mittelbach GG, Oberdorff T, O´Brien EM, Turner JRG (2009) Spatial species-richness gradients across scales: a meta-analysis. J Biogeogr 36:132–147. https://doi.org/10.1111/j.1365-2699.2008.01963.x
Galetti M, Rodarte RR, Neves CL, Moreira M, Costa-Pereira R (2016) Trophic niche differentiation in rodents and marsupials revealed by stable isotopes. PLoS One 11:e0152494. https://doi.org/10.1371/journal.pone.0152494
Gómez-Rodríguez C, Baselga A (2018) Variation among European beetle taxa in patterns of distance decay of similarity suggests a major role of dispersal processes. Ecography. https://doi.org/10.1111/ecog.03693
Goslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19. https://doi.org/10.18637/jss.v022.i07
Graham CH, Storch D, Machac A (2018) Phylogenetic scale in ecology and evolution. Glob Ecol Biogeogr 27:175–187. https://doi.org/10.1111/geb.12686
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. https://doi.org/10.1002/joc.1276
Hill MJ, Heino J, Thornhill I, Ryves DB, Wood PJ (2017) Effects of dispersal mode on the environmental and spatial correlates of nestedness and species turnover in pond communities. Oikos 126:1575–1585. https://doi.org/10.1111/oik.04266
Legendre P (2014) Interpreting the replacement and richness difference components of beta diversity. Glob Ecol Biogeogr 23:1324–1334. https://doi.org/10.1111/geb.12207
Legendre P, Legendre L (2012) Numerical ecology, 3rd edn. Elsevier, New York
López-González C, Presley SJ, Lozano A, Stevens RD, Higgins CL (2015) Ecological biogeography of Mexican bats: the relative contributions of habitat heterogeneity, beta diversity, and environmental gradients to species richness and composition patterns. Ecography 38:261–272. https://doi.org/10.1111/ecog.00813
Lyons SK, Willig MR (1997) Latitudinal patterns of range size: methodological concerns and empirical evaluations for New World bats and marsupials. Oikos 79:568–580. https://doi.org/10.2307/3546901
Maestri R, Patterson BD (2016) Patterns of species richness and turnover for the South American rodent fauna. PLoS One 11:e0151895. https://doi.org/10.1371/journal.pone.0151895
Muylaert RL, Stevens RD, Esbérard CEL, Mello MAR, Garbino GST, Varzinczak LH, Faria D, Weber MM, Rogeri PK, Regolin AL, Oliveira HFM, Costa LDM, Barros MAS, Sabino-Santos G Jr, Morais MAC, Kavagutti VS, Passos FC, Marjakangas EL, Maia FGM, Ribeiro MC, Galetti M (2017) Atlantic bats: a data set of bat communities from the Atlantic Forests of South America. Ecology 98:3227. https://doi.org/10.1002/ecy.2007
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Nekola JC, White PS (1999) The distance decay of similarity in biogeography and ecology. J Biogeogr 26:867–878. https://doi.org/10.1046/j.1365-2699.1999.00305.x
Norberg UM, Rayner JMV (1987) Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philos Trans R Soc B Biol Sci 316:335–427. https://doi.org/10.1098/rstb.1987.0030
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Michin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHM, Szoecs E, Wagner H (2017) Vegan: Community ecology package. R package version 2.4-4. http://CRAN.Rproject.org/package=vegan. Accessed 20 Feb 2017
Oliveira-Filho AT, Fontes AL (2000) Patterns of floristic differentiation among Atlantic Forests in Southeastern Brazil and the influence of climate. Biotropica 32:793–810. https://doi.org/10.1111/j.1744-7429.2000.tb00619.x
Qian H (2009) Beta diversity in relation to dispersal ability for vascular plants in North America. Glob Ecol Biogeogr 18:327–332. https://doi.org/10.1111/j.1466-8238.2009.00450.x
Qian H, Ricklefs RE (2012) Disentangling the effects of geographic distance and environmental dissimilarity on global patterns of species turnover. Glob Ecol Biogeogr 21:341–351. https://doi.org/10.1111/j.1466-8238.2011.00672.x
R Core Team (2016) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. https://www.R-project.org/. Accessed 30 Nov 2016
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021
Simard M, Pinto N, Fisher JB, Baccini A (2011) Mapping forest canopy height globally with spaceborne lidar. J Geophys Res Biogeosciences 116:1–12. https://doi.org/10.1029/2011JG001708
Soininen J, McDonald R, Hillebrand H (2007) The distance decay of similarity in ecological communities. Ecography 30:3–12. https://doi.org/10.1111/j.2006.0906-7590.04817.x
Soininen J, Heino J, Wang J (2017) A meta-analysis of nestedness and turnover components of beta diversity across organisms and ecosystems. Glob Ecol Biogeogr 27:96–109. https://doi.org/10.1111/geb.12660
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett 17:866–880. https://doi.org/10.1111/ele.12277
Stevens RD (2004) Untangling latitudinal richness gradients at higher taxonomic levels: familial perspectives on the diversity of New World bat communities. J Biogeogr 31:665–674. https://doi.org/10.1111/j.1365-2699.2003.01042.x
Stevens RD (2013) Gradients of bat diversity in Atlantic Forest of South America: environmental seasonality, sampling effort and spatial autocorrelation. Biotropica 45:764–770. https://doi.org/10.1111/btp.12056
Stevens RD, Tello JS (2012) Do desert rodents form metacommunities? J Mamm 93:1029–1041. https://doi.org/10.1644/11-MAMM-A-292.2
Stevens RD, López-González CL, Presley SJ (2007) Geographical ecology of Paraguayan bats: spatial integration and metacommunity structure of interacting assemblages. J Anim Ecol 76:1086–1093. https://doi.org/10.1111/j.1365-2656.2007.01288.x
Trabucco A, Zomer RJ (2009) Global aridity index (global-aridity) and global potential evapo-transpiration (Global-PET) geospatial database. http://www.csi.cgiar.org Accessed 15 Dec 2016
Trabucco A, Zomer RJ (2010) Global soil water balance geospatial database.http://www.cgiar-csi.org Accessed 15 Dec 2016
Tuomisto H, Ruokolainen K, Yli-Halla M (2003) Dispersal, environment, and floristic variation of Western Amazonian forests. Science 299:241–244. https://doi.org/10.1126/science.1078037
USGS (2003) Global 30-arc-second elevation data set (GTOPO30). http://lta.cr.usgs.gov/GTOPO30 Accessed 25 Nov 2015
Varzinczak LH, Lima CS, Moura O, Passos FC (2018) Relative influence of spatial over environmental and historical processes on the taxonomic and phylogenetic beta diversity of Neotropical phyllostomid bat assemblages. J Biogeogr 45:617–627. https://doi.org/10.1111/jbi.13150
Vavrek M (2015) Fossil: palaeoecological and palaeogeographical analysis tools. R package version 0.3.7
Whittaker RH (1960) Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr 30:279–338
Acknowledgements
We thank Sidney F. Gouveia for suggestions on an early version of this manuscript. We extend our most sincere acknowledgements to all researchers that collected data on these mammal assemblages in the field, and to all researchers that jointed efforts to make these data available as part of the “Atlantic-datasets: beyond distribution maps” project.
Funding
LHV and CSL received scholarships from the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES). MOM (309154/2014-6) and FCP (307303/2017-9) received support from the Brazilian National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Contributions
LHV, MOM and FCP conceived the ideas. LHV and CSL collected the data. LHV performed statistical analysis. LHV led the writing with substantial contribution from all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study formal consent is not required.
Additional information
Communicated by Mathew Samuel Crowther.
Testing hypotheses with community data in a macroecological context and based on solid theory is essential to understand the factors underlying the community structure along geographical gradients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Varzinczak, L.H., Moura, M.O., Lima, C.S. et al. How do bat, rodent, and marsupial communities respond to spatial and environmental gradients? Insights from a deconstruction of mammal beta diversity from the Atlantic Forest of South America. Oecologia 189, 851–861 (2019). https://doi.org/10.1007/s00442-018-4288-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4288-y