Abstract
Trade-offs among plant functional traits indicate diversity in plant strategies of growth and survival. The leaf economics spectrum (LES) reflects a trade-off between short-term carbon gain and long-term leaf persistence. A related trade-off, between foliar growth and anti-herbivore defense, occurs among plants growing in contrasting resource regimes, but it is unclear whether this trade-off is maintained within plant communities, where resource gradients are minimized. The LES and the growth-defense trade-off involve related traits, but the extent to which these trade-off dimensions are correlated is poorly understood. We assessed the relationship between leaf economic and anti-herbivore defense traits among sunlit foliage of 345 canopy trees in 83 species on Barro Colorado Island, Panama. We quantified ten traits related to resource allocation and defense, and identified patterns of trait co-variation using multivariate ordination. We tested whether traits and ordination axes were correlated with patterns of phylogenetic relatedness, juvenile demographic trade-offs, or topo-edaphic variation. Two independent axes described ~ 60% of the variation among canopy trees. Axis 1 revealed a trade-off between leaf nutritional and structural investment, consistent with the LES. Physical defense traits were largely oriented along this axis. Axis 2 revealed a trade-off between investments in phenolic defenses versus other foliar defenses, which we term the leaf defense spectrum. Phylogenetic relationships and topo-edaphic variation largely did not explain trait co-variation. Our results suggest that some trade-offs among the growth and defense traits of outer-canopy trees may be captured by the LES, while others may occur along additional resource allocation dimensions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A resource-based theory of allocation hypothesizes that plants must make trade-offs in the allocation of finite internal resources to competing plant functions such as growth, survival, and reproduction (Bloom et al. 1985; Bazzaz et al. 1987). Trade-offs occur at many levels, from physiological trade-offs within plant organs to demographic trade-offs within and between ecological communities (Reich et al. 2003; Shipley et al. 2006; Adler et al. 2014). Different optimizations, or strategies, along trade-off axes may contribute to the functional and biological diversity within ecological communities, but the clearest evidence for resource-mediated trade-offs is often found in comparisons between plant communities at contrasting endpoints of a resource gradient (Westoby and Wright 2006; Fine et al. 2006; Wright et al. 2010). For example, a trade-off between traits related to rapid plant growth and defense against herbivory is observed between tropical plant communities occupying high- and low-resource environments (Kursar and Coley 2003; Fine et al. 2006). This trade-off may be related to a more fundamental trade-off observed among plants, in the allocation of carbon and foliar nutrients towards key leaf functions, known as the leaf economics spectrum (LES; Wright et al. 2004). Along the LES, species with ‘acquisitive’ strategies allocate carbon resources sparingly towards the rapid construction of nutrient-rich, metabolically active leaves that turnover quickly. In contrast, ‘conservative’ species allocate more carbon resources towards the durable construction of individual leaves, betting on long-term returns-on-investment from leaves which are well-defended against threats (both abiotic and biotic) to their survival (Wright et al. 2004; Shipley et al. 2006). Interspecific variation along the LES is associated with life-history variation and plant performance among rainforest trees in gaps and understory environments (Poorter and Bongers 2006; Wright et al. 2010). However, less is understood about how the LES or other trade-off axes are represented within plant communities, where resource gradients may be minimized.
Within plant communities, trade-off patterns may be more complex than a simple one-dimensional relationship. In the absence of a single strong resource constraint, such as light, water, or key macronutrients, multiple limiting resources may contribute dynamically to species co-existence, and allocation trade-offs may change with plant ontogeny (Tilman 2004; Silvertown 2004; Kitajima and Poorter 2008). Moreover, if one or several potentially limiting resources exist in surplus within a community, allocation towards multiple plant functions may be possible without trade-offs (e.g., Dominy et al. 2003). This latter point has rarely been addressed, as few plant communities are conceivably “free” (in a relative sense) of resource constraints. One exception may be the community of adult tropical canopy trees that comprise the outermost surface of lowland tropical rainforests, which owing to their size, position, and location, are arguably among most resource-rich terrestrial plants on the planet.
Canopy trees represent an important, but poorly understood, component of tropical rainforest biodiversity. As the largest organisms in the forest, canopy, and emergent trees have a disproportionate influence on forest productivity, carbon storage, and nutrient cycling, as well as providing the physical architecture and much of the base of the trophic pyramid in tropical forests (Basset et al. 1992; Clark and Clark 1992, 1996). The foliage of canopy trees differs from that of understory trees (including both species which complete their lifecycle in the understory and juvenile canopy trees) in several distinctive ways: fully sunlit canopy foliage tends to be thicker, tougher, and has a higher light saturation point and higher leaf mass per unit area (LMA) than shaded understory foliage (Givnish 1988; Dominy et al. 2003; Kitajima and Poorter 2008). Yet, tropical canopy tree communities are biologically and functionally diverse, suggesting that a wide range of ecological strategies may be viable among trees that have attained the canopy (Clark and Clark 1992; Condit 2000; Asner et al. 2014). However, little is known about patterns of variation within adult canopy communities, including whether trade-offs among plant functions, or functional traits, occur among co-existing canopy trees (but see Clark and Clark 1992; Kitajima et al. 2005; Asner and Martin 2011).
If carbon limitation is not the constraining paradigm of the adult canopy tree community, axes of functional trait variation among canopy foliage may reflect trade-offs beyond those related to carbon uptake and investment. Leaf investment strategies may respond to other limiting resources, such as soil nutrients that may be unevenly distributed across landscapes, or modifications of the costs and benefits of a given investment strategy imposed by fitness losses due to herbivory (Coley et al. 1985; Clark et al. 1999; Baltzer and Thomas 2010). Variation in plant responses to foliar herbivores (e.g., to tolerate or defend against herbivory) has largely been considered as an extension of resource allocation theory, suggesting that the optimal level of foliar defense is linked to the ideal rate of leaf turnover: where it is advantageous for leaves to be long-lived, they should also be well-defended against herbivores (Coley 1988; Fine et al. 2006; Mason and Donovan 2015). If this is true, then plant defense traits should be largely correlated with LES traits. Alternatively, multiple limiting resources affecting plant growth may contribute to variation over several key dimensions, with traits varying over multiple axes corresponding to the resources to which they relate (Bloom et al. 1985; Tilman 2004; Ackerly 2004). Thus, if soil resources or even ‘avoided herbivory’ function as additional constraints on resource allocation for canopy trees, interspecific variation in foliar traits may be organized over multiple trade-off axes, and a single dimension alone may fail to account for the majority of functional variation within a canopy community. Jointly investigating patterns among leaf economic and anti-herbivore defense traits among canopy trees may, therefore, provide insight into constraints on tropical tree functional diversity, beyond the primary dimension of light availability.
Here, we assess the relationship between leaf economic and anti-herbivore defense traits within a diverse community of tropical canopy trees at Barro Colorado Island (BCI), Panama. We focus our analysis on traits with well-established relationships with the LES, indicating variation among foliar strategies of carbon uptake and investment, as well as a limited number of individual anti-herbivore defense traits that, albeit an incomplete characterization of plant defense, represent a range of characteristics relevant to the overall costs and benefits of defense (Feeny 1976; Koricheva 2002; Wright et al. 2004; Agrawal and Fishbein 2006). We ask whether trade-offs among foliar resource allocation strategies and anti-herbivore defense occur among ten foliar traits for a phylogenetically diverse group of 345 upper canopy trees in 83 species, which span the topo-edaphic conditions present at BCI. We then explore several plausible mechanisms that may contribute to the generation of patterns observed, including (1) phylogenetic dependency of trait variation among closely related species, (2) ontogenetic links between current patterns of functional trait co-variation and juvenile demographic relationships, and (3) the influence of topo-edaphic variation, which may contribute to spatial variation in resource availability across BCI.
Materials and methods
Site description and sampling design
Field sampling was conducted in January 2013 at BCI (9°9′N, 79°51′W; Fig. 1). The island has an area of roughly 15 km2 with a maximum elevation of 137 m above Lake Gatun. BCI is classified as tropical moist forest (Holdridge 1947) with mean annual temperature of 26.9 °C, mean annual precipitation of 2551 mm, and a dry season lasting from December into April (Leigh 1999). Soils on BCI vary with underlying geology and topology, but are primarily brown fine loams and clays with high concentrations of many key nutrients (e.g., N, P, Ca, and S) and high cation-exchange capacity (Yavitt and Wieder 1988; Yavitt 2000; Baillie et al. 2007; Messmer et al. 2014).
Canopy tree locations overlaid on topo-edaphic gradients and forest age map of Barro Colorado Island, Panama (BCI). Centerpoints of 345 canopy tree crowns included in study are shown as black circles, overlaid upon maps of a slope, b soil wetness index, and c elevation. BCI is located in Gatun Lake, on the Isthmus of Panama, as shown in inset
We sampled 345 outer-canopy trees randomly distributed along transects running west to east across the island (Fig. 1). Outer-canopy trees, defined here as mature individuals whose crowns have unobstructed exposure to the sky, included both large canopy and emergent trees and were carefully selected following the procedure of Asner et al. (2011). Individuals are distributed across 83 species, 65 genera, and 35 families. Our sampling included 60 of the 87 most common species of canopy and emergent trees from 30 out of 34 families, as determined in a comprehensive study by Condit et al. (1996), and spans the geological conditions and forest age classes on BCI. Leaf chemistry and LMA are sensitive to vertical light gradients within forests (e.g., Poorter et al. 2009), thus only fully sunlit, fully expanded, top-of-canopy foliage was collected to avoid combining sun and shade leaves, which could obscure meaningful chemical and trait distinctions both within and among species (Asner and Martin 2011). For each individual, 2–3 intact branches and an additional 25–50 full leaves were harvested and immediately stored on ice. Trait measurements were made on fresh or flash-frozen/lyophilized leaf tissue, and all processing/stabilization was conducted within ~ 6 h of collection. Visual examination of leaf voucher specimens informed outlier removal for individual trees, where foliar samples were clearly in the juvenile or senescent stages. Three species containing high proportions of very young foliage were omitted from the analysis (Jacaranda copaia, Trattinickia aspera, and Platypodium elegans). The final data set comprised 330 individuals across 80 species, 65 genera, and 35 families.
Determination of foliar functional traits
We measured ten leaf traits with well-established relationships with the LES/foliar carbon investment strategies and/or anti-herbivore defense for individual trees (Table 1). Leaf nitrogen (N; % dry weight), leaf phosphorus (P; % dry weight), and leaf water content (LWC; % total weight) are foliar nutrients which are found in high concentrations in species with acquisitive LES strategies (Wright et al. 2004). Leaf mass per unit area (LMA; g m−2), has a positive relationship with leaf lifespan, and high LMA indicates a high carbon investment, consistent with a conservative LES strategy (Westoby et al. 2002). Leaf density (LD; mg mm−3) and leaf toughness (LT; kN m−1) contribute to leaf longevity by protecting against abiotic hazards such as high wind and falling debris, as well as against herbivory by reducing the palatability or nutrition of foliar tissue (Kitajima et al. 2012). Leaf latex (presence/absence) constitutes a physical defense against herbivory, and also commonly contains a variety of bioactive or toxic compounds, including terpenoids, phenolics, proteins, and alkaloids (Konno 2011). Cyanogenic glycosides (CG, presence/absence), are a toxic, highly potent defense against herbivores (Lechtenberg and Nahrstedt 1999).
We included three measures of phenolic compounds in foliar tissue. The total concentration of phenolic compounds (TP; mg g−1 dry weight) is a frequently reported metric of chemical defense investment. Phenolics are phenol-containing plant secondary metabolites, with a range of anti-herbivore and antibiotic defense functions, as well as protective functions against abiotic stress such as UV radiation (Appel 1993; Mazza et al. 2000). Condensed tannins (CT; mg g−1 dry weight) are a subset of TP with anti-digestive or anti-nutritive properties, primarily through their ability to irreversibly bind with ingested or endogenous proteins (Ayres et al. 1997). However, many folivorous insects may be largely immune to the effects of CT, as the protein-precipitating activity occurs only under acidic to neutral conditions, and the digestive environments of most insects are alkaline (Bi and Felton 1995; Barbehenn and Constabel 2011). In contrast, insects may be much more vulnerable to the defensive activity of another category of TP, the hydrolysable tannins (HT; mg g−1 dry weight). HT have a dose-dependent effect on oxidative stress in the digestive environments of many insect herbivores, which stems from their capacity to propagate free radicals (Ayres et al. 1997; Salminen and Karonen 2011).
Leaf N, leaf P, LWC, and LMA were measured according to the Carnegie Spectranomics Project protocols (http://spectranomics.stanford.edu) and are briefly summarized here. Total N and P were determined from oven-dried, powdered leaf tissue. Total N was determined by combustion–reduction elemental analysis on a Costech CHN Analyzer (Costech Analytical Technologies). Elemental P was determined by inductively coupled plasma optical emission spectroscopy on an IRIS/AP Plasma Spectrometer (Thermo Jarrell Ash). LWC was calculated as the percent mass loss of fresh leaves after oven-drying for 72 h. LMA was determined via measurements of leaf area and dry mass on leaves (petioles and large midribs removed, leaves cut when necessary) that filled a scan area of 21 × 25 cm on a 600 dpi flatbed scanner, which were weighed immediately after scanning, and again after 72 h of oven-drying. To obtain LD, we measured leaf thickness for three leaves per individual as the laminar thickness between secondary veins adjacent to the mid-rib, two-thirds of the length from leaf tip to leaf base. LD was calculated as the quotient of LMA divided by leaf thickness. LT was measured concurrently as the critical mass required to puncture the laminar tissue of the fresh leaf using a penetrometer. LT and LD were collected for 184 out of 345 individuals, due to limitations on the number of measurements that could be made on fresh leaves—priority was given to obtaining a minimum of three individuals of each species rather than to intensively sampling the most common species at the expense of under sampling of rare species.
We measured the concentration of CG for all individuals via the colorimetric determination of cyanide (CN), following the method of Gleadow et al. (2011). CN is liberated following the hydrolysis of the glycoside and is trapped in a well containing 1 M NaOH. Freeze-dried, ground plant tissue (10–20 mg) was incubated for 12–15 h at 37 °C in with 1 mL of 0.1 M citrate–HCl (pH 5.5) containing β-glucosidase from almonds in excess (Sigma G0395), within a sealed vial containing the inner NaOH well. The addition of exogenous β-glucosidase was taken as a precautionary step as the freeze-drying process may de-activate endogenous glucosidase. The concentration of NaCN trapped in NaOH was measured using König color reactions (Lambert et al. 1975), and the absorbance at 595 nm was read on an Infinite M1000 Pro microplate reader (Tecan, Austria) with NaCN as the standard. This method can detect concentrations as low as 5 µg L−1 and is relatively specific for CN (Gleadow et al. 2011).
We quantified total phenolics (TP) via the Folin–Ciocalteu (F–C) assay using freeze-dried leaf tissue and following the protocol of Ainsworth and Gillespie (2007). The F–C assay is colorimetric, based on the transfer of electrons from phenolic compounds to the F–C reagent (a mixture of sodium molybdate, sodium tungstate, and other reagents), resulting in the formation of blue complexes which can be quantified spectroscopically. Absorbance at 765 nm is linearly related to TP concentration using gallic acid as the standard. This method is not strictly specific to phenolic compounds, as the F–C reagent will react with other oxidative substrates, but provides a robust quantitative estimate of total phenols, as they comprise the vast majority of oxidative substrates in plant extracts (Huang et al. 2005; Ainsworth and Gillespie 2007; Everette et al. 2010). Importantly, concentration is reported in ‘gallic acid equivalents’ (GAE), and may equivalently be interpreted as the total antioxidant potential of the sample, as structural variation among specific polyphenolic compounds may influence their reactivity (Appel 1993). However, from an herbivore perspective, this functional definition may be more defensible, as it is this molecular activity that confers defense against a given herbivore (Barbehenn and Constabel 2011).
Condensed tannins (CT) and hydrolysable tannins (HT) were quantified as the fractions of TP to bind with protein or oxidize under alkaline conditions, respectively. These derivations of CT and HT are also based on their functional properties, as all substrates which precipitate protein under acidic conditions or auto-oxidize under alkaline conditions and react with the F–C reagent would be included in this method of quantification. However, the large majority of these will be phenolics, and furthermore, the qualities being measured correspond with the molecular mechanism of defense conferred by CT and HT, respectively (Salminen and Karonen 2011). CT confer defense by irreversibly binding with ingested proteins in the digestive tracts of mammals and some orders of insects (e.g., Coleoptera) with acidic to neutral midgut environments, rendering such proteins indigestible (Hagerman 1992; Barbehenn and Constabel 2011). However, CT are ineffective at precipitating protein under alkaline conditions. Conversely, at pH > ~ 9, HT are known to take radical forms and to propagate harmful reactive oxygen species (ROS), but may serve as beneficial antioxidants at neutral pHs (Salminen and Karonen 2011; Barbehenn and Constabel 2011). The supplement contains further details on the molecular activities of TP, CT, and HT.
The quantification of CT utilized a modification of the Folin–Ciocalteu (F–C) assay to first allow for the precipitation of protein–CT complexes using polyvinylpolypropylene (Toth and Pavia 2001; Makkar et al. 2007). The concentration of CT is given as the difference in concentration of TP, quantified using the unmodified F–C assay, described previously, and the concentration of TP detected from the modified F–C assay after the protein precipitation step. HT were also measured using a modification of the F–C assay to quantify the oxidative capacity of phenols (Salminen and Karonen 2011). This procedure works by exploiting the behavior of a defining chemical constituent of HT, o-dihydroxy polyphenols, which are irreversibly oxidized in an alkaline buffer solution (pH 10) to yield o-quinones (Quideau et al. 1995; Feldman et al. 1999; Chen and Hagerman 2005). Similar to the quantification of CT, the concentration of HT is determined from the difference between TP measured for oxidized and non-oxidized replicates for each sample (Salminen and Karonen 2011). As fractions of TP, both CT and HT concentrations are reported in GAE. We binned the HT data into intervals of 3 mg g−1 as a pre-processing measure to reduce the effects of minor observation errors associated with the assay, as internal standards showed a standard error of approximately 10%, likely due to the chemical complexity of phenolic compounds and other oxidative substrates in the samples (Everette et al. 2010). Values within the bin range were replaced with the central value of each bin, resulting in 27 bins ranging from 0 to 79 mg g−1. All traits were measured at the individual scale, with the exception of leaf latex, which was assessed qualitatively for species, and confirmed with data from the literature (Croat 1978; Condit et al. 2011).
Trait distributions were examined for normality across all individuals and within species. Outlier detection and removal was performed using modified Z-scores (Iglewicz and Hoaglin 1993). Data were aggregated at the species level for some analyses using species mean trait values. The number of individuals per species utilized for species-level means varied from 1 to 22, with a mean of four individuals per species. A high ratio of interspecific-to-intraspecific variation across all traits permitted the use of species with low representation in this data set (consistent with their representation in the BCI tropical canopy tree community; Table 1). Both individual and species-level variables were transformed where necessary to improve normality: N, P, LMA, and LT were log-transformed and LD, CT, and HT were square-root transformed within the individual-level data set; N, P, and LT were log-transformed and TP and HT were square-root transformed in the species-level data set. For multivariate trait analyses, continuous trait data were standardized to have a mean of zero and unit variance.
Interspecific patterns of trait variation and co-variation
To assess whether traits varied more between species than within species, we performed one-way analyses of variance (ANOVA) at the species level for all traits, using data for all individuals. We also calculated the ratio of the coefficient-of-variation (CV) between species to the mean CV within species for all species for which we collected at least three individuals, which provides an estimate of the relative importance of interspecific-to-intraspecific variation. To determine the interdependency of traits, we calculated pairwise correlation among traits at the species and individual levels, as well as among phylogenetically independent contrasts (PICs, discussed below). The significance of pairwise correlations was corrected for multiple hypotheses testing using the Bonferroni adjustment method (Legendre and Legendre 1998).
We utilized two multidimensional scaling methods, principal component analysis (PCA) and principal coordinate analysis (PCoA), to examine multivariate patterns of trait variation at two levels: among all individuals and using species mean values. PCA is based on a q × q matrix of associations between q variables and requires complete, continuous data for all traits. PCoA is based on an n × n matrix of associations between n subjects and allows for a more flexible analysis including missing and categorical data (Gower 1966). The PCA included 56 species and 185 individuals with complete data for eight continuously distributed foliar traits (N, P, LWC, LMA, LD, LT, CT, and HT). In addition, the PCoA included two additional binary traits, CG and latex (the latter at the species level only), and 80 species and 331 individuals with missing data for some traits. PCoA does not produce trait ‘loadings’ as in PCA; however, linear correlations between scores along the PCoA axes and original variable values can be used as a measure of each variable’s contribution to a given PCoA axis (Legendre and Legendre 1998). TP was excluded from all ordination analyses, because it was used to calculate both CT and HT.
Phylogenetic influence on trait variation
Shared evolutionary histories among co-existing canopy trees may contribute to patterns of trait variation among species. To understand the extent to which patterns of trait variation and co-variation could be attributed to the phylogenetic relationships among canopy trees, we quantified phylogenetic signal for all traits and axes of trait co-variation and used phylogenetically corrected means of assessing trait co-variation. We utilized a molecular phylogeny, derived from the DNA-barcode community phylogeny established by Kress et al. (2009) for 281 species of woody trees and shrubs within the 50-ha Forest Dynamics Plot on BCI (Supplementary Fig. 1). The Kress phylogeny is a maximum likelihood phylogenetic tree that was reconstructed as a supermatrix tree from a multilocus DNA-barcode library using three markers (rbcL, matK, and trnH-psbA; detailed method in Kress et al. 2009). This barcode phylogeny has been used for numerous BCI studies (e.g., Wright et al. 2010, Westbrook et al. 2011). Three species (Enterolobium cyclocarpum, Vatairea erythrocarpa, and Zanthoxylum panamense) were not present in the Kress phylogeny and thus were omitted from all phylogenetic analyses.
Phylogenetic signal (PS), the tendency of related species to resemble one another, was quantified using Blomberg’s K statistic for continuously distributed traits and PCA and PCoA ordination axes (Blomberg et al. 2003). A K value of zero indicates no phylogenetic signal and a high value of K (approaching or greater than one) indicates increasing phylogenetic conservatism of trait values (Blomberg et al. 2003; Losos 2008; Crisp and Cook 2012). We followed the tip randomization procedure of Blomberg et al. (2003) to test for significance against a null hypothesis of no phylogenetic signal (K = 0) and used a parametric bootstrapping approach to test the null hypothesis of perfectly Brownian motion evolution (K = 1). For two binary traits (CG and latex), we calculated the D statistic, which is analogous to K for binary data. D ranges from − 1 (strong phylogenetic conservatism) to 1 (no phylogenetic signal). We tested D for significance against null models of no phylogenetic signal (D = 1) and Brownian motion evolution (D = 0; Fritz and Purvis 2010).
We tested for phylogenetic structure in patterns of trait co-variation, utilizing pairwise correlations of phylogenetically independent contrasts (PICs) and phylogenetic PCA (Felsenstein 1985; Revell 2009). PICs were calculated for each trait using standardized trait data and log-transformed branch lengths, which better standardized the PICs than raw molecular branch lengths from the Kress phylogeny (Garland et al. 1992). As the calculation of PICs requires a fully bifurcating tree, 11 polytomies were represented by internal branches of zero length (Diaz-Uriarte and Garland 1999). We then calculated pairwise correlations of PICs, which were forced through the origin, following Garland et al. (1992). Phylogenetic PCA was performed to evaluate the extent to which multivariate trait patterns observed among species were driven by shared phylogenetic histories (Revell 2009). Phylogenetic PC axes and scores were calculated from an evolutionary correlation matrix of traits assuming a Brownian motion model of evolution (Revell 2009). Resulting phylogenetic PCA axes are evolutionarily independent—that is, they reflect the residual variation among traits, once phylogenetic co-variation has been accounted for (Polly et al. 2013). Although phylogenetic PCA is constructed to find orientation axes that are independent of species’ shared evolutionary histories, the positions of species along these axes still may reflect their phylogenetic non-independence (Revell 2009); therefore, we calculated phylogenetic signal for phylogenetic PC axes in addition to the conventional PC axes.
Juvenile demographic niches
Interspecific variation in LES strategies among adult canopy trees may be concordant with differences in their light requirements as seedlings and saplings, as fitness may be most tightly linked to environmental variation during the regeneration phase (Grubb 1977; Stearns 1992; Poorter 2007). In a study of the demographic trade-offs of tropical trees on BCI, Wright et al. (2010) found evidence for a strong trade-off between growth rates of the fastest-growing saplings (95th percentile relative growth rates for census interval; RGR95) and mortality rates of the slowest growing saplings (the 25% of individuals with the smallest RGR in the census interval; M25; r2 = 0.69), suggesting that light availability may exert a performance filter on regenerating species with contrasting carbon investment strategies. We utilized species positions along the RGR95—M25 trade-off axis as a continuous measure of juvenile demographic niche variation for 28 species which were also included in our canopy tree data set. To assess whether interspecific patterns of foliar functional trait co-variation among adult canopy trees tracked patterns of demographic variation among conspecific juveniles, we measured the correlation between species rankings along the RGR95—M25 trade-off axis and along the first two ordination axes from PCA and PCoA.
Topo-edaphic variables
BCI has highly fertile soils (Vitousek 1984) with soil macronutrients relatively evenly distributed across the landscape (Leigh 1999; Yavitt 2000). P and Ca availabilities—both key constraints on tropical forest productivity (Vitousek and Sanford 1986)—are much higher here than in most tropical soils (Vitousek 1984; Yavitt and Wieder 1988). However, topographic features may contribute to spatially arranged variation in the distribution of abiotic resources, such as soil moisture, light, and nutrient availability, and thus may influence the functional traits of vegetation occupying different positions along topo-edaphic gradients. We evaluated the relationship of five topo-edaphic variables (slope, elevation, soil wetness, eastness, and northness) with individual foliar traits and trait trade-off axes.
Spatially explicit topographic data were obtained from the Smithsonian Tropical Research Institute (STRI) GIS Portal (http://mapserver.stri.si.edu/). Topographic information was obtained from the 5-m resolution digital elevation model of BCI based on a 1:25,000 topographic map of the island (Kinner et al. 2002). Slope, aspect, and soil wetness index (SWI) were calculated in ArcGIS v.9.3. SWI was calculated for each pixel i as the natural log of the ratio between the upslope area of that pixel (A i ) to the tangent of the local slope angle in radians (α i ) (Beven and Kirkby 1979). Aspect was further derived as northness and eastness, as the cosine and sine of aspect in radians, respectively. The correlation between topo-edaphic variables and foliar functional traits was assessed at the crown level, based on sample location using mixed-effects modeling with species identity as the random (intercept) effect. These relationships were assessed using linear regression for all continuous traits. As noted previously, latex was determined at the species level, and thus was excluded from this analysis. CG was also omitted, as the low number of positive occurrences among individuals (19 out of 345 individuals) is likely to introduce substantial bias in the maximum likelihood estimation of the logistic regression model (King and Zeng 2001).
All statistical analyses were conducted in the statistical language R (R Core Team 2015) using the packages ‘ape’ (Paradis et al. 2004), ‘caper’ (Orme et al. 2013), ‘MASS’ (Venables and Ripley 2002), ‘phytools’ (Revell 2012), and ‘picante’ (Kembel et al. 2010).
Results
Patterns of foliar trait variation
We quantified ten leaf traits related to foliar investment strategies and anti-herbivore defense for 225–328 individual canopy trees in 61–80 species (Table 1). Three defense traits were detected in only some of the species studied: hydrolysable tannins (HT) were present in 60 of 71 species sampled, cyanogenic glycosides (CG) were present in 3 out of 80 species, and latex was present in 12 out of 80 species (see Supplement). One-way ANOVA was significant for species-level differences for all traits, and coefficients of variation (CV) between species were 1.2–2.4 times greater than CV within species, with the lowest ratio for leaf density (LD) and the highest for leaf N (Table 1). Traits related to foliar resource investment (N, P, LWC, and LMA) varied among species from two- to fourfold, while defense traits (LD, LT, CT, HT, and TP) varied from roughly fourfold for LD to nearly 30-fold for non-zero occurrences of HT.
Pairwise relationships among traits
Pairwise analyses revealed substantial co-variation among species mean trait values related to foliar resource investment and uptake (N, P, LWC, and LMA), and between these traits and physical defenses of leaf density (LD) and leaf toughness (LT) among species (Table 2, Supplementary Fig. 2). Fewer relationships were observed between traits related to foliar resource investment and chemical defenses (TP, CT, HT, CG, and latex), and among anti-herbivore defense traits themselves. The foliar nutrients N and P were positively correlated, but only P was associated with leaf water content (LWC). However, all three foliar nutrients (N, P, and LWC) were negatively correlated with LMA. Leaf P and LWC exhibited a significant negative association with leaf density (LD), while leaf N was negatively associated with leaf toughness (LT). Both leaf N and P were inversely related to total phenolic content (TP), but only N showed a negative association with condensed tannins (CT). No foliar resource investment strategy traits (N, P, LWC, and LMA) showed an association, positive or negative, with the defense traits of latex, cyanogenic glycosides (CG), or hydrolysable tannins (HT). However, both physical defense traits (LD and LT) were positively correlated with the occurrence of latex in leaves, and there was a tri-variate correlation among the phenolic defenses (TP and the two phenolic subsets, CT and HT). The presence of CG within leaves was apparently independent of variation of all other traits included in this study.
Among individuals, we found more significant pairwise relationships than among species, although the direction and magnitude of relationships remained largely unchanged (Supplementary Table 1). This may be due to the larger sample size and statistical non-independence of conspecific individuals. However, among individuals we found a significant association of leaf N with all traits at the individual level, including negative associations with LT and all phenolic defenses (CT, HT, and TP) and a positive association with CG. Negative associations of leaf P with LT and HT were also significant among individuals. LMA was positively correlated with HT and TP, and negatively correlated with CG. LD and TP were also positively correlated. Finally, CG was negatively correlated with CT and TP.
Multivariate patterns of trait co-variation
Ordination analyses consistently indicated two major gradients of trait variation among canopy trees (Fig. 2, Supplementary Tables 2 and 3). The first two ordination axes for species-level PCA and PCoA explained 60.0 and 54.5% of interspecific variation, respectively. The first axis explained 42.3% (PCA) or 37.6% (PCoA) of total variation, and was consistent with strategic variation in foliar carbon investment along the LES, ranging from a conservative strategy of slow carbon returns-on-investment (as indicated by high values for LMA and low values for N, P, and LWC) to an acquisitive strategy of rapid carbon returns-on-investment (as indicated by opposing trait values). Two physical defense traits, LT and LD, were oriented along this axis. However, all the other defense traits varied independently of this axis. Correlations between trait values and PCo 1 were significant for LMA, LD, LT, N, P, and LWC and insignificant for latex, CG, CT, or HT (Supplementary Table 2).
Biplots of a PCA among species, b phylogenetic PCA among species, c PCoA among species, and d PCoA among individuals. The percentage of variance explained by each axis is reported with axes labels. Histograms for the distribution of scores along each axis are depicted above each plot for the first ordination axis, and to the right of plots for the second ordination axis. The orientation of trait loadings is depicted with red arrows. Variables are defined in the caption to Table 1
The second axis explained 17.7% (PCA) or 16.9% (PCoA) of total variation among species, indicating a gradient of phenolic defense investment (PCA) and a trade-off between investment into phenolic defenses and investment into latex or leaf toughness (PCoA). We refer to this axis as a leaf defense spectrum (LDS), as it incorporated several anti-herbivore defenses. The orientation of traits along the LDS differed between the PCA and PCoA analyses, as neither latex nor CG was included in the PCA. PCo 2 revealed a continuum characterized by high foliar investment into CT and HT on the positive side and investment into latex and LT on the negative side. LT was the only trait to show a significant correlation to both PCo axes (Supplementary Table 2). The LDS was most closely correlated with interspecific variation in CT concentration, while LT exhibited the strongest negative correlation with this axis. A visual examination of the pairwise relationships among LDS-related traits revealed that high mean CT values were absent among species with high mean toughness values and vice versa (Supplementary Fig. 2). We further explored the relationship between mean species values of CT and LT by performing quantile regression to assess the correlation between CT and LT for increasing percentiles of the CT distribution (Koenker 2005). We found a significant negative association of CT and LT at and above the 80th percentile of mean species CT values (p < 0.05; Supplementary Fig. 3).
However, low CT concentration was also observed among non-latex producing species and at low levels of leaf toughness. Variation in CG was not explained by either of the first two PCoA axes, which is unsurprising given that only three species were found to be cyanogenic. Linear regression of PCA and PCoA results revealed strong correlations between PC 1 and PCo 1 and between PC 2 and PCo2 (Supplementary Fig. 4); thus, only species-level PCoA scores and axes were utilized in subsequent analyses of potential determinants of interspecific trait co-variation, as they permitted the use of all available data.
At the individual level, PCA and PCoA results also indicated two primary ordination axes of trait co-variation, accounting for 60.8 and 54.8% of the variation among individuals, respectively. Individual-scale PC 1 and PCo 1 largely reflected the same trade-off among traits as indicated by species levels PC 1 and PCo 1; however, there was a significant correlation between PCo1 with CT and HT among individuals (Fig. 2; Supplementary Table 2). However, we found a difference in the trait loadings on the second ordination axis for individual-level PCA and PCoA compared to species-level analyses. Among individuals, PC 2 and PCo 2 reflected a greater contribution of LES-related traits, namely N, LWC, LD, LMA, and toughness. Individual-level PCo 2 additionally indicated a negative association with CG. Thus, a second axis for individual-level PCoA reflected a trade-off between investment into CT and HT and to a lesser extent LWC, with investment into LD, LT, N, LMA, and CG. The omission of leaf latex from individual-level analyses may contribute to this discrepancy between individual and species-level analyses, as may the uneven representation of species across all individuals.
Influence of phylogenetic relatedness on trait variation
Phylogenetic comparative methods suggested that phylogenetic structure has limited influence on the observed patterns of interspecific trait variation and co-variation. Analyses of phylogenetic signal revealed that several traits (N, LT, CG, latex, CT, and TP) and PC1 exhibited K values significantly greater than zero, which is the null expectation for no influence of phylogeny on interspecific trait variation. However, only two traits (CG and latex) exhibited phylogenetic signal at a level high enough to be consistent with an expectation of traits varying according to Brownian evolution (Table 3; Supplementary Fig. 5). All other traits and the first two ordination axes of both PCA and PCoA showed significantly less phylogenetic signal than was predicted by a null model of Brownian evolution, indicating that shared evolutionary history is insufficient to explain patterns of variation in trait values among canopy tree species.
No pairwise correlations of phylogenetically independent contrasts (PICs) of traits were significantly different from their respective pairwise trait associations, indicating a lack of phylogenetic structure in patterns of trait co-variation (Table 2). Moreover, phylogenetic PCA revealed consistent patterns of trait co-variation and explained similar levels of variation as the conventional PCA (Fig. 2; Supplementary Table 2), suggesting that the multivariate trade-off axes are not an artifact of species’ shared evolutionary history, but rather reflect convergent trait strategies. However, a limited amount of phylogenetic signal (more than zero and less than Brownian) was still observed among species positions along the first PC of both the conventional and phylogenetically corrected PCA (Table 3).
Influence of juvenile demographic niche on trait variation
Species demographic niches, as their positions along a juvenile growth-mortality trade-off axis (RGR95—M25), showed an association with LES variation among adult canopy trees, such that adult canopy species with high-LMA, low-nutrient, “conservative” foliage also tended to have juvenile conspecifics with low rates of mortality (Fig. 3). However, this relationship was only significant when utilizing the results of the PCoA analysis, which included more species than the PCA analysis (Supplementary Table 4). The latter required complete trait data for all observations, as previously discussed. It is interesting to note that several species with a low-LMA, high-nutrient, “acquisitive” foliar investment strategy also showed low rates of mortality among juvenile conspecifics. This may suggest that ontogenetic concordance may be expected for some canopy species, while shifts in allocation strategies are the expectation for others. No significant relationship was found between the LDS and this growth-mortality trade-off axis, for either PCA or PCoA results (Supplementary Table 4).
Pearson’s correlation of 28 species scores for the first ordination axes of principal coordinates analysis for all continuous traits included in this study (PCo 1) and principal components analysis for the relationship between growth rates and mortality rates of the fastest and slowest growing saplings, respectively, from Wright et al. (2010; Wright PC 1). PCo 1 ranges from species with high foliar investments into growth traits (N, P, and LWC) on the negative end to species with high investments into longevity and physical defense traits (LMA, LD, and LT) on the positive end. Wright PC 1 ranges from species with low rates of juvenile mortality in shaded environments on the negative end to high rates of juvenile growth in gap environments on the positive end. Full species names are given in the Supplement
Influence of topo-edaphic variation on trait variation
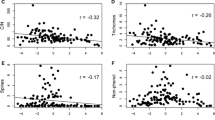
Topo-edaphic variables (elevation, slope, SWI, eastness, and northness) mostly did not explain patterns of trait variation or co-variation among tropical canopy trees axes (Supplementary Table 5). Mixed-effects models yielded very few significant fixed effects (i.e., trait–environment associations). Significant associations were observed only between LD and elevation, and the second orientation axis of PCoA, or the LDS, with slope. Rather, a large proportion of residual variation (42–82%) was attributed to species identify, included as a random intercept term.
Discussion
We found evidence for two independent axes of trait co-variation which together accounted for approximately 60% of variation among 80 canopy tree species in multidimensional trait space (8 and 10 trait dimensions, for PCA and PCoA, respectively; Fig. 2). The first trade-off axis alone accounted for roughly 40% of variation and depicted a continuum of acquisitive to conservative foliar investment strategies across six traits, with investment in foliar nutrition (N, P, and LWC) trading-off against investment in leaf structure and physical defense (LMA, LD, and LT), a pattern indicative of a leaf economics spectrum (LES). Two physical defense traits, LT and LD, were correlated with LES variation. Both leaf toughness and density have been shown to positively correlate with leaf lifespan among saplings, and a trade-off between growth-related traits and physical defenses may be maintained by physiological constraints on rapid leaf expansion versus dry mass investment (Wright et al. 2004; Poorter et al. 2009; Kitajima and Poorter 2010). The second trade-off axis described an additional 17% of variation and reflected a gradient of phenolic defense investment as well as an apparent trade-off between high CT investment and investment into other anti-herbivore defenses, such as LT and/or latex. We refer to this pattern as a leaf defense spectrum (LDS), as it characterizes co-variation among traits with primarily anti-defense functions.
Interspecific variation for most defense traits was independent of LES variation, suggesting that anti-herbivore defense may not be regulated by the same factors which constrain foliar resource allocation within this canopy tree community. More specifically, although physical leaf defenses may be related to an overall foliar investment strategy, all other defense traits included in this study were decoupled from this primary axis of variation. This finding contrasts with the resource availability hypothesis, which suggests that foliar investment in anti-herbivore defenses should trade-off against investment into rapid leaf growth, and, in particular, contrasts with previous research from BCI which finds a growth-defense trade-off among juvenile canopy trees (Coley et al. 1985; Coley 1988). For the fully sunlit foliage of adult canopy trees, a universal growth-defense trade-off does not appear to best describe the allocation of foliar resources towards growth and defense functions. High-resource availability experienced by canopy trees may instead permit the simultaneous allocation of foliar resources towards both growth and defense.
Patterns generating a leaf defense spectrum
Trade-offs among plant defense traits may arise from constraints on the simultaneous production of defenses, or where traits, or suites of traits, represent alternative adaptive strategies towards herbivory (Herms and Mattson 1992; Agrawal and Fishbein 2006). The most striking trade-off relationship depicted along the LDS indicated that species which invest heavily in LT do not make large investments in CT (Supplementary Fig. 3). On one side of the LDS, tannins are a generalist defense against herbivores through deterrence and/or toxicity (Barbehenn and Constabel 2011). On the other side of the LDS, LT is an effective deterrent against many types of herbivores, and leaf latex constitutes a physical barrier which often contains high concentrations of defensive chemical compounds (Agrawal and Konno 2009; Konno 2011). Species which produced latex or were cyanogenic also appeared to invest relatively little in CT (Supplementary Fig. 2). However, the inverse was apparently not true: low CT concentrations were also observed at low levels of LT (and among non-latex-producing and non-cyanogenic species). This suggests that, to the extent to which high concentrations of CT versus high investment in LT constitute alternative anti-herbivore defense strategies, there are likely to be additional strategies involving defense traits not included here. Nevertheless, this trade-off relationship may indicate strategies that are mutually exclusive, due to evolutionary or physiological constraints, or represent equally viable anti-herbivore defense responses among the species within the BCI canopy tree community. We discuss the influence that evolutionary and environmental determinants may have in contributing to strategic variation along both the LES and LDS among canopy tree species in the following sections.
Phylogenetic basis for LES and LDS
Phylogenetic relationships among species did not appear to drive patterns of trait co-variation underlying the LES and LDS, and we found no evidence for phylogenetic conservatism (sensu Losos 2008) among individual traits and trade-off axes (Fig. 2b; Table 3). Trade-offs among traits contributing to the LES are widespread across plant lineages, and may result in part from physiological constraints on growth and differentiation processes at the cellular level (Wright et al. 2004; Shipley et al. 2006; Poorter et al. 2009). The LDS, primarily driven by variation among CT and HT, also did not appear to be phylogenetically organized. Specialized plant defense traits, namely, latex and cyanogenic glycosides (CG), were the most phylogenetically conserved traits. The presence of these traits was restricted to specific taxonomic clades, with latex found in all observed members of three families (Apocynaceae, Moraceae, and Sapotaceae) and CG restricted to the two Tabebuia species within the Bignoniaceae family and one of three species of Ocotea (Ocotea puberula; Supplementary Fig. 5). Phylogenetic conservatism may be particularly common among specialized plant defense traits, as the evolution of complex biosynthetic pathways required to produce various defenses is not likely to have occurred more than once or a few times (Agrawal 2007). However, as the presence of CG in the distantly related genera of Tabebuia and Ocotea suggests, convergent evolution of particular classes of defenses or of multivariate defense strategies may also occur. Such defenses may vary in their precise composition while being functionally similar in their mode of defense (Wink 2003, 2010; Agrawal and Fishbein 2006).
Phylogenetic constraints on interspecific variation among both LES- and LDS-related traits may be obscured by phenotypic plasticity, and defense traits in particular are known to vary across a range of temporal scales, from compositional changes in defense strategies across ontogenetic stages to induced production of some types of defense following herbivore attacks (Schultz 1988; Boege and Marquis 2005). Although interspecific variation exceeded intraspecific variation for all traits, plastic responses of species to the canopy environment may have contributed to the low phylogenetic signal of both LES- and LDS-related traits. Phylogenetic PCA results indicated that the orthogonality of these trade-off axes was maintained when phylogenetic structure was accounted for. This suggests that the independence of LES and LDS is maintained across the diverse evolutionary lineages represented within this canopy tree community.
Juvenile demographic niche associations and adult patterns of trait co-variation
Our results suggest that the demographic associations of juvenile trees along a growth-mortality trade-off axis may were broadly associated with conspecific positions along an LES axis among adult canopy trees (Fig. 3). Species with juvenile demographic niches indicative of a pioneer lifestyle, with high growth rates in in gap environments and high mortality in shaded environments appear to maintain acquisitive LES strategies as canopy trees. However, species which showed the lowest rates of mortality in shaded environments and the slowest growth in gaps spanned a wide range of LES positions as adult canopy trees. The previous authors have noted that ontogenetic trajectories of canopy trees may be complex, with some species existing as “whole-life” light demanders or shade tolerants but others switching between these two categorical distinctions throughout their ontogenies (Clark and Clark 1992; Kitajima and Poorter 2008). One notable example is Alseis blackiana, which has been found to exhibit a seed germination pattern and early life history consistent with a pioneer species, but shows remarkable persistence in the understory as a sapling (Dalling et al. 2001). This species was further identified as a low-mortality, low-growth sapling by Wright et al. (2010), but was oriented towards the acquisitive end of the LES in the current study—evidently returning to its pioneer-like origins. Our results suggest that the light habitat specializations of juvenile trees may help to maintain these trade-offs beyond the regeneration phase, and highlight the importance of including all ontogenetic stages, from germination to mature adult, in studies of life history (Poorter 2007). Alternately, physical defense traits (LT and LD) may contribute to the link between juvenile demographic niches and adult LES strategies, as these defenses may be considered a ‘first line of defense’ against a wide range of herbivores (Lucas 2000; Peeters et al. 2007; Onoda et al. 2011).
In contrast, no significant relationship was found between the LDS and a juvenile growth-mortality trade-off axis. Among saplings of the 41 most common canopy tree species on BCI, Coley (1988) noted a trade-off between height growth rates and levels of foliar defense investment. Our results indicate that physical defenses, which are a part of the LES, may contribute to this trade-off, but that chemical defenses (phenolics, latex, and cyanogenic glycosides) may not. Ontogenetic trajectories for plant responses to herbivory are expected to be at least as complex, if not more, than for strategies of carbon acquisition and allocation, as resource availability critically influences the costs and benefits of herbivore responses (Mooney et al. 1983; Coley et al. 1985; Bazzaz et al. 1987). Thus, the lack of resource constraints among canopy trees may contribute to a lack of correlation between juvenile demographic niches and adult defense investment, even if juvenile defense investment was concomitant with a growth-mortality trade-off.
Topo-edaphic variation and patterns of trait co-variation
Spatial variation in five topo-edaphic variables (elevation, slope, soil water content, northness, and eastness) largely did not appear to contribute to patterns of trait variation or co-variation among canopy trees (Supplementary Table 4). Variations in soil fertility are known to be an important second dimension of foliar functional variation in more nutrient-limited tropical forest communities, contributing in particular to variation in foliar P concentrations (Baltzer and Thomas 2010). Moreover, spatial variation in soil fertility may influence habitat specialization of plants with diverging herbivore responses, with low-resource specialists investing more heavily in anti-herbivore defenses than their high-fertility counterparts (Fine et al. 2006). Relatively fertile soils across BCI compared to many tropical forest communities may contribute to the lack of strong trait–environmental relationships (Vitousek and Sanford 1986), and the vast root networks of canopy trees may enable them to access soil nutrients over a large spatial extent. However, the topo-edaphic variables included in this analysis are undoubtedly crude proxies for soil fertility, and a closer look at associations of foliar traits with specific soil macro- and micro-nutrients may provide a deeper insight into the role of edaphic constraints on LES and LDS variation (e.g., Steidinger 2015).
Foliar resource allocation along an axis of leaf defense strategies
Interspecific variation along a leaf defense spectrum (LDS) appears to indicate a trade-off among two suites of anti-herbivore defense traits (phenolic defenses versus other defenses, including latex and to some extent leaf toughness). This variation does not appear to be a consequence of phylogenetic relationships among species, nor does it appear to reflect juvenile demographic niches or topo-edaphic variation among canopy trees. However, this trade-off is neither fully independent of a leaf economics spectrum, as demonstrated by the orientation of LT on Axis 1, nor fully determined by it, as no relationship was found between CT or latex and Axis 1. In particular, species which invest the most heavily in leaf toughness do not appear to invest the most heavily in condensed tannins. Classical plant defense theory posits that trade-offs should occur among traits which utilize the same limited resources and/or provide the same type of anti-herbivore defense (Herms and Mattson 1992), yet studies of toughness and phenolics more broadly have failed to show any evidence of a trade-off among these two defense types (e.g., Read et al. 2009; Endara and Coley 2011). Wide variation in the structure and function of phenolic compounds may permit the simultaneous allocation of foliar resources to some types of phenolics and to leaf toughness, particularly where defense trait combinations enhance plant fitness through synergistic interactions or by providing comprehensive defense against a range of herbivores (Agrawal and Fishbein 2006). Hydrolysable tannins, for example, are relatively low molecular weight phenolic compounds that appear to be preferentially located within plant cell walls, which may allow for them to act synergistically with cell wall components that contribute to leaf toughness, and may not be expected to trade-off (Grundhöfer et al. 2001). In contrast, CT are high molecular weight phenolic compounds with a dose-dependent effect on herbivores, and thus may compete with the carbon-rich constituents of leaf toughness for foliar carbon resources. Notably, the presence of cyanogenic glycosides, a highly toxic, non-dose-dependent, chemical defense, was not associated with either PCo Axis 1 or Axis 2. These findings suggest that the LDS may reflect foliar resource allocation trade-offs among carbon-rich, dose-dependent defenses, and that defenses which utilize different resources or utilize the same resources differently may not be expected to trade-off along this dimension. Incorporation of a wider range of defenses, including physical defenses (e.g., trichomes, epicuticular waxes) as well as tactical means of defense (e.g., synchronous leaf flushing, ant mutualism) may result in the identification of additional dimensions and greater diversity of defense investment.
This study represents the first landscape-scale analysis of multiple plant defense traits across a tropical forest canopy tree community. We found that for the canopy tree community of BCI, functional variation among ten traits related to foliar resource allocation and anti-herbivore defense was not correlated, as is predicted by the resource availability hypothesis, but was instead distributed across two independent axes related to leaf economic and leaf defense strategies. These strategies may be responding to independent limiting resources, including not only those which presently act upon mature canopy trees, but also those which may have been imposed by selection at the survival bottlenecks of prior ontogenetic stages. The elucidation of an LDS as a second axis of functional variation suggests that multiple independent limiting resources may be contributing to a greater dimensionality to functional trait co-variation than would be suggested by a simple growth-defense trade-off. This greater dimensionality may be an important contributor to the functional and biological diversity of this canopy tree community.
References
Ackerly D (2004) Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol Monogr 74:25–44. https://doi.org/10.1890/03-4022
Adler PB, Salguero-Gómez R, Compagnoni A et al (2014) Functional traits explain variation in plant life history strategies. Proc Natl Acad Sci USA 111:740–745. https://doi.org/10.1073/pnas.1315179111
Agrawal AA (2007) Macroevolution of plant defense strategies. Trends Ecol Evol 22:103–109. https://doi.org/10.1016/j.tree.2006.10.012
Agrawal AA, Fishbein M (2006) Plant defense syndromes. Ecology 87:S132–S149. https://doi.org/10.1890/0012-9658(2006)87[132:PDS]2.0.CO;2
Agrawal AA, Konno K (2009) Latex: a model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu Rev Ecol Evol Syst 40:311–331. https://doi.org/10.1146/annurev.ecolsys.110308.120307
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Appel HM (1993) Phenolics in ecological interactions: the importance of oxidation. J Chem Ecol 19:1521–1552. https://doi.org/10.1007/BF00984895
Asner GP, Martin RE (2011) Canopy phylogenetic, chemical and spectral assembly in a lowland Amazonian forest. New Phytol 189:999–1012. https://doi.org/10.1111/j.1469-8137.2010.03549.x
Asner GP, Martin RE, Tupayachi R et al (2011) Taxonomy and remote sensing of leaf mass per area (LMA) in humid tropical forests. Ecol Appl 21:85–98. https://doi.org/10.1890/09-1999.1
Asner GP, Martin RE, Tupayachi R et al (2014) Amazonian functional diversity from forest canopy chemical assembly. Proc Natl Acad Sci 111:5604–5609. https://doi.org/10.1073/pnas.1401181111
Ayres MP, Clausen TP, MacLean SF et al (1997) Diversity of structure and antiherbivore activity in condensed tannins. Ecology 78:1696–1712. https://doi.org/10.1890/0012-9658(1997)078[1696:DOSAAA]2.0.CO;2
Baillie I, Elsenbeer H, Barthold F et al (2007) Semi-detailed soil survey of Barro Colorado Island. Smithsonian Tropical Research Institute. Panama City, Panama
Baltzer JL, Thomas SC (2010) A second dimension to the leaf economics spectrum predicts edaphic habitat association in a tropical forest. PLoS One 5:e13163. https://doi.org/10.1371/journal.pone.0013163
Barbehenn RV, Constabel CP (2011) Tannins in plant-herbivore interactions. Phytochemistry 72:1551–1565. https://doi.org/10.1016/j.phytochem.2011.01.040
Basset Y, Aberlenc HP, Delvare G (1992) Abundance and stratification of foliage arthropods in a lowland rain forest of Cameroon. Ecol Entomol 17:310–318. https://doi.org/10.1111/j.1365-2311.1992.tb01063.x/full
Bazzaz FA, Chiariello NR, Coley PD, Pitelka LF (1987) Allocating resources to reproduction and defense. Bioscience 37:58–67
Beven KJ, Kirkby MJ (1979) A physically based, variable contributing area model of basin hydrology. Hydrol Sci Bull 24:43–69. https://doi.org/10.1080/02626667909491834
Bi JL, Felton GW (1995) Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J Chem Ecol 21:1511–1530. https://doi.org/10.1007/BF02035149
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution (NY) 57:717–745. https://doi.org/10.1111/j.0014-3820.2003.tb00285.x
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants—an economic analogy. Annu Rev Ecol Syst 16:363–392
Boege K, Marquis RJ (2005) Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol Evol 20:441–448. https://doi.org/10.1016/j.tree.2005.05.001
Chen Y, Hagerman AE (2005) Reaction pH and protein affect the oxidation products of β-pentagalloyl glucose. Free Radic Res 39:117–124
Clark DA, Clark DB (1992) Life history diversity of canopy and emergent trees in a neotropical rain forest. Ecol Monogr 62:315. https://doi.org/10.2307/2937114
Clark DB, Clark DA (1996) Abundance, growth and mortality of very large trees in neotropical lowland rain forest. For Ecol Manag 80:235–244. https://doi.org/10.1016/0378-1127(95)03607-5
Clark DB, Palmer MW, Clark DA (1999) Edaphic factors and the landscape-scale distribution of tropical rain forest trees. Ecology 80:2662–2675. https://doi.org/10.1890/0012-9658(1999)080[2662:EFATLS]2.0.CO;2
Coley PD (1988) Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia 74:531–536. https://doi.org/10.1007/BF00380050
Coley PD, Bryant JP, Chapin FS (1985) Resource availability and plant antiherbivore defense. Science 230:895–899. https://doi.org/10.1126/science.230.4728.895
Condit R (2000) Spatial patterns in the distribution of tropical tree species. Science 288:1414–1418. https://doi.org/10.1126/science.288.5470.1414
Condit R, Hubbell SP, Foster RB (1996) Assessing the response of plant functional types to climatic change in tropical forests. J Veg Sci 7:405–416. https://doi.org/10.2307/3236284
Condit R, Pérez R, Daguerre N (2011) Trees of Panama and Costa Rica. Princeton University Press, Princeton
Crisp MD, Cook LG (2012) Phylogenetic niche conservatism: what are the underlying evolutionary and ecological causes? New Phytol 196:681–694. https://doi.org/10.1111/j.1469-8137.2012.04298.x
Croat TB (1978) Flora of Barro Colorado Island. Stanford University Press, Stanford
Dalling JW, Winter K, Nason JD et al (2001) The unusual life history of Alseis blackiana: a shade-persistent pioneer tree? Ecology 82:933–945. https://doi.org/10.1890/0012-9658(2001)082[0933:TULHOA]2.0.CO;2
Diaz-Uriarte R, Garland T (1999) Polytomies and phylogenetically independent contrasts: examination of the bounded degrees of freedom approach. Syst Biol 48:547–558. https://doi.org/10.1080/106351599260139
Dominy NJ, Lucas PW, Wright SJ (2003) Mechanics and chemistry of rain forest leaves: canopy and understorey compared. J Exp Bot 54:2007–2014
Endara M-J, Coley PD (2011) The resource availability hypothesis revisited: a meta-analysis. Funct Ecol 25:389–398. https://doi.org/10.1111/j.1365-2435.2010.01803.x
Everette JD, Bryant QM, Green AM et al (2010) Thorough study of reactivity of various compound classes toward the Folin–Ciocalteu reagent. J Agric Food Chem 58:8139–8144. https://doi.org/10.1021/jf1005935
Feeny P (1976) Plant apparency and chemical defense. Recent Adv Phytochem 10:1–40
Feldman KS, Sahasrabudhe K, Quideau S et al (1999) Prospects and progress in ellagitannin synthesis. In: Gross GG, Hemingway RW, Yoshida T, Branham SJ (eds) Plant polyphenols 2. Springer, New York, pp 101–125
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution (NY) 39:783–791
Fine PVA, Miller ZJ, Mesones I et al (2006) The growth-defense trade-off and habitat specialization by plants in Amazonian forests. Ecology 87:S150–S162. https://doi.org/10.1890/0012-9658(2006)87[150:TGTAHS]2.0.CO;2
Fritz SA, Purvis A (2010) Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conserv Biol 24:1042–1051. https://doi.org/10.1111/j.1523-1739.2010.01455.x
Garland T, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol 41:18–32. https://doi.org/10.1093/sysbio/41.1.18
Givnish TJ (1988) Adaptation to sun and shade: a whole-plant perspective. Aust J Plant Physiol 15:63. https://doi.org/10.1071/PP9880063
Gleadow R, Bjanrholt N, Jorgensen K et al (2011) Detection, identification and quantitative measurement of cyanogenic glycosides. In: Narwal SS, Szajdak L, Sampietro DA (eds) Soil allelochemicals. Stadium Press, Houston, pp 283–310
Gower JC (1966) A Q-technique for the calculation of canonical variates. Biometrika 53:588–590
Grubb PJ (1977) The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biol Rev 52:107–145. https://doi.org/10.1111/j.1469-185X.1977.tb01347.x
Grundhöfer P, Niemetz R, Schilling G, Gross GG (2001) Biosynthesis and subcellular distribution of hydrolyzable tannins. Phytochemistry 57:915–927. https://doi.org/10.1016/S0031-9422(01)00099-1
Hagerman AE (1992) Tannin-protein interactions. In: Ho C, Lee CY, Huang M (eds) Phenolic compounds in food and their effects on health I. American Chemical Society, Washington, DC, pp 236–247
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Holdridge LR (1947) Determination of world plant formations from simple climate data. Science 105:367–368
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856. https://doi.org/10.1021/jf030723c
Iglewicz B, Hoaglin DC (1993) How to detect and handle outliers. ASQC Quality Press, Milwaukee
Kembel SW, Cowan PD, Helmus MR et al (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464
King G, Zeng L (2001) Logistic regression in rare events data. Polit Anal 9:137–163. https://doi.org/10.1093/oxfordjournals.pan.a004868
Kinner D, Mixon D, Stallard R, Wahl S (2002) BCI GIS version 1.3
Kitajima K, Poorter L (2008) Functional basis for resource niche partitioning by tropical trees. In: Carson WP, Schnitzer SA (eds) Tropical forest community ecology. Blackwell Publishing, Oxford, pp 160–181
Kitajima K, Poorter L (2010) Tissue-level leaf toughness, but not lamina thickness, predicts sapling leaf lifespan and shade tolerance of tropical tree species. New Phytol 186:708–721. https://doi.org/10.1111/j.1469-8137.2010.03212.x
Kitajima K, Mulkey SS, Wright SJ (2005) Variation in crown light utilization characteristics among tropical canopy trees. Ann Bot 95:535–547. https://doi.org/10.1093/aob/mci051
Kitajima K, Llorens A-M, Stefanescu C et al (2012) How cellulose-based leaf toughness and lamina density contribute to long leaf lifespans of shade-tolerant species. New Phytol 195:640–652. https://doi.org/10.1111/j.1469-8137.2012.04203.x
Koenker R (2005) Quantile regression. Cambridge University Press, Cambridge, UK
Konno K (2011) Plant latex and other exudates as plant defense systems: roles of various defense chemicals and proteins contained therein. Phytochemistry 72:1510–1530. https://doi.org/10.1016/j.phytochem.2011.02.016
Koricheva J (2002) Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83:176–190. https://doi.org/10.1890/0012-9658(2002)083[0176:MAOSOV]2.0.CO;2
Kress WJ, Erickson DL, Jones FA et al (2009) Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc Natl Acad Sci USA 106:18621–18626. https://doi.org/10.1073/pnas.0909820106
Kursar T, Coley P (2003) Convergence in defense syndromes of young leaves in tropical rainforests. Biochem Syst Ecol 31:929–949. https://doi.org/10.1016/S0305-1978(03)00087-5
Lambert JL, Ramasamy J, Paukstelis JV (1975) Stable reagents for the colorimetric determination of cyanide by modified Koenig reactions. Anal Chem 47:916–918. https://doi.org/10.1021/ac60356a036
Lechtenberg M, Nahrstedt A (1999) Cyanogenic glycosides. In: Ikan R (ed) Naturally occurring glycosides. Wiley, Chichester, pp 147–191
Legendre P, Legendre L (1998) Numerical ecology. Elsevier Science, Amsterdam
Leigh EG (1999) Tropical forest ecology: a view from Barro Colorado Island. Oxford University Press, Oxford
Losos JB (2008) Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett 11:995–1003. https://doi.org/10.1111/j.1461-0248.2008.01229.x
Lucas P (2000) Mechanical defences to herbivory. Ann Bot 86:913–920. https://doi.org/10.1006/anbo.2000.1261
Makkar HPS, Siddhuraju P, Becker K (2007) Plant secondary metabolites
Mason CM, Donovan LA (2015) Does investment in leaf defenses drive changes in leaf economic strategy? A focus on whole-plant ontogeny. Oecologia 177:1053–1066. https://doi.org/10.1007/s00442-014-3177-2
Mazza CA, Boccalandro HE, Giordano CV et al (2000) Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiol 122:117–126. https://doi.org/10.1104/PP.122.1.117
Messmer T, Elsenbeer H, Wilcke W (2014) High exchangeable calcium concentrations in soils on Barro Colorado Island, Panama. Geoderma 217–218:212–224. https://doi.org/10.1016/j.geoderma.2013.10.021
Mooney HA, Gulmon SL, Johnson ND (1983) Physiological constraints on plant chemical defenses. In: Hedin P (ed) Plant resistance to insects. American Chemical Society, Washington, DC., pp 21–36
Onoda Y, Westoby M, Adler PB et al (2011) Global patterns of leaf mechanical properties. Ecol Lett 14:301–312. https://doi.org/10.1111/j.1461-0248.2010.01582.x
Orme D, Freckleton R, Thomas G et al (2013) Caper: comparative analyses of phylogenetics and evolution in R. R Packag Vers 0.5.2 5:1
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Peeters PJ, Sanson G, Read J (2007) Leaf biomechanical properties and the densities of herbivorous insect guilds. Funct Ecol 21:246–255. https://doi.org/10.1111/j.1365-2435.2006.01223.x
Polly PD, Lawing AM, Fabre A-C, Goswami A (2013) Phylogenetic Principal Components Analysis and Geometric Morphometrics. Hystrix. Ital. J. Mammal. 24:33–41
Poorter L (2007) Are species adapted to their regeneration niche, adult niche, or both? Am Nat 169:433–442. https://doi.org/10.1086/512045
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87:1733–1743. https://doi.org/10.1890/0012-9658(2006)87[1733:LTAGPO]2.0.CO;2
Poorter H, Niinemets Ü, Poorter L et al (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588. https://doi.org/10.1111/j.1469-8137.2009.02830.x
R Core Team (2015) R: A language and environment for statistical computing
Quideau S, Feldman KS, Appel HM (1995) Chemistry of gallotannin-derived o-quinones: reactivity toward nucleophiles. J Org Chem 60:4982–4983. https://doi.org/10.1021/jo00121a012
Read J, Sanson GD, Caldwell E et al (2009) Correlations between leaf toughness and phenolics among species in contrasting environments of Australia and New Caledonia. Ann Bot 103:757–767. https://doi.org/10.1093/aob/mcn246
Reich PB, Wright IJ, Cavender-Bares J et al (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164:S143–S164. https://doi.org/10.1086/374368
Revell LJ (2009) Size-correction and principal components for interspecific comparative studies. Evolution 63:3258–3268. https://doi.org/10.1111/j.1558-5646.2009.00804.x
Revell LJ (2012) Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223
Salminen J-P, Karonen M (2011) Chemical ecology of tannins and other phenolics: we need a change in approach. Funct Ecol 25:325–338. https://doi.org/10.1111/j.1365-2435.2010.01826.x
Schultz JC (1988) Plant responses induced by herbivores. Trends Ecol Evol 3:45–49. https://doi.org/10.1016/0169-5347(88)90047-X
Shipley B, Lechowicz MJ, Wright I, Reich PB (2006) Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 87:535–541. https://doi.org/10.1890/05-1051
Silvertown J (2004) Plant coexistence and the niche. Trends Ecol Evol 19:605–611. https://doi.org/10.1016/j.tree.2004.09.003
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Steidinger B (2015) Qualitative differences in tree species distributions along soil chemical gradients give clues to the mechanisms of specialization: why boron may be the most important soil nutrient at Barro Colorado Island. New Phytol. https://doi.org/10.1111/nph.13298
Tilman D (2004) Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA 101:10854–10861
Toth GB, Pavia H (2001) Removal of dissolved brown algal phlorotannins using insoluble polyvinylpolypyrrolidone (PVPP). J Chem Ecol 27:1899–1910. https://doi.org/10.1023/A:1010421128190
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Vitousek PM (1984) Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298. https://doi.org/10.2307/1939481
Vitousek PM, Sanford RL (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167. https://doi.org/10.1146/annurev.es.17.110186.001033
Westbrook JW, Kitajima K, Burleigh JG et al (2011) What makes a leaf tough? Patterns of correlated evolution between leaf toughness traits and demographic rates among 197 shade-tolerant woody species in a neotropical forest. Am Nat 177:800–811. https://doi.org/10.1086/659963
Westoby M, Wright IJ (2006) Land-plant ecology on the basis of functional traits. Trends Ecol Evol 21:261–268. https://doi.org/10.1016/j.tree.2006.02.004
Westoby M, Falster DS, Moles AT et al (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33:125–159. https://doi.org/10.1146/annurev.ecolsys.33.010802.150452
Wink M (2003) Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64:3–19. https://doi.org/10.1016/S0031-9422(03)00300-5
Wink M (2010) Introduction: Biochemistry, physiology, and ecological functions of secondary metabolites. In: Wink M (ed) Annual plant reviews, volume 40: biochemistry of plant secondary metabolism, 2nd edn. Blackwell Publishing, Oxford, pp 1–19
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Wright SJ, Kitajima K, Kraft NJB et al (2010) Functional traits and the growth–mortality trade-off in tropical trees. Ecology 91:3664–3674. https://doi.org/10.1890/09-2335.1
Yavitt JB (2000) Nutrient dynamics of soil derived from different parent material on Barro Colorado Island, Panama. Biotropica 32:198–207
Yavitt JB, Wieder RK (1988) Nitrogen, phosphorus, and sulfur properties of some forest soils on Barro Colorado Island, Panama. Biotropica 20:2–10
Acknowledgements
We thank K. Kryston, B. Tsang, F. Sinca, R. Tupayachi, N. Jaramillo, J.A. Vega, R. Mena-Werth, and C. Mong for their indispensable help with logistics, field work, and laboratory analyses. The Carnegie Spectranomics Project (http://spectranomics.ciw.edu) is supported by the John D. and Catherine T. MacArthur Foundation.
Author information
Authors and Affiliations
Contributions
KMC, GPA, and CBF conceived and designed this study. GPA, REM, and KMC developed methodology. GPA and SJW collaborated on sampling design. KMC, REM, and GPA conducted fieldwork and chemical analyses. WJK generated the molecular phylogeny. KMC wrote the manuscript; other authors provided editorial advice.
Corresponding author
Ethics declarations
Human/animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Jennifer Thaler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chauvin, K.M., Asner, G.P., Martin, R.E. et al. Decoupled dimensions of leaf economic and anti-herbivore defense strategies in a tropical canopy tree community. Oecologia 186, 765–782 (2018). https://doi.org/10.1007/s00442-017-4043-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-4043-9