Abstract
Trade-offs between juvenile survival and the development of sexually selected traits can cause ontogenetic conflict between life stages that constrains adaptive evolution. However, the potential for ecological interactions to alter the presence or strength of these trade-offs remains largely unexplored. Antagonistic selection over the accumulation and storage of resources could be one common cause of environment-specific trade-offs between life stages: higher condition may simultaneously enhance adult ornament development and increase juvenile vulnerability to predators. We tested this hypothesis in an ornamented dragonfly (Pachydiplax longipennis). Higher larval body condition indeed enhanced the initial development of its intrasexually selected wing coloration, but was opposed by viability selection in the presence of large aeshnid predators. In contrast, viability selection did not oppose larval body condition in pools when aeshnids were absent, and was not affected when we manipulated cannibalism risk. Trade-offs between larval survival and ornament development, mediated through the conflicting effects of body condition, therefore occurred only under high predation risk. We additionally characterized how body condition influences several traits associated with predator avoidance. Although body condition did not affect burst distance, it did increase larval abdomen size, potentially making larvae easier targets for aeshnid predators. As high body condition similarly increases vulnerability to predators in many other animals, predator-mediated costs of juvenile resource accumulation could be a common, environment-specific limitation on the elaboration of sexually selected traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite sexual selection favoring continuous exaggeration of secondary sexual traits (Andersson 1994), these traits often exhibit tremendous diversity in size, shape, and intensity across natural populations (e.g., Moczek and Nijhout 2003; Svensson et al. 2004; Martin et al. 2014). Evaluating the environmental and developmental causes of this variation can highlight important factors that promote and constrain phenotypic diversification. For example, spatiotemporal variation in the strength of mate competition or the efficiency of signal transmission can drive diversification by altering the strength or form of sexual selection (Endler 1992; Miller and Svensson 2014). Spatiotemporal differences in natural selection opposing secondary sexual traits can further shape the extent of elaboration by causing trade-offs between survival and mate acquisition (Endler 1980; Andersson 1994; Zuk et al. 2006; Heinen-Kay et al. 2015). Moreover, as sexually selected traits commonly require resources that are accumulated throughout the life cycle (Kasumovic 2013; Morehouse 2014), viability selection may also oppose features of their development in non-reproductive seasons or life stages (Jennions et al. 2001; Kotiaho 2001). In these cases, the trait values that improve reproductive success in one life stage ultimately reduce survival in another, and there is “ontogenetic conflict” over the development of the sexually selected trait (sensu Sinervo and Calsbeek 2003; Calsbeek and Goedert 2017; see also Chippindale et al. 2001). However, the potential for ecological and/or developmental factors to moderate such fitness trade-offs between life stages or seasons has received only limited attention.
Diversification of sexual phenotypes may be especially influenced by variation in the strength of trade-offs between the development of sexually selected traits and juvenile survival (Jennions et al. 2001; Cornwallis and Uller 2010; Mojica and Kelly 2010). For instance, differences in predators, pathogens, or other sources of mortality among juvenile habitats could alter survival costs of sexual phenotype development. Then, for a genotype that produces an advantageous sexual phenotype, the likelihood of ever reaching maturity and even being exposed to sexual selection would differ widely among juvenile environments (Hadfield 2008; Mojica and Kelly 2010; Johnson and Hixon 2011). Indeed, such trade-offs between life stages are known to maintain genetic variation in secondary sexual traits within natural populations (Brooks 2000; Robinson et al. 2008) and inhibit phenotypic exaggeration under even strong artificial selection (Hine et al. 2011). Examining how the juvenile environment modifies the strength, or even presence, of trade-offs between juvenile survival and sexual phenotype development could, therefore, illuminate important, but perhaps overlooked, factors underlying the diversity of secondary sexual traits. Yet, the potential for these environment-dependent trade-offs between life stages remains largely unexplored.
As predators preferentially consume prey that are easier to capture and handle (Gosler et al. 1995; Van Buskirk et al. 1997; Mikolawjewski et al. 2006), environment-specific trade-offs between juvenile survival and sexual phenotype development could commonly arise over the resource accumulation used in the production of sexually selected traits. For example, higher juvenile body condition often enhances the development of sexually selected traits (Kasumovic 2013; Morehouse 2014), but also increases vulnerability to predators by making individuals slower and/or easier targets (Gosler et al. 1995; Zamora-Camacho et al. 2014). The strength of the resulting ontogenetic conflict over the development of secondary sexual traits, mediated by this potential functional constraint, would then depend on the predators in the juvenile environment. Here, we experimentally test for predator-mediated variation in trade-offs between larval survival and adult ornament development using the territorial dragonfly, Pachydiplax longipennis (Burmeister). This broadly distributed, North American dragonfly inhabits ponds with a wide range of aquatic predators as a larva (McCauley et al. 2008), and produces intrasexually selected melanin wing ornaments that vary substantially in size and intensity across its range (Paulson 2012, Moore and Martin 2016, MPM, unpublished data). Wing pigmentation in odonates is well suited for examining such patterns, because these ornaments have long been a model system for understanding trade-offs between survival and reproduction (e.g., Grether 1996a; Svensson et al. 2004), and aspects of melanin-synthesis pathway are strongly affected by the larval environment (Stoks and Córdoba-Aguilar 2012; Debecker et al. 2015). In P. longipennis, this wing coloration is essential during male–male competition over the reproductive territories in which males copulate with females and into which females oviposit (Moore and Martin 2016). If condition-mediated trade-offs between larval survival and adult ornament development depend on predation risk, then high-condition larvae will have improved adult ornament development if they emerge, but also lower survival in the presence of larval predators.
Methods
Focal species and study overview
Pachydiplax longipennis (Burmeister) is a medium-sized dragonfly with a wide distribution across North America (Paulson 2012). In the northern extent of the range, where this study was conducted, P. longipennis is univoltine (Wissinger 1988; MPM, personal observation). After overwintering, the larvae of P. longipennis and most other pond-dwelling dragonflies move towards the shore and rapidly complete the final three-to-four development stages before emergence (Wissinger 1988). Larval dragonfly assemblages subsequently exhibit tremendous hetero- and conspecific size asymmetries during this period, leading to extremely high rates of intraguild predation and cannibalism (Wissinger 1992; Hopper et al. 1996; Crumrine et al. 2008).
In this study, we first examined how larval body condition influences ornament development and how environmental context modifies selection on larval body condition by rearing uniquely marked individuals in outdoor wading pools that differed in intraguild predator and cannibalism regime. We then assessed several performance and morphological correlates of larval condition that can affect vulnerability to predators (Mikolawjewski et al. 2006; Strobbe et al. 2009): escape distance, abdomen size, and cuticle darkness.
Aim 1: ornament development and viability selection
We collected P. longipennis larvae from a pond at Case Western Reserve University’s Squire Valleevue Farm (Hunting Valley, Ohio, USA), at three different times (16–18 May, 28–29 May, and 31 May 2016). We maintained larvae in 473 mL opaque plastic cups filled with dechlorinated water at a 15:9 L:D photoperiod in a laboratory at Case Western Reserve University (Cleveland, Ohio, USA). We uniquely marked each larva by injecting a coded wire tag (Northwest Marine Technology Inc., Shaw Island, Washington, USA) ventrally into its abdomen (Catania and McCauley 2015). We measured head width, a proxy for body size (Corbet 1999) using an ocular micrometer. We blot dried larvae, gently induced them to expel water from their rectal branchial chambers, and measured mass to the nearest 0.0001 g using an electronic balance. To assess the repeatability of this method, we massed 86 larvae twice with a week between measurements. Repeatability was high (R = 0.921, F84,85 = 24.27, P < 0.001; Lessells and Boag 1987). We estimated larval body condition for each individual using the residuals from a loge–loge regression of body mass on head size (Jakob et al. 1996). We provide a detailed rationale for the validity of this condition estimate in Appendix 1 of the Electronic Supplementary Material. Briefly, in P. longipennis, this metric satisfies the requirements for a proxy of an individual’s accumulated resources that are available for future growth, development, or physiological maintenance, because it: (1) is independent of head size (r = − 8.125 × 10−11, t476 = 0, P > 0.999); (2) increases with food quantity received (0.064 ± 0.025, F1,42 = 6.72, P = 0.013, n = 44); (3) is positively associated with producing an energetically costly melanin immune response (75.529 ± 30.755, F1,40 = 5.90, P = 0.020, n = 44), and is positively associated with time until emergence overall and enables developmental acceleration under high predation risk (Moore et al. 2018). In addition, this metric is positively correlated with fat stores in odonates with highly similar and very disparate body shapes (see Appendix 1 of Electronic Supplementary Material).
To investigate ornament development and viability selection, we raised these marked and measured larvae in outdoor wading pools, where we manipulated predation risk by altering the presence/absence of Anax junius and the size variation of our focal P. longipennis larvae (n = 4 pools per A. junius × conspecific size variation combination). For pools with A. junius (Anax present, n = 8 total pools), we introduced two ultimate instar A. junius larvae 1 h after the release of our focal larvae, and replaced them when they were emerged (n = 6 larvae) or were found dead (n = 3 larvae). To manipulate conspecific size variation, we divided focal larvae into four groups within each collection period: relatively large and small ultimate instars and relatively large and small penultimate instars. We randomly assigned larvae within each group to either high- or low-size variation treatments, where high-size variation pools had a 2:1 ratio of large-to-small ultimate instars and a 1:2 ratio of large to small penultimate instars, and low-size variation pools had the opposite (Fig. S3). Because the available larvae differed among the collection periods, the ratio of instars differed slightly among blocks (Table S1), but was the same between treatments within a block. Between treatments, mean head sizes were equal (mean ± SD: low = 4.961 ± 0.107; high = 4.972 ± 0.151; F1,13 = 0.04, P = 0.840), and head size variation differed by ~ 24% (mean coefficient of variation ± SD: low = 0.116 ± 0.015, high = 0.144 ± 0.023; F1,13 = 7.62, P = 0.0162).
Three days after marking, we introduced 30 P. longipennis larvae by their assigned treatment each to 100 L plastic wading pools that we filled with aged water, ~ 0.003 m3 of aquatic vegetation, ~ 0.011 m3 of leaf litter, and ~ 1400 cm3 of benthic substrate. To provide a self-sustaining prey base for the larvae, we introduced 100 mL of concentrated Daphnia magna 10 days before releasing P. longipennis larvae. We also permitted the natural colonization of prey species (e.g., Culicidae, Chironomidae, and Hylidae spp.) up until the day prior to dragonfly introduction, after which we covered pools with window screen. We checked for adults daily and sacrificed them in a freezer set to − 22.8 °C immediately upon recovery. As coded wire tags could be found in adults or their exoskeletons, we collected both when possible. After 20 days, we recovered surviving larvae, and stored them in 95% ethanol in the same freezer. Two larvae accidentally were not measured prior to release into the wading pools at the beginning of the experiment and were excluded from all analyses. In addition, five Anax larvae had to be replaced from one Anax-present pool, and we have, therefore, excluded it. However, all results are robust to its inclusion.
Ornament development
To characterize how larval body condition influences the initial stages of ornament development, we gently removed the wings of the sacrificed males that successfully emerged from our experimental wading pools (n = 26 males), and took pictures of them in a dark box against a standard white background. We assessed if larval body condition influenced the initiation of ornament development on the day of emergence (1 = initiated development, 0 = had not initiated development) using a generalized linear-mixed-effects model with a binomial error distribution and pool as a random effect. Given that males without coloration are largely unable to defend breeding territories, males that initiate ornament development earlier will be able to occupy and hold territories earlier in their adult lifespans (Sherman 1983; Moore and Martin 2016). As fixed effects, we included larval body condition, head size, Anax presence, conspecific size variation, and the interactions between the traits and the treatments. Models including interactions between Anax presence and head size would not converge, likely due to relatively few males successfully emerging from the Anax-present treatment (n = 6 males), and we thus only included the main effect of Anax presence and its interaction with larval body condition. We report overall condition-dependent patterns of emergence elsewhere (Moore et al. 2018).
Larval survival
We evaluated how predator treatment modified viability selection on larval body condition using a generalized linear-mixed-effects model with each individual’s survival as the response (Lande and Arnold 1983; Chenoweth et al. 2012). We designated all individuals, whose tags were not recovered as dead. In some cases, adults emerged successfully from their exoskeletons, but died after falling into the water, and we scored these individuals as “survivors”, because they likely would have survived if not for the window screen covering the tanks. We included body condition, head size, the quadratic effects of body condition and head size, Anax presence, conspecific size variation, and all interactions as fixed effects, and pool nested within block as a random effect. We scaled individuals’ phenotypes to a mean of zero and standard deviation of one within each pool (Lande and Arnold 1983). When a significant trait by treatment interaction was observed, we compared the strength and direction of the linear and non-linear selection coefficients within each of the treatments that differed (Chenoweth et al. 2012). We also computed the opportunity for selection in such treatments (variance in survival dived by its squared mean), a standardized measure of fitness variation that quantifies the upper potential limit to the strength of selection (Arnold and Wade 1984). We calculated selection coefficients and their standard errors with the logistic regression approach (Janzen and Stern 1998; Mitchell et al. 2013) and used projection pursuit regression to visualize the fitness surface (Schluter and Nychka 1994). Mixed-effects models were fit with ‘lme4’ (Bates et al. 2014) and projection pursuit regression with ‘stats’ (R Core Team 2014). Selection coefficients and standard errors that were alternatively calculated from generalized additive mixed-effects models (Morrissey and Sakrejda 2013; ‘gsg’ package’, Morrissey and Sakrejda 2014) were highly similar (Table S2).
Aim 2: performance, morphological, and physiological correlates of body condition
We also quantified the effects of larval body condition on several traits known to affect vulnerability to predators. We first evaluated how body condition (calculated using residuals from loge–loge regression as described above) affected escape performance using 60 ultimate instar larvae that we collected in July 2016 from the same source pond as for the wading pool experiment. We placed each larva next to a ruler in the center of a clear plastic box (34.6 cm L × 21 cm W × 12.4 cm H) filled with 1 L of aged water, gently prodded its abdomen, and measured the distance (mm) of its initial escape burst three times. This performance metric should reflect an individual’s ability to move out of the attack radius of other dragonfly larvae (Corbet 1999). We used a general linear model to consider how mean burst distance (loge transformed) varied with body condition and head size.
We also characterized how body condition influences morphological traits that affect predation risk. We collected larvae in Oct 2016 from the same source pond as the wading pool study, and reared 44 of them in the laboratory in 473 mL plastic cups under a 13:11 L:D photoperiod (to match conditions at time of this experiment). To generate additional variation in condition, we provided larvae with a 1.5 mL aliquot of concentrated D. magna (mean ± SD 15.9 ± 3.8) either 6 (high food) or 2 (low food) times per week (see also Appendix 1 in Electronic Supplementary Material). After 21 days, we took digital photographs of each larva against a brightfield background with a dissecting microscope. We calculated relative abdomen size using the residuals from a loge–loge regression of dorsal area (measured from digital photographs in Image J; Rasband 2012) on head size. From the photographs, we also scored cuticle darkness, as 255 (maximum white value)—the mean grey value of the abdomen (e.g., Fedorka et al. 2013). Darker cuticles are typically thicker and thus more difficult to puncture (Corbet 1999). We used separate regressions to assess the relationships between larval body condition and relative abdomen size and cuticle darkness. We also included food treatment in models to account for any effects that acted in addition to its direct influence on body condition.
Results
Aim 1: ornament development and viability selection
Ornament development
The probability of a male initiating ornament development on the day that it emerged increased with larval body condition (parameter estimate ± SE, hereafter: 1.939 ± 0.974, \(\chi_{1}^{2}\) = 6.46, P = 0.011, Fig. 1, n = 26), but was not influenced by larval head size, Anax treatment, conspecific size variation treatment, or any interactions (all \(\chi_{1}^{2}\) < 2.01, P > 0.156).
Pachydiplax longipennis males with higher larval body condition were more likely to initiate ornament development on the day of emergence (n = 26). Each point represents whether or not a male began developing ornamentation (1 = initiated ornament production, 0 = did not initiate ornament production), and points are jittered vertically by 0.1 to improve visual clarity
Larval survival
When considering how predator regime influenced viability selection, we detected a significant interaction among head size, body condition, and Anax presence (\(\chi_{1}^{2}\) = 4.75, P = 0.029; Fig. 2, n = 448), indicating that Anax presence altered selection on head size and body condition. Conspecific size variation did not affect viability selection (all χ2 < 1.11, P > 0.292), and there was no evidence of stabilizing or disruptive selection on either trait (both χ2 < 0.29, P > 0.591). We therefore compared selection on larval condition and head size between pools with and without Anax predators. Anax presence decreased overall survival (present 46.2%, absent 60.8%), resulting in ~ 80% greater among-individual fitness variation (present 1.6876, absent 0.9319). When Anax were present, larval survival increased strongly with head size and decreased strongly with body condition (Fig. 2a, b, Table 1, n = 208). In contrast, in pools without A. junius, the fitness landscape showed marginal evidence of correlational selection that favored large larvae with intermediate body condition and intermediately sized larvae with very high body condition (Fig. 2c, d, Table 1, n = 240). However, in these pools without A. junius, larval survival did not decrease with larval body condition overall, and also the positive relationship between larval survival and head size was > 37% weaker (Table 1). Qualitative comparisons of just high-condition larvae between these two predator treatments revealed similar patterns of survival. Larvae with a body condition 1 SD above average had 42% (15/36) and 26% (8/31) survival in the Anax-absent pools and Anax-present pools, respectively. Moreover, 71% (5/7) and 0% (0/5) of larvae with a body condition 2 SD above average survived in the Anax-absent pools and Anax-present pools, respectively. The average selection gradient calculated within each pool also showed that directional selection opposed body condition in pools with, but not without, A. junius (see Appendix 2 in Electronic Supplementary Material). Together, this broadly supports differences in directional selection on body condition between predator treatments.
Fitness surfaces for Pachydiplax longipennis larvae in pools (a, b; n = 208) with or (c, d; n = 240) without predatory Anax junius larvae. Surfaces were calculated with projection pursuit regression using cubic splines estimated by generalized cross validation. In contour plots showing the fitness surfaces in two dimensions (b, d), points represent individuals that either survived (circles) or died (x’s), and lines illustrate regions of relative fitness. A color version of this figure is available online
Aim 2: performance and morphological correlates of body condition
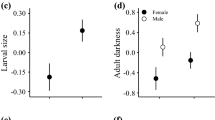
Mean escape distance was not associated with larval body condition (F1,57 = 1.40, P = 0.241, Fig. 3a, n = 60), but tended to increase with head size (1.800 ± 0.969, F1,57 = 3.45, P = 0.068). Relative abdomen size increased with body condition (0.571 ± 0.097, F1,40 = 34.87, P < 0.001, Fig. 3b, n = 44), but was not additionally influenced by food treatment or the interaction with condition (both F1,40 < 0.90, P > 0.349). Cuticle darkness marginally increased with body condition (24.166 ± 12.744, F1,40 = 3.66, P = 0.063, n = 44), but was not additionally affected by food treatment or the interaction with condition (both F1,40 < 1.72, P > 0.197).
Discussion
While the evolution of sexually selected traits may be substantially influenced by differences in the strength of trade-offs between their development and juvenile survival (Jennions et al. 2001; Robinson et al. 2008; Mojica and Kelly 2010; Hine et al. 2011), the potential for, and ecological causes of, variation in the resulting ontogenetic conflict between the juvenile and adult life stages remains largely unexplored. Using an ornamented dragonfly, we examined how the larval environment could influence trade-offs between juvenile survival and the development of sexually selected male wing coloration, which is vital to an important adult fitness component (territorial success). Specifically, we considered: (1) how larval body condition influenced the development of intrasexually selected wing ornaments and (2) how viability selection on larval body condition varied with larval predation risk. Higher larval body condition improved the early development of the intrasexually selected wing ornaments (Fig. 1), and was directly opposed by viability selection only in pools with predatory A. junius (Fig. 2a, b). The accumulation of resources used for producing intrasexually selected adult wing coloration is, therefore, associated with lower larval survival when larval predation risk is high. Conversely, when the large predatory A. junius was not present, viability selection did not directly oppose larval body condition overall (Fig. 2c, d). Thus, trade-offs between larval survival and the development of intrasexually selected wing coloration can depend on the larval predation regime. This highlights the potential for context dependence in trade-offs between adult and juvenile fitness components, and further indicates the importance of considering antagonistic selection over features of ornament development prior to maturity (Jennions et al. 2001; Cornwallis and Uller 2010).
The extent to which ontogenetic conflict between the juvenile and adult life stages will limit the adaptive evolution of sexually selected traits depends largely on the relative magnitudes of the fitness costs incurred through lower juvenile survival versus the fitness benefits gained through improvements to adult fitness components (Mojica and Kelly 2010; Johnson and Hixon 2011). Although we did not directly estimate the relationship between early ornament development and territorial success in this study, producing coloration sooner after emergence will enable a male to begin holding breeding territories earlier in the adult stage (Moore and Martin 2016), which in turn will greatly enhance its opportunity to acquire mates over its adult lifespan (Sherman 1983; see also Koenig and Albano 1987; Moore 1990; Suhonen et al. 2008). In pools with A. junius, males with a larval body condition that was 1 SD above average had 38% lower larval survival than those males that were 1 SD below average (Table 1, Fig. 2a, b). Conversely, the probability of beginning ornament development on the day of emergence was 63.8% greater for those high-condition males than the low-condition males (Fig. 1). All else being equal (van Tienderen 2000), the benefits of this earlier ornament development for those high-condition males would have to improve territorial success by 59.6% to offset the observed survival costs—equivalent to an additional 1.7 days over which a male holds a territory in our population (mean number of days holding a territory ± SD = 2.8 ± 2.2; Moore and Martin 2016). While increases to territorial success of this magnitude are certainly plausible (Sherman 1983; Grether 1996b), and other adult fitness benefits of high larval body condition may further offset these costs (e.g., female fecundity, Stoks and Córdoba-Aguilar 2012), our results indicate that predator-mediated viability selection against larval body condition can substantially limit the net benefits of the early development of an intrasexually selected trait.
Any costs of developing or possessing sexually selected characters that cannot be mitigated by increasing body condition impose strong constraints on the elaboration of these traits (Jennions et al. 2001; Kotiaho 2001; Hine et al. 2011; Morehouse 2014). In pools with A. junius, high-condition larvae had lower survival (Fig. 2a, b), despite high-condition larvae tending to actually have darker, thicker cuticles (Fig. 3c) and being able to accelerate development in the presence of A. junius (Moore et al. 2018). Previous work has shown that aeshnid dragonfly larvae preferentially capture and consume prey with bodies that are easier to grab and hold, such as other odonates with relatively large abdominal spines (Mikolawjewski et al. 2006) and tadpoles with relatively wide and deep bodies (Van Buskirk et al. 1997). Consequently, it may have been the relatively large abdomens of high-condition larvae (Fig. 3b) that increased vulnerability to predation by providing an easier target for the extendable, grasping mouthparts of A. junius. Although other aspects of being large overall could improve performance and decrease predation risk, larger abdomens are unlikely to enhance other features of performance to offset any predator-mediated costs of being an easier target. Given the role of aeshnids as predators to other aquatic invertebrates (Strobbe et al. 2009), amphibians (Anderson and Semlitsch 2016), and even small fish (Marchinko 2009), this potential functional constraint of high body condition could similarly constrain the benefits of sexual phenotypes in many animals. Furthermore, as the accumulation of large energetic stores often increases vulnerability to predators by making animals slower (Zamora-Camacho et al. 2014) and/or easier to catch and handle (Gosler et al. 1995), environment-specific trade-offs between juvenile survival and the development of sexually selected traits, mediated through the effects of body condition, could be common.
While our results show that differences in juvenile predation regime can modify the survival costs of developing sexually selected traits, they also indicate that differences in predators’ foraging ability may have important consequences for variation in ontogenetic conflict. For instance, despite cannibalism rates typically increasing with size asymmetry in dragonflies (Crumrine et al. 2008) and other animals with considerable among-individual size differences (e.g., fish: Persson et al. 2003; salamanders: Wissinger et al. 2010), a 24% increase in conspecific size variation did not change the strength or form of viability selection on larval body condition or head size—perhaps because of the gape limitations of P. longipennis (Wissinger 1992; Corbet 1999). Consequently, in contrast with the presence or the absence of A. junius, which can consume any naturally occurring larval P. longipennis, differences in conspecific size variation are unlikely to modify the juvenile survival costs of the resource accumulation used in ornament production. This suggests that functional differences among putative predators, such as differences in gape limitation or foraging strategy (see also Miehls et al. 2014), could crucially affect spatiotemporal variation in trade-offs. More generally, as our understanding of the effects of ecological agents of selection continues to expand (Moore et al. 2016; Siepielski et al. 2017; Caruso et al. 2017), considering variation within simplified habitat categories (e.g., predation or competition) could further illuminate the shared and unique components of the ecological causes of diversification (Langerhans and DeWitt 2004; Oke et al. 2017).
Trade-offs between life stages are likely to be an important constraint on adaptive evolution generally (Schluter et al. 1991; Marshall and Morgan 2011), and the elaboration of secondary sexual traits specifically (Jennions et al. 2001). However, their underlying ecological causes have received only limited attention (see also Crean et al. 2011; Monro and Marshall 2014). Overall, our results highlight (1) how viability selection against body condition can lead to trade-offs between larval survival and the development of sexually selected traits and (2) how variation in an important ecological factor, predation, could alter the strength of these trade-offs. Although many animals accumulate resources for the production of the adult phenotype primarily during the juvenile stage (Morehouse 2014), the potential for ecological interactions during this stage to generate fitness variation that constrains the benefits of sexually selected traits has been largely ignored (Jennions et al. 2001; Cornwallis and Uller 2010; Kasumovic 2013). Further examination of how ecological factors across the life cycle influence trade-offs between juvenile survival and sexual phenotype development therefore could ultimately yield exciting, novel insights into the evolution of secondary sexual traits, and the optimization of trade-offs between life stages more broadly.
References
Anderson TL, Semlitsch RD (2016) Top predators and habitat complexity alter an intraguild predation module in pond communities. J Anim Ecol 85:548–588
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Arnold SJ, Wade MJ (1984) On the measurement of natural and sexual selection — theory. Evolution 38:709–719
Bates D, Machler M, Bolker B, Walker S (2014) lme4: linear mixed-effects models using Eigen and S4. R package version 1.0-6. http://CRAN.R-project.org/packagel/4lme4. Accessed 14 Jan 2015
Brooks R (2000) Negative genetic correlation between male sexual attractiveness and survival. Nature 406:67–70
Calsbeek R, Goedert D (2017) Performance tradeoffs, ontogenetic conflict, and multisport athletes: how is an ironman triathlete like a frog? Integr Comp Biol 57:207–216
Caruso CM, Martin RA, Sletvold N, Morrissey MB, Wade MJ, Augustine KE et al (2017) What are the environmental determinants of phenotypic selection? A meta-analysis of experimental studies. Am Nat 190:363–376
Catania SVL, McCauley SJ (2015) Evaluating the use of coded-wire tags in individually marking Odonata larvae. Can Entomol 148:371–374
Chenoweth SF, Hunt J, Rundle HD (2012) Analyzing and comparing the geometry of individual fitness surfaces. In: Svensson EI, Calsbeek R (eds) The adaptive landscape in evolutionary biology. Oxford University Press, Oxford, pp 126–149
Chippindale AK, Gibson JR, Rice WR (2001) Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc Natl Acad Sci USA 98:1671–1675
Corbet PS (1999) Dragonflies: behavior and ecology of Odonata. Cornell University Press, Ithaca
Cornwallis CK, Uller T (2010) Towards an evolutionary ecology of sexual traits. Trends Ecol Evol 25:145–152
Crean AJ, Monro K, Marshall DJ (2011) Fitness consequences of larval traits persist across the metamorphic boundary. Evolution 65:3079–3089
Crumrine PW, Switzer PV, Crowley PH (2008) Structure and dynamics of odonate communities: assessing habitat, responding to risk, and enabling reproduction. In: Córdoba-Aguilar A (ed) Dragonflies and damselflies: model organisms for ecological and evolutionary research. Oxford University Press, Oxford, pp 21–38
Debecker S, Sommaruga R, Maes T, Stoks R (2015) Larval UV exposure impairs adult immune function through trade-off with larval investment in cuticular melanin. Funct Ecol 29:1292–1299
Endler JA (1980) Natural selection on color patterns in Poecilia reticulata. Evolution 34:76–91
Endler JA (1992) Signals, signal conditions, and the direction of evolution. Am Nat 139:S125–S153
Fedorka KM, Lee V, Winterhalter WE (2013) Thermal environment shapes cuticle melanism and melanin-based immunity in the ground cricket Allonemobius socius. Evol Ecol 27:521–531
Gosler AG, Greenwood JJD, Perrins C (1995) Predation risk and the cost of being fat. Nature 377:621–623
Grether GF (1996a) Sexual selection and survival selection on wing coloration and body size in the rubyspot damselfly Hetaerina americana. Evolution 50:1939–1948
Grether GF (1996b) Intrasexual selection alone favors a sexually dimorphic ornament in the rubyspot damselfly Hetaerina americana. Evolution 50:1949–1957
Hadfield JD (2008) Estimating evolutionary parameters when viability selection is operating. Proc R Soc B 275:723–734
Heinen-Kay JL, Morris KE, Ryan NA, Byerley SL, Venezia RE, Peterson MN, Langerhans RB (2015) A tradeoff between natural and sexual selection underlies diversification of a sexual signal. Behav Ecol 26:533–542
Hine E, McGuigan K, Blows MW (2011) Natural selection stops the evolution of male attractiveness. Proc Natl Acad Sci USA 108:3659–3664
Hopper KR, Crowley PH, Kielman D (1996) Density dependence, hatching synchrony, and within-cohort cannibalism in young dragonfly larvae. Ecology 77:191–200
Jakob EM, Marshall SE, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77:61–67
Janzen FJ, Stern HS (1998) Logistic regression for empirical studies of multivariate selection. Evolution 52:1564–1571
Jennions MD, Møller AP, Petrie M (2001) Sexually selected traits and adult survival: a meta-analysis. Q Rev Biol 76:3–36
Johnson DW, Hixon MA (2011) Sexual and lifetime selection on body size in a marine fish: the importance of life-history trade-offs. J Evol Biol 24:1653–1663
Kasumovic MM (2013) The multidimensional consequences of the juvenile environment: towards an integrative view of the adult phenotype. Anim Behav 85:1049–1059
Koenig WD, Albano SS (1987) Lifetime reproductive success, selection, and the opportunity for selection in the white-tailed skimmer Plathemis lydia (Odonata: Libellulidae). Evolution 41:22–36
Kotiaho JS (2001) Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol Rev 76:365–376
Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37:1210–1226
Langerhans RB, DeWitt TJ (2004) Shared and unique features of evolutionary diversification. Am Nat 164:335–349
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Marchinko KB (2009) Predation’s role in repeated phenotypic and genetic divergence of armor in threespine stickleback. Evolution 63:127–138
Marshall DJ, Morgan SG (2011) Ecological and evolutionary consequences of linked life-history stages in the sea. Curr Biol 21:R718–R725
Martin RA, Riesch R, Heinen-Kay JL, Langerhans RB (2014) Evolution of male coloration during a post-pleistocene radiation of Bahamas mosquitofish (Gambusia hubbsi). Evolution 68:397–411
McCauley SJ, Davis CJ, Relyea RA, Yurewicz KL, Skely DK, Werner EE (2008) Metacommunity patterns in larval odonates. Oecologia 158:329–342
Miehls AJ, Peacor SD, McAdam AG (2014) Gape-limited predators as agents of selection on the defensive morphology of an invasive invertebrate. Evolution 68:2633–2643
Mikolawjewski DJ, Johansson F, Wohlfahrt B, Stoks R (2006) Invertebrate predation selects for the loss of a morphological antipredator trait. Evolution 60:1306–1310
Miller CW, Svensson EI (2014) Sexual selection in complex environments. Annu Rev Entomol 59:427–445
Mitchell TS, Warner DA, Janzen FJ (2013) Phenotypic and fitness consequences of maternal nest-site choice across multiple early life stages. Ecology 94:336–345
Moczek AP, Nijhout HF (2003) Rapid evolution of a polyphenic threshold. Evol Dev 5:259–268
Mojica JP, Kelly JK (2010) Viability selection prior to trait expression is an essential component of natural selection. Proc R Soc B 277:2945–2950
Monro K, Marshall DJ (2014) Faster is not always better: selection on growth rate fluctuates across life history and environments. Am Nat 183:798–809
Moore AJ (1990) The evolution of sexual dimorphism by sexual selection: the separate effects of intrasexual selection and intersexual selection. Evolution 44:315–331
Moore MP, Martin RA (2016) Intrasexual selection favours an immune-correlated color ornament in a dragonfly. J Evol Biol 29:2256–2265
Moore MP, Riesch R, Martin RA (2016) The predictability and magnitude of life-history divergence to ecological agents of selection: a meta-analysis in livebearing fishes. Ecol Lett 19:435–442
Moore MP, Lis C, Martin RA (2018) Larval body condition regulates predator-induced life-history variation in a dragonfly. Ecology 99:224–230
Morehouse NI (2014) Condition-dependent ornaments, life histories, and the evolving architecture of resource-use. Integr Comp Biol 54:591–600
Morrissey MB, Sakrejda K (2013) Unification of regression-based methods for the analysis of natural selection. Evolution 67:2094–2100
Morrissey MB, Sakrejda K (2014) gsg: calculation of selection coefficients. R package version 2.0. http://CRAN.R-project.org/package=gsg. Accessed 17 Aug 2017
Oke KB, Rolshausen G, LeBlond C, Hendry AP (2017) How parallel is parallel evolution? A comparative analysis in fishes. Am Nat 190:1–16
Paulson D (2012) Dragonflies and damselflies of the East. Princeton University Press, Princeton
Persson L, De Roos A, Claessen D, Byström P, Lövgren J, Sjögren S, Svanbäck R, Wahlström E, Westman E (2003) Gigantic cannibals driving a whole-lake trophic cascade. Proc Natl Acad Sci USA 100:4035–4039
Rasband WS (2012) ImageJ. U.S. National Institutes of Health, Bethesda. http://imagej.nih.gov/ij/
Robinson MR, Pilkington JG, Clutton-Brock TH, Pemberton JM, Kruuk LEB (2008) Environmental heterogeneity generates fluctuating selection on a secondary sexual trait. Curr Biol 18:751–757
Schluter D, Nychka D (1994) Exploring fitness surfaces. Am Nat 143:597–616
Schluter D, Price TD, Rowe L (1991) Conflicting selection pressures and life history trade-offs. Proc R Soc B 246:11–17
Sherman KJ (1983) The adaptive significance of postcopulatory mate guarding in a dragonfly, Pachydiplax longipennis. Anim Behav 31:1107–1115
Siepielski AM, Morrissey MB, Buoro M, Carlson SM, Caruso CM, Clegg SM et al (2017) Precipitation drives global variation in natural selection. Science 355:959–962
Sinervo B, Calsbeek R (2003) Physiological epistasis, ontogenetic conflict and natural selection on physiology and life history. Integr Comp Biol 43:419–430
Stoks R, Córdoba-Aguilar A (2012) Evolutionary ecology of Odonata: a complex life cycle perspective. Annu Rev Entomol 57:249–265
Strobbe F, McPeek MA, De Block M, De Meester L, Stoks R (2009) Survival selection on escape performance and its underlying phenotypic traits: a case of many-to-one mapping. J Evol Biol 22:1172–1182
Suhonen J, Rantala MJ, Honkavaara J (2008) Territoriality in odonates. In: Córdoba-Aguilar A (ed) Dragonflies and damselflies: model organisms for ecological and evolutionary research. Oxford University Press, Oxford, pp 203–217
Svensson EI, Kristofferson L, Oskarsson K, Bensch S (2004) Molecular population divergence and sexual selection on morphology in the banded demoiselle (Calopteryx splendens). Heredity 93:423–433
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Van Buskirk J, McCollum SA, Werner EE (1997) Natural selection for environmentally induced phenotypes in tadpoles. Evolution 51:1983–1992
van Tienderen PH (2000) Elasticities and the link between demographic and evolutionary dynamics. Ecology 81:666–679
Wissinger SA (1988) Spatial distribution, life history, and estimates of survivorship in a fourteen-species assemblage of larval dragonflies (Odonata: Anisoptera). Freshw Biol 20:329–340
Wissinger SA (1992) Niche overlap and the potential for competition and intraguild predation between size-structured populations. Ecology 73:1431–1444
Wissinger SA, Whiteman HH, Denoël M, Mumford ML, Aubee CB (2010) Consumptive and nonconsumptive effects of cannibalism in fluctuating age-structured populations. Ecology 91:549–559
Zamora-Camacho FJ, Reguera S, Rubiño-Hispán MV, Moreno-Rueda G (2014) Effects of limb length, body mass, gender, gravidity, and elevation on escape speed lizard Psammodromus algirus. Evol Biol 41:509–517
Zuk M, Rotenberry JT, Tinghitella RM (2006) Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol Lett 2:521–524
Acknowledgements
This work could not have been completed without technical assistance from C. Lis, L. Robinson, A. Wiecek, and H. Rollins. We thank A. Gilmore for generously sharing data on fat stores. We also thank A. Locci and the Squire Valleevue Farm staff, especially S. Brown and J. Miller, for providing access to all field sites and maintaining an exceptionally well-mowed area for our wading pools. Critical feedback from M. Dugas, S. Diamond, L. Chick, A. Perez, S. Peacor, several anonymous reviewers, and the Case Western Reserve University Ecology and Evolution reading group greatly improved the manuscript. MPM was funded in part by a GAANN fellowship.
Author information
Authors and Affiliations
Contributions
MPM designed the experiment, collected and analyzed all data, and wrote the manuscript. RAM discussed experimental design, analyses, and manuscript revisions.
Corresponding author
Ethics declarations
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Scott D Peacor.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moore, M.P., Martin, R.A. Trade-offs between larval survival and adult ornament development depend on predator regime in a territorial dragonfly. Oecologia 188, 97–106 (2018). https://doi.org/10.1007/s00442-018-4171-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4171-x