Abstract
Predators influence communities through either consuming prey (consumptive effects, CEs) or altering prey traits (non-consumptive effects, NCEs), which has cascading effects on lower trophic levels. CEs are well known to decrease in physically stressful environments, but NCEs may be reduced at physically benign levels that affect the ability of prey to detect and respond to predators (i.e., sensory stress). We investigated the influence of physical and sensory stressors created by spatial and temporal differences in tidal flow on predator controls in a tritrophic system. We estimated mud crab reactive ranges to blue crab NCEs by evaluating mud crab CEs on juvenile oysters at different distances away from caged blue crabs across flow conditions. Mud crab reactive ranges were large at lower physical and sensory stress levels and blue crabs had a positive cascading effect on oyster survival. Blue crab NCEs were not important at higher flow conditions. Oyster survival was a complicated function of both types of stressors. Physical stress (i.e., current speed) had a positive effect on oyster survival by physically limiting mud crab CEs at high current speeds. Sensory stress (i.e., turbulence) interfered with the propagation of blue crab chemical cues used by mud crabs for predator detection, which removed blue crab NCEs. Mud crab CEs increased as a result and had a negative effect on oyster survival in turbulent conditions. Thus, environmental properties, such as fluid flow, can inflict physical and sensory stressors that have distinct effects on basal prey performance through impacts on different predator effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predators promote species coexistence and influence community structure (Paine 1966; Lubchenco 1978; Ripple et al. 2014). Traditionally, predators were thought to control lower trophic levels by reducing prey density through direct consumption (consumptive effects, CEs), but the alteration of prey behavior and phenotype in response to predation risk (non-consumptive effects, NCEs) can impact communities, as well (Werner and Peacor 2003; Peckarsky et al. 2008; Suraci et al. 2016). Many studies suggest that the effect of cascading NCEs can be as strong as or stronger than CEs (Preisser et al. 2005). Full understanding of the role of predators requires understanding how CEs and NCEs are modulated by environmental context. Although it is well known that physical stress imposed by harsh environmental conditions can reduce the strength of CEs (Menge 1978; Menge and Sutherland 1987; Leonard et al. 1998; Bertness et al. 2002; Shears et al. 2008), how environmental conditions affect NCEs in communities is less well studied (Weissburg et al. 2014; but see Van de Meutter et al. 2005; Smee and Weissburg 2006; Large et al. 2011).
Direct and indirect predator CEs can be modulated by environmental gradients, particularly those that have the capacity to cause injury or damage. Consumer stress models postulate that physically harsh conditions may interfere with predator motility, and the release of prey from predation may cascade to affect other organisms (Menge and Sutherland 1987). For example, the intensity of crab predation on dog whelks in tidal estuaries decreased at sites with higher water flow, resulting in increased dog whelk abundances and higher growth rates due to potentially increased consumption of their preferred prey, barnacles (Leonard et al. 1998).
Current environmental stress models generally only consider physical stress constraining CEs, but some environmental conditions can diminish the ability of animals to collect information about prevailing conditions. Such “sensory stress” can occur at physically benign levels, but still may interfere with sensing the smells, sounds, and sights associated with predation (Munoz and Blumstein 2012; Weissburg et al. 2014). In turn, reduced predator sensing can modify interactions between these prey and other organisms (i.e., NCEs such as behaviorally mediated trophic cascades; Schmitz et al. 2004). The maximum distance at which prey detect and respond to predators, which we refer to as prey reactive range, sets the spatial limits of NCEs (Turner and Montgomery 2003).
The physical environment alters prey reactive range (Robinson et al. 2007; Smee et al. 2008) and thus can modulate when and where NCEs may be important. For instance, acoustic cues from predatory bats are attenuated in forested areas compared to in open fields. This diminishes the ability of moths to detect predators and increases predation rates on moths by bats (Jacobs et al. 2008). Similarly, visual perception can be hindered in aquatic environments by water clarity. Antipredator responses of fish to visual predator cues are reduced in turbid compared to clear waters (Hartman and Abrahams 2000). These and other examples (Weissburg et al. 2014) indicate that the environment can interfere with sensory perception in conditions that are not noticeably stressful physically, and these sensory stressors may modify cascades produced by prey responses to risk.
Certain environmental gradients within a system can impose both physical and sensory stresses, which complicates predicting the importance of predator effects on community regulation. For example, fluid flow can simultaneously impose physical stress on locomotion due to hydrodynamic forcing and sensory stress on chemosensory abilities through turbulent mixing (Weissburg et al. 2003). Physical stress has been shown to limit crustacean foraging abilities at high flow conditions in tidally driven estuaries, which decreases the importance of predator CEs (Leonard et al. 1998). Yet, flow is also important in modulating chemical perception in aquatic systems (Weissburg and Zimmer-Faust 1993; Finelli et al. 2000; Jackson et al. 2007; Webster and Weissburg 2009). Turbulence creates greater cue mixing within odor plumes, which reduces information available for crustacean predators and reduces foraging success (Weissburg and Zimmer-Faust 1993; Powers and Kittinger 2002; Jackson et al. 2007). However, in contrast to physical stressors, the impact of sensory stress is contingent on the proximity of predators to prey. Greater fluid mixing may reduce the effectiveness of signals over larger, but not smaller distances, whereas a predator affected by physical stress is simply unable to forage.
CEs and NCEs are important in a variety of species interactions within oyster reefs that are exposed to tidally driven flows (Grabowski et al. 2008; Byers et al. 2014; Hughes et al. 2014). Blue crabs (Callinectes sapidus) are important predators of salt marsh crustaceans and bivalves (Micheli 1997; Smee and Weissburg 2006; Hill and Weissburg 2013a). Mud crabs (Panopeus herbstii) are small, cryptic xanthid crabs that reside inside oyster beds (Lee and Kneib 1994; Hollebone and Hay 2007) and prey on juvenile oysters and other bivalve species (Bisker and Castagna 1987; Silliman et al. 2004; Toscano and Griffen 2012). Chemical cues from top predator blue crabs suppress the foraging of intermediate mud crab consumers on juvenile oysters (Crassostrea virginica) (Hill and Weissburg 2013b; Weissburg et al. 2016). Yet, hydrodynamic conditions vary spatially and temporally in tidally driven salt marshes (Wilson et al. 2013), which suggests that the importance of environmental (i.e., physical and sensory) stressors on modulating blue crab–mud crab–oyster interactions may be context-dependent.
We investigated the effect of physical (i.e., current speed) and sensory stress (i.e., turbulence) on oyster survival through potential alterations of blue crab cascading NCEs and mud crab direct CEs. Specifically, we estimated the reactive range of mud crabs to blue crabs by quantifying mud crab consumption of juvenile oysters in the presence of blue crab risk cues. We examined how oyster survival changes as a function of distance between blue crab sources of aversive chemical cues and mud crabs in different flow regimes. This allowed us to estimate the spatial extent of blue crab NCEs. We predicted that predator effects shift from blue crab NCEs to mud crab CEs as flow increases. NCEs should be greatest when low flow environments permit large reactive ranges in mud crabs. Greater turbulence initially compromises sensing and reduces mud crab reactive range to blue crab cues, but mud crab foraging ultimately declines at high flow speeds despite limited ability to sense predators from a distance. Understanding the environmental conditions where each stressor exerts affects lends insights into the spatial and temporal variance of predator effects, given their different mode of operation.
Methods
Animal collection and maintenance
Both blue crabs and mud crabs were collected from Wassaw Sound (Savannah, GA, USA) and associated tributaries. Blue crabs were caught using baited crab pots. Mud crabs were collected by hand from natural oyster reefs during low tide. Collections were permitted under a scientific collecting permit obtained from the Georgia Department of Natural Resources. Blue crabs and mud crabs were maintained in separate flow-through seawater systems at the Skidaway Institute of Oceanography (SkIO). Mud crabs were sorted and housed separately according to carapace width (CW) size classes: 15–20, 20–25, and 25–30 mm. Mud crabs were fed every 2 days a diet of ab libitum oyster spat to prevent starvation. Blue crabs (12–16 cm CW) were housed individually and 48 h prior to experiment fed an ad libitum diet of crushed mud crabs daily. Blue crabs fed strictly mud crab diets which induce greater reductions in mud crab foraging (Weissburg et al. 2016) and this diet was chosen to maximize blue crab NCEs. Oyster spat (10–16 mm hinge length) were obtained from local commercial hatcheries. Oysters were maintained in a separate flow-through seawater system prior to field experiments.
Site description
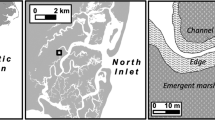
The field experiments described below were performed at sites located in Skidaway and Wilmington Rivers, which are estuarine rivers that flow into Wassaw Sound. Skidaway Narrows site was located along the Skidaway River, which flows into the Wilmington River where Priest Landing site was located (Fig. 1). Both sites are characterized by mudflats bordered by Spartina alterniflora salt marshes. Priest Landing contained a higher density of loose oyster clusters and isolated patches of oyster reefs, but ambient blue crab predation threat level was equivalent at each site based on consumption rates of tethered mud crabs in the field (Fig. S1).
Flow measurement analysis
The previous extensive flow measurements by Wilson et al. (2013) demonstrated that flow parameters vary significantly between these sites. These flow characteristics have been highly conserved across multiple years of monitoring and are strongly predicted by tidal height and range (Wilson 2011; Wilson et al. 2013). We used, and further analyzed, this extensive data set to categorize the flow properties during different tidal types at our sites based on tidal height. Briefly, current speed and turbulent kinetic energy (TKE) were measured using acoustic Doppler velocimeters (ADVs; Nortek) for multiple consecutive tidal cycles. The ADV measurements were taken 10 cm above the substrate, which is within the vertical boundary layer experienced by benthic estuarine organisms. See the Supplementary Material for a more descriptive summary of the methods by Wilson et al. (2013). We characterized the probability density functions of current speed, (|ū|), and turbulent kinetic energy (TKE) using these data. We also used the relationships between tidal range and flow properties provided by this data to estimate the flow properties during our experiments, as described fully below.
Field experiment
We evaluated oyster survivorship in the presence of mud crab predation at different distances away from caged blue crabs and across spatial and temporal flow environments. Mud crab enclosures were 2.2 m by 0.75 m by 0.3 m (L × W × H) and constructed out of 1 cm2 vexar mesh and PVC frame. An oyster reef was created at one end of the enclosure to serve as a refuge for mud crabs. The oyster reef consisted of four natural sun bleached oyster clusters (~ 30 cm dia.) and four artificial oyster clusters. The artificial oyster clusters were constructed by gluing together 4–6 sun bleached oyster shells to create small clusters of approximately 6 cm diameter. The artificial oyster clusters were interspersed within the oyster reef and four additional artificial oyster clusters were placed 25–30 cm away from the oyster reef (Fig. 2). Top predator cages (0.3 m dia. by 0.3 m tall, 1 cm2 vexar mesh) contained an individual blue crab to produce predation risk cues and were placed at varying distances away from the center of the oyster reef (see below).
Diagram of the mud crab (Panopeus herbstii) enclosure design. The refuge contained four artificial clusters (black; “ART”) interspersed within four natural clusters (gray; “Natural”). Four additional artificial clusters were placed outside the refuge as well. Juvenile oyster spat (Crassostrea virginica) were epoxied to the surface of the artificial clusters. Blue crab (Callinectes sapidus) cages were placed on both sides of the refuge, with one cage inside the enclosure (shown) and another outside the enclosure (not shown) (Hill and Weissburg 2013b; Weissburg and Beauvais 2015)
Enclosures were staked down on intertidal mudflats parallel to tidal flow and approximately one tidal foot below mean low water. Four juvenile oyster spat (10–16 mm hinge length) were attached to the surface of each artificial cluster with marine epoxy, so that there was a total of 16 oyster spat inside and outside the refuge (32 spat total). Mud crabs were placed within the oyster reef that was inside the enclosure. Fifteen mud crabs (8 mud crabs 15–20 mm CW, 4 mud crabs 20–25 mm CW, and 3 mud crabs 25–30 mm CW), which reflects the natural field density and size distribution of mud crabs (Hill and Weissburg 2013b), were placed in the oyster reef within the enclosure. Mud crabs were marked with fluorescent paint prior to field deployment to distinguish them from potential immigrating mud crabs. However, most cages (> 90%) lacked any immigration and only 7 out of 215 cages had more than 1 immigrant. Top predator cages contained a blue crab (12–16 cm CW) and were placed at either 0.25, 0.5, 1.0, 1.5, or 2.0 m away from the center of the oyster reef at each end of the cage along the direction of tidal flow. One top predator cage was placed inside the enclosure and the other outside (Fig. 2) to take into account the opposing effects of the cage mesh on flow; Hill and Weissburg (2013b) demonstrated that current speed was slightly weakened by the cage mesh, but the mesh also enhanced turbulence. The overall effect is that TKE remained the same or slightly increased inside the enclosure relative to the ambient flow, but conditions within the cages are well within the range of ambient conditions measured outside the cage. Control treatments consisted of empty top predator cage placed 0.25 m from the reef.
The number of oysters consumed inside and outside the refuge was measured after 24 h. Each 24 h block had two replicates of each distance treatment and no-blue crab cage control, placed at least 5 m apart in random order. Only one site at a certain tidal type could be tested at a time due to distance between sites and the limited time mudflats were exposed during low tide when experiments could be set up and taken down. Tidal type (mean or spring) was defined according to the average low tide height during each deployment. Mean tide low tide heights were between − 0.067 m and 0.033 m, and spring tide low tide heights were between − 0.33 m and − 0.17 m for both sites (Table S1).
We deployed 7 experiments in 2014, 10 in 2015, and 6 in 2016 between the months of June and August. Two blocks, one for Priest Landing at mean tide in 2016 and another for Skidaway Narrows at mean tide in 2015, were removed from the analysis due to extreme heat during the experiment, in which the water temperatures were above 30 °C and air temperatures were above 37 °C. Replicates were also removed if one or more blue crabs were found dead or missing after 24 h. However, blue crab survival generally was high (~ 92%) and only 11 out of 200 distance replicates were omitted due to blue crab death.
Statistical analysis
To provide an estimate of the ability of mud crabs to sense blue crab chemical cues, we analyzed the effect of site, tidal type, and distance of blue crabs from mud crabs on normalized oyster consumption and refuge use. As noted, distance is defined relative to the artificial reef where mud crabs take refuge. Detecting the effects of blue crab chemical cues on mud crabs is facilitated by normalizing consumption to the controls in each block, because the no-blue crab control represents the response of mud crabs in the absence of blue crab chemical cues. Thus, data for total oyster consumption were normalized by dividing the total oyster consumption in a given distance treatment over the average total oyster consumption in the controls in that block. Refuge use was defined as the proportion of oysters consumed within the oyster reef. Data were analyzed using a mixed model analysis. Fixed effects were site, tidal type, and distance treatment. Distance treatment was designated as a categorical factor, so that the no-blue crab control could be included as a distance treatment. Block date was designated as a random effect. The model was fit using a restricted maximum-likelihood (REML) approach, which is appropriate for unbalanced data (Kenward and Roger 1997).
Individual mixed-effects models fit by REML were conducted for each site and tidal type combination to approximate the mud crab reactive range for each site and tidal type, using the number of oysters consumed. The fixed effect was distance from blue crab, including the no-blue crab control, and the random effect was block date. Planned contrast t tests were used to compare the control treatment to each distance treatment, if there was a significant distance treatment main effect. Mud crab reactive range was interpreted as the farthest distance treatment at which oyster consumption differed from the no-blue crab control.
We also analyzed the relationship between oyster survival over 24 h and flow properties in the presence of blue crab risk cues to understand how predator effects change along physical and sensory stress gradients. We regressed oyster survival in the presence of blue crab chemical cues against current speed and TKE, separately. The flow properties during our experiments were estimated for each site and tidal type block based on the predictive relationship between tidal range and flow properties from Wilson (2011). Regression equations on the relationship between tidal range and either mean current speed or average TKE were derived from the flow data for each site, respectively, as obtained by Wilson (2011) and Wilson et al. (2013) (Table S2).
All data analyses were performed in R version 3.3.1 (R Core Team 2016), using the lme4 package for mixed-effects model analysis (Bates et al. 2015). Degrees of freedom and P values for the mixed-effects models were based on Kenward–Roger approximations using the lmerTest package (Kuznetsova et al. 2016).
Results
Flow measurement analysis
Site and tidal type had strong effects on flow conditions. Site- and tide-specific regressions showed robust relationships between tidal range and both mean current speed and turbulent kinetic energy (TKE) at these sites (Table S2). In general, data collected by Wilson et al. (2013) showed that mean current speed and TKE were higher during spring tide relative to mean tide, regardless of site (Table S3). However, between sites, Priest Landing (PL) had greater mean TKE and slower mean current speed compared to Skidaway Narrows (SN), which had faster speeds and lower TKEs (Table S3). The distribution of these parameters was consistent with these trends; the distribution of TKE at PL skewed to higher values but current speed to lower values compared to SN (Fig. S2). A more exhaustive description of the flow characteristics is found in the Supplementary Material.
Field experiment
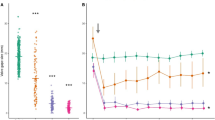
Chemical cues from blue crab top predators reduced normalized oyster consumption (consumption relative to no-blue crab control), but only during mean tide and with site-specific patterns (Table 1; Fig. 3). Tidal type had a significant effect on mud crab normalized oyster consumption, with less normalized consumption at mean versus spring tide (Table 1). Normalized consumption during spring tide appeared similar to the no-blue crab controls across distance treatments, whereas the average-normalized consumption in the blue crab distance treatments at mean tide was 0.637 ± 0.035 (mean ± SE). There also was an effect of blue crab distance treatment on normalized oyster consumption that was site-dependent (Table 1), which seems to result from site-specific consumption patterns during mean tides. During mean tides, normalized oyster consumption was lower than the no-blue crab control in each blue crab predator distance treatment at PL, but consumption was only reduced in the distance treatments up to 1.5 m at SN.
Normalized oyster consumption by mud crabs (mean ± SE) at different distances away from caged blue crabs during mean tide (closed symbols, solid lines) and spring tide (open symbols, dashed lines) at a Priest Landing (PL; n for mean tide = 64, n for spring tide = 49) and b Skidaway narrows (SN; n for mean tide = 61, n for spring tide = 48) Asterisks denote the mud crab reactive range for PL and SN at mean tide based on the farthest distance in which oyster consumption was significantly lower than the control (Table 2). There was no difference in oyster consumption in the distance treatments and the controls at PL and SN during spring tide
Tidal type also had a significant effect on refuge use by mud crabs, with mud crabs consuming a larger proportion of oysters inside the refuge during mean tide (Fig. S3, Table S4). Approximately 80% of the total oysters consumed by mud crabs during mean tide were inside the refuge, compared to only 67% at spring tide. Distance treatment also affected refuge use, but unlike normalized oyster consumption was not site specific (Table S4). Tukey post hoc test revealed that only the 0.25 m distance treatment differed in refuge use from the control. Refuge use was uniformly higher in each distance treatment compared to the no-blue crab control at mean tide for both sites. Yet, during spring tide, the proportion of oysters consumed inside the refuge was highest at 0.25 m and declined linearly, but weakly, as distance away from blue crab increased (Fig. S3).
Individual mixed-effects models within each site and tidal type revealed spatial and temporal differences in the mud crab reactive ranges inferred from oyster survival. During spring tide at both sites, the mud crab reactive range was 0 m and oyster consumption was similar across all treatments (Fig. 3; PL: F5,39 = 1.625, P = 0.176; SN: F5,32 = 0.926, P = 0.477). There was an effect of blue crab distance treatment at SN during mean tide (F5,50 = 3.978, P = 0.004) and oyster consumption was suppressed ~ 39% compared to the no-blue crab control in all distance treatments except at 2 m (Table 2; Fig. 3b). The mud crab reactive range was 1.5 m (Table 2). There also was an effect of distance treatment at PL during mean tide (F5,53 = 3.781, P = 0.005), despite some variation in the reduction of consumption. There was no significant difference in oyster consumption at the 1.5 m distance compared to the no-blue crab control. However, consumption was significantly lower in the other distance treatments, including the 2 m distance where oyster consumption was decreased by 52% relative to the no-blue crab control (Table 2; Fig. 3a).
The relationships between oyster survival, mud crab reactive range, and flow parameters varied between the physical (current speed) and sensory stress (turbulence) gradients (Fig. 4). Oyster survival and mud crab reactive ranges were highest at both sites during mean tides where estimated current speeds and TKE were lowest. Oyster survival was also high at the highest estimated current speed (SN-spring), which corresponded to low mud crab reactive ranges. In contrast, oyster survival was lowest during PL-spring tide conditions where mud crab reactive range was also 0. Here, current speed was intermediate between mean tide conditions at both sites and SN-spring tide conditions. Thus, at speeds < 11 cm s−1, low TKE is associated with large mud crab reactive ranges, suggesting that foraging suppression from perception of blue crab chemical cues enhances oyster survival. At speeds above 11 cm s−1, higher TKEs are coincident with low mud crab reactive range and oyster survival is low (PL-spring) until speeds exceed 13 cm s−1, suggesting physical stress limits mud crab foraging (SN-spring). These complex relationships result in no association between average estimated current speed and oyster survival (F1,182 = 0.006; P = 0.940; r2 < 0.001), and a significant but weak relationship between estimated TKE and oyster survival (F1,182 = 6.757, P = 0.010, r2 = 0.036).
Average oyster survival in the presence of blue crab risk cues (z-axis) at different mean current speeds (cm s−1; x-axis) and turbulent kinetic energies (TKE; m2 s−2; y-axis). Current speeds and TKEs correspond to estimated flow conditions during each trial block derived from each site and tidal type based on regression equations (Table S2). Labels above points denote site (PL Priest Landing, SN Skidaway narrows) and tidal type (mean tide, spring tide), and mud crab reactive range determined for that site and tidal type combination (Table 2; n = 55 for PL: Mean, n = 42 for PL: Spring, n = 51 for SN: Mean, n = 36 for SN: Spring)
Discussion
Environmental forces can inflict either physical or sensory stressors that alter predator direct and indirect effects, which in turn influence the abundance and spatial distribution of basal resources. Our results suggest that within a tritrophic system, both these stressors can interact to produce distinct patterns of predator control. We found that mud crab reactive ranges were large under conditions when ambient flows were likely relatively slow and less turbulent. Here, oyster survival was high suggesting mud crabs foraged less, resulting in a classic behaviorally mediated cascade. Mud crab response to blue crab cues declined under tidal conditions that were predicted to increase current speed and turbulence, and blue crab NCEs were not important at higher physical and sensory stress levels. There was a positive effect of flow on oyster survival at the highest mean speed, because foraging was likely physically constrained in mud crabs, but a negative effect at the highest turbulence, because mud crabs apparently no longer responded to blue crab chemical cues, but could still consume oysters. The difference in the sign of these effects suggests hydrodynamics affected oyster survival through different stressors.
Blue crab NCEs had a positive indirect effect on oyster survival when estimated current speeds and TKEs were lowest, which occurred during mean tide at both sites (Fig. 4). The estimated mud crab reactive range at SN during mean tide was 1.5 m and at least 2 m (the farthest distance tested) at PL during mean tide (Table 2). Reactive ranges of prey are important, since they define the landscape of fear perceived by prey, but relatively a few studies have measured prey reactive ranges (but see Turner and Montgomery 2003; Morgan et al. 2016). Habitat heterogeneity creates areas of risky versus refuge space (i.e., “landscape of fear”, Laundré et al. 2001), which alters NCE strength and the distribution of basal resources across landscapes (Creel et al. 2005; Matassa and Trussell 2011; Burkholder et al. 2013). These sensory landscapes that govern the strength of NCEs are strongly affected by the physical environment, and, as shown here, are constant neither in time nor space. More estimates of prey reactive ranges are needed to understand the spatial extent of NCEs under natural conditions.
Chemical cues from blue crabs did not influence mud crab-oyster consumption at PL during spring tide, which had the highest estimated TKEs (Fig. 4). Oyster survival was greatly reduced during spring tide compared to mean tide at this site and mud crab refuge use was also lower (Fig. S3). Thus, turbulence appears to act as a sensory stressor by interfering with mud crabs’ ability to detect blue crab chemical cues, which decreased reactive range to zero in conditions where turbulence was expected to be greater and removed blue crab NCEs. Increased turbulent mixing creates odor plumes that contain short, highly intermittent burst of chemical signals at lower peak concentrations (Koehl 2006; Jackson et al. 2007). Alteration of plume structure due to turbulence has been shown to reduce odor-mediated foraging success in blue crabs (Weissburg and Zimmer-Faust 1994; Powers and Kittinger 2002; Jackson et al. 2007). Clam reactive ranges to predators also decreased when turbulence was increased while holding velocity constant (Smee et al. 2008). Mud crabs are known to decrease the strength of antipredator responses when presented with lower concentrations of blue crab chemical cues, either due to decreased predator biomass or diet amount (Hill and Weissburg 2013a; Weissburg and Beauvais 2015; Weissburg et al. 2016). Thus, mud crabs may not have detected blue crab cues in flows estimated to have higher turbulences, or the reduction in cue concentration at higher turbulences was perceived as a less risky environment where foraging suppression was not warranted (Chivers et al. 2001).
Mud crab consumption of oysters was not affected by blue crab NCEs at SN during spring tide, where estimated current speeds were greatest, but oyster survival was higher than that seen at PL during spring tide (Fig. 4). Analysis of data obtained by Wilson et al. (2013) shows that, although mean current speed was only 2 cm s−1 faster at SN during spring tide than at PL, the mode was 11 cm s−1 higher (Table S3; Fig. S2a). In addition, these differences in flow between the two sites at spring tide may have been even greater during the field experiments than when flow was measured, because the tidal range for ADV deployment at PL during spring tide was larger than the tidal ranges for SN and the field experiments during spring tide, potentially overrepresenting flow values for PL at spring tide (Table S1; Supplementary material). Thus, the most likely explanation is that physical forcing reduced mud crab foraging abilities in conditions indicative of higher current speeds, which decreased mud crab CEs. Hydrodynamic forces, such as lift and drag, inflict physical limitations on animal locomotion and foraging abilities (Denny 1988; Weissburg et al. 2003). Drag force increases at higher flow velocities creating more environmentally stressful environments (Weissburg et al. 2003). Physical stress from increased current speeds of 15 cm s−1 compared to 3 cm s−1 has been shown to increase handling time in green crabs (Carcinus maenas; Robinson et al. 2011).
The physical environment modulated when and where certain predator effects were important, which had distinct effects on oyster survival. Oyster survival was negatively affected by sensory stress due to reduced importance of blue crab NCEs. This suggests turbulence impaired mud crab’s ability to detect blue crabs, which enhanced negative mud crab CEs and removed the positive cascading blue crab NCE seen at lower sensory stress conditions. Physical stress had a positive effect on oyster survival by possibly physically constraining mud crab foraging, which decreased mud crab CEs. However, oyster survivorship was dependent on the interaction between physical and sensory stressors (as discussed below) and should be included in existing environmental stress models.
We created a simple conceptual model based on our results that incorporates the distinct effects of physical and sensory stressors on predator controls that simultaneously interact to create different impacts on basal resources across environmental gradients (Fig. 5). Top predator NCEs are important at low physical and sensory stress conditions, because intermediate prey can detect and respond to top predators. Cascading NCEs will have positive effects on basal prey survival, because top predators decrease intermediate prey foraging rates.
A conceptual model of basal prey survivorship in a tritrophic system across an environmental gradient that imposes both physical and sensory stress. Top predator NCEs initiate behavioral trophic cascades at low physical and sensory stress levels, because intermediate prey detect and respond to top predators. As sensory stress increases, intermediate prey no longer detect top predators as easily, which reduces positive cascading NCEs on basal prey (lower left panel). Here, the decline of intermediate prey reactive range also creates spatial variation in NCEs. Sensory stress interferes with intermediate prey ability to detect basal prey at high sensory stress levels, which decreases intermediate prey CEs (upper left panel). This also produces a spatially non-uniform pattern of basal prey survival. However, regardless of sensory stress levels, physical forcing reduces intermediate prey foraging at high physical stress levels, which removes intermediate prey CEs and results in uniformly high basal prey survival across space (right panel)
As sensory stress increases, but physical stress remains low, sensory abilities of intermediate prey diminish and NCEs decline until intermediate prey no longer detect and respond to top predators (Fig. 5; lower left panel). Note this implies that indirect effects on basal resources will be spatially variable, because intermediate prey can perceive their predators if they are very close; basal prey survival will depend on the distance away from the source of aversive cues. We found that the reactive range was 0.5 m shorter at SN during mean tide, which had lower estimated TKEs, compared to PL, but reactive ranges were zero during spring tide in both sites which had higher estimated turbulence. Large et al. (2011) documented a similar pattern where predator avoidance by Nucella snails increased at intermediate turbulences along a flow gradient before declining, possibly because moderate turbulence increases the spatial coverage of the predator cue plume without diluting concentrations sufficiently to affect perception. Despite some variation in responses at low estimated TKEs, sensory stress clearly reduced the ability of mud crabs to detect blue crabs at the highest estimated turbulence level. Thus, at higher sensory stress, intermediate prey CEs increase, because they are released from top predator NCEs and basal prey survivorship decreases as a result. Larger reactive ranges at low levels of sensory stress will produce a more coarse-grained spatial pattern of basal prey survival compared to that produced when higher levels of sensory stress reduce reactive ranges.
Although not seen in our study, as sensory stress continues to increase, intermediate prey sensory detection of basal resources may erode, and sensory stress can have an indirect positive effect on basal prey abundances by reducing intermediate prey CEs (Fig. 5; upper left panel). For example, along a turbidity gradient, zooplanktivorous fish foraging rates increased as turbidity increased, due to suspected decreases in the importance of piscivorous fish NCEs (Pangle et al. 2012). Yet, zooplanktivore foraging rates decreased at higher turbidity levels due to a decline in visually mediated foraging abilities (Pangle et al. 2012).
Regardless of sensory stress, physical stress hinders intermediate prey motility and so foraging declines as physical stress increases. Like predictions in the traditional models (consumer stress model: Menge and Sutherland 1987; Menge and Olson 1990), CEs are not important in high physical stress environments and basal prey are released from intermediate consumer control (Fig. 5; right panel). In this study, at the tide-site combination where we estimated current speed to be the highest, blue crab NCEs were not important and consumer stress models predicted mud crab and oyster interactions (i.e., SN during spring tide). We saw a positive effect of abiotic conditions on oyster survival due to reduced mud crab CEs. However, unlike sensory stress, a given level of physical stress produces a spatially homogenous effect on basal prey survival.
Our model was influenced by results from this study in which prey chemosensory detection was modified by hydrodynamics. However, the interaction between sensory and physical stressors likely is general and this conceptual model can be used to predict predator controls and indirect effects in other environmental contexts. Odor cues are also transported as filamentous plumes by turbulent air flow in terrestrial habitats, which affects the spatial and temporal distribution of chemical signals (Koehl 2006). Thus, wind may affect chemoperception of predators by prey while also imposing physical limitations on walking and flying, which could inhibit prey ability to respond to predators (Cherry and Barton 2017). Mechanosensory detection, which is important in predator detection for arthropod prey in both aquatic and terrestrial habitats (Casas and Dangles 2010), can be hindered in high flow environments due to decreased signal-to-noise ratio (Robinson et al. 2007). Related environmental properties that impose different stressors should both be considered when determining how predator effects vary across environmental gradients.
References
Bates D, Machler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bertness MD, Trussell GC, Ewanchuk PJ, Silliman BR (2002) Do alternate stable community states exist in the Gulf of Maine rocky intertidal zone? Ecology 83:3434–3448. https://doi.org/10.2307/3072092
Bisker R, Castagna M (1987) Predation on single spat oysters Crassostrea virginica by blue crabs Callinectes sapidus and mud crabs Panopeus herbstii. J Shellfish Res 6:37–40
Burkholder DA, Heithaus MR, Fourqurean JW, Wirsing A, Dill LM (2013) Patterns of top-down control in a seagrass ecosystem: could a roving apex predator induce a behaviour-mediated trophic cascade? J Anim Ecol 82:1192–1202. https://doi.org/10.1111/1365-2656.12097
Byers JE, Smith RS, Weiskel HW, Robertson CY (2014) A non-native prey mediates the effects of a shared predator on an ecosystem service. PLoS ONE 9:e93969. https://doi.org/10.1371/journal.pone.0093969
Casas J, Dangles O (2010) Physical ecology of fluid flow sensing in arthropods. Annu Rev Entomol 55:505–520. https://doi.org/10.1146/annurev-ento-112408-085342
Cherry MJ, Barton BT (2017) Effects of wind on predator-prey interactions. Food Webs 13:92–97. https://doi.org/10.1016/j.fooweb.2017.02.005
Chivers DP, Mirza RS, Bryer PJ, Kiesecker JM (2001) Threat-sensitive predator avoidance by slimy sculpins: understanding the importance of visual versus chemical information. Can J Zool 79:867–873. https://doi.org/10.1139/cjz-79-5-867
Creel S, Winnie J, Maxwell B, Hamlin K, Creel M (2005) Elk alter habitat selection as an antipredator response to wolves. Ecology 86:3387–3397. https://doi.org/10.1890/05-0032
Denny MW (1988) Biology and mechanics of wave-swept environment. Princeton University Press, Princeton
Finelli CM, Pentcheff ND, Zimmer RK, Wethey DS (2000) Physical constraints on ecological processes: a field test of odor-mediated foraging. Ecology 81:784–797. https://doi.org/10.1890/0012-9658(2000)081[0784:pcoepa]2.0.co;2
Grabowski JH, Hughes AR, Kimbro DL (2008) Habitat complexity influences cascading effects of multiple predators. Ecology 89:3413–3422. https://doi.org/10.1890/07-1057.1
Hartman EJ, Abrahams MV (2000) Sensory compensation and the detection of predators: the interaction between chemical and visual information. Proc Biol Sci 267:571–575. https://doi.org/10.1098/rspb.2000.1039
Hill JM, Weissburg MJ (2013a) Habitat complexity and predator size mediate interactions between intraguild blue crab predators and mud crab prey in oyster reefs. Mar Ecol Prog Ser 488:209–219. https://doi.org/10.3354/meps10386
Hill JM, Weissburg MJ (2013b) Predator biomass determines the magnitude of non-consumptive effects (NCEs) in both laboratory and field environments. Oecologia 172:79–91. https://doi.org/10.1007/s00442-012-2488-4
Hollebone AL, Hay ME (2007) Population dynamics of the non-native crab Petrolisthes armatus invading the South Atlantic Bight at densities of thousands m(-2). Mar Ecol Prog Ser 336:211–223. https://doi.org/10.3354/meps336211
Hughes AR, Mann DA, Kimbro DL (2014) Predatory fish sounds can alter crab foraging behaviour and influence bivalve abundance. Proc Biol Sci 281:20140715. https://doi.org/10.1098/rspb.2014.0715
Jackson JL, Webster DR, Rahman S, Weissburg MJ (2007) Bed-roughness effects on boundary-layer turbulence and consequences for odor-tracking behavior of blue crabs (Callinectes sapidus). Limnol Oceanogr 52:1883–1897. https://doi.org/10.4319/lo.2007.52.5.1883
Jacobs DS, Ratcliffe JM, Fullard JH (2008) Beware of bats, beware of birds: the auditory responses of eared moths to bat and bird predation. Behav Ecol 19:1333–1342. https://doi.org/10.1093/beheco/arn071
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997. https://doi.org/10.2307/2533558
Koehl MAR (2006) The fluid mechanics of arthropod sniffing in turbulent odor plumes. Chem Senses 31:93–105. https://doi.org/10.1093/chemse/bjj009
Kuznetsova A, Brockhoff PB, Christensen RHB (2016). lmerTest: tests in linear mixed models. R package version 2.0–32. http://CRAN.R-project.org/package=lmerTest
Large SI, Smee DL, Trussell GC (2011) Environmental conditions influence the frequency of prey responses to predation risk. Mar Ecol Prog Ser 422:41–49. https://doi.org/10.3354/meps08930
Laundré JW, Hernández L, Altendorf KB (2001) Wolves, elk, and bison: reestablishing the “landscape of fear” in Yellowstone National Park, USA. Can J Zool 79:1401–1409. https://doi.org/10.1139/cjz-79-8-1401
Lee SY, Kneib RT (1994) Effects of biogenic structure on prey consumption by the xanthid crabs Eurytium limosum and Panopeus herbstii in salt marsh. Mar Ecol Prog Ser 104:39–47. https://doi.org/10.3354/meps104039
Leonard GH, Levine JM, Schmidt PR, Bertness MD (1998) Flow-driven variation in intertidal community structure in a Maine estuary. Ecology 79:1395–1411
Lubchenco J (1978) Plant species diversity in a marine intertidal community: importance of herbivore food preference and algal competitive abilities. Am Nat 112:23–39. https://doi.org/10.1086/283250
Matassa CM, Trussell GC (2011) Landscape of fear influences the relative importance of consumptive and nonconsumptive predator effects. Ecology 92:2258–2266
Menge BA (1978) Predation intensity in a rocky intertidal community: relation between predator foraging activity and environmental harshness. Oecologia 34:1–16. https://doi.org/10.1007/bf00346237
Menge BA, Olson AM (1990) Role of scale and environmental factors in regulation of community structure. Trends Ecol Evol 5:52–57. https://doi.org/10.1016/0169-5347(90)90048-i
Menge BA, Sutherland JP (1987) Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am Nat 130:730–757. https://doi.org/10.1086/284741
Micheli F (1997) Effects of predator foraging behavior on patterns of prey mortality in marine soft bottoms. Ecol Monogr 67:203–224
Morgan SG, Gravem SA, Lipus AC, Grabiel M, Miner BG (2016) Trait-mediated indirect interactions among residents of rocky shore tidepools. Mar Ecol Prog Ser 552:31–46. https://doi.org/10.3354/meps11766
Munoz NE, Blumstein DT (2012) Multisensory perception in uncertain environments. Behav Ecol 23:457–462. https://doi.org/10.1093/beheco/arr220
Paine RT (1966) Food web complexity and species diversity. Am Nat 100:65–75. https://doi.org/10.1086/282400
Pangle KL, Malinich TD, Bunnell DB, DeVries DR, Ludsin SA (2012) Context-dependent planktivory: interacting effects of turbidity and predation risk on adaptive foraging. Ecosphere 3:114. https://doi.org/10.1890/es12-00224.1
Peckarsky BL, Abrams PA, Bolnick DI, Dill LM, Grabowski JH, Luttbeg B, Orrock JL, Peacor SD, Preisser EL, Schmitz OJ, Trussell GC (2008) Revisiting the classics: considering nonconsumptive effects in textbook examples of predator-prey interactions. Ecology 89:2416–2425. https://doi.org/10.1890/07-1131.1
Powers SP, Kittinger JN (2002) Hydrodynamic mediation of predator-prey interactions: differential patterns of prey susceptibility and predator success explained by variation in water flow. J Exp Mar Biol Ecol 273:171–187. https://doi.org/10.1016/s0022-0981(02)00162-4
Preisser EL, Bolnick DI, Benard MF (2005) Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86:501–509. https://doi.org/10.1890/04-0719
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, Berger J, Elmhagen B, Letnic M, Nelson MP, Schmitz OJ, Smith DW, Wallach AD, Wirsing AJ (2014) Status and ecological effects of the world’s largest carnivores. Science 343:1241484. https://doi.org/10.1126/science.1241484
Robinson HE, Finelli CM, Buskey EJ (2007) The turbulent life of copepods: effects of water flow over a coral reef on their ability to detect and evade predators. Mar Ecol Prog Ser 349:171–181. https://doi.org/10.3354/meps07123
Robinson EM, Smee DL, Trussell GC (2011) Green crab (Carcinus maenas) foraging efficiency reduced by fast flows. PLoS ONE 6:e21025. https://doi.org/10.1371/journal.pone.0021025
Schmitz OJ, Krivan V, Ovadia O (2004) Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol Lett 7:153–163. https://doi.org/10.1111/j.1461-0248.2003.00560.x
Shears NT, Babcock RC, Salomon AK (2008) Context-dependent effects of fishing: variation in trophic cascades across environmental gradients. Ecol Appl 18:1860–1873. https://doi.org/10.1890/07-1776.1
Silliman BR, Layman CA, Geyer K, Zieman JC (2004) Predation by the black-clawed mud crab, Panopeus herbstii, in Mid-Atlantic salt marshes: further evidence for top–down control of marsh grass production. Estuaries 27:188–196. https://doi.org/10.1007/bf02803375
Smee DL, Weissburg MJ (2006) Clamming up: environmental forces diminish the perceptive ability of bivalve prey. Ecology 87:1587–1598. https://doi.org/10.1890/0012-9658(2006)87[1587:cuefdt]2.0.co;2
Smee DL, Ferner MC, Weissburg MJ (2008) Alteration of sensory abilities regulates the spatial scale of nonlethal predator effects. Oecologia 156:399–409. https://doi.org/10.1007/s00442-008-0995-0
Suraci JP, Clinchy M, Dill LM, Roberts D, Zanette LY (2016) Fear of large carnivores causes a trophic cascade. Nat Commun 7:10698. https://doi.org/10.1038/ncomms10698
Toscano BJ, Griffen BD (2012) Predatory crab size diversity and bivalve consumption in oyster reefs. Mar Ecol Prog Ser 445:65–74. https://doi.org/10.3354/meps09461
Turner AM, Montgomery SL (2003) Spatial and temporal scales of predator avoidance: experiments with fish and snails. Ecology 84:616–622. https://doi.org/10.1890/0012-9658(2003)084[0616:satsop]2.0.co;2
Van de Meutter F, De Meester L, Stoks R (2005) Water turbidity affects predator-prey interactions in a fish-damselfly system. Oecologia 144:327–336. https://doi.org/10.1007/s00442-005-0050-3
Webster DR, Weissburg MJ (2009) The hydrodynamics of chemical cues among aquatic organisms. Annu Rev Fluid Mech 41:73–90. https://doi.org/10.1146/annurev.fluid.010908.165240
Weissburg M, Beauvais J (2015) The smell of success: the amount of prey consumed by predators determines the strength and range of cascading non-consumptive effects. Peerj 3:e1426. https://doi.org/10.7717/peerj.1426
Weissburg MJ, Zimmer-Faust RK (1993) Life and death in moving fluids: hydrodynamic effects on chemosensory-mediated predation. Ecology 74:1428–1443. https://doi.org/10.2307/1940072
Weissburg MJ, Zimmer-Faust RK (1994) Odor plumes and how blue crabs use them in finding prey. J Exp Biol 197:349–375
Weissburg MJ, James CP, Smee DL, Webster DR (2003) Fluid mechanics produces conflicting constraints during olfactory navigation of blue crabs, Callinectes sapidus. J Exp Biol 206:171–180. https://doi.org/10.1242/jeb.00055
Weissburg M, Smee DL, Ferner MC (2014) The sensory ecology of nonconsumptive predator effects. Am Nat 184:141–157. https://doi.org/10.1086/676644
Weissburg M, Poulin RX, Kubanek J (2016) You are what you eat: a metabolomics approach to understanding prey responses to diet-dependent chemical cues released by predators. J Chem Ecol 42:1037–1046. https://doi.org/10.1007/s10886-016-0771-2
Werner EE, Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84:1083–1100. https://doi.org/10.1890/0012-9658(2003)084[1083:arotii]2.0.co;2
Wilson ML (2011) Sensory landscape impacts on odor-mediated predator-prey interactions at multiple spatial scales in salt marsh communities. PhD dissertation, School of Biology, Georgia Institute of Technology, Atlanta, Georgia, USA
Wilson ML, Webster DR, Weissburg MJ (2013) Spatial and temporal variation in the hydrodynamic landscape in intertidal salt marsh systems. Limnol Oceanogr 3:156–172
Acknowledgements
The authors would like to thank Jeff Beauvais, Alex Draper, and Holly Nichols for field assistance. We also appreciate the help and support of the staff at the Skidaway Institute of Oceanography. This work was funded by NSF grant Bio-OCE #1234449 awarded to MJW.
Author contribution statement
JLP and MJW conceived, designed, and performed the experiments. JLP analyzed the data. JLP and MJW wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Pablo Munguia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pruett, J.L., Weissburg, M.J. Hydrodynamics affect predator controls through physical and sensory stressors. Oecologia 186, 1079–1089 (2018). https://doi.org/10.1007/s00442-018-4092-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4092-8