Abstract
Predator–prey interactions are often size-structured and focused on smaller vulnerable size classes. Predators are also predicted to sort prey communities according to relative vulnerabilities. Increased system productivity and juvenile growth may benefit some species more than others, making relative vulnerability non-static and growth-mediated. We hypothesized that increased system productivity would weaken juvenile-stage predation generally, and potentially shift the community sorting effects of a predator. Using replicated wetland mesocosms we quantified the effects of a generalist size-specific crayfish predator (Procambarus fallax) on juveniles of two species of apple snails (Pomacea spp.) under two levels of system productivity (low vs. high). After 6 weeks of exposure, we quantified predator and productivity effects on snail survival, biomass, and composition of the assemblage. Crayfish depressed the final density and biomass of snails, and sorted the assemblage, selectively favoring survival of the native P. paludosa over the intrinsically more vulnerable invasive P. maculata. Both snails grew faster at higher productivity, but growth differentially increased survival of the invasive snail in the presence of crayfish and weakened the sorting effect. The native P. paludosa hatches at a larger less vulnerable size than the invasive P. maculata, but higher productivity reduced the relative advantage of P. paludosa. Our results are inconsistent with predictions about the sorting effects of predators across productivity gradients, because the more vulnerable prey dominated at low productivity. Our findings highlight that the relative vulnerabilities of prey to a common predator are not always fixed, but can be growth-mediated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation plays a fundamental role in shaping the structure of ecological communities. Predators with strong limiting effects on a fraction of the prey species in a community (often termed sorting effects; Holt et al. 1994; Leibold 1989, 1996) ultimately produce communities of relatively invulnerable prey (Brooks and Dodson 1965; Sih et al. 1985; Wellborn et al. 1996). Determining which prey species will dominate a community, therefore, requires an understanding of which species are more vulnerable to common predators. The vulnerability of any given prey species can be determined by the rate at which predators can successfully attack and handle individuals of that prey (Holling 1959, 1961). Together, a predator’s attack rates and handling times for a given prey species describe the amount of time that predators need to locate, pursue, consume, and digest that prey (Holling 1959). Predator attack rates and handling times on different prey species can vary with interspecific differences in prey life history traits (e.g., Wellborn et al. 1996; Klecka and Boukal 2013; Sarma et al. 2013).
Predator attack rates on prey are often mediated during prey ontogeny via body size (Vucic-Pestic et al. 2009; Brose 2010; McCoy et al. 2011). Many prey species grow by several orders of magnitude from birth to adulthood, and predator attack rates are generally determined by predator–prey body size ratios (Aljetlawi et al. 2004; Brose et al. 2006; Vucic-Pestic et al. 2009). Predator attack rates are typically lower on prey that are too large for predators to successfully handle (Vucic-Pestic et al. 2009), and in some cases, prey can grow largesimilar for treatments enough that they reach invulnerable sizes (i.e., a size refuge; Osenberg and Mittelbach 1989; Chase 1999). Size-structured predation theory predicts that the survivorship of a cohort of growing prey will be determined by its growth rate, because growth rate will determine the amount of time that those prey spend in vulnerable size classes (Werner and Gilliam 1984; Vonesh and Bolker 2005; McCoy et al. 2011).
The extent to which predators sort prey communities by selectively consuming more vulnerable species has long been thought to be sensitive to environmental contexts such as primary productivity. Theory suggests that as system productivity increases, predator sorting effects also increase. In other words, as productivity increases, prey species that are resistant to predators become more abundant while more vulnerable species are reduced or even eliminated (Leibold 1989, 1996). In communities where predation is size-structured (e.g., Chase 1999, 2003a, b), these predictions become naturally somewhat complicated because prey vulnerability itself can be sensitive to productivity. Per capita survival of a given prey species should be enhanced if environmental productivity simply increases juvenile growth rates and shortens the window of vulnerability, as proposed by Werner and Gilliam (1984) and McCoy et al. (2011).
Less is known about how productivity will affect predator effects on multi-species, size-structured prey communities. In these types of communities, the degree to which predators sort prey community structure may depend on the relative change in vulnerability that each prey species undergoes across a productivity gradient. If the vulnerability of all prey within a community changes proportionally as productivity increases, then the vulnerability of each prey species relative to one another goes unchanged. In this case, productivity does not influence the sorting effect of the predator. On the other hand, it is possible that some prey will benefit disproportionately from increased productivity. For example, if those prey are more vulnerable than other prey because they are born at smaller and, therefore, vastly more susceptible sizes, their vulnerability to predation may decrease relatively more than alternative prey that start life at a larger size. In this case, predator sorting of the community may weaken because those more vulnerable prey will increase in abundance relative to other prey as productivity increases, contrary to predictions made by Leibold (1989, 1996). We tested these predictions using crayfish predators and a two-species assemblage of apple snail (Pomacea spp.) prey.
Two large-bodied apple snails (Ampullaridae) inhabit freshwaters in Florida—including lakes, canals, and wetlands, including the Everglades—the invasive Pomacea maculata and the native P. paludosa. The two species have overlapping distributions in some wetlands, but can also be found separately in different wetlands and canals or in different parts of the same wetland (Dorn and Hafsadi 2016). Although P. maculata has been present in the southern Florida wetlands for > 15 years, it has not widely invaded the oligotrophic wetlands present in the southern Everglades (Everglades National Park) and is more prevalent near canals in the central Everglades. Interest in the spread and establishment of P. maculata has grown in recent years. Apple snails of both species constitute the primary diet of the endangered Everglades snail kite (Rostrhamus sociabilis plumbeus), and breeding populations of kites have become established in areas dominated by P. maculata (Cattau et al. 2014, 2016; Wilcox and Fletcher 2016). Environmental factors limiting adult apple snail densities are poorly understood, but adult densities are most likely limited by juvenile survival. Potential sources of juvenile mortality may include poor availability of high quality food in oligotrophic wetlands such as those present in southern Florida (Ruehl and Trexler 2011) or predation (Dorn and Hafsadi 2016).
Body size is a critical trait influencing the vulnerability of many invertebrates to their predators (Paine 1976; Sousa 1993; Vucic-Pestic et al. 2009) and hatchling and juvenile-sized apple snails are vulnerable to a host of predators including turtles, aquatic insects, sunfish and crustaceans (Procambarus spp.; Carlsson et al. 2004; Dorn and Hafsadi 2016). While both species of Pomacea exhibit similar breeding habits, laying eggs above the water line in warmer months with rising or stable water levels (Barnes et al. 2008; Rogevich et al. 2009), the two species differ notably in the size and number of their eggs and hatchlings. Despite their large adult sizes (> 25 mm shell length; SL), both species of apple snails produce small hatchlings (< 4 mm SL). Pomacea paludosa typically produces clutches of 20–40 large eggs that yield 3–4 mm SL hatchlings (Dorn and Hafsadi 2016). On the other hand, P. maculata produces significantly larger clutches (300–4000 eggs, avg. = 2100) of smaller eggs that produce 1.5–2 mm SL hatchlings (Barnes et al. 2008).
Past research suggests that these differences in hatchling sizes produce interspecific differences in mortality to crayfish (Dorn and Hafsadi 2016; Davidson and Dorn 2017). Slough crayfish (Procambarus fallax) are common predators in wetlands in south Florida (Turner et al. 1999; Dorn and Trexler 2007; Hagerthey et al. 2014; Dorn and Cook 2015; Dorn and Trexler 2007). Procambarus fallax has been implicated as an important predator of snails in the Everglades (Dorn 2013; Ruehl and Trexler 2015), and P. fallax may be an especially important predator of apple snails (Dorn and Hafsadi 2016). The risk P. fallax poses to individual apple snails varies both with snail size and crayfish size (Davidson and Dorn 2017). Both species of apple snails eventually reach a size refuge from crayfish predation such that even large adult P. fallax (> 25 mm carapace length; CL), cannot consume sub-adult (> 12 mm SL) or larger apple snails (Davidson and Dorn 2017). The survivorship of cohorts of P. maculata in the presence of crayfish is worse than the survivorship of cohorts of P. paludosa (Dorn and Hafsadi 2016), because crayfish attack rates on hatchling P. maculata are > 15 × higher than those on hatchling P. paludosa (Davidson and Dorn 2017). While this intrinsic difference in hatchling vulnerability is primarily due to size, P. maculata reared to the same shell length as hatchling P. paludosa remain more vulnerable because they also have thinner shells (Davidson and Dorn 2017). Dorn and Hafsadi (2016) predicted that the presence or absence of crayfish predators in wetlands mediates the relative success of the two species. Crayfish will sort communities of juvenile apple snails to favor higher abundances of native P. paludosa when crayfish are present, while invasive P. maculata, with larger clutches (Barnes et al. 2008) and competitive superiority (Posch et al. 2013), should be favored in the absence of crayfish. These predictions could be altered or reversed, however, in enriched wetlands if growth-mediated vulnerability is important for P. maculata.
Natural wetlands in Florida can vary widely in nutrient availability (total phosphorous in soils ranging from 100 to > 700 mg P/kg soil; see Childers et al. 2003). According to size-structured predation theory, both species will have increased growth at high productivity, and thus spend less time vulnerable to predation, but P. maculata will still spend more time vulnerable due to its smaller hatchling size. Thus, P. maculata should still be more heavily suppressed by the presence of crayfish than P. paludosa. P. maculata may, however, benefit from increased productivity disproportionately more than P. paludosa for two primary reasons. P. maculata produces clutches that are on average 70 × larger than those produced by P. paludosa, so even modest gains in survivorship at higher productivity will yield more individual P. maculata than P. paludosa. Additionally, not only are P. maculata hatchlings more vulnerable to crayfish than P. paludosa hatchlings because they hatch smaller, they are also initially more intrinsically vulnerable than P. paludosa due to their thinner shells. Therefore, P. maculata stand to gain relatively more benefit from higher growth environments than P. paludosa. In this case, contrary to predictions laid out both by Chase (1999) and Leibold (1996) and size-structured predation theory, P. maculata may dominate at high productivity due to interspecific differences in other life history traits relating to vulnerability.
The objective of this study was to test size-structured hypotheses about how productivity influences the strength of predation and the degree to which predators influence community structure for a community of mixed prey species that can grow to a size refuge. We predicted that higher productivity would weaken predator control of total apple snail abundance and biomass and may also reduce the effects of crayfish predators on community structure by disproportionately benefiting P. maculata. Both species of apple snail grow faster at higher productivity, improving their survivorship where crayfish are present, but if P. maculata benefits more strongly from increased system productivity than P. paludosa, P. maculata will dominate at high productivity despite its smaller hatchling size. We tested these predictions by manipulating crayfish presence across two productivity levels in experimental wetland communities.
Methods
Experimental design
To assess how the strength of crayfish predation on juvenile apple snails varies under differing productivities, we conducted a mesocosm experiment manipulating both predator presence and primary productivity in a factorial design by crossing crayfish presence and absence with low and high productivity conditions. The four treatments were interspersed in 24 1.1 m2 × 50 cm deep round mesocosms (n = 6 replicates). The array was located out-of-doors in a grassy space on the edge of the FAU Davie campus near a small wetland in Broward County, FL (Knorp and Dorn 2016). Each mesocosm was filled with a five cm layer of peat soil. We established two different productivity levels in the mesocosms in August 2015 by adding a pellet slow-release fertilizer (Osmocote, © The Scotts Company) to bring nutrient levels up to 400 mg P/kg soil in half of the mesocosms (high productivity) and 100 mg P kg−1 in the other half (low productivity). As most freshwater wetlands in southern Florida are P-limited (McCormick et al. 2002), nutrient availability was treated as a proxy for primary productivity. Wetland productivity naturally varies in Florida; soil nutrient levels in Florida’s nutrient-enriched stormwater treatment areas (large nutrient remediation wetlands) have average phosphorus concentrations of 615 ± 396 mg P kg−1 (mean ± std. error; Reddy et al. 2011). Natural Everglades peat soils without nutrient enrichment are typically much lower in phosphorous (total phosphorus ranging from 100 to 300 mg P kg−1; Childers et al. 2003). While the pellet fertilizer also introduced nitrogen and trace amounts of other micronutrients (e.g., iron, magnesium) to the mesocosms, the primary goal was to create two different phosphorous levels to mimic different wetland conditions.
Mesocosms were filled using water from a nearby pond with relatively low nutrient content (total phosphorus < 10 ppm, unpublished data). To simulate typical vegetation assemblages that could be found in shallow freshwater marshes in Florida, we added small pots (942 cm3) of coastal spikerush (Eleocharis cellulosa) and six 30 cm strands of the macroalgae Chara vulgaris. Large apple snails have been documented feeding on C. vulgaris (Baker et al. 2010; Morrison and Hay 2011), but small juveniles presumably feed on periphytic algae (Shuford et al. 2005). We seeded populations of mosquitofish (Gambusia holbrooki) and one species of smaller gastropod (Planorbella duryi) in each of the mesocosms. These species are common in Florida wetlands, and the presence of mosquitofish also helps limit colonization by insects such as dragonflies (Knorp and Dorn 2016) that can potentially prey on smaller juvenile apple snails (Yusa et al. 2006). Crayfish will consume P. duryi, but at the end of this study most P. duryi in all replicates of all treatments were relatively large (> 10 mm shell length) and, therefore, we suspect the majority of the population was not particularly vulnerable (complete refuge = 13 mm SL).
The vegetation in the mesocosm established from August 2015 to April 2016 (approx. 8 months), and the experiment was conducted over 49 days in the early wet season (late April to early June). Throughout the course of the experiment, we indirectly measured the effects of fertilization on primary productivity using vegetative growth as a proxy. At the start of the experiment E. cellulosa stem density was approximately twice as high in the high productivity mesocosms (173.8 ± 12.8 stems m−2) than in the low productivity mesocosms (84.2 ± 4.6 stems m−2). By the end of the experiment, E. cellulosa stem density was almost three times higher in the high productivity mesocosms (265.2 ± 31.8 E. cellulosa stems m−2) than in the low productivity mesocosms (92.3 ± 7.3 stems m−2). Throughout the course of the 49-day experiment, we also added 10 mg P in the form of sodium phosphate weekly to the water in high productivity mesocosms to maintain higher nutrient availability for algae. We chose to use 10 mg P/week to approximate the phosphorous loading rates observed in eutrophic storm-water treatment wetlands in southern Florida in 2011 (Reddy et al. 2011).
To establish predator treatments, we collected medium-sized juvenile male crayfish (Procambarus fallax; 14.25 ± 0.32 mm CL, mean ± SE) from the wetlands in Water Conservation Area 3A (26.0702°N, 80.6771°W). Two crayfish were added to half of the experimental mesocosms creating predator densities of 1.8 m−2. We added the crayfish 5 days before the addition of apple snails and crayfish were assigned to mesocosms such that the average size of the individuals was equal in all mesocosms. Natural crayfish densities in Florida wetlands can range from 1 to 10 m−2 depending on location, season, and year (Dorn and Trexler 2007; Dorn and Cook 2015) so the density used was on the low end of the observed range. Recent observations indicated that crayfish as small as 14 mm CL will consume hatchling P. paludosa at low rates (0.06 snails h−1; Davidson and Dorn 2017).
Apple snail egg masses were collected from the Water Conservation Area 3A wetlands and hatched out in trays in the greenhouse. Equal total biomasses of hatchlings (< 2 days old) of both species were stocked into the mesocosms. A single P. paludosa hatchling is approximately 8 × more massive (mg total wet mass) than a P. maculata hatchling (Dorn and Hafsadi 2016), so mesocosms were stocked with 30 P. paludosa hatchlings (27.3 m−2 or ~ 1 clutch m−2; mean sizes ± SE: 4.0 ± 0.06 mm SL) and 240 P. maculata hatchlings (218.2 m−2 or ~ 0.11 clutches m−2; mean sizes ± SE: 1.7 ± 0.02 mm SL) in each mesocosm. Both species were stocked in groups over the course of 10 days as the hatchlings became available, but biomasses were matched for each species each day until the target stocking densities were reached.
Data collection
The experiment ended when snails stocked on the median stocking date had been in the mesocosms for 49 days. All vegetation was removed from the mesocosms and systematically rinsed and snails were removed from the walls of the mesocosms, mosquitofish were collected via dip net, and all of the soil and water from the mesocosms was poured through a bar seine (2 mm mesh) to collect any remaining fish, crayfish, and snails. All of the animals were euthanized (MS-222 was used for fish) and preserved with solutions of formaldehyde followed by 70% ethanol.
Apple snails were counted and identified to species using morphological differences. Larger juvenile to adult snails (> 15 mm SL) were identified using characteristics of the aperture. P. paludosa possess an aperture lip that meets the body of the shell at a right angle, while the aperture lip on P. maculata curves anteriorly as it reaches the body of the shell (personal obs.). For smaller snails (< 15 mm SL) we measured the length of the shell spire; Pomacea maculata possesses a proportionally longer spire than P. paludosa (Davidson 2016) such that we identified snails with spires longer than 10% of the total shell length of the animal as P. maculata and snails with spires shorter than 7.5% of the total shell length of the animal as P. paludosa. Identifications of 48 (3%) of the surviving snails in the intermediate size range (6–21 mm) were confirmed with genetic tests of the cytochrome c oxidase subunit I gene (Dr. J. Baldwin, Florida Atlantic University, unpublished technique). We recorded the shell length of all surviving apple snails and calculated total apple snail biomass in each mesocosm using regressions of dry soft tissue mass to shell length (Davidson 2016). To compare growth conditions in the productivity treatments we calculated growth rates (mm SL day−1) as the difference in shell length from hatchling size divided by 49 days. We recorded the final size of all crayfish in low and high productivity conditions.
Data analysis
We hypothesized that snail growth rates would be important to survival by limiting the amount of time apple snails spend at vulnerable sizes. To determine whether our treatments created different growth environments for apple snails, growth rates and average final sizes (mm SL) were compared from the crayfish-free mesocosms using one-way analysis of variance. In addition to prey growth rates, predator growth rates might also be enhanced by increased productivity. Because crayfish kill rates increase with carapace length (Davidson and Dorn 2017) faster growing crayfish might have greater capita effects on apple snail survival. We compared the final size of crayfish (mm CL) using one-way analysis of variance.
To test for effects of increased productivity and crayfish presence on apple snail survival, we performed a multivariate analysis of variance on the logit-transformed proportional survival (Warton and Hui 2011) of both P. paludosa and P. maculata with productivity and crayfish presence as factors. We contrasted the treatment effects on P. paludosa and P. maculata survival individually using two separate two-way analyses of variance with planned contrasts. We tested for the effects of crayfish and productivity on total apple snail biomass and individual species biomass by comparing the apple snail biomass (g dry soft tissue mass) across treatments using three separate two-way analyses of variance.
Theoretical predictions about how predators sort prey communities often also predict the total impact of predators on the overall prey community. Models that incorporate static or size-dependent differences in prey vulnerability (e.g. Leibold 1989, 1996; Chase 1999, 2003a) often predict that predator effects will weaken as productivity increases and less vulnerable species become more dominant. We compared predator effect sizes on total apple snail biomass across productivity levels using one-way analysis of variance to determine whether productivity altered predator effect sizes on total apple snail biomass. We calculated effect sizes as ln(Ncrayfish/Ncontrol), where Ncontrol was the mean apple snail biomass where crayfish were absent.
Dorn and Hafsadi (2016) predicted that the presence or absence of crayfish predators in wetlands will mediate the relative success of the two species; crayfish will sort assemblages to favor higher relative densities or biomass of P. paludosa when crayfish are present. Apple snail vulnerability varies with snail size (Davidson and Dorn 2017) and, therefore, greater productivity may weaken crayfish predation on P. maculata by enhancing growth rates and limiting the time juvenile P. maculata spend vulnerable. This may weaken the ability of crayfish to sort snail assemblages, producing assemblages that are still dominated by P. maculata regardless of crayfish presence. To test these hypotheses, we calculated the logit-transformed proportion of the apple snail assemblage that was composed of P. paludosa and compared it across treatments using two-way analysis of variance.
To consider potential effects on apple snail assemblages in natural wetlands we also made projections of the survivorship of individual egg clutches for both species incorporating clutch size estimates (Barnes et al. 2008; Rogevich et al. 2009; Posch et al. 2012) and empirical survival estimates from this study. Hatchling biomass in this experiment was equal, and thus we could not simultaneously account for clutch size differences, but P. maculata clutches average 2100 eggs (Barnes et al. 2008) whereas P. paludosa clutches average only 30 (Posch et al. 2012). To make a projection for heuristic purposes, similar to Dorn and Hafsadi (2016), we used the survival rates from each experimental combination to estimate the effects of crayfish on a single clutch of eggs of either species by calculating the number of surviving juveniles after 49 days under each set of conditions.
For all analyses, we tested for violations of model assumptions by visually assessing and testing the residuals using the Shapiro–Wilk test and checking for homogeneity of variance using Levene’s test. In some cases, the residuals were not normally distributed due to the presence of outliers in some treatments. Re-running the analyses without these outliers produced normally distributed residuals, but did not qualitatively change the interpretation so we have reported the full analyses in the results.
Results
Increased productivity accelerated the growth rates of both P. paludosa and P. maculata, but accelerated growth affected the survival of both species differentially. Pomacea maculata survival was strongly impacted by the addition of crayfish, but increased productivity diminished this effect. On the other hand, P. paludosa survival was only weakly affected by crayfish and did not realize any survival benefits at high productivity. This led to snail assemblages that were composed of higher relative abundances of P. maculata where crayfish were absent or productivity was high.
Prey and predator growth rates
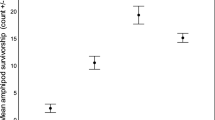
Increased productivity improved overall apple snail growth rates of both species (MANOVA: F1,10 = 27.60, p < 0.001; one-way ANOVAs: P. maculata F1,10 = 50.45, p < 0.001; P. paludosa F1,10 = 38.32, p < 0.001, controls shown in Fig. 1). Pomacea maculata reared at low productivity grew to only 7.3 ± 0.5 mm SL (mean ± SE), whereas at high productivity P. maculata grew more than twice as fast, reaching 16.3 ± 1.2 mm SL. The native P. paludosa reared at low productivity reached sizes of 13.0 ± 0.6 mm SL and at high productivity, P. paludosa grew 60% faster, reaching 18.6 ± 0.7 mm. Crayfish presence did not influence the average growth rate of surviving snails within a productivity level (P. paludosa: F1,20 = 0.09, p = 0.763; P. maculata: F1,20 = 2.57, p = 0.126) and interactions between predator and productivity level were not significant for either species (P. paludosa: F1,20 = 0.85, p = 0.367; P. maculata: F1,20 = 3.00, p = 0.1). Surviving crayfish grew to an average of 30.4 ± 0.5 mm CL regardless of productivity level (F1,10 = 0.0, p = 0.994).
Mean apple snail growth rates (mm SL day−1) when reared together in low and high productivity wetland mesocosms in the absence of predatory crayfish. Errors bars represent the standard error of the mean (n = 6). Mean growth rates were similar for treatments including crayfish (see “Results”)
Prey survival and biomass
Apple snail survival was significantly reduced by crayfish predation (MANOVA: F1,20 = 25.74, p < 0.001) and increased by productivity (F1,20 = 9.43, p < 0.001), and the interaction between the two (F1,20 = 4.99, p = 0.018). Crayfish reduced P. maculata survival (F1,20 = 49.03, p < 0.001; Fig. 2a), but higher productivity increased survival (F1,20 = 19.77, p < 0.001) such that high productivity diminished the effects of crayfish predation (interaction: F1,20 = 34.37, p = 0.005, Fig. 2a). Crayfish reduced survival of P. maculata by an average of 96.6% at low productivity (p < 0.001) and 72.2% at high productivity (p = 0.014). In the presence of crayfish, greater productivity significantly (p < 0.001) increased the average number of surviving P. maculata from near zero (2 ± 1.2 ind./mesocosm) to an average of 27.2 ± 0.1 ind./mesocosm. Increased productivity did not significantly affect P. maculata survivorship where crayfish were absent (p = 0.377). The treatment effects were qualitatively equivalent for final P. maculata biomass (analysis not shown).
Crayfish reduced P. paludosa survival (F1,20 = 8.58, p = 0.008; Fig. 2b), but there were no effects of productivity (productivity: F1,20 = 0.52, p = 0.48; interaction: F1,20 = 0.94, p = 0.343). Crayfish reduced the survival of juvenile P. paludosa by an average of 18%. Final P. paludosa biomass was 2.5 × greater at high productivity (F1,20 = 48.98, p < 0.001), but was unaffected by crayfish (crayfish: F1,20 = 0.55, p = 0.47; interaction: F1,20 = 0.65, p = 0.43).
Total apple snail biomass was directly increased 5.5 × by higher productivity (F1,20 = 23.99, p < 0.001) and decreased by 54.2% with crayfish (F1,20 = 6.87, p = 0.016). The interaction between crayfish and productivity on total apple snail biomass was marginal (F1,20 = 3.84, p = 0.064) and suggested a slight weakening of total predator effects with increasing productivity.
The density of Planorbella duryi present in the mesocosms was enhanced by productivity (F1,20 = 10.61, p = 0.004), but crayfish did not significantly reduce their densities in this experiment (F1,20 = 0.23, p = 0.64) at either productivity level (interaction: F1,20 = 1.36, p = 0.25).
Effects of productivity on predator effect size
Productivity did not change the effect size of crayfish on total apple snail biomass (i.e., both species) measured with a log-ratio response (F1,10 = 0.03, p = 0.869; Fig. 3).
Predator sorting effects
The proportion of the final assemblage that was composed of native P. paludosa was affected by crayfish (F1,20 = 41.57, p < 0.001) and productivity (F1,20 = 14.96, p < 0.001), with productivity level modifying the effect of crayfish (interaction: F1,20 = 5.80, p = 0.026; Fig. 4). Background mortality of P. maculata reduced the relative density of P. maculata from 89% at initial stocking (240 P. maculata: 30 P. paludosa) to an average of 69.5% where crayfish were absent (Fig. 4). Crayfish shifted the assemblage towards higher relative abundances of P. paludosa regardless of productivity level (Fig. 4), but the sorting effect of the predator was 1.8 × greater at low productivity than at high productivity (productivity contrast with crayfish: p < 0.001; Fig. 4). At low productivity where crayfish were present, the apple snail assemblage was on average 90.5% P. paludosa. At high productivity the assemblage was almost evenly divided (mean = 48.3% P. paludosa) (Fig. 4). Increased productivity alone did not affect the proportion of P. paludosa in the final assemblage (productivity contrast without crayfish: p = 0.314; Fig. 4).
Mean proportion of the overall apple snail assemblage composed of P. paludosa at two productivities in the presence and the absence of crayfish. Initial stocking density of P. paludosa was 0.11 across all treatments, whereas biomass was equal between the species. Errors bars represent one standard error (n = 6)
When clutch size differences were accounted for, projected survivors per clutch of each species over a 49-day period indicated that P. paludosa was slightly favored over P. maculata in low productivity wetlands with crayfish (Table 1) and that P. maculata juveniles were projected to be favored under all other conditions (Table 1).
Discussion
In our experiment, increased productivity reduced interspecific differences in the vulnerability of two prey to a shared predator and reduced predator sorting of the prey assemblage. Increased productivity increased the growth rates of both prey, but because differences in vulnerability were largely growth-mediated (and thus sensitive to productivity), the smaller and intrinsically more vulnerable prey, P. maculata, gained relatively more resistance to predators than the less vulnerable P. paludosa. Because the less vulnerable prey (P. paludosa) dominated the assemblage at lower productivity, our results depart from prior investigations (empirical: Chase 2003b; theoretical: Leibold 1996). Our results are most consistent with a hypothesis of growth-mediated vulnerability suggested by size-dependent functional response models (McCoy et al. 2011) and provide an alternative expectation for the effects of enrichment on community-level sorting by predators. Despite differences in community sorting effects, the total predator effects on prey biomass remained similar across productivity levels. Our results also suggest a set of interacting environmental conditions that may influence the distribution and abundance of the invasive P. maculata.
Size-structured predation theory predicts that factors that increase prey growth rate will improve survivorship by limiting the amount of time spent at smaller, more vulnerable sizes (Werner and Gilliam 1984; McCoy et al. 2011). While both species of apple snail grew faster with increased productivity, thus reaching a size refuge in a shorter time, only the more vulnerable P. maculata realized significant gains in survivorship in the presence of crayfish. This is likely to have occurred for two primary reasons. First, nutrient enrichment acted differentially on the growth of the two apple snail species, causing P. maculata to grow proportionally faster than P. paludosa. Second, due to intrinsic differences in the vulnerability of P. maculata and P. paludosa, P. maculata had proportionally more to gain than P. paludosa by growing faster. Predation rates in size-structured predator–prey interactions depend on the ratio of the predator’s body size to its prey’s body size—smaller predator–prey size ratios tend to produce weaker predator–prey interactions (Vucic-Pestic et al. 2009). Juvenile P. maculata are, therefore, intrinsically more vulnerable to predation than juvenile P. paludosa because of their smaller hatchling sizes (i.e., larger predator–prey size ratio; Vucic-Pestic et al. 2009; Davidson and Dorn 2017). However, by growing proportionally faster than P. paludosa at high productivity, P. maculata’s predator–prey size ratio shrank faster and to a higher degree than P. paludosa’s, weakening crayfish predation comparatively more. Crayfish feeding rates on 4 mm SL apple snails are already low (Davidson and Dorn 2017), so by hatching at 4 mm, P. paludosa has less to gain from growing faster. Growth-mediated decreases in predator–prey size ratios may ultimately have been more important to our result than the presence of a strict size refuge for either prey species. At low productivity, surviving P. maculata did not reach size refuge (~ 12 mm), only attaining an average size of 7.3 mm SL. At high productivity, most P. maculata did reach size refuge in the course of an estimated 31–32 days.
Predation rates can also be weakened by high prey densities (i.e., with type II or III functional responses; McCoy et al. 2011) and predator satiation remains a potential alternative explanation if high productivity conditions increased background P. maculata survival. We find this to be unlikely as a stand-alone explanation for two reasons. First, although the mean number of surviving snails may appear different in Table 1, productivity did not significantly influence survival of P. maculata or P. paludosa in the absence of crayfish. Second, previous observations suggest predation rates on juvenile snails are sufficiently high to eliminate any small background survival differences that might have existed. In a previous mesocosm experiment, 1–3 crayfish consumed 161 juvenile P. maculata (sizes: 1.6–4.9 mm SL) over a similar timeframe (Dorn and Hafsadi 2016). In the lab, small adult crayfish (22 mm CL) fed on P. maculata at per capita rates of 9.5 day−1 (4-mm juveniles) or 48 day−1 (1.6-mm hatchlings; Davidson and Dorn 2017). An increase of 20–40 available snails, without any dramatic change in snail sizes, would not obviously pose a handling time/satiation problem for the crayfish in our experiment. While we are suggesting that predation rates were reduced under high productivity, a simple increase in snail density alone does not appear to be responsible; in other words, any mechanistic explanation of our results will necessarily have to invoke to a size-dependent satiation (McCoy et al. 2011) which leads back to growth-mediated vulnerability of P. maculata.
In contrast to the benefits P. maculata gained with greater productivity, the effects of crayfish on P. paludosa survival were unaffected by productivity level. Species with high juvenile survival rates with predators will be less affected by changes to their juvenile growth rates (Werner and Gilliam 1984). Pomacea paludosa hatches at larger sizes and with thicker shells, thus P. paludosa hatches in a more predator-resistant condition (Davidson and Dorn 2017) and gains proportionally less from enhanced growth. The survival differences of the two species are consistent with theoretical predictions that benefits of increased growth for cohorts of prey facing a size-limited predator depend on the relative vulnerability of the different ontogenetic stages (Werner and Gilliam 1984; McCoy et al. 2011). It is also possible that crayfish predation rates on P. paludosa were somewhat muted in the presence of alternative P. maculata prey. Crayfish feed selectively on P. maculata over P. paludosa when provided a choice, and selectivity remains even when snail size is constant because P. paludosa have heavier shells (Dorn and Hafsadi 2016; Davidson and Dorn 2017). Pomacea paludosa might also benefit from decreases in growth-mediated vulnerability, but at a lower range of growth rates. 67–78% P. paludosa survived with crayfish in this study while in a previous study with lower productivity (lower P. paludosa growth) only 43% survived with crayfish (Dorn and Hafsadi 2016). While the survival rates seem consistent with that interpretation, demonstrating a growth benefit for P. paludosa would require additional experiments.
Our results appear inconsistent with Leibold (1996) and Chase’s (1999) predictions about how productivity alters predation strength and predator sorting of a mixed-prey community. Models predicting predator structuring of prey communities across productivity gradients (Leibold 1996; Chase 1999) come to various conclusions depending on their assumptions about prey vulnerability. The keystone predation model (Leibold 1996) assumes prey vulnerability is essentially static and predicts that predation will favor resistant (less vulnerable) prey at high productivity. Chase (1999) included size-refuges for some prey and also predicted that larger-bodied resistant prey would dominant at higher productivity. In both models, prey that are more vulnerable to predators—but better competitors—dominate at low productivity while prey that can reach a size refuge dominate at high productivity. In our study, the assemblage was dominated by the intrinsically more resistant prey species at low productivity, but was evenly split at high productivity. While both prey species could reach a size refuge, the interspecific difference in vulnerability was growth-mediated (not static), and was reduced with increased productivity. It is important to note that without the differences in propagule numbers—which favor the more fecund P. maculata—the assemblages would still favor P. paludosa at high productivity because differences in per capita differences survivorship were reduced, but not eliminated, at high productivity.
In our study, predator effect sizes on total prey biomass were constant across a productivity gradient. Borer et al. (2006) demonstrated that predator effects on prey biomass are largely unaffected by productivity, but in the studies they reviewed, productivity also had no consistent effect on herbivore (prey) biomass. Borer et al. (2006) concluded that this was because bottom-up fertilization effects enhanced plant biomass but attenuated too quickly to influence herbivore biomass, even when predators were absent. In our study, increased nutrients enhanced juvenile apple snail growth rates and final biomass in controls; even so, crayfish limitation of total apple snail biomass did not vary with productivity level. Although this overall result is consistent with predator effect sizes in Borer et al. (2006), the mechanism cannot be explained as rapid attenuation of bottom-up effects. Instead, the survivorship and biomass of the relatively invulnerable prey, P. paludosa, was only weakly affected by crayfish at either productivity level and made up a large fraction (≥ 60% on average) of the final prey biomass in all replicates. This was especially true at low productivity where crayfish were present, where P. paludosa biomass made up on average > 90% of the total prey biomass. Therefore, the effect of the predator on apple snail final biomass was dominated by the predator’s relatively weak effects on P. paludosa, limiting the extent to which increased productivity could further weaken predator effect sizes at high productivity.
Classical predator–prey population models (e.g., modified Lotka-Volterra models such as Oksanen et al. 1981) include numerical responses of both predators and their prey. The connection between our experiment and theoretical community predictions is somewhat strained because our experiment did not allow for numerical responses of the predator or prey, but we believe our results and the extrapolation to success per clutch (Table 1) are still instructive about the potential interactive effects of productivity and predation on prey community assembly. Our results suggest that crayfish dramatically affect P. maculata survival and may promote mixed assemblages or P. paludosa dominance under low nutrient conditions. This implies, conversely, that eutrophic conditions may facilitate invasion by P. maculata even in the presence of predators such as crayfish. However, the degree of this shift in assemblage structure appears to be dependent on interspecific differences in clutch size. When clutch size was accounted for, the only conditions that were projected to favor higher relative abundances of P. paludosa than P. maculata were low productivity wetlands with crayfish; in all other cases survival of juveniles favored P. maculata, such that there were 10.3–32.8 × more surviving P. maculata than P. paludosa per clutch (Table 1).
Incorporating reproductive traits such as clutch size and reproductive rate will be important to determining the outcome of this predator–prey interaction. Primary productivity may affect both snail and crayfish population sizes, and without further studies it is unknown which will increase faster. Crayfish densities are often higher in enriched wetlands (Hagerthey et al. 2014), but their population densities appear strongly limited by fish predators feeding primarily on juvenile crayfish (Kellogg and Dorn 2012; Dorn and Cook 2015; NJD personal observations). If crayfish vulnerability to fish predators is also growth-mediated it is still possible crayfish densities could increase somewhat under high productivity conditions because larger crayfish are less vulnerable to predators (van der Heiden and Dorn 2017). Higher densities of crayfish and other snail predators at greater productivity might limit the survivorship benefit P. maculata gains with high productivity conditions, but available evidence suggests crayfish densities will not typically exceed 4–5 large (> 19 mm CL) individuals per m2 (Hagerthey et al. 2014).
In oligotrophic wetlands like those present in southern Florida, gastropod survival and growth may be limited by the availability of high quality food (Ruehl and Trexler 2011, 2015), and this has been suggested as another possible mechanism that could explain part of the distribution and abundance of native and invasive apple snails in south Florida (Turner et al. 1999; Ruehl and Trexler 2011). Dorn and Hafsadi (2016) suggested that P. paludosa may be more tolerant of poor food quality present in oligotrophic interior marshes far from canals where P. maculata were relatively rare. In our study, P. maculata survival was improved under high productivity conditions, but only where crayfish were present. Low productivity conditions alone did not limit P. maculata survivorship when compared to high productivity replicates. This suggests that high productivity conditions (e.g., high phosphorus levels like those near canals in Florida, Rehage et al. 2006) should favor high densities of P. maculata apple snails, but only by weakening predator effects on juvenile apple snails and increasing the number of snails that survive through the predation window.
Predators sort prey communities by preferentially consuming prey according to their vulnerability, and environmental contexts such as productivity can alter this vulnerability by increasing prey growth rates and decreasing predator–prey size ratios. If different prey species benefit differentially from productivity then interspecific differences in vulnerability will be productivity or growth mediated. This can produce scenarios where species that are more vulnerable at low productivity (low growth) increase in relative abundance at high productivity (high growth). We encourage others to make a careful evaluation of how prey vulnerability to common predators varies with life history, prey ontogeny, and the environmental factors that affect prey development rates.
Change history
29 March 2018
There was an error in the abstract of the original publication. The 9th sentence of abstract should be:
“Our results are inconsistent with predictions about the sorting effects of predators across productivity gradients because the more resistant prey dominated at low productivity.”
References
Aljetlawi AA, Sparrevik E, Leonardsson K (2004) Prey-predator size-dependent functional response: derivation and rescaling to the real world. J Anim Ecol 73:239–252
Baker P, Zimmanck F, Baker SM (2010) Feeding rates of an introduced freshwater gastropod Pomacea insularum on native and nonindigenous aquatic plants in Florida. J Mollus Stud 76:138–143
Barnes MA, Fordham RK, Burks RL, Hand JT (2008) Fecundity of the exotic apple snail, Pomacea insularum. J N Am Benthol Soc 27:738–745
Borer ET, Halpern BS, Seabloom EW (2006) Asymmetry in community regulation: effects of predators and productivity. Ecology 87:2813–2820
Brooks JL, Dodson SI (1965) Predation, body size, and composition of plankton. Science 150(3692):28–35
Brose U (2010) Body-mass constraints on foraging behaviour determine population and food-web dynamics. Funct Ecol 24(1):28–34
Brose U, Jonsson T, Berlow EL, Warren P, Banasek-Richter C, Bersier LF, Blanchard JL, Brey T, Carpenter SR, Blandenier MF, Cushing L, Dawah HA, Dell T, Edwards F, Harper-Smith S, Jacob U, Ledger ME, Martinez ND, Memmott J, Mintenbeck K, Pinnegar JK, Rall BC, Rayner TS, Reuman DC, Ruess L, Ulrich W, Williams RJ, Woodward G, Cohen JE (2006) Consumer- resource body- size relationships in natural food webs. Ecology 87:2411–2417
Carlsson N, Kestrup Å, Mårtensson M, Nystrӧm P (2004) Lethal and non-lethal effects of multiple indigenous predators on the invasive golden apple snail (Pomacea canaliculata). Freshw Biol 49:1269–1279
Cattau CE, Darby PC, Fletcher RJ Jr, Kitchens WM (2014) Reproductive responses of the endangered snail kite to variations in prey density. J Wildl Manag 78:620–631
Cattau CE, Fletcher RJ Jr, Reichert BE, Kitchens WM (2016) Counteracting effects of a non-native prey on the demography of a native predator culminate in positive population growth. Ecol Appl 26:1952–1968
Chase JM (1999) Food web effects of prey size refugia: variable interactions and alternative stable equilibria. Am Nat 154:559–570
Chase JM (2003a) Strong and weak trophic cascades along a productivity gradient. Oikos 101:187–195
Chase JM (2003b) Experimental evidence for alternative stable equilibria in a benthic pond food web. Ecol Lett 6(8):733–741
Childers DL, Doren RF, Jones R, Noe GB, Rugge M, Scinto LJ (2003) Decadal change in vegetation and soil phosphorus pattern across the Everglades Landscape. J Environ Qual 32:344–362
Davidson AT (2016) Predator impacts of crayfish on apple snails (Pomacea paludosa and P. maculata). Masters Thesis, Department of Biology, Florida Atlantic University, Davie, FL
Davidson AT, Dorn NJ (2017) Life history traits determine vulnerability of native and invasive apple snails (Pomacea spp.) to a shared juvenile predator. Aquat Ecol. https://doi.org/10.1007/s10452-017-9620-9
Dorn NJ (2013) Consumptive effects of crayfish limit snail populations. Freshw Sci 32:1298–1308
Dorn NJ, Cook MI (2015) Hydrological disturbance diminishes predator control in wetlands. Ecology 96:2984–2993
Dorn NJ, Hafsadi M (2016) Native crayfish consume more exotic than native apple snails. Biol Invasions 18:159–1679
Dorn NJ, Trexler JC (2007) Crayfish assemblage shifts in a large drought-prone wetland: the roles of hydrology and competition. Freshw Biol 52:2399–2411
Hagerthey SE, Cook MI, Kobza RM, Newman S, Bellinger BJ (2014) Aquatic faunal responses to an induced regime shift in the phosphorus-impacted Everglades. Freshw Biol 59:1389–1405
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398
Holling CS (1961) Principles of insect predation. Annu Rev Entomol 6:163–182
Holt RD, Grover J, Tilman D (1994) Simple rules for interspecific dominance in systems with exploitative and apparent competition. Am Nat 144:741–777
Kellogg CM, Dorn NJ (2012) Consumptive effects of fish reduce wetland crayfish recruitment and drive species turnover. Oecologia 168:1111–1121
Klecka J, Boukal DS (2013) Foraging and vulnerability traits modify predator–prey body mass allometry: freshwater macroinvertebrates as a case study. J Anim Ecol 82:1031–1041
Knorp NE, Dorn NJ (2016) Mosquitofish predation and aquatic vegetation determine emergence patterns of dragonfly assemblages. Freshw Sci 35(1):114–125
Leibold MA (1989) Resource edibility and the effects of predators and productivity on the outcome of trophic interactions. Am Nat 134:922–949
Leibold MA (1996) A graphical model of keystone predators in food webs: trophic regulation of abundance, incidence, and diversity patterns in communities. Am Nat 147:784–812
McCormick PV, Newman S, Miao S, Gawlik DE, Marley D, Reddy KR, Fontaine TD (2002) Effects of anthropogenic phosphorus inputs on the Everglades. In: Porter JW, Porter KG (eds) The Everglades, Florida Bay, and coral reefs of the Florida Keys: an ecosystem sourcebook. CRC Press, Boca Raton, pp 83–126
McCoy MW, Bolker BM, Warkentin KM, Vonesh JR (2011) Predicting predation through prey ontogeny using size-dependent functional response models. Am Nat 177(6):752–7661
Morrison WE, Hay ME (2011) Feeding and growth of native, invasive and non-invasive alien apple snails (Ampullariidae) in the United States: invasives eat more and grow more. Biol Invasions 13:945–955
Oksanen L, Fretwell SD, Arruda J, Niemela P (1981) Exploitation ecosystems in gradients of primary productivity. Am Nat 118:240–261
Osenberg CW, Mittelbach GG (1989) Effects of body size on the predator-prey interaction between pumpkinseed sunfish and gastropods. Ecol Monograph 59:405–432
Paine RT (1976) Size-limited predation: an observational and experimental approach with the Mytilus-Pisaster interaction. Ecology 57:858–873
Posch H, Garr AL, Pierce R, Davis M (2012) The effect of stocking density on the reproductive output of hatchery-reared Florida apple snails, Pomacea paludosa. Aquaculture 360–361:37–40
Posch H, Garr AL, Reynolds E (2013) The presence of an exotic snail, Pomacea maculata, inhibits growth of juvenile Florida apple snails, Pomacea paludosa. J Mollus Stud. https://doi.org/10.1093/mollus/eyt034
Reddy KR, Newman S, Osborne TZ, White JR, Fitz HC (2011) Phosphorous cycling in the Greater Everglades ecosystem: legacy phosphorous implications for management and restoration. Crit Rev Env Sci Tech 41:149–186
Rehage JS, Trexler JC (2006) Assessing the net effect of anthropogenic disturbance on aquatic communities in wetlands: community structure relative to distance from canals. Hydrobiologia 569:359–373
Rogevich EC, Hoang TC, Rand GM (2009) Effects of sublethal chronic copper exposure on the growth and reproductive success of the Florida apple snail. Arch Environ Contam Toxicol 56:450–458
Ruehl CB, Trexler JC (2011) Comparison of snail density, standing stock, and body size between Caribbean karst wetlands and other freshwater ecosystems. Hydrobiologia 665:1–13
Ruehl CB, Trexler JC (2015) Reciprocal transplant reveals trade-off of resource quality and predation risk in the field. Oecologia 179(1):117–127
Sarma SSS, Jiménez-Contreras J, Fernández R et al (2013) Functional responses and feeding rates of Mesocyclops pehpeiensis Hu (Copepoda) fed different diets (rotifers, cladocerans, alga and cyanobacteria). J Nat Hist 47:841–852
Shuford RBE III, McCormick PV, Magson J (2005) Habitat related growth of juvenile Florida apple snails (Pomacea paludosa). Fla Sci 68(1):11–19
Sih A, Crowley P, McPeek M, Petranka J, Strohmeier K (1985) Predation, competition, and prey communities: a review of field experiments. Annu Rev Ecol Syst 16(1):269–311
Sousa WP (1993) Size-dependent predation on the salt-marsh snail Cerithidea californica Haldeman. J Exp Mar Biol Ecol 166:19–37
Turner AM, Fetterolf SA, Bernot RJ (1999) Predator identity and consumer behavior: differential effects of fish and crayfish on the habitat use of a freshwater snail. Oecologia 118:242–247
van der Heiden CA, Dorn NJ (2017) Benefits of adjacent habitat patches to the distribution of a crayfish population in a hydro-dynamic wetland landscape. Aquat Ecol. https://doi.org/10.1007/s10452-016-9612-1
Vonesh JR, Bolker BM (2005) Compensatory larval responses shift trade-offs associated with predator-induced hatching plasticity. Ecology 86(6):1580–1591
Vucic-Pestic O, Rall BC, Kalinkat G, Brose U (2009) Allometric functional response model: body masses constrain interaction strengths. J Anim Ecol 79:249–256
Warton DI, Hui FKC (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27(1):337–363
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Evol Syst 15:393–425
Wilcox RC, Fletcher RJ II (2016) Experimental test of preferences for an invasive prey by an endangered predator: implications for conservation. PLoS One 11(11):e0165427. https://doi.org/10.1371/journal.pone.0165427
Yusa YN, Sugiura N, Wada T (2006) Predatory potential of freshwater animals on an invasive agricultural pest, the apple snail Pomacea canaliculata (Gastropoda: Ampullariidae), in southern Japan. Biol Invasions 8:137–147
Acknowledgements
We thank E. Noonburg, B. Benscoter, S. Gonzalez, E. Binkley, and two anonymous reviewers for making helpful comments on earlier versions of this manuscript. We also thank P. Polpornvitoon and M. Finn for assistance in the lab.
Funding
This study was funded internally by Florida Atlantic University.
Author information
Authors and Affiliations
Contributions
ATD and NJD conceived, designed, and performed the experiments. ATD analyzed the data. ATD wrote the manuscript; NJD provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Bryan Brown.
Rights and permissions
About this article
Cite this article
Davidson, A.T., Dorn, N.J. System productivity alters predator sorting of a size-structured mixed prey community. Oecologia 186, 1101–1111 (2018). https://doi.org/10.1007/s00442-018-4099-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4099-1