Abstract
Recent evidence suggests that plant performance can be influenced by the phylogenetic diversity of neighboring plants. However, no study to date has examined the effect of such phylogenetic density dependence on the transition from seed to seedling. Using 6 years of data on seedling recruitment and seed rain of 13 species from 130 stations (one 0.5 m2 seed trap and three adjacent 1 m2 seedling plots) in a subtropical evergreen forest, we asked: (1) Does negative density dependence act across seed to seedling stages? (2) Is there evidence for phylogenetic density dependence during the seed to seedling transition? (3) Does the strength of density dependence vary among years? Generalized linear mixed-effects models were used to model seed to seedling transition as a function of conspecific seed and seedling densities, heterospecific seed and seedling densities, and mean phylogenetic distance of heterospecific seeds and seedling. Conspecific seed density had a significant negative effect on seedling transition rates for 12 of 13 focal species. In contrast, conspecific seedling density had a positive effect for 7 species, suggesting species-specific habitat preferences. Few species were significantly affected by the density or phylogenetic relatedness of heterospecific seeds and seedlings. Only conspecific seed density effects varied among years for most focal species. Overall, our results reveal that conspecific seed and seedling densities play a more important role than the density or relatedness of heterospecific seeds and seedlings during the seed to seedling stage, suggesting that species-specific seed predators, along with habitat preferences, may contribute to diversity maintenance in this forest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanisms that maintain high tree diversity in tropical forests have long fascinated biologists (Denslow 1987; Wright 2002). One of the best known models of tropical tree species coexistence is the Janzen–Connell model (J–C), which assumes that due to the high seed rain beneath adult trees, density- or distance-responsive predators and pathogens tend to concentrate near parent trees, resulting in high conspecific seed and seedling mortality and permitting the successful recruitment by other tree species (Janzen 1970; Connell 1971). Accumulated evidence suggests that such conspecific negative density dependence (NDD), particularly at early life stages (Zhu et al. 2015), is widespread in tropical tree communities (e.g., Harms et al. 2000; Comita and Hubbell 2009; Comita et al. 2010; Swamy et al. 2011), and thus may be important for slowing competitive exclusion and maintaining tropical forest diversity. A growing number of studies have demonstrated that Janzen–Connell effects are not restricted to tropical forests but are also common in other systems, including subtropical (Chen et al. 2010; Zhu et al. 2010; Liu et al. 2012) and temperate tree communities (Hille Ris Lambers et al. 2002; Johnson et al. 2012; LaManna et al. 2016). However, the strength of conspecific density dependence can vary geographically, such as with respect to latitude (Johnson et al. 2012) or precipitation (Comita et al. 2014). In addition, even at a single site, the strength of density dependence has been shown to vary temporally (Wright et al. 2005; Comita et al. 2009; Lin et al. 2012; Bachelot et al. 2015), potentially due to variation in natural enemy behavior (e.g., predator satiation) or climate (e.g., seasonal or annual variation in rainfall).
At the same time, there is increasing recognition that the identity of heterospecific neighbors can play an important role in determining their impact on focal individuals (Bagchi et al. 2010; Metz et al. 2010; Paine et al. 2012; Lebrija-Trejos et al. 2016). Previous studies usually divided neighbors into conspecific and heterospecific individuals, thereby ignoring the wide variation in the likely effect of heterospecific neighbors on focal plants (Uriarte et al. 2004; Webb et al. 2006). However, both evolutionary theory and empirical results have shown that the phylogenetic relatedness of neighbors can affect the performance and survival of individuals within a local neighborhood (e.g., Webb et al. 2006; Metz et al. 2010; Paine et al. 2012; Zhu et al. 2015). In general, ecologists expect greater negative interactions among individuals of species that are phylogenetically more closely related, because they are more likely to compete for more similar resources and/or share seed predators, herbivores, or pathogens (Novotny et al. 2002; Narwani et al. 2013; Fritschie et al. 2013; Venail et al. 2014; Naughton et al. 2015; Wu et al. 2016). Consistent with this idea, a number of studies have found a decrease in focal plant growth or survival with closer phylogenetic distance of neighboring plants (e.g., Webb et al. 2006; Metz et al. 2010). Conversely, other recent studies reported an increase in survival when neighbors were more closely related (Lebrija-Trejos et al. 2014; Zhu et al. 2015), suggesting that facilitation (e.g., via shared mutualists) and/or phylogenetically conserved habitat preferences are more important than natural enemies in some cases.
While a number of recent studies have looked at phylogenetic density-dependent seedling survival (Webb et al. 2006; Bagchi et al. 2010; Metz et al. 2010; Paine et al. 2012; Lebrija-Trejos et al. 2014; Wu et al. 2016), no study to date has examined phylogenetic density dependence across the seed to seedling stages. Patterns found for seedlings and later life stages may not generalize to the seed to seedling transition stage. Effects of mutualists (e.g., mycorrhizal fungi) are likely more limited at the seed stage compared to later stages when individuals are more dependent on the local environment, rather than maternal reserves, for resource acquisition (Fenner 1985). At both the seed and seedling stages, the effect of phylogenetic relatedness of neighbors will depend, at least in part, on the host-specificity of natural enemies. Insect seed predators usually display some level of host-specificity. For example, in a study of insect seed predators in a Costa Rican deciduous forest, Janzen (1980) found that polyphagous beetle species tended to attack seeds of plant species that were phylogenetically closely related. Therefore, the seed to seedling transition rate could be phylogenetic density-dependent if insect seed predators are the dominant cause of seed mortality. In contrast, vertebrate seed predators, such as rodents, tend to be generalists (Hanski and Hansson 1991). Thus, in systems where vertebrates are the dominant seed predators, the seed to seedling transition is likely related to the local density, but not relatedness, of heterospecific seeds.
Previous studies of density dependence during the seed to seedling transition have focused exclusively on seed densities (e.g., Harms et al. 2000). However, seedling neighbor density may also influence seed germination and seedling recruitment. For example, high densities of established conspecific seedlings may increase the local density of host-specific pathogens that may kill newly emerging seedlings. On the other hand, higher seed to seedling transition rates could be found in sites with higher conspecific seedling density because of species-specific habitat preferences that favor recruitment in similar conditions. At the same time, high densities of heterospecific seedlings could decrease the detection of seeds by predators and increase the probability of seed to seedling transition (e.g., species-herd protection; Wills 1996).
Here, we examine density- and phylogenetic-dependent rates of transition from the seed to seedling stage using data from a long-term, community-level study of seed rain and seedling recruitment in a subtropical forest in eastern China. Using data from 13 focal tree species collected over 6 years, we address three main questions: (1) Does conspecific NDD act across the seed to seedling transition in this subtropical forest? (2) Is there evidence for phylogenetic density dependence during the seed to seedling transition? (3) Is there year to year variation in the strength of density dependence? We predicted that there were strong negative effects of local conspecific seed and seedling densities on transition rates due to species-specific natural enemies, but also negative effects of local heterospecific seed densities due to polyphagous natural enemies, with the effect of heterospecifics decreasing with phylogenetic distance.
Materials and methods
Study site
The study was conducted at the Gutianshan (GTS) 24-ha permanent forest plot (29°15′N, 118°07′E), located in the old-growth subtropical forest of Gutianshan National Nature Reserve (GNNR), Kaihua County, Zhejiang Province in eastern China. GNNR covers a total area of 8107 ha and the topography is characterized by mountains with steep slopes. The elevation in the plot ranges from 446.3 to 714.9 m a.s.l., and the mean slope is approximately 37.5° and ranges from 12.8° to 62°. The mean annual temperature is 15.3 °C. The hottest month is July with a mean temperature of 27.9 °C, and the coldest is January with a mean temperature of 4.3 °C. The mean annual precipitation is 1787 mm, most of which falls between March and July. The mean annual number of frost-free days is 250. The dominant vegetation type in GNNR is subtropical evergreen broad-leaved forest dominated by Castanopsis spp., Cyclobalanopsis spp. (both Fagaceae) and Schima superba (Theaceae) (Hu et al. 2003). Approximately, 140,000 individuals ≥1 cm diameter at breast height (DBH 1.3 m) of 159 species, 103 genera, and 49 families have been recorded in the plot, including 26 shrub, 70 understory tree, and 63 canopy tree species (Zhu et al. 2010).
Establishing and monitoring census stations
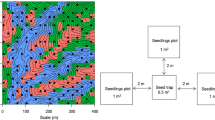
To monitor seed rain and seedling recruitment in the plot, a total of 130 census stations were established in a stratified random design along trails in May 2006. Each station consisted of one 0.5-m2 seed trap for collecting seeds and three 1-m2 seedling plots for monitoring seedling dynamics (N = 130 seed traps, 390 seedling plots). Each seed trap consisted of a square PVC frame supporting an open-topped, 1-mm nylon mesh bag suspended 0.8 m above the ground. All seeds and fruits that fell into the traps were identified to species every week. The total number of seeds of each species in each trap was based on a count of actual seeds in the trap or, in cases where entire fruits fell, calculated based on the mean number of seeds per fruit (calculated using ≥30 fruits) for that species.
The three seedling plots were placed 2 m away from each of three sides of each seed trap. In each seedling plot, all woody plants ≤1 cm DBH were tagged and identified to species. Survivors were checked in subsequent censuses and new recruits were tagged and identified. We censused seedling plots three times per year (May, August and November) in 2006 and 2007, and twice per year (May and August) since 2008.
We explored the relationship between recruitment and seedfall using 6 years of data. Seedfall data and recruit data were matched by year and station. We then used data from all stations and all year cohorts (seedfall from August 2006 to July 2012; recruitment from November 2006 to August 2012) to examine the overall relationship between local recruit and seedfall density for each species. Most seed rain in the study site falls between September and the following January, with peak seed fall in November and minimal seed fall from May through July (Du and Ma 2012), and germination and seedling recruitment occurs between February and July in this region (Shi et al. 2014). Therefore, we grouped weekly observations of seed fall from August to the following July and matched those data to the number of new seedling recruits tagged in the May and August censuses of each year. For the first 2 years when there was an additional seedling census in November, seedlings tagged in the November census were counted as new recruits in the subsequent May census if they were still alive (i.e., to be consistent with recruit counts and timing in the subsequent years). The detailed match of seed fall period and seedling recruitment period during the 6-year study can be found in Table S1. To examine the effects of existing seedlings on seed to seedling transition rates, we calculated the number of live conspecific and heterospecific seedlings that were recruited in previous years.
Study species
A total of 78 species were recorded from the traps and 117 species in the seedling plots. Species were excluded from analyses if (1) seeds passed through the 1-mm trap mesh or (2) seeds were recorded at fewer than 25 stations in 6 years. This left 13 focal species for analysis (Table S2). Seed numbers for each focal species and ranges of all predictor variables are included in Table S2.
Phylogenetic tree
We constructed a phylogenetic tree for the GTS forest plot species (159 species) following the methodology of Kress et al. (2009). The phylogeny was constructed using a DNA super matrix composed of three sequence regions: rbcL, matK, and trnH-psbA. Leaf tissue was collected from three individuals of each species at the plot and desiccated with silica gel. The phylogeny was constructed using the following steps: (1) total DNA was extracted from samples of leaf tissue with the CATB method (Khanuja et al. 1999); (2) three DNA barcode loci (rbcL, matK and trnH-psbA) were amplified and sequenced using Polymerase Chain Reaction (PCR); (3) each sequence was compared with the GenBank using Blast (Altschul et al. 1997); (4) The matK and rbcL loci were globally aligned and the trnH-psbA sequences were aligned within orders using MUSCLE software (Edgar 2004); (5) a super matrix was matched and built with the R package ‘Phylotools’ (Zhang et al. 2010); (6) three division GTR + GAMMA models were set to apply to the three DNA regions using RAxML software (Stamatakis 2006); (7) the approval rating of every node was confirmed by 1000 rapid bootstrap tests (see Fig. S1); (8) an ultrametric tree was finally obtained using software ‘r8s’ with non-parametric rate smoothing method (Sanderson 2003). Non-parametric rate smoothing was implemented in r8s assuming the age for the most recent common ancestor of seed plants at 180.0 MYR, of the Angiosperms 140 years, of the Fagaceae at 34.0 MYR, and the Rubiaceae at 56.0 MYR (2001). A more detailed description of the phylogeny for the GTS plot can be found in Feng et al. (2014) and Mi et al. (2016).
We measured the relative phylodiversity using the square-root of mean phylogenetic distance (in units of millions of years) from the focal species to all other (n−1) species in the quadrat (Letten and Cornwell 2015). We calculated the standard effect sizes of observed distances given an expected phylogenetic distance for a specified number of species, because phylogenetic mean distance may be influenced by species richness (Webb et al. 2006). We used a null model to calculate expected phylogenetic distance, generating 10,000 random neighborhoods at a given species richness and calculating the mean and standard deviation of the pairwise phylogenetic distances among species for each neighborhood. Species were drawn from the pool of all species in the seed traps for seed APd’ and from the pool of all species in the seedling plots for seedling APd’ in each year. We refer to the resulting metrics as relative average phylodiversity, APd’. Larger positive values of APd’ indicate communities whose species are less closely related to the focal species (Webb et al. 2006).
Data analyses
For each species, we used generalized linear mixed-effects models (GLMMs) with binomial errors to model the probability of transition from seed to seedling. We estimated this probability for each station and year combination by comparing the number of seeds in the trap to the number of subsequent recruits in the three adjacent plots. An equal number of seedlings and seeds would give a transition probability of 1; half the number of seedlings to seeds would give a transition probability of 0.5.
For each of the 13 species, we modeled this transition as a function of local conspecific seed density (CON), heterospecific seed density (HET), phylodiversity of heterospecific seeds (APd1), conspecific seedling density (Con.seedling; i.e., seedlings already present when the seeds fell), heterospecific seedling density (Het.seedling), phylodiversity of heterospecific seedlings (APd2), as well as the interaction between heterospecific seed density and APd1 and between heterospecific seedling density and APd2 as follows:
where p it is the odds (the ratio of the probability of a seed becoming a seedling to the probability of a seed not becoming a seedling) for station i and year t. Station (ɸ i ) and year (θt) were included as random effects to account for spatial and temporal variation in transition rates. The interaction terms with heterospecific density and APd were included in the model to allow for the possibility that heterospecific density would have a stronger effect if neighbors were more closely related. To aid model convergence, CON, HET, Con.seedling, and Het.seedling were log-transformed, and then all six independent variables (including APd1 and APd2) were standardized by subtracting the mean and dividing by the standard deviation of that variable.
Because seed arrival into seed traps may differ from seed arrival into the adjacent seedling plots, it is possible for values of seed density to be lower than seedling recruit density, leading to impossible (i.e., negative) transition rates. In the few cases when this occurred, we set seed density equal to recruit density, following Hille Ris Lambers et al. (2002) and Wright et al. (2005). This correction introduces a conservative bias against detecting conspecific NDD by increasing seed numbers whenever observed seed density was less than observed recruit density.
To test whether the effects of our model covariates varied across the 6 years, we ran models with a random slope term that allowed the effect of conspecific seed density, heterospecific seed density, APd1, conspecific seedling density, heterospecific seedling density, or APd2 to vary among years. We tested each term individually and compared a model with year-to-year variation in that term to the model without year-to-year variation using a likelihood ratio test.
All analyses were performed in the R 3.0.3 statistical software package (R Development Core Team 2014) with GLMMs run using the ‘lme4 1.1-7’ package (Bates et al. 2012).
Results
A total of 108,334 seeds and 6061 seedling recruits of the 13 focal species were recorded in the 130 seed traps and 390 seedling plots, respectively, over the 6-year period.
In agreement with our prediction, there was a significant negative effect of conspecific seed density on the seed-to-seedling transition probability for 12 out of the 13 focal species (Table 1; Fig. 1; Fig. S2). The effect of an additional log (1) unit of conspecific seeds decreased the log odds of transition by up to an order of magnitude, ranging from −0.23 in Fraxinus insularis to an order −4.38 in Neolitsea aureata.
Relationships between the recruitment probability of the most dominant species, Castanopsis eyrei, and conspecific seed density (a), heterospecific seed density (b), phylodiversity of heterospecific seeds (c), conspecific seedling density (d), heterospecific seedling density (e), and phylodiversity of heterospecific seedlings (f). Each symbol represents observed data from a seed–seedling pair consisting of one 0.5-m2 seed trap and one 1-m2 seedling plot in the same census station in each year. Symbol size is proportional to the number of stations with identical values of seeds and recruitment proportion. The solid lines represent the fitted lines of model predictions based on generalized linear mixed-effects models (GLMMs) with binomial errors that model with random intercepts for year and station. Only significant fitted lines are presented
In contrast to our prediction, only one species (Corylopsis glandulifera) showed a significant negative effect of heterospecific seed density on the seed-to-seedling transition (Table 1). The other 12 species showed no significant effect.
In contrast to our prediction, significant effects of mean phylogenetic distance of seeds were found for only four species (Table 1). All these effects were negative, i.e., increasing seed APd’ decreased seed-to-seedling transition rates. Only three species showed significant interactions between phylodiversity of seeds and heterospecific seed density (Table 1), with the direction of the effect varying among species.
We predicted a strong negative effect of conspecific seedling density on seed-to-seedling transition. However, seven focal species showed a significant effect and all were positive, with a greater probability of seed-to-seedling transition at higher conspecific seedling density (Table 1; Fig. S2). Four species showed a significant effect of heterospecific seedling density, with three showing positive effects and one showing a negative effect (Table 1).
Significant effects of phylodiversity of heterospecific seedlings were found for only two species (Quercus serrate and Alniphyllum fortunei), and the effects were positive for both (i.e., increasing seedling APd’ increased seed-to-seedling transition rates). Two species showed significant positive interactions between seedling APd’ and heterospecific seedling density (Table 1).
Annual variation in these parameters was significant for conspecific seed density (nine species) and conspecific seedling density (four species), but in only two species for heterospecific seed density and one species for heterospecific seedling density, and phylodiversity of both seeds and seedlings (Table 2).
Discussion
In our analysis of density dependence during the seed to seedling transition, we tested for effects of conspecific seed and seedling densities, heterospecific seed and seedling densities, and the phylogenetic diversity of seeds and seedlings on seed-to-seedling transition rates in 13 tree species in a subtropical forest. Our results revealed a significant and strong negative effect of local conspecific seed density in almost all focal species. In contrast, many species showed positive effects of conspecific seedling density on transition rates, and there was limited evidence for effects of the density or phylogenetic diversity of heterospecific seeds or seedlings. Thus, density-dependent seedling recruitment in the Gutianshan forest appears to be driven predominantly by negative effects of specialist natural enemies and/or intraspecific competition. At the same time, the positive relationship between transition rates and density of existing conspecific seedlings suggests that recruitment can also be influenced by habitat filtering and/or facilitation.
Effects of conspecific seed and seedling densities on seedling recruitment
Our results indicate that pervasive negative conspecific seed density dependence characterizes post-dispersal seed survival and seedling emergence in the Gutianshan subtropical evergreen forest. This is consistent with previous studies of density dependence across seed to seedling stages in a number of tropical and temperate forests (Harms et al. 2000; Hille Ris Lambers et al. 2002; Masaki et al. 2007; Muscarella et al. 2013; Umaña et al. 2016). Negative density-dependent recruitment of seedlings, that is, seeds of a given species are less likely to become established seedlings if the local density of that species is high, may be an important mechanism contributing to the maintenance of diversity in plant communities by preventing any one species from becoming dominant (Harms et al. 2000; Hille Ris Lambers et al. 2002). Ultimately, the influence of NDD at the seed to seedling stage on the structure and diversity of the forest will depend on whether such effects are reinforced or canceled out by processes occurring at later life stages, as well as how the strength of those effects varies temporally, spatially, and among species (e.g., Wright et al. 2005; Comita et al. 2010; Bachelot et al. 2015). In the Gutianshan forest, negative density-dependent mortality has also been documented for established seedlings (Chen et al. 2010), although habitat effects appear to play a larger role in shaping spatial patterns of adult trees (Zhu et al. 2013). This is consistent with a recent study from tropical forest in Panama, which found negative effects of conspecific neighbor density at early life stages (e.g., seedlings and saplings), but positive effects for adult trees suggesting a greater influence of habitat at later life stages (Zhu et al. 2015).
Despite the pervasive negative effects of conspecific seed density on seedling recruitment in our study, the local density of conspecific seedlings had either a neutral or positive effect during the seed to seedling transition for the focal species. This is in contrast to multiple studies that have shown strong negative effects of conspecific seedling density on seedling survival (e.g., Queenborough et al. 2007; Comita et al. 2010; Metz et al. 2010). Our results suggest that areas of high seedling densities are sites that are good for both germination and continued seedling survival, and that this habitat effect outweighed any negative effects of high conspecific seedling density (i.e., shared natural enemies or competition for resources).
We also found evidence that the strength of conspecific density dependence (including conspecific seeds and seedlings) varied among years. With only 6 years of data, we cannot yet link this variation to specific variables. However, previous studies at other sites suggest that year to year variation in density dependence can result from predator satiation in years of high seed production (e.g., Wright et al. 2005). In addition, studies have found that the strength of conspecific density dependence can vary with temporal variation in climate (Ibanez et al. 2007; Lin et al. 2012; Bachelot et al. 2015; Inman-Narahari et al. 2016) or changes in abiotic variables following disturbance (e.g., Comita et al. 2009). With continued monitoring, we will be able to test whether the variation in conspecific seed density effects at our site are driven by climate and/or fruit production (e.g., masting). Additional years of data will also allow us to include more species in our analysis. Although our study examined 76% of the seed rain, we were only able to model seed to seedling transition for a small proportion of the tree species present at our site due to sample size limitations.
NDD can be generated both by intraspecific resource competition and by the action of specialized natural enemies (Bell et al. 2006). However, density-dependent recruitment from seed to seedling stage is less likely to result from resource competition between germinating seedlings, since they have maternal reserves for germination and are, therefore, less dependent on local resources. As we matched seeds and recruits annually, some new recruits might still depend on maternal resources while others do not anymore and might be competing for resources. Therefore, intraspecific resource competition might play a role in driving NDD for some species. Even after establishment, resources competition between seedlings is thought to be negligible in forests where strong asymmetric competition with large trees limits seedling densities (Wright 2002; Paine et al. 2008). The density-dependent recruitment observed in our study is more likely caused by specialized natural enemies, such as insect herbivores or soil pathogens, though natural enemy effects could be offset to some degree by positive habitat effects or mutualists (Morris et al. 2007; Liang et al. 2015). While we did not assess causes of mortality in the present study, a growing number of studies have demonstrated conspecific density dependence mediated by host-specific pathogens, herbivores, and insect seed predators (e.g., Janzen 1980; Comita et al. 2010; Metz et al. 2010; Liu et al. 2012; Bagchi et al. 2014), although the effect of NDD could be counteracted by arbuscular mycorrhizal fungi (Liang et al. 2015). Experimental manipulations to differentially exclude different classes of natural enemies (i.e., insects, fungal pathogens; sensu Bagchi et al. 2014) are the next step for assessing the mechanisms underlying conspecific density-dependent seed to seedling transition rates at our study site. In addition, our results reflect only post-dispersal seed predation. Although we have no data from Gutianshan, pre-dispersal seed predation rates could also be high and density dependent, with such predators attracted either by high densities of fruits on individual adult plants or to areas with high densities of fruiting individuals (Janzen 1970; Jones and Comita 2010). Future studies examining pre-dispersal seed predation in the Gutianshan forest would contribute to a more complete understanding of how density dependence influences seedling recruitment.
Effects of heterospecific seed density on seed to seedling transition rates
In our study, seedling recruitment was unrelated to heterospecific seed input for almost all species tested, indicating that the effect of conspecific seed density on seed to seedling transition is greater than the effect of heterospecific seed density. Our results are consistent with one similar study that examined density dependence in seven temperate tree species and found greater effects of conspecific seed density on mortality than those of heterospecific seed density (Hille Ris Lambers et al. 2002). Other studies also showed stronger effects of conspecific neighbors than heterospecific neighbors, although these studies looked at the seedling stage (e.g., Comita and Hubbell 2009; Metz et al. 2010; Zhu et al. 2015).
Density effects of heterospecific seeds were found for only one species in our study. The negative effect of heterospecific seed density for that species suggests that density-responsive generalist seed predators might play a role in driving patterns of seedling recruitment. However, their effects could be offset by positive effects of generalist mutualists. It is possible that seed densities of particular species—specifically those that are attacked by the same seed predators, rather than all heterospecifics combined—may influence seed to seedling transition rates (e.g., Garzon-Lopez et al. 2015).
The seed to seedling transition was also positively correlated with heterospecific seedling density for three out of four focal species, suggesting that habitat effects are not always species specific. At the same time, strong interspecific associations of reproductive adults can contribute to positive interspecific associations in seed arrival and seedling recruitment (Wright et al. 2016). The positive effects of heterospecific seedling density could also be caused by ‘species herd protection’, i.e., high densities of heterospecific seedlings could decrease the detection of seeds by predators and increase the probability of seed to seedling transition (Wills 1996).
In addition, we found little evidence of year-to-year variation in the effect of heterospecific seeds and seedlings, indicating when such effects occur they are not highly variable over time. This could be caused by the fact that effect of heterospecific seeds and seedlings on the seed-to-seedling transition is very limited for most species. Monitoring of recruitment for >6 years may be necessary to detect temporal variation in these relatively weak effects.
Phylogenetic density dependence during the seed to seedling transition
We originally hypothesized that seed to seedling transition rates would be lower when co-occurring seeds were of closely related species, due to reports of phylogenetic signal in the host range of natural enemies (i.e., Novotny et al. 2002; Gilbert and Webb 2007). However, we found that phylodiversity (APd’) of seeds did not influence seed to seedling transition rates for the majority of species. In the four cases where significant effects of phylodiversity were detected, seed to seedling transition rates were higher when heterospecific seeds were more closely related to the focal species, contrary to expectations. The phylodiversity of heterospecific seedlings did positively influence seed to seedling transition rates for two species, suggesting that stronger competition and/or shared natural enemies between more closely related species may occur, but is not pervasive. For these two species, there was a significant interaction between phylodiversity and density of heterospecifics, suggesting that the phylogenetic relatedness of neighbor heterospecifics becomes more important at high heterospecific densities.
Our results contribute to a growing body of literature on phylogenetic neighborhood effects (e.g., Webb et al. 2006; Bagchi et al. 2010; Metz et al. 2010; Paine et al. 2012; Lebrija-Trejos et al. 2014; Zhu et al. 2015), which taken together suggest that the strength and direction of such effects vary among sites, life stages, and spatial scales. For example, Webb et al. (2006) found no effect of neighborhood phylodiversity for seedling survival at smaller scales (0.25 and 1 m2), but found significant effects of phylodiversity at relatively larger scales (4 and 36 m2) in a tropical forest in Southeast Asia. In Ecuador, Metz et al. (2010) found no evidence that a more diverse local seedling neighborhood reduced the per capita risk of first year seedling mortality, but did find that greater phylodiversity in the seedling neighborhood enhanced survival of established seedlings beyond their first year. In contrast, Zhu et al. (2015) found that established seedling survival was enhanced by closely related heterospecific neighbors, while the probability of survival of larger juvenile and adult trees was significantly reduced when heterospecific neighbors were closely related in the BCI forest. In the only previous study of phylogenetic density dependence in a subtropical forest, Liu et al. (2012) found both observational and experimental evidence indicating that seedling survival decreased with increasing phylogenetic distance to neighboring trees, with effects driven by soil pathogens. These conflicting effects of neighborhood phylogenetic relatedness observed both within and among study sites likely result from differences in host ranges of density-responsive natural enemies, as well as differences in the relative importance of natural enemies versus phylogenetically conserved habitat preferences for individual plant survival.
In addition, recent studies suggest that density effects in general can be spatially and temporally heterogeneous even at a single site (Comita et al. 2009; Lin et al. 2012; Bachelot et al. 2015; LaManna et al. 2016). However, in our study, we found very limited evidence of year-to-year variation in the effect of mean phylogenetic relatedness of heterospecific seeds or seedlings over the 6 years of the study.
Conclusion
As the first study to date to examine phylogenetic density dependence across the seed to seedling transition, our study contributes to a better understanding of the role of evolutionary history in shaping species interactions and to an increasing recognition of the complexity of neighborhood interactions in diverse plant communities. Our results show that conspecific NDD during the seed to seedling transition is pervasive at Gutianshan subtropical evergreen forest, while effects of heterospecific seed and seedling density and phylogenetic relatedness of seeds and seedlings are negligible for the majority of species. In addition, our results indicate that negative conspecific effects are typically driven by seed rather than seedling densities. These patterns suggest that seed and early seedling survival are likely driven by natural enemies that are species- and stage-specific, rather than generalist, at least in their effects on host survival. The strong conspecific NDD across seed to seedling stages may act as an important stabilizing mechanism (sensu Chesson 2000) for promoting species coexistence and maintaining diversity in the Gutianshan subtropical forest.
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25:3389–3402. doi:10.1093/nar/25.17.3389
Bachelot B, Kobe RK, Vriesendorp C (2015) Negative density-dependent mortality varies over time in a wet tropical forest, advantaging rare species, common species, or no species. Oecologia 179:853–861. doi:10.1007/s00442-015-3402-7
Bagchi R, Swinfield T, Gallery RE, Lewis OT, Gripenberg S, Narayan L, Freckleton RP (2010) Testing the Janzen-Connell mechanism: pathogens cause overcompensating density dependence in a tropical tree. Ecol Lett 13:1262–1269. doi:10.1111/j.1461-0248.2010.01520.x
Bagchi R, Gallery RE, Gripenberg S, Gurr SJ, Narayan L, Addis CE, Freckleton RP, Lewis OT (2014) Pathogens and insect herbivores drive rain forest plant diversity and composition. Nature 506:85–88. doi:10.1038/nature12911
Bates D, Maechler M, Bolker B (2012) lme4: Linear mixed-effects models using s4 classes. R Package Version 3.0.3. http://CRAN.R-project.org/package=lme4. Accessed 22 June 2012
Bell T, Freckleton RP, Lewis OT (2006) Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecol Lett 9:569–574. doi:10.1111/j.1461-0248.2006.00905.x
Chen L, Mi XC, Comita LS, Zhang LW, Ren HB, Ma KP (2010) Community-level consequences of density dependence and habitat association in a subtropical broad-leaved forest. Ecol Lett 13:695–704. doi:10.1111/j.1461-0248.2010.01468.x
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Evol Syst 31:343–366. doi:10.1146/annurev.ecolsys.31.1.343
Comita LS, Hubbell SP (2009) Local neighborhood and species’ shade tolerance influence survival in a diverse seedling bank. Ecology 90:328–334. doi:10.1890/08-0451.1
Comita LS, Uriarte M, Thompson J, Jonckheere I, Canham CD, Zimmerman JK (2009) Abiotic and biotic drivers of seedling survival in a hurricane-impacted forest. J Ecol 97:1346–1359. doi:10.1111/j.1365-2745.2009.01551.x
Comita LS, Muller-Landau HC, Aguilar S, Hubbell SP (2010) Asymmetric density dependence shapes species abundances in a tropical tree community. Science 329:330–332. doi:10.1126/science.1190772
Comita LS, Queenborough SA, Murphy S, Eck JL, Xu KY, Krishnadas M, Beckman N, Zhu Y (2014) Testing predictions of the Janzen-Connell hypothesis: a meta-analysis of experimental evidence for distance and density-dependent seed and seedling survival. J Ecol 102:845–856. doi:10.1111/1365-2745.12232
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and rain forest trees. In: der Boer PJ, Gradell GR (eds) Dynamics of numbers in populations. Center for Agricultural Publishing and Documentation, Wageningen, pp 298–312
Denslow JS (1987) Tropical rain forest gaps and tree species diversity. Annu Rev Ecol Evol Syst 18:431–451. doi:10.1146/annurev.es.18.110187.002243
Du YJ, Ma KP (2012) Temporal and spatial variation of seedfall in a broad-leaved evergreen forest in Gutianshan nature reserve of Zhejiang Province, China. Chin J Plant Ecol 36:717–728. doi:10.3724/SP.J.1258.2012.00717
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res 32:1792–1797. doi:10.1093/nar/gkh340
Feng G, Svenning J, Mi XC, Jia Q, Rao MD, Ren HB, Bebber DP, Ma KP (2014) Anthropogenic disturbance shapes phylogenetic and functional tree community structure in a subtropical forest. For Ecol Manag 313:188–198. doi:10.1016/j.foreco.2013.10.047
Fenner M (1985) Seed Ecology. Chapman and Hall, New York. doi:10.1007/978-94-009-4884-0
Fritschie KJ, Cardinale BJ, Alexandrou MA, Oakley TH (2013) Evolutionary history and the strength of species interactions: testing the phylogenetic limiting similarity hypothesis. Ecology 95:1407–1417. doi:10.1890/13-0986.1
Garzon-Lopez CX, Ballesteros-Mejia L, Ordoñez A, Bohlman SA, Olff H, Jansen PA (2015) Indirect interactions among tropical tree species through shared rodent seed predators: a novel mechanism of tree species coexistence. Ecol Lett 18:752–760. doi:10.1111/ele.12452
Gilbert GS, Webb CO (2007) Phylogenetic signal in plant pathogen-host range. Proc Natl Acad Sci USA 104:4979–4983. doi:10.1073/pnas.0607968104
Hanski I, Hansson Henttonen L (1991) Specialist predators, generalist predators, and the microtine rodent cycle. J Ecol 60:353–367. doi:10.2307/5465
Harms KE, Wright SJ, Calderon O, Hernandez A, Herre EA (2000) Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature 404:493–495. doi:10.1038/35006630
Hille Ris Lambers J, Clark JS, Beckage B (2002) Density-dependent mortality and the latitudinal gradient in species diversity. Nature 417:732–735. doi:10.1038/nature00809
Hu ZH, Yu MJ, Ding BY, Fang T, Qian HY, Chen QC (2003) Types of evergreen broadleaved forests and the species diversity in Gutianshan Mountain nature reserve. Chin J Appl Environ Biol 9:341–345
Ibanez I, Clark JS, LaDeau S, Hille Ris Lambers J (2007) Exploiting temporal variability to understand tree recruitment response to climate change. Ecol Monogr 77:163–177. doi:10.1890/06-1097
Inman-Narahari F, Ostertag F, Hubbell SP, Giardina CP, Cordell S, Sack L (2016) Density-dependent seedling mortality varies with light availability and species abundance in wet and dry Hawaiian forests. J Ecol 104:773–780. doi:10.1111/1365-2745.12553
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528. doi:10.1086/282687
Janzen DH (1980) Specificity of seed-attacking beetles in a Costa Rican deciduous forest. J Ecol 68:929–952. doi:10.2307/2259466
Johnson D, Beaulieu WT, Bever JD, Clay K (2012) Conspecific negative density dependence and forest diversity. Science 336:904–907. doi:10.1126/science.1220269
Jones FA, Comita LS (2010) Density-dependent pre-dispersal seed predation and fruit set in a tropical tree. Oikos 119:1841–1847. doi:10.1111/j.1600-0706.2010.18547.x
Khanuja SPS, Shasany AK, Darokar MP, Kumar S (1999) Rapid isolation of DNA from dry and freshsamples of plants producing large amounts of secondary metabolites and essential oils. Plant Mol Biol Rep 17:74. doi:10.1023/A:1007528101452
Kress WJ, Erickson DL, Jones FA, Swenson NG, Perez R, Sanjur O, Bermingham E (2009) Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc Natl Acad Sci USA 106:18621–18626. doi:10.1073/pnas.0909820106
LaManna JA, Walton ML, Turner BL, Myers JA (2016) Negative density dependence is stronger in resource-rich environments and diversifies communities when stronger for common but not rare species. Ecol Lett 19:657–667. doi:10.1111/ele.12603
Lebrija-Trejos E, Wright SJ, Hernandez A, Reich PB (2014) Does relatedness matter? Phylogenetic density-dependent survival of seedlings in a tropical forest. Ecology 95:940–951. doi:10.1890/13-0623.1
Lebrija-Trejos E, Reich PB, Hernandez A, Wright SJ (2016) Species with greater seed mass are more tolerant of conspecific neighbours: a key driver of early survival and future abundances in a tropical forest. Ecol Lett 19:1071–1080. doi:10.1111/ele.12643
Letten AD, Cornwell WK (2015) Trees, branches and (square) roots: why evolutionary relatedness is not linearly related to functional distance. Methods Ecol Evol 6:439–444. doi:10.1111/2041-210X.12237
Liang M, Liu X, Etienne RS, Huang F, Wang Y, Yu S (2015) Arbuscular mycorrhizal fungi counteract the Janzen-Connell effect of soil pathogens. Ecology 96:562–574. doi:10.1890/14-0871.1
Lin LX, Comita LS, Zheng Z, Cao M (2012) Seasonal differentiation in density-dependent seedling survival in a tropical rainforest. J Ecol 100:905–914. doi:10.1111/j.1365-2745.2012.01964.x
Liu XB, Liang MX, Etienne RS, Wang YF, Staehelin C, Yu SX (2012) Experimental evidence for a phylogenetic Janzen-Connell effect in a subtropical forest. Ecol Lett 15:111–118. doi:10.1111/j.1461-0248.2011.01715.x
Masaki T, Osumi K, Takahashi K, Hoshizaki K, Matsune K, Suzuki W (2007) Effects of microenvironmental heterogeneity on the seed-to-seedling process and tree coexistence in a riparian forest. Ecol Res 22:724–734. doi:10.1007/s11284-006-0308-1
Metz MR, Sousa WP, Valencia R (2010) Widespread density-dependent seedling mortality promotes species coexistence in a highly diverse Amazonian rain forest. Ecology 91:3675–3685. doi:10.1890/08-2323.1
Mi XC, Swenson NG, Jia Q, Rao MD, Feng G, Ren HB, Bebber DP, Ma KP (2016) Stochastic assembly in a subtropical forest chronosequence: evidence from contrasting changes of species, phylogenetic and functional dissimilarity over succession. Sci Rep 6:32596. doi:10.1038/srep32596
Morris WF, Hufbauer RA, Agrawal AA, Bever JD, Borowicz VA, Gilbert GS, Maron J, Mitchell CE, Parker IM, Power AG, Torchin ME, Vazquez DP (2007) Direct and interactive effects of enemies and mutualists on plant performance: a meta-analysis. Ecology 88:1021–1029. doi:10.1890/06-0442
Muscarella R, Uriarte M, Forero-Montaña J, Comita LS, Swenson NG, Thompson J, Nytch CJ, Jonckheere I, Zimmerman JK (2013) Life-history trade-offs during the seed-to-seedling transition in a subtropical wet forest community. J Ecol 101:171–182. doi:10.1111/1365-2745.12027
Narwani A, Alexandrou MA, Oakley TH, Carroll IT, Cardinale BJ (2013) Experimental evidence that evolutionary relatedness does not affect the ecological mechanisms of coexistence in freshwater green algae. Ecol Lett 16:1373–1381. doi:10.1111/1365-2745.12027
Naughton HR, Alexandrou MA, Oakley TH, Cardinale BJ (2015) Phylogenetic distance does not predict competition in green algal communities. Ecosphere 6:1–19. doi:10.1890/ES14-00502.1
Novotny V, Basset Y, Miller SE, Weiblen GD, Bremer B, Cizek L, Drozd P (2002) Low host specificity of herbivorous insects in a tropical forest. Nature 416:841–844. doi:10.1038/416841a
Paine CET, Harms KE, Schnitzer SA, Carson WP (2008) Weak competition among tropical tree seedlings: implications for species coexistence. Biotropica 40:432–440. doi:10.1111/j.1744-7429.2007.00390.x
Paine CET, Norden N, Chave J, Forget PM, Fortunel C, Dexter KG, Baraloto C (2012) Phylogenetic density dependence and environmental filtering predict seedling mortality in a tropical forest. Ecol Lett 15:34–41. doi:10.1111/j.1461-0248.2011.01705.x
Queenborough SA, Burslem DFRP, Garwood NC, Valencia R (2007) Neighborhood and community interactions determine the spatial pattern of tropical tree seedling survival. Ecology 88:2248–2258. doi:10.1890/06-0737.1
R Development Core Team (2014) R: a Language and environment for statistical computing. R Foundation for Statistical Computing. Available at http://www.R-project.org. Accessed 6 Mar 2014
Sanderson MJ (2003) r8 s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19:301–302. doi:10.1093/bioinformatics/19.2.301
Shi LL, Luo ZR, Xia JT, Zhao WJ, Wu YG, Ding BY (2014) Woody seedling dynamics and the correlation between habitat and regeneration mortality in a subtropical evergreen broad-leaved forest in China. Acta Ecol Sin 34:6510–6518. doi:10.5846/stxb201302150268
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi:10.1093/bioinformatics/btl446
Swamy V, Terborgh J, Dexter KG, Best BD, Alvarez P, Cornejo F (2011) Are all seeds equal? Spatially explicit comparisons of seed fall and sapling recruitment in a tropical forest. Ecol Lett 14:195–201. doi:10.1111/j.1461-0248.2010.01571.x
Umaña MN, Forero-Montaña J, Muscarella R, Nytch CJ, Uriarte M, Zimmerman JK, Swenson NG (2016) Inter-specific functional convergence and divergence and intra-specific negative density dependence underlie the seed-to-seedling transition in tropical trees. Am Nat 187:99–109. doi:10.1086/684174
Uriarte M, Condit R, Canham CD, Hubbell SP (2004) A spatially explicit model of sapling growth in a tropical forest: does the identity of neighbours matter? J Ecol 92:348–360. doi:10.1111/j.0022-0477.2004.00867.x
Venail PA, Narwani A, Fritschie K, Alexandrou MA, Oakley TH, Cardinale BJ (2014) The influence of phylogenetic relatedness on species interactions among freshwater green algae in a mesocosm experiment. J Ecol 102:1288–1299. doi:10.1111/1365-2745.12271
Webb CO, Gilbert GS, Donoghue MJ (2006) Phylodiversity-dependent seedling mortality, size structure, and disease in a Bornean rain forest. Ecology 87:123–131. doi:10.1890/0012-9658(2006)87
Wills C (1996) Safety in diversity. N Sci 149:38–42
Wright SJ (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130:1–14. doi:10.1007/s004420100809
Wright SJ, Muller-Landau HC, Calderon O, Hernandez A (2005) Annual and spatial variation in seed fall and seedling recruitment in a neotropical forest. Ecology 86:848–860. doi:10.1007/s004420100809
Wright SJ, Calderon O, Hernandez A, Detto M, Jansen PA (2016) Interspecific associations in seed arrival and seedling recruitment in a neotropical forest. Ecology 97:2780–2790. doi:10.1002/ecy.1519
Wu JJ, Swenson NG, Brown C, Zhang CC, Yang J, Ci XQ, Li J, Sha LQ, Cao M, Lin LX (2016) How does habitat filtering affect the detection of conspecific and phylogenetic density dependence? Ecology 97:1182–1193. doi:10.1890/14-2465.1
Zhang JL, Mi XC, Pei NC (2010) Phylotools: phylogenetic tools for ecologists. R package version 0.0.7.4. 201019
Zhu Y, Mi XC, Ren HB, Ma KP (2010) Density dependence is prevalent in a heterogeneous subtropical forest. Oikos 119:109–119. doi:10.1111/j.1600-0706.2009.17758
Zhu Y, Getzin S, Wiegand S, Ren HB, Ma KP (2013) The relative importance of Janzen-Connell effects in influencing the spatial patterns at the Gutianshan subtropical forest. PLoS One 8(9):e74560. doi:10.1371/journal.pone.0074560
Zhu Y, Comita LS, Hubbell SP, Ma KP (2015) Conspecific and phylogenetic density-dependent survival differs across life stages in a tropical forest. J Ecol 103:957–966. doi:10.1111/1365-2745.12414
Acknowledgements
We thank Dr. Lebrija-Trejos for sharing R code for calculating phylodiversity and Jinlong Zhang for building the phylogenetic tree. Yanjun Du was financially supported by Research Division of Biodiversity and Conservation Ecology (80006F2005) and National Natural Science Foundation of China (Y32H3A1001). Yanjun Du is appreciative of the visiting scholar program at Yale University and the China Scholarship Council, which supported his visit.
Author contribution statement
YJD, KPM and LSC developed the original idea; LC, XCM and YJD conducted the fieldwork. YJD, YQW, and LSC analyzed the data. YJD, SAQ, LSC and KPM wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Daniel Laughlin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, Y., Queenborough, S.A., Chen, L. et al. Intraspecific and phylogenetic density-dependent seedling recruitment in a subtropical evergreen forest. Oecologia 184, 193–203 (2017). https://doi.org/10.1007/s00442-017-3842-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3842-3