Abstract
Intermittent breeding may be adaptive for long-lived species subjected to large accessory reproductive costs, but it may also reflect reduced adaptation to the environment, reducing population growth. Nevertheless, environmental influences on breeding propensity, particularly that of predation risk, remain poorly understood and difficult to study, because non-breeders are typically not identified. Female eiders Somateria mollissima from the Baltic Sea provide an excellent testbed, because nesting females have been exposed to intensifying predation and growing male bias that may increase female harassment. We based our study on long-term data (14 years) on females captured and marked at the nest, and females individually identified at sea irrespective of capture status. We hypothesized that breeding propensity decreases with increasing predation risk and male bias, and increases with breeder age. Consistent with our hypotheses, females nesting on islands with higher nest predation risk were more likely to skip breeding, and breeding probability increased with age. In contrast, the steep temporal decline in breeding propensity could not be reliably attributed to annual adult sex ratio or to the abundance of white-tailed sea eagles (Haliaeetus albicilla), the main predator on females, at the nearby Hanko Bird Observatory. Breeding probability showed significant consistent individual variation. Our results demonstrate that spatiotemporal variation in predation risk affects the decision to breed and high incidence of non-breeding was associated with low fledging success. The increased frequency of intermittent breeding in this declining population should be explicitly considered in demographic models, and emphasis placed on understanding the preconditions for successful reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The evolution of intermittent breeding—non-breeding of sexually mature adults with prior breeding experience—is enigmatic, because intermittent breeders face the risk of a dual fitness disadvantage: the loss of current reproduction and the risk of dying before the next chance at reproduction. However, long-lived species are expected to favour survival over current reproduction to maximize their future reproduction (Stearns 1992; Gaillard et al. 1998). Consequently, intermittent breeding may evolve in species with ‘slow’ life histories inhabiting temporally variable environments if breeding conditions fall below a certain threshold (Erikstad et al. 1998; Cubaynes et al. 2011; Shaw and Levin 2013; Jean-Gagnon et al. 2017). However, if environmental change exceeds a critical rate, this may lead to reduced adaptation to local conditions, and a concomitant increase in the incidence of intermittent breeding. As breeding propensity is a critical demographic parameter determining population growth (Nichols et al. 1994; Cam et al. 1998; Lee et al. 2017), such environment-induced changes in breeding behaviour may play an important role in population declines. Despite this importance, the impacts of environmental and ecological drivers on breeding propensity still remain understudied compared to the internal physiological and physical cues associated with the decision to breed (Bradley et al. 2000; Sergio and Hiraldo 2008). This is unfortunate, since we need to understand both external factors and intrinsic attributes underlying variation in breeding propensity (Hoy et al. 2016; Jean-Gagnon et al. 2017).
Life-history theory suggests that intermittent breeding should be particularly likely to evolve in long-lived species, in which reproduction entails an accessory cost in terms of survival, time, or energy beyond the direct investment into gametes or fertilization (Shaw and Levin 2013). Environmental cues that enable individuals to anticipate food availability and to make facultative decisions about whether or not to breed are well-documented (reviewed in White 2008). The same is true for the previous experience of breeding that positively affects future breeding prospects (Grieco et al. 2001; Brommer et al. 2004; Desprez et al. 2011; Warren et al. 2014). In contrast, demonstrating the indirect impact of predation risk on the decision to skip breeding has proven to be challenging under natural conditions, for both conceptual and practical reasons. First, the strategy of intermittent breeding requires substantial accessory costs of reproduction to evolve (Morbey and Shuter 2013) and also that reliable predictive cues about predation risk are available to breeders prior to the onset of breeding (Reed et al. 2015). Second, detection of non-breeders is difficult and sometimes even impossible, because non-breeders are simply not present at the breeding grounds (Gimenez et al. 2008; Desprez et al. 2011). Sampling is often limited to a single occasion per breeding season only involving the actively breeding segment of the population, which provides only limited scope to differentiate the probability of being present from that of being detected given presence (Reed et al. 2004; Gimenez et al. 2008).
Eider ducks (Somateria mollissima) breeding in the Baltic Sea are long-lived animals (estimated life expectancy of 21 years; Coulson 1984) and provide an ideal testbed to address the role of external cues associated with the decision to forgo breeding. First, breeding philopatry is high (Öst et al. 2011) and non-breeders are present and equally conspicuous as breeders at and around the breeding colonies. Second, we had access to ancillary information about the breeding status of individually colour-ringed females outside the actual nest-capture occasions, owing to our long-term observational data (14 years) on all females encountered at sea during the brood-rearing season. Third, this population has recently experienced rapidly increased predation by a recovering population of an apex predator, the white-tailed sea eagle Haliaeetus albicilla (Jaatinen et al. 2011; Ekroos et al. 2012a; this study), concomitant with an increasing population-wide bias towards males (Lehikoinen et al. 2008). This allowed us to assess both the impact of spatial and temporal variation in predation risk and any effects of surplus unpaired males on breeding propensity, as these males may interfere with female preparations for breeding (Steele et al. 2007). Finally, we included female age estimates (based on ringing history; Jaatinen and Öst 2011) in our analysis: individual reproductive responses to changes in extrinsic conditions may depend on intrinsic attributes (Jean-Gagnon et al. 2017), foremost among which are age and breeding experience (Desprez et al. 2011; Warren et al. 2014). This is because individuals are expected to increasingly favour their current reproductive attempt with advancing age, to compensate for the decline in future breeding prospects (Stearns 1992). We tested the following hypotheses: (1) increasing predation pressure is associated with a higher incidence of intermittent breeding; (2) an increasing male bias reduces breeding propensity; and (3) breeding probability generally increases with age. Finally, we explored the connection between breeding propensity and population productivity, measured as fledging success.
Materials and methods
Study area and female capture and observation protocol
This study was conducted in Tvärminne (59°50′N, 23°15′E), western Gulf of Finland, in 2003–2016. The 31 study islands were either small and treeless with scattered stands of juniper (Juniperus communis; N = 16, referred to as open islands; mean area ± SD = 0.52 ± 0.40 ha) or larger and covered mainly by pine forest (Pinus sylvestris) (N = 15, referred to as forested islands; mean area ± SD = 5.54 ± 4.42 ha). Open islands have a higher predation pressure on incubating females (Ekroos et al. 2012a; this study) and habitat types may also differ regarding perceived predation risk from a female eider’s perspective, wherefore island type was included as a covariate in the statistical analysis. Female eiders were captured with hand nets predominantly during the end of incubation. On capture, the females were ringed with a standard metal ring, and uniquely colour-ringed on their leg(s) with plastic ring(s) for individual recognition at a distance (up to ca 600 m using a spotting scope under good light conditions). Females were also equipped with a temporary wing flag (lasting up to 1 month) with a unique combination to ease recognition while swimming at sea (recognition distance ca 800 m using a spotting scope). Because all females irrespective of capture status were colour-ringed and females showed no signs of aberrant behaviour apparently ignoring their markings, we consider it unlikely that our marking techniques would have affected female survival or decisions about whether or not to breed. The number of years since the bird was first ringed was used as an estimate of minimum age (Öst and Steele 2010; Jaatinen and Öst 2011). We acknowledge inevitable measurement error in this variable stemming from variation in the age at first breeding (typically 3 years, range 2–5 years; Hario and Rintala 2009). Nonetheless, this variable can still be considered a reasonably accurate indicator of minimum age. This is due to the facts that we trapped the majority of the successfully breeding females each year (Jaatinen and Öst 2011), and that females are very site-faithful to their previous breeding location (mean breeding dispersal distances are on the scale of tens of metres; Öst et al. 2011). Age-related reproductive senescence is unlikely to significantly affect breeding propensity in the current study. This is because most observed females were at their prime reproductive age, with very few individuals reaching the theoretical expected lifespan of about 21 years (Coulson 1984) or the age at which senescence effects on fecundity start to become apparent (> 17 years of age; Baillie and Milne 1982). Based on our capture success of all incubating females on the study islands, we also calculated the year-specific proportion of trapped females for each island (mean ± SD = 0.57 ± 0.25, N = 292), for use as a covariate (see ‘Statistical Analysis’). This proportion excluded nests encountered as depredated at first encounter (see ‘Estimating predation risk’ below), since re-nesting, although highly unlikely, may still be possible after nest failure at an early stage.
During daily observations made by a team of two-to-five observers equipped with spotting scopes, we tried to locate all individually identifiable females in the entire study area, from the first appearance of a brood until the young were close to independence (~ 30 days after hatching) (observation period late May until late June) (Jaatinen and Öst 2013). At each sighting of an individually marked female, we recorded her identity, whether she was attending a brood, the number of ducklings in the brood, and, if present, the number of other females in the brood. Each focal female was followed long enough to ensure correct assessment of her brood-rearing status. This assessment is straightforward in our study area, as non-tending females are not tolerated within broods and are promptly chased away by the tending female(s) (Öst et al. 2003). Based on all annual observations of a focal female, we grouped each individual into two distinct classes: solitary females never seen associated with young, and brood-tending females associated with young at least once during the brood-rearing season.
Spatial and temporal variation in predation risk
Predation risk was estimated using two indices that were specifically designed to separate the effects of spatial and temporal variation in predation risk on breeding propensity. The first index, the annual island-specific proportion of depredated nests (Jaatinen et al. 2014) was calculated as the number of depredated nests at first encounter divided by the total number of nesting attempts (including depredated nests at first encounter and nests in which the ducklings had already hatched) on each island in 2003–2016 (mean ± SD = 0.21 ± 0.22, N = 292). Clutches are depredated mainly by hooded crows (Corvus corone cornix), ravens (Corvus corax), and large gulls (Larus spp.), but they may also become depredated as a by-product of attacks on the nesting females (for predators on adults, see below). Only depredated nests found during our first visit to each part of the islands were considered (Öst et al. 2011), because additional visits may induce nest depredation and abandonment. The nest censuses on all study islands were done at a phenologically equivalent time in each year. For the statistical analysis, annual island-specific proportions of depredated nests were standardized within years (mean = 0, variance = 1) to obtain a time-detrended predation index only estimating spatial variation in predation risk among islands.
The second index measured the annual abundance of white-tailed sea eagles at Hanko Bird Observatory (HALIAS, 59°49′N, 22°54′E), situated ca 20 km west of the Tvärminne study area (Jaatinen et al. 2011). This index was calculated by dividing the total sum of daily numbers of resident white-tailed sea eagles observed during 1 April–15 June in 2003–2016 (corresponding to the breeding season of eiders) with the number of annual observation days during the same period (mean ± SD = 3.84 ± 1.84, N = 14 years). The eagle abundance index showed a steep increase over time (log-linear regression: 13.4% annual increase, CI95% = 9.4–17.5%, N = 14 years).
We also documented temporal trends in adult predation risk at Tvärminne. To this end, we recovered all incubating females killed at their nests during nest censuses in 1994–2016 (N = 493). The killer could be determined for 191 freshly killed carcasses according to the way the females had been killed and devoured (see Jaatinen et al. 2011).
Adult sex ratio
The overall adult sex ratio in the entire Gulf of Finland can be assessed by observing migrating birds at HALIAS located at the entrance of the Gulf, acting as a major migration funnel (Kilpi et al. 2003). HALIAS is manned year-round by professional observers using a standardized daily observation protocol and spring-migrating eiders pass close and in small flocks that allow accurate recording of the sex ratio in the group. Here, we determined the overall annual sex ratio in 2003–2016 during a 15-day period around peak migration (Lehikoinen et al. 2008). Because the timing of peak migration depends on the severity of the preceding winter (Lehikoinen et al. 2006), we selected the 15-day peak migration period separately for each year. This was done by selecting the first clear 5-day migration peak and adding, respectively, subtracting, 5 days to/from that period (Lehikoinen et al. 2008). The data on the adult sex ratio were based on a total count of 177,525 spring-migrating eiders (annual mean ± SD = 12,680 ± 6267 birds, range 5351–24,443, N = 14 years), with an average (± SD) sex ratio of 60.9% (± 4.26%) males (range 53.3–66.6%, N = 14 years).
Fledging success
Breeding success at Tvärminne was determined annually during large-scale brood counts at the turn of June and July (ca. 6 weeks after peak hatching), from fixed vantage points distributed evenly across the entire study area (Lehikoinen et al. 2006). The total number of ducklings and females (sum of brood-caring and solitary adult females) was recorded during these counts, and the ratio of ducklings per adult female was used as an annual index of duckling production.
Statistical analysis
Variation in predation pressure
First, we compared the predation risk on nests and adult breeding females between island types (open vs. forested islands). This was done using a logistic regression on the island-specific proportion of depredated nests and killed females relative to the total number of nests on each island over the study period (2003–2016 and 1994–2016 for nest predation and adult predation, respectively; see “Spatial and temporal variation in predation risk”).
Temporal trends in white-tailed sea eagle abundance and observed cases of eagle- and mink-induced predation were investigated using year as a predictor in log-linear and Poisson regressions (log link, quasi-Poisson errors), respectively. The average proportion and temporal trend in the proportion of eagle vs. mink predation was investigated using a logistic regression (logit link, quasi-binomial errors), with centralized year as the explanatory variable (at 50:50, the intercept is expected to be 0). To filter out confounding temporal trends and autocorrelation, correlation analyses between any two time series were conducted on the first differences of both variables involved.
Breeding propensity
To determine the incidence of intermittent breeding, we used data from 2004 to 2016 on resighted colour-ringed females at sea and recaptured females on the nest. A female was considered to be a breeder if it was caught on the nest during the incubation stage and/or if it was observed and identified at sea associated with ducklings at least once. To reduce bias, we included only females known to be both marked and recruited into the breeding population in earlier breeding seasons. In other words, we excluded (1) all first-time breeders, because females observed at sea in the year of their first capture had, by definition, been nesting in that season as evidenced by their earlier capture at the nest and (2) all records from 2003 when the colour-ringing scheme was initiated. After this selection, the data set included 1650 records of 698 females observed during the brood-rearing period (range = 1–10 annual resightings, i.e., all resightings of a female within a year were pooled) and associated with one of the breeding islands in 2004–2016.
We used generalized linear mixed models with binomial errors and logit link to analyse the probability to breed, with the binary response variable being the presumed breeding status. The explanatory variables in all these analyses were standardized by subtracting the mean and dividing by the standard deviation to make effect sizes directly comparable. The following explanatory variables were modelled as fixed effects: island type (factor; open/forested), female minimum years of maternal experience (quantitative; hereafter ‘minimum age’), annual islandwise proportion of successfully trapped females (quantitative; ‘trapping success’), and the annual island-specific proportion of depredated nests (quantitative; ‘predation risk’, see above for variable descriptions). The factor variables female identity, island identity, and year identity were included in the model as random effects on the intercept. The model was fitted using maximum likelihood, with Laplace approximation of the likelihood function, optimizer “bobyqa”, and a maximum of 20,000 function evaluations.
We assessed a null model with the structure described above, which effectively assumes no temporal trend. Apart from the null model, we evaluated seven models also involving all combinations of the following annual-level explanatory variables: year (quantitative variable; uniform logit-linear annual trend), annual abundance of white-tailed sea eagles, and annual adult sex ratio—all being variables with clear temporal trends (ESM Table S1). We assessed the relative support for the resulting eight competing models of temporal pattern using information-theoretic model selection. We applied the Akaike information criterion (AIC), which evaluates the degree of model support, optimizing the trade-off between underfitting and overfitting (Johnson and Omland 2004). Lower AIC scores indicate a better-fitting model with respect to its complexity. We expect that any strong driver of intermittent breeding should provide a higher ranked model compared to the year-only-model.
We present the fixed effect coefficients (± SE) of the lowest AIC model. The statistical significance of the fixed effect coefficients is based on z tests.
To test the null hypothesis of no consistent individual variation in breeding propensity, we performed a Monte Carlo test with 10,000 repetitions, where we for each trial simulated a situation with no individual variation and refitted the model. Similar to parametric bootstrapping, we generated new data sets by drawing all random components from their assumed distributions, given the fitted model parameters, however, excluding the individual level random effect. The P value is simply the proportion of larger-than-observed estimates of individual SD among the 10,000 simulation trials.
Female body condition
Finally, we indirectly assessed the influence of female body condition on breeding decisions. Body condition is an important confounding factor, because a minimum threshold body mass is required to initiate reproduction (see “Discussion”), yet this variable is unquantifiable for the non-nesting females included in our study. However, we may draw indirect inferences about the body condition dynamics in non-nesting birds by analysing temporal trends in body condition of breeding birds. This is because the ‘reproductive suppression model’ (Wasser and Barash 1983) predicts that the condition threshold for initiating breeding may increase under unfavourable conditions, which typically delay the onset of breeding. To this end, we analysed the body condition at hatching and timing of breeding for nesting females at Tvärminne during 2003–2016. Body condition was estimated as size-corrected residual body mass at hatching; the detailed procedure for deriving these indices has been described elsewhere (Öst and Steele 2010). The estimated hatching date was calculated based on egg floatation at capture (Kilpi and Lindström 1997). For analysing both response variables, we used linear mixed models (LMMs) with Gaussian errors and based on restricted maximum likelihood estimation, and with female identity included as a random effect. All statistical analyses were performed using R 3.3.1 (R Core Team 2016).
Results
Variation in predation pressure and correlation between adult and egg predation
Out of the totally 1176 nests depredated at first encounter in 2003–2016, 799 were found on forested islands (nest depredation rate per nest 0.19, N = 4215 nests on 15 islands) and 377 on open islands (nest depredation rate per nest 0.224, N = 1682 nests on 16 islands). The probability of nest depredation was significantly lower on forested than on open islands (logistic regression: b = 0.21 ± 0.07 SE, z29 = 3.00, P = 0.003). Correspondingly, out of the 493 females found killed at their nests in 1994–2016, 325 were nesting on forested islands (predation rate per nest 0.081, N = 4025 nests on 15 islands) and 168 on open islands (predation per nest 0.134, N = 1256 nests on 27 islands). The probability of a female being killed was significantly lower on forested than on open islands (logistic regression: b = 0.56 ± 0.10 SE, z40 = 5.58, P < 0.001).
The two most important predators on adult females were the white-tailed sea eagle (44.5% of kills, N = 85) and the American mink (Neovison vison) (37.2% of kills, N = 71), while predation by the eagle owl (Bubo bubo) (11.5% of kills, N = 22), the raccoon dog (Nyctereutes procyonoides) (6.3% of kills, N = 12), and the goshawk (0.5% of kills, N = 1) was more uncommon or occasional. The absolute numbers of annual kills steeply increased in the two main predators. Based on Poisson regression, the annual increase in white-tailed sea eagle-caused mortality was 14.2% (CI95% = 8.2–20.5%) and the increase in predation by minks was 11.0% (CI95% = 3.7–18.9%). In the less important predators, data were not sufficient for testing such trends. The relative proportion of eagle predation vs. mink predation did not differ significantly from 50:50 (logistic regression, intercept: 0.083 ± 0.233 SE, t17 = 0.36, P = 0.73) and there was no significant temporal trend in the proportion of eagle predation (logistic regression: 0.027 ± 0.037 SE, t17 = 0.73, P = 0.48). Annual predation risks on incubating females and nests were strongly positively correlated (based on first-differenced time series: r = 0.751, CI95% = 0.462–0.896, N = 20).
Breeding propensity and its connections to population productivity
We ran seven models with different additive combinations of the explanatory variables and compared these to the null model that included island type, minimum age, trapping success, and predation risk as fixed effects (see “Statistical analysis”; ESM Table S1). All candidate models with annual-level explanatory variables (describing trends) fitted the data better than the null model with no additional predictors (ΔAIC = 15.29). The best-ranked model, with lowest AIC score, was the one including only year added to the null model, describing an unspecified annual trend (marginal R2 = 0.19, conditional R2 = 0.31). We found no support for replacing year in the best model with either annual adult sex ratio (ΔAIC = 12.14) or with the annual white-tailed sea eagle index (ΔAIC = 8.01). Similarly, adding simultaneous effects of eagles or adult sex ratio to the top-ranked model with year, did not provide better models (range of ΔAIC = 1.09–9.88). After ignoring models with uninformative parameters sensu Arnold (2010), only the model with lowest AIC score was considered relevant. Therefore, we concentrate on the parameter estimates from that model.
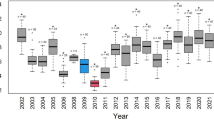
Investigation of the explanatory variables in the top-ranked model revealed that after correcting for island-specific trapping success (b = 0.23 ± 0.08, z = 2.96, P = 0.003), breeding propensity did not differ depending on island type (z = 0.80, P = 0.42). Females associated with islands with higher predation risk were more likely to skip breeding (Fig. 1, predation risk, b = − 0.43 ± 0.07, z = − 6.39, P < 0.001). There was a strong annual trend, where a female’s probability to breed decreased over the course of the study period (Fig. 1, annual trend, b = − 0.85 ± 0.14, z = − 5.90, P < 0.001). Older females were more likely to breed than younger ones (Fig. 2, b = 0.22 ± 0.07, z = 3.01, P < 0.003). The model intercept was 1.32 ± 0.19.
Probability of presumed breeding in female eiders as a function of time-detrended annual island-specific proportion of depredated nests (for definitions, see text). The lines illustrate the model fit for 2004–2016 (2-year intervals; lighter grey indicate more recent time) when all non-displayed variables are set to their averages. The data points are displayed as open circles in grey (darker colour meaning more overlap) and with added jitter along the y-axis to facilitate viewing the distribution of raw data (actual data are zeros and ones)
Probability of presumed breeding in female eiders (for definition, see text) as a function of minimum age (years since first capture as a breeding bird). The black solid line is the model fit when all non-displayed variables are set to their averages, while the dashed lines are 95% CIs. The data points are displayed as open circles in grey (darker colour meaning more overlap). Jitter is added to the raw data (zeros and ones) along the x- and y-axes to facilitate viewing their distribution
There was also evidence for consistent variation between individual females in their propensity to breed (random effect, female ID, estimated SD = 0.47). The Monte Carlo test for zero individual SD revealed that the observed individual variation differed significantly from zero (P = 0.032; ESM Fig. S1). However, even in the case of zero individual SD (simulated), the bimodal sampling distribution has a second peak around SD = 0.25 (ESM Fig. S1), suggesting that this quantitative result should be interpreted with caution (rather qualitatively; rejection of the null hypothesis). In addition, a female’s breeding island explained her propensity to breed (breeding island, SD = 0.34), and annual variation in breeding propensity was high (factor year, SD = 0.43).
Both fledgling production and proportion of non-breeding showed large annual variation during the study period; fledgling production ranged between 0.13 and 1.82 fledged young per adult female (mean ± SD = 0.82 ± 0.50, N = 13 years), while the annual proportion of non-breeding ranged between 0.072 and 0.53 (mean ± SD = 0.23 ± 0.15, N = 13). The annual proportion of presumed non-breeding females and fledgling production showed a negative correlation (based on first-differenced time series: r = − 0.620, CI95% = − 0.881 to − 0.072, N = 13).
Time trends in female body condition and breeding schedule
Our ancillary analysis of time trends in female body condition at hatching and timing of breeding revealed that female body condition at hatching increased over time (LMM: b = 0.034, t = 6.60, P < 0.001, N = 2523 observations on 1326 females). There was also a temporal shift towards later timing of breeding (LMM: b = 0.29, t = 9.55, P < 0.001, N = 2523 observations on 1326 females).
Discussion
Our results provided support for our first and third hypotheses (skipping breeding was more common under high predation risk and among younger breeders), but not for our second hypothesis (increasing male bias results in a higher incidence of intermittent breeding). We also detected a strong temporal increase in the incidence of intermittent breeding, with average estimated breeding propensity decreasing from 95.2% in 2004 to only 53.8% in 2016 (Fig. 1). The estimated rate of temporal decrease in breeding propensity may, in fact, be conservative, given the exclusion of (presumed) first-time breeders from our analysis and the fact that declining population size in eiders has been linked to a later onset of first breeding (Hario and Rintala 2009). Breeding propensity also showed a strong negative correlation with population productivity. With respect to the effects of predation risk, breeding in a high predation risk area (indexed by the time-detrended annual island-specific proportion of depredated nests) had a strong negative association with the probability of breeding. This finding agrees with the theoretical prediction that individuals should refrain from breeding as the mortality cost of reproduction increases (Shaw and Levin 2013). Though in itself, this idea is not new—e.g., Coulson (1984) proposed that eiders refrain from breeding in years of low adult survival—predation risk has not before been invoked as a variable underlying the decision to forgo breeding in this species. Thus, Coulson (2010) ascribed the periodically high incidence of intermittent breeding observed in a sedentary British eider population to food shortage for unspecified reasons. Perhaps surprisingly, we found that annual abundance of white-tailed sea eagles had no independent explanatory effect on the probability of breeding. One possibility is that annual-based indices of eagle abundance 20 km away may not capture local variation in predation pressure. Furthermore, the functional form of the relationship between the two variables may be more complicated than expected here. It is also noteworthy that the eagle abundance index showed a dramatic increase over time (see “Spatial and temporal variation in predation risk”), and therefore, it is conceivable that the likewise very strong temporal increase in intermittent breeding could have masked any effects of eagle abundance per se on breeding propensity. Although predation on breeding eider females by eagles was the single most important cause of female mortality during the breeding season, increasing markedly over time, there was a corresponding temporal increase in predation events by mink. Furthermore, we could not detect any temporal trend in the proportion of predation events by these two predators. Although predation by eagles is likely to affect the incidence of intermittent breeding in this population (see also Ekroos et al. 2012a), the effect of other important predators may act to lessen the importance of a predation index solely estimating eagle abundance in explaining eider breeding propensity.
As our results are based on correlative evidence alone, there is a need to consider alternative explanations. We cannot rule out the possibility that some females observed at sea but not captured at the nest actually nested outside the study area. However, we consider it very unlikely that a significant segment of the breeding population would have settled elsewhere to breed, for two reasons. First, females show a high level of breeding philopatry to specific nesting islands (Öst et al. 2011). Although predator-induced nest failure increases breeding dispersal distances in the subsequent breeding season, these movements occur at a very fine spatial scale (tens of metres), only rarely involving island switching (Öst et al. 2011, Ekroos et al. 2012a). Second, adult females irrespective of their breeding status occur aggregated close (typically < 1 km) to their nesting island throughout the brood-rearing season in this population (Öst and Kilpi 2000).
Intermittent breeding as a response to predation risk is only likely to evolve given substantial survival costs of reproduction and the presence of predictive cues on predation risk prior to the onset of breeding. These two conditions are likely to be met in our study system. First, the apparent survival of breeding eider females in this population is the lowest recorded in this species, which has been attributed to increased predation during incubation (Ekroos et al. 2012a). This, in turn, is believed to be the main reason for the progressively increasing male bias in the entire Baltic/Wadden Sea flyway population (Lehikoinen et al. 2008). Second, nest success shows moderate spatial predictability at the island level (Öst et al. 2011). The main predators on incubating females, in particular the day-active white-tailed sea eagle, are conspicuous elements in the archipelago year-round. Although we were unable to confirm a direct relationship between white-tailed sea eagle-induced predation risk and breeding propensity, prevailing predation risk, nonetheless, affects the nest-site decisions of female eiders in several contexts. For example, breeding females disperse farther following nest predation, which delays their breeding schedule in the subsequent season (Öst et al. 2011). Second, large spatiotemporal variation in predation risk—as observed in our study population—may in itself favour the evolution of intermittent breeding, and promote annual and individual variability in breeding propensity. Thus, theoretical and empirical work suggests that individuals inhabiting more variable environments tend to show a higher average frequency of intermittent breeding (Nevoux et al. 2010), pronounced inter-annual variation in the extent of intermittent breeding (Cayuela et al. 2016), as well as large individual differences in breeding propensity (Shaw and Levin 2013). Indeed, our results revealed that there was significant variation between individuals in their propensity to breed, and breeding propensity showed annual variation not captured by a simple time trend.
Our correlative approach prevents us from drawing conclusions about the mechanisms by which predation risk may suppress reproduction. However, one possibility, supported by a growing body of research, is that predatory stress encountered prior to breeding onset could cause abandonment of the current breeding attempt. Predation risk may demonstrably trigger physiological adjustments that induce reproductive suppression. Although the majority of the existing evidence of such hormonal regulation comes from mammals (Sheriff et al. 2009; Cherry et al. 2016), pre-breeding stress can also suppress ovarian function in seabirds through increased glucocorticoid (corticosterone) secretion (Goutte et al. 2010a). Incubating females having elevated baseline corticosterone levels have lower nest success (Jaatinen et al. 2013) and pre-breeding eider females with higher baseline corticosterone levels have a later breeding phenology (Hennin et al. 2016). It is, therefore, conceivable that predator-induced stress may also affect the fundamental decision of whether or not to breed. However, testing this hypothesis would require manipulation of predation risk and monitoring of stress hormone concentrations in pre-breeding females, which is logistically challenging in a natural population. Our results also showed that breeding propensity increased with age. This result may also fit the notion of predator stress-induced suppression of reproduction, as younger individuals are often more susceptible to stressors than prime-aged breeders (Goutte et al. 2010b, 2011).
One important confounding factor is body condition, because a minimum threshold body mass is required to initiate reproduction (Drent and Daan 1980; Rowe et al. 1994; Warren et al. 2014; Legagneux et al. 2016; also see “Statistical analysis”). According to the ‘reproductive suppression model’ (Wasser and Barash 1983), long-lived species challenged by unfavourable conditions are expected to maximize their lifetime reproductive success by suppressing their reproduction until a more favourable time. Empirical tests of this model have shown that experimentally challenged individuals refrain from breeding only in unfavourable years (as indexed by nest success) (Griesser et al. 2017). Consequently, only individuals of high quality and/or condition may opt to breed under unfavourable conditions, a prediction recently corroborated in eiders (Jean-Gagnon et al. 2017). Indeed, the observed increase in the body condition of breeding females at Tvärminne appears to match this prediction (see “Results”). Furthermore, the potential deterioration of breeding conditions is reflected in a concomitant temporal shift towards later timing of breeding. The environment may have become less favourable due to intensifying predation, reduced nutrient load affecting mussel stocks (Laursen and Møller 2014), and/or a shift in the relative importance of wintering vs. breeding areas for acquiring the energy reserves needed for reproduction. The increasing mean body condition in the breeding pool is perhaps surprising, given that excess body mass may jeopardize escape performance (Freed 1981; Norberg 1981). However, apparently such effects, if present, are overshadowed by the generally positive relationship between body condition (reflecting individual quality) and survival in this population (Ekroos et al. 2012a). The change in climate forcing, in turn, may be associated with warming winters, which are related to blue mussels of lower nutritional value for wintering eiders (Waldeck and Larsson 2013). Such conditions may cause greater reliance on food resources gathered at the breeding grounds, forcing females to breed later (Jaatinen et al. 2016). Regardless of the reason for the time trend in body condition of breeding females, an increasing fraction of potential breeders may be unable to build up sufficient body reserves for successful breeding under current conditions. To conclude, temporal changes in the energetic requirements for successful reproduction may have contributed to the steep increase in the incidence of intermittent breeding over time (Fig. 1).
In this study, we have demonstrated that spatiotemporal variation in predation risk and breeder age had a profound influence on breeding propensity, which also showed substantial annual and individual variation. The current unprecedented high level of intermittent breeding should cause serious management concern, as this species, although still common, is now classified as endangered in Europe (BirdLife International 2015) due to the recent steep decline over the entire Baltic region (Ekroos et al. 2012b; Öst et al. 2016). Failing to account for the pool of non-breeders may lead us to seriously overestimate the effective reproductive output per mature female, which may obscure alarmingly low levels of population growth (Lee et al. 2017). Consistent with this notion, we found that high incidence of non-breeding was associated with low fledging success. As for the next steps in this research, we suggest population-wide modelling of the relative role of increased intermittent breeding vs. changes in fecundity and offspring survival in contributing to the population-wide decline of eiders in the Baltic Sea. At the individual level, it would be a logistically challenging, yet important, endeavour to develop non-invasive means to monitor the body condition of pre-laying females that skip breeding. Furthermore, it would be illuminating to explore whether the observed between-female variation in breeding propensity is linked to personality traits such as risk-taking, and whether females skipping breeding in dangerous years really achieve a fitness benefit compared to those birds nesting on a more regular basis.
References
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s Information Criterion. J Wildl Manag 74:1175–1178. https://doi.org/10.1111/j.1937-2817.2010.tb01236.x
Baillie SR, Milne H (1982) The influence of female age on breeding in the eider Somateria mollissima. Bird Study 29:55–66. https://doi.org/10.1080/00063658209476738
BirdLife International (2015) European Red List of Birds. Office for Official Publications of the European Communities, Luxembourg
Bradley JS, Wooller RD, Skira IJ (2000) Intermittent breeding in the short-tailed shearwater Puffinus tenuirostris. J Anim Ecol 69:639–650. https://doi.org/10.1046/j.1365-2656.2000.00422.x
Brommer JE, Karell P, Pietiäinen H (2004) Supplementary fed Ural owls increase their reproductive output with a one year time lag. Oecologia 139:354–358. https://doi.org/10.1007/s00442-004-1528-0
Cam E, Hines JE, Monnat J-Y, Nichols JD, Danchin E (1998) Are adult nonbreeders prudent parents? The kittiwake model. Ecology 79:2917–2930. https://doi.org/10.1890/0012-9658(1998)079[2917:aanppt]2.0.co;2
Cayuela H, Arsovski D, Thirion JM, Bonnaire E, Pichenot J, Boitaud S, Brison AL, Miaud C, Joly P, Besnard A (2016) Contrasting patterns of environmental fluctuation contribute to divergent life histories among amphibian populations. Ecology 97:980–991. https://doi.org/10.1890/15-0693.1
Cherry MJ, Morgan KE, Rutledge BT, Conner LM, Warren RJ (2016) Can coyote predation risk induce reproduction suppression in white-tailed deer? Ecosphere 7:e01481. https://doi.org/10.1002/ecs2.1481
Core Team R (2016) R: a language and environment for statistical computing, 3.3.0 edn. R Foundation for Statistical Computing, Vienna
Coulson JC (1984) The population dynamics of the eider duck Somateria mollissima and evidence of extensive non-breeding by adult ducks. Ibis 126:525–543. https://doi.org/10.1111/j.1474-919X.1984.tb02078.x
Coulson JC (2010) A long-term study of the population dynamics of common eiders Somateria mollissima: why do several parameters fluctuate markedly? Bird Study 57:1–18. https://doi.org/10.1080/00063650903295729
Cubaynes S, Doherty PF, Schreiber EA, Gimenez O (2011) To breed or not to breed: a seabird’s response to extreme climatic events. Biol Lett 7:303–306. https://doi.org/10.1098/rsbl.2010.0778
Desprez M, Pradel R, Cam E, Monnat JY, Gimenez O (2011) Now you see him, now you don’t: experience, not age, is related to reproduction in kittiwakes. Proc R Soc B 278:3060–3066. https://doi.org/10.1098/rspb.2011.0189
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 68:225–252
Ekroos J, Öst M, Karell P, Jaatinen K, Kilpi M (2012a) Philopatric predisposition to predation-induced ecological traps: habitat-dependent mortality of breeding eiders. Oecologia 170:979–986. https://doi.org/10.1007/s00442-012-2378-9
Ekroos J, Fox AD, Christensen TK, Petersen IK, Kilpi M, Jonsson JE, Green M, Laursen K, Cervencl A, de Boer P, Nilsson L, Meissner W, Garthe S, Öst M (2012b) Declines amongst breeding eider Somateria mollissima numbers in the Baltic/Wadden Sea flyway. Ornis Fenn 89:81–90
Erikstad KE, Fauchald P, Tveraa T, Steen H (1998) On the cost of reproduction in long-lived birds: the influence of environmental variability. Ecology 79:1781–1788. https://doi.org/10.1890/0012-9658(1998)079[1781:OTCORI]2.0.CO;2
Freed LA (1981) Loss of mass in breeding wrens: stress or adaptation? Ecology 62:1179–1186. https://doi.org/10.2307/1937282
Gaillard J-M, Festa-Blanchet M, Yoccoz NG (1998) Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol Evol 13:58–63. https://doi.org/10.1016/S0169-5347(97)01237-8
Gimenez O, Viallefont A, Charmantier A, Pradel R, Cam E, Brown CR, Anderson MD, Bomberger Brown M, Covas R, Gaillard J-M (2008) The risk of flawed inference in evolutionary studies when detectability is less than one. Am Nat 172:441–448. https://doi.org/10.1086/589520
Goutte A, Angelier F, Clément Chastel C, Trouvé C, Moe B, Bech C, Gabrielsen GW, Chastel O (2010a) Stress and the timing of breeding: glucocorticoid-luteinizing hormones relationships in an arctic seabird. Gen Comp Endocrinol 169:108–116. https://doi.org/10.1016/j.ygcen.2010.07.016
Goutte A, Antoine E, Weimerskirch H, Chastel O (2010b) Age and the timing of breeding in a long-lived bird: a role for stress hormones? Funct Ecol 24:1007–1016. https://doi.org/10.1111/j.1365-2435.2010.01712.x
Goutte A, Kriloff M, Weimerskirch H, Chastel O (2011) Why do some adult birds skip breeding? A hormonal investigation in a long-lived bird. Biol Lett 7:790–792. https://doi.org/10.1098/rsbl.2011.0196
Grieco F, van Noordwijk AJ, Visser ME (2001) Evidence for the effect of learning on timing of reproduction in blue tits. Science 296:136–138. https://doi.org/10.1126/science.1068287
Griesser M, Wagner GF, Drobniak SM, Ekman J (2017) Reproductive trade-offs in a long-lived bird species: condition-dependent reproductive allocation maintains female survival and offspring quality. J Evol Biol 30:782–795. https://doi.org/10.1111/jeb.13046
Hario M, Rintala J (2009) Age of first breeding in the common eider Somateria m. mollissima population in the northern Baltic Sea. Ornis Fenn 86:81–88
Hennin HL, Bêty J, Legagneux P, Gilchrist HG, Williams TD, Love OP (2016) Energetic physiology mediates individual optimization of breeding phenology in a migratory Arctic seabird. Am Nat 188:434–445. https://doi.org/10.1086/688044
Hoy SR, Millon A, Petty SJ, Whitfield DP, Lambin X (2016) Food availability and predation risk, rather than intrinsic attributes, are the main factors shaping the reproductive decisions of a long-lived predator. J Anim Ecol 85:892–902. https://doi.org/10.1111/1365-2656.12517
Jaatinen K, Öst M (2011) Experience attracts: the role of age in the formation of cooperative brood-rearing coalitions in eiders. Anim Behav 81:1289–1294. https://doi.org/10.1016/j.anbehav.2011.03.020
Jaatinen K, Öst M (2013) Brood size matching: a novel perspective on predator dilution. Am Nat 181:171–181. https://doi.org/10.1086/668824
Jaatinen K, Öst M, Lehikoinen A (2011) Adult predation risk drives shifts in parental care strategies: a long-term study. J Anim Ecol 80:49–56. https://doi.org/10.1111/j.1365-2656.2010.01757.x
Jaatinen K, Seltmann MW, Hollmén T, Atkinson S, Mashburn K, Öst M (2013) Context dependency of baseline glucocorticoids as indicators of individual quality in a capital breeder. Gen Comp Endocrinol 191:231–238. https://doi.org/10.1016/j.ygcen.2013.06.022
Jaatinen K, Seltmann MW, Öst M (2014) Context-dependent stress responses and their connections to fitness in a landscape of fear. J Zool 294:147–153. https://doi.org/10.1111/jzo.12169
Jaatinen K, Öst M, Hobson KA (2016) State-dependent capital and income breeding: a novel approach to evaluating individual strategies with stable isotopes. Front Zool 13:24. https://doi.org/10.1007/s00442-017-4002-5
Jean-Gagnon F, Legagneux P, Gilchrist G, Bélanger S, Love OP, Bêty J (2017) The impact of sea ice conditions on breeding decisions is modulated by body condition in an arctic partial capital breeder. Oecologia. https://doi.org/10.1007/s00442-017-4002-5 (in press)
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108. https://doi.org/10.1016/j.tree.2003.10.013
Kilpi M, Lindström K (1997) Habitat-specific clutch size and cost of incubation in common eiders, Somateria mollissima. Oecologia 111:297–301. https://doi.org/10.1007/s004420050238
Kilpi M, Öst M, Lehikoinen A, Vattulainen A (2003) Male sex bias in eiders Somateria mollissima during spring migration into the Gulf of Finland. Ornis Fenn 80:137–142
Laursen K, Møller AP (2014) Long-term changes in nutrients and mussel stocks are related to numbers of breeding eiders Somateria mollissima at a large Baltic colony. PLoS One 9:e95851. https://doi.org/10.1371/journal.pone.0095851
Lee AM, Reid JM, Beissinger SR (2017) Modelling effects of nonbreeders on population growth estimates. J Anim Ecol 86:75–87. https://doi.org/10.1111/1365-2656.12592
Legagneux P, Hennin H, Williams TD, Gilchrist HG, Love OP, Bêty J (2016) Unpredictable perturbation reduces breeding propensity regardless of reproductive readiness in a partial capital breeder. J Avian Biol 47:880–886. https://doi.org/10.1111/jav.00824
Lehikoinen A, Kilpi M, Öst M (2006) Winter climate affects subsequent breeding success of common eiders. Glob Chang Biol 12:1–11. https://doi.org/10.1111/j.1365-2486.2006.01162.x
Lehikoinen A, Christensen TK, Öst M, Kilpi M, Saurola P, Vattulainen A (2008) Large-scale change in the sex-ratio of a declining eider Somateria mollissima population. Wildl Biol 14:288–301. https://doi.org/10.2981/0909-6396(2008)14[288:LCITSR]2.0.CO;2
Morbey YE, Shuter BJ (2013) Intermittent breeding in the absence of a large cost of reproduction: evidence for a non-migratory, iteroparous salmonid. Ecosphere 4:150. https://doi.org/10.1890/ES13-00259.1
Nevoux M, Forcada J, Barbraud C, Croxall J, Weimerskirch H (2010) Bet-hedging response to environmental variability, an intraspecific comparison. Ecology 91:2416–2427. https://doi.org/10.1890/09-0143.1
Nichols JD, Hines JE, Pollock KH, Hinz RL, Link WA (1994) Estimating breeding proportions and testing hypotheses about costs of reproduction with capture-recapture data. Ecology 75:2052–2065. https://doi.org/10.2307/1941610
Norberg RA (1981) Temporary weight decrease in breeding birds may result in more fledged young. Am Nat 118:838–850. https://doi.org/10.1086/283874
Öst M, Kilpi M (2000) Eider females and broods from neighboring colonies use segregated local feeding areas. Waterbirds 23:24–32
Öst M, Steele BB (2010) Age-specific nest-site preference and success in eiders. Oecologia 162:59–69. https://doi.org/10.1007/s00442-009-1444-4
Öst M, Ydenberg R, Kilpi M, Lindström K (2003) Condition and coalition formation by brood-rearing common eider females. Behav Ecol 14:311–317. https://doi.org/10.1093/beheco/14.3.311
Öst M, Lehikoinen A, Jaatinen K, Kilpi M (2011) Causes and consequences of fine-scale breeding dispersal in a female-philopatric species. Oecologia 166:327–336. https://doi.org/10.1007/s00442-010-1855-2
Öst M, Ramula S, Lindén A, Karell P, Kilpi M (2016) Small-scale spatial and temporal variation in the demographic processes underlying the large-scale decline of eiders in the Baltic Sea. Popul Ecol 58:121–133. https://doi.org/10.1007/s10144-015-0517-y
Reed ET, Gauthier G, Giroux JF (2004) Effects of spring conditions on breeding propensity of greater snow goose females. Anim Biodivers Conserv 27:35–46
Reed T, Harris M, Wanless S (2015) Skipped breeding in common guillemots in a changing climate: restraint or constraint? Front Ecol Evol 3:1. https://doi.org/10.3389/fevo.2015.00001
Rowe L, Ludwig D, Schluter D (1994) Time, condition, and the seasonal decline of avian clutch size. Am Nat 143:698–722. https://doi.org/10.1086/285627
Sergio F, Hiraldo F (2008) Intraguild predation in raptor assemblages: a review. Ibis 150:132–145. https://doi.org/10.1111/j.1474-919X.2008.00786.x
Shaw AK, Levin SA (2013) The evolution of intermittent breeding. J Math Biol 66:685–703. https://doi.org/10.1007/s00285-012-0603-0
Sheriff MJ, Krebs CJ, Boonstra R (2009) The sensitive hare: sublethal effect of predator stress on reproduction in snowshoe hares. J Anim Ecol 78:1249–1258. https://doi.org/10.1111/j.1365-2656.2009.01552.x
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Steele BB, Lehikoinen A, Öst M, Kilpi M (2007) The cost of mate guarding in the common eider. Ornis Fenn 84:49–56
Waldeck P, Larsson K (2013) Effects of winter water temperature on mass loss in Baltic blue mussels: implications for foraging sea ducks. J Exp Mar Biol Ecol 444:24–30. https://doi.org/10.1016/j.jembe.2013.03.007
Warren JM, Cutting KA, Takekawa JY, De La Cruz SE, Williams TD, Koons DN (2014) Previous success and current body condition determine breeding propensity in lesser scaup: evidence for the individual heterogeneity hypothesis. Auk 131:287–297. https://doi.org/10.1642/AUK-13-236.1
Wasser SK, Barash DP (1983) Reproductive suppression among female mammals: implications for biomedicine and sexual selection theory. Q Rev Biol 58:513–538. https://doi.org/10.1086/413545
White TCR (2008) The role of food, weather and climate in limiting the abundance of animals. Biol Rev 83:227–248. https://doi.org/10.1111/j.1469-185X.2008.00041.x
Acknowledgements
We thank numerous people participating in fieldwork at Tvärminne, in particular Kim Jaatinen for his hard work. Tvärminne Zoological Station provided excellent facilities. Handling of birds was approved by the National Animal Experiment Board (Permit numbers HY-85-2003, ESLH-2009-02969/Ym-23, ESAVI/1697/04.10.03/2012, ESAVI/2831/04.10.07/2015) and complied with the regulations of Tvärminne Zoological Station. This work was supported by the Swedish Cultural Foundation in Finland (Grant nos. 17/3317, 16/1476, 15/3296, 14/2657, 13/2654 and 138139) (to MÖ) and the Academy of Finland (#309992 to PK, #285746 to SR). We are also grateful to two anonymous reviewers for their helpful comments, and Aleksi Lehikoinen for enlightening us on migratory strategies of eiders.
Author information
Authors and Affiliations
Contributions
All authors jointly formulated the original idea, MÖ conducted fieldwork, AL analysed the data, MÖ wrote the manuscript, and other authors provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Ola Olsson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Öst, M., Lindén, A., Karell, P. et al. To breed or not to breed: drivers of intermittent breeding in a seabird under increasing predation risk and male bias. Oecologia 188, 129–138 (2018). https://doi.org/10.1007/s00442-018-4176-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4176-5