Abstract
In forests, negative density/distance-dependent seedling mortality (NDD) caused by natural enemies plays a key role in maintaining species diversity [Janzen–Connell (J–C) model]. However, the relative importance of natural enemies in mediating NDD under heterogeneous light conditions has remained unclear. We examined the relative importance of pathogens (i.e., soil pathogens, leaf diseases) on seedling performance in forest understories (FUs) and gaps (gaps) during a 3-year period (results of first year of our study have been previously reported). For the hardwood, Prunus grayana, we investigated seedling mortality, morbidity agents, growth, and root infection by arbuscular mycorrhizal fungi (AMF) beneath conspecific and heterospecific adults in FUs and gaps. Seedling mortality was higher beneath conspecific than heterospecific adults throughout 3 years at both sites, mainly due to continuous leaf disease (i.e., angular leaf spot), whereas damping-off diseases caused mortality only in the first year. Beneath each adult, seedling mortality was higher in FUs than in gaps until second year, but it did not differ between two habitat types in the third year, because leaf diseases caused severe damage even in gaps. Seedling mass was significantly lower beneath conspecific adults. AMF infection of seedlings was also lower beneath conspecific adults, while it was higher in gaps than in FUs beneath both adults. This study demonstrates that the J–C model in a hardwood tree, P. grayana is mainly driven by high NDD seedling mortality caused by airborne leaf diseases, which continuously attack seedlings in a NDD manner regardless of environmental light conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Janzen–Connell (J–C) model is commonly used to explain species diversity in forest communities (Janzen 1970; Connell 1971). Specially, it proposed that negative density- and/or distance-dependency (NDD) in juvenile mortality caused by species-specific natural enemies was a key factor in maintaining high tree diversity. There is an increasing body of evidence to support NDD seedling mortality in both tropical and temperate forests (e.g., Harms et al. 2000; Packer and Clay 2000; Hyatt et al. 2003; Kulmatiski et al. 2008; Mangan et al. 2010; Konno et al. 2011; McCarthy-Neumann and Ibanez 2013; Bagchi et al. 2014; Comita et al. 2014), but few studies have considered all causes of NDD simultaneously.
Numerous studies in tropical forests have revealed that soil pathogens and insect herbivores are important agents in determining NDD (Wright 2002; Terborgh 2012). Fungicide experiments, which have been the major tool employed to explore NDD seedling mortality in both tropical and temperate forests, have revealed the important role of soil-borne pathogens in determining NDD (e.g., Bell et al. 2006; Kotanen 2007). Furthermore, a recent study on pesticide application in a tropical forest demonstrated that soil pathogens were more important in determining NDD than insect herbivores, which affected only the abundance of tree species (Bagchi et al. 2014). These studies on pesticide application assumed that NDD seedling mortality was caused primarily by soil-borne pathogens (e.g., fungi, Oomycota), which cause damping-off diseases in young seedlings. Recent field studies in temperate forests, however, reported that seedling mortality caused by soil-borne damping-off diseases occurred primarily during the early growing season; thereafter, leaf diseases caused mortality in seedlings in an NDD manner (Seiwa et al. 2008; Yamazaki et al. 2009; Bayandala et al. 2016). Furthermore, a reciprocal seed sowing experiment revealed that leaf diseases caused more severe mortality compared with the damping-off diseases in an NDD manner in the current-year seedlings of two hardwood species, Cornus controvera and Prunus grayana (Bayandala et al. 2016). The synergistic negative effects of soil pathogens and leaf diseases may reinforce the J–C mechanism through significantly stronger NDD compared with the single effects of soil-borne pathogens. However, the duration of and the extent to which leaf diseases continue to affect the NDD and subsequent J–C mechanisms remain to be studied.
It has been thought that the damage caused by soil-borne damping-off diseases may be greater during the early stage of seedling growth and decrease with increasing plant maturity, because the infection of large and old plants is often limited to the fine roots (Sahashi et al. 1995; Martin and Loper 1999; but see Packer and Clay 2003). In contrast, leaf diseases would be expected to significantly reduce plant performance throughout the juvenile stage irrespective of plant age (see Seiwa et al. 2008), because each year the leaves of adult trees infected by air-borne pathogens generally fall on juveniles in the vicinity of the adults (Konno and Seiwa, unpublished data). It remains unexplored how and the extent to which relative influence of damping-off pathogens and leaf diseases on seedling mortality changes with the growth stage of juveniles. As the cumulative impact of NDD effects likely increases with the amount of time that an individual is exposed to natural enemies, longer-term studies would be more likely than shorter ones to detect significant effects of NDD on survival (see Terborgh 2012; Comita et al. 2014). In this study, we explored the relative importance of the two types of pathogens (i.e., soil-borne damping-off diseases and air-borne leaf diseases) in determining NDD seedling mortality until the end of the third growing season.
Although abiotic environments are remarkably heterogeneous along the gap—understory continuum in forest ecosystems (Denslow 1987; Seiwa 1998), the J–C hypothesis has been intensively tested in shaded forest understories (FUs; Packer and Clay 2000; Tomita et al. 2002; Mangan et al. 2010; Alvare-Loayza and Terborgh 2011), but it has rarely been studied in light-abundant gaps (gaps). Studies assessing the J–C hypothesis under both types of light conditions have demonstrated stronger NDD caused by soil pathogens under shaded than under high-light conditions (Augspurger 1983, 1984; O’Hanlon-Manners and Kotanen 2004; McCarthy-Neumann and Ibanez 2013). In contrast, in a reciprocal seed-sowing experiment, leaf diseases attacked the current-year seedlings in an NDD manner not only in FUs but also in gaps (Bayandala et al. 2016); however, the absolute survival rate in gaps was relatively high (55.7%), even beneath conspecific adults at the end of the first growing season. If such high survivorship continues after this stage, is it not applicable at high light levels. Multi-year studies, beyond first year survival are necessary to evaluate whether the J–C model is valid in gaps.
Exposed light conditions often enhance the infection rate of mycorrhizal fungi, which have positive effects on seedling performance near adults (Dickie et al. 2002; Hood et al. 2004; Nara and Hogetsu 2004). If the occurrence of the negative and positive effects of microbial communities on seedling performance is largely influenced by the environmental light conditions, it would be useful to study the mechanism by which and the extent to which the relative negative effects of accumulating antagonists (e.g., damping-off pathogens and leaf diseases) versus the relative positive effects of accumulating mutualists (e.g., mycorrhizae) change according to environmental light conditions.
In this study, we explore whether the J–C model is applicable in gaps using the reciprocal seed-sowing experiment described in Bayandala et al. (2016). To evaluate the ontogenetic changes in the relative importance of damping-off diseases and leaf diseases in determining seedling mortality of P. grayana seedlings beneath conspecific vs. heterospecific (i.e., C. controvera) adults, we measured the causes of mortality in the second year in addition to in the first growing season reported in Bayandala et al. (2016). Furthermore, we investigated the infection of mycorrhizal fungi and seedling mass in the third growing season to evaluate the effects of mutualists on seedling performance beneath conspecific vs. heterospecific adults.
Materials and methods
Species and forest studied
This study was conducted in a deciduous broad-leaved forest in the experimental forest of the Field Science Centre (FSC) of Tohoku University in northeastern Japan (38°48′N, 140°44′E, altitude 500–610 m). Mean monthly temperatures ranged from −2.4 °C (January) to 23.7 °C (July) during the study years (2011–2013). Mean annual temperature and rainfall were 10.3 °C and 1616 mm, respectively. During winter (December–April), the maximum snow depth is ~1.0 m. Trees in the study area have re-established after the clear-cutting of fuel forests or the abandonment of pasture 40–60 years ago, and the area has not been influenced by human activity for at least the past 40 years. The canopy layer is dominated by Quercus mongolica var. grossesrrata and Fagus crenata as mosaic patches, and the total basal area of the two species is ~48% (Terabaru et al. 2004). Other major canopy trees are Castanea crenata, Aesculus turbinata, Carpinus laxifolia, Acer mono, Quercus serrata, and Acer palmatum var. matsumurae. The relative basal area of the two study species is less than 0.97%.
The tree species, Prunus grayana and Cornus controversa (hereafter referred to by their genus names), are deciduous broad-leaf monoecious trees, common in cool-temperate regions of Japan. In both species, flowering occurs from May to June, and fruits ripen from September to October. Prunus seeds are usually dispersed by birds, although most are disseminated beneath the canopy (Seiwa et al. 2008), and the seeds germinate the following spring. Large fruit crops occur every 2–3 years, and dense seedling mats often form the following year (Seiwa et al. 2008). In shaded forest understories, Prunus juveniles persist for more than 20 years with relatively short (maximum height = 3 m) monolayer canopies with shrubby multi-stems (Hara et al. 1991; Seiwa et al. 2008). After gap formation, they rapidly grow into the canopy layer and start to reproduce (Hara et al. 1991).
Seed-sowing experiment
In a 50-ha area of the FSC, we selected 14 Cornus and 13 Prunus adult trees located at least 25 m from the nearest Cornus or Prunus adult (Bayandala et al. 2016). Two light-level treatments were designed: leaving the forest understory intact (FU) and opening the canopy near the focal tree (gap). For the gap-treatment, we randomly selected seven focal trees of each species and created a 7-m-radius gap around the tree bole by felling all trees and shrubs. The total number of Gap-treated focal adults was seven for each species, and the total number of FU-treated focal adults was six and seven for Prunus and Cornus, respectively. Three quadrats (35 × 45 cm) were randomly established within a 5-m range of each focal adult bole. In each of the three quadrats, 70 Prunus seeds were sown. The total number of Prunus seeds sown was 5460. To avoid severe seed predation by small mammals during the winter, the quadrats were covered with 0.2 × 0.2-cm mesh nets, whose edge was buried to a depth of 10 cm. The height of the nets was 10 cm. The nets were removed when seedlings began to emerge the following spring. Canopy openness (%) was lower in FUs than in gaps beneath the Cornus (FUs: 13.29 ± 0.25; gaps: 21.73 ± 0.73) and Prunus adults (FUs: 3.73 ± 0.92; gaps: 24.22 ± 0.83), whereas few differences were observed in soil water, pH, or concentrations of carbon (C), phosphorus (P), and nitrogen (N); however, the soil water content was lower in FUs than in gaps beneath Cornus adults (Table S1). Further details of the experimental design are provided in Bayandala et al. (2016).
Seedling survival and causes of mortality
All newly emerging Prunus seedlings in the two quadrats were tagged and monitored for survivorship until the end of the third growing season under each focal tree. The number of seedlings observed was provided in Table S2. All sources of seedling mortality were investigated during the first (2011; Bayandala et al. 2016) and second (2012) growing seasons (a total of five times) for all seedlings. The causes of mortality were classified as follows: (1) damping-off disease (initial necrosis near the soil level, leaf wilt, and finally, dislodging from the weakened stem); (2) leaf disease (for further identification, see below); (3) invertebrate herbivores (seedling death caused by predation of hypocotyls, >80% of leaf area and/or roots); (4) vertebrate herbivores [severing of main stems by mammals such as wood mice (Apodemus speciosus, A. argenteus), rabbits, and Japanese serows; we distinguished symptoms from vertebrates from those of pathogens and invertebrates based on the traces from mammals, such as the shape of teeth marks and cut edges, animal droppings and soil that had been turned by mammals]; (5) physical damage (uprooting, drying, fallen branches, and snow pressure); and (6) unknown.
Foliar pathogens were identified as follows: beneath Prunus adults, the death of Prunus seedlings was characterized by early shedding of leaves showing necrotic spots (5 mm in radius) with observable conidia and conidiophores characteristic of Phaeoisariopsis. Using these symptoms, we identified the pathogen as an angular leaf spot caused by Phaeoisariopsis prunigrayanae Sawada (Seiwa et al. 2008; Bayandala et al. 2016). Beneath Cornus adults, the most common form of seedling morbidity was characterized by early shedding of leaves, demonstrating zonate necrosis with discoid propagules on both the upper and lower surfaces caused by a pathogen that possessed conidia and conidiophores characteristic of Haradamyces (Masuya et al. 2009). We identified the pathogen as zonate leaf blight disease caused by Haradamyces foliicola (Masuya et al. 2009; Bayandala et al. 2016).
Seedling mass and root colonization by arbuscular mycorrhizal fungi
To estimate colonization of arbuscular mycorrhizal fungi (AMF) and seedling mass, 2-year-old Prunus seedlings were harvested (a total of 54 and 60 in FUs and gaps, respectively, beneath Cornus adults; and a total of 42 and 47 in FUs and gaps, respectively, beneath Prunus adults) on October 29, 2013. Seedlings were carefully removed from the ground by hand and, using a cutlass, washed with water; a total of 0.03 g (wet mass) of roots were then cut to estimate AMF colonization. The seedlings were dried at 70 °C for 60 h before weighing.
The roots were rinsed with tap water and cleaned by heating in 10% KOH at 90 °C for at least 1–2 h. The cleaned roots were rinsed with distilled water and bleached in 0.1% H2O2 for 15 min. The bleached roots were then rinsed with tap water and fixed in 2% HCl. The fixed roots were stained with Trypan blue and stored in lactoglycerol (lactic acid 875 mL, glycerin 63 mL, made up to a volume of 1 L with distilled water; Phillips and Hayman 1970; Gehring and Connell 2006; Fukasawa 2012). Root samples (1–3-cm segments of roots from each plant) were mounted on slides and viewed under a compound microscope at 200× magnification (McGonigle et al. 1990) to determine the percentage of the root length colonized by fungal structures. The presence of arbuscular mycorrhizal fungal arbuscules, vesicles, and coils was scored at 150 randomly selected intersections of root and reticle line per plant.
Statistical analysis
Since the data about seedling survival a binomial, the binomial logistic analysis was performed as follows:
where a i (i = 0‒2) is coefficient and p t indicates seedling survival (alive = 1 and dead = 0) at year t (2011‒2013), respectively. The terminal term indicates a random effect, and quadrate is nested within adult. Adult species and light level are categorical variables to explain the difference in the adult tree species above the quadrats (“heterospecific” or “conspecific”) and the light condition [“forest understoreys (FUs)” or “gaps”]. Logit-link function was used, since the binomial distribution was assumed for the model.
To evaluate the biotic environmental condition that causes the seedling mortality [leaf disease (LD), invertebrate herbivores (IH), and vertebrate herbivores (VH)], the multinomial logistic analysis was performed as follows:
where b i (i = 0‒2) is coefficient. P Cause indicates seedling mortality caused by LD, IH, and VH, whereas, p IN is the mortality of survived seedlings. In this model, the mortality of survived seedlings is the baseline-category, that is, the relative contribution of both adult species and light level to the seedling mortality against with the survived seedlings (IN). Here, dumping-off was eliminated from the analysis because of few data (n = 2). Multinomial distribution was assumed for the model.
Effects of adult species and light level on the infection of AMF and seedling mass were evaluated by the linear mixed model (LMM) as follows:
where c i (i = 0‒2) is coefficient. The terminal term indicates a random effect, and quadrate is nested within adult. Gaussian distribution was assumed for the model. Akaike’s information criterion (AIC) was used to select the best-fit model (Anderson and Burnham 2001). Fixed variables were successively deleted from the full model, including all variables mentioned above. We used R ver. 3.3.1 (The R Foundation for Statistical Computing 2016) with glmer function in the package lme4 for the binomial logistic model and LMM, and with vglm function in the package VGAM for the multinomial logistic model. Random effects were omitted if their inclusion did not improve the fit of the model. We used glm, instead of glmer, if random effects were not included.
Results
Seedling survival
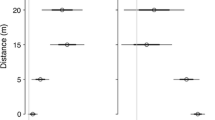
The seedling survival of Prunus was greater beneath Cornus than beneath Prurnus adults across all sampling times (Fig. 1), and in fact, the model with adult species was selected in the binomial logistic analysis for consecutive 3 years (Table 1).
Beneath the adults of both species, seedlings survival was generally higher in gaps than in FUs until the end of the second growing season (Table 1; Fig. 1). But light level was excluded from the model for 2013 (Table 1; Fig. 1), immediately means that the effect of light condition on the seedling survival vanished until the end of the third growing season (Fig. 1). It is noted that the model with the intercept only is the case of ‘heterospecific’ with ‘FUs’; i.e., the seedling survival beneath Cornus in the forest understorey. The size of intercept decreased year-to-year, immediately corresponds to the decrease of seedling survival beneath Cornus in the forest understorey.
Causes of seedling mortality
For 1-year-old Prunus seedlings, the most morbidity agent was leaf diseases, followed by vertebrate herbivores (mainly wood mice), whereas no seedlings were killed by damping-off diseases in either FUs or gaps beneath Cornus and Prunus adults (Fig. 2). For the relative probability of morbidity caused by leaf diseases, |z| value of adult species (+) and light level (−) was >2 indicating that the seedling mortality was higher beneath Prunus than beneath Cornus (Table 2) and was higher in FUs and in gaps (Table 2). Seedling mortality caused by vertebrate herbivores was also higher beneath Prunus than Cornus adults (z = 7.109), but it did not differ between the light levels (Table 2).
Mortality of 1-year-old Prunus grayana seedlings caused by a leaf diseases, b invertebrates, c vertebrates, and d physical damage in forest understories (FUs) and gaps (gaps) beneath heterospecific (Cornus controversa) and conspecific (P. grayana) adults. Values are mean ± SE (n = 272, 420, 330, 478, respectively)
Similar to Table 1, the model with the intercept only is the case of ‘heterospecific’ with ‘FUs’. The result suggests that the seedling mortality beneath Cornus in the forest understorey decreased in the order of ‘leaf disease’, ‘invertebrate’, and ‘vertebrate’.
The most prevalent leaf diseases beneath Cornus and Prunus adults were zonate leaf blight and angular leaf spot caused by the airborne pathogenic fungi Haradamyces foliicola and Phaeoisariopsis pruni-grayanae, respectively. Beneath Cornus adults, other leaf diseases were also observed, but their occurrence relative to zonate leaf blight and angular leaf spot was negligible.
Infection of AMF and seedling mass
GLM analysis revealed that both adult species and light level contributed to the percent infection of AMF and seedling mass (Table 3). The coefficient of adult species was negative indicating that the percent infection of AMF in Prunus seedlings was higher beneath Cornus than beneath Prunus (Fig. 3a). On the other hand, the coefficient of light level was positive indicating that the percent infection of AMF in Prunus seedlings was higher in gaps than in FUs.
Similar tendency was observed for the seedling mass, particularly in gaps (Fig. 3b). The seedling dry mass increased with the increasing percentage colonization of AMF beneath both adult species, but the increasing rate was very low beneath Prunus adults (Fig. S1).
Discussion
The experimental study of gaps in a temperate forest in northern Japan clearly demonstrated that the J–C hypothesis was valid even in light-abundant gaps (gaps), in addition to shaded forest understories (FUs), in a hardwood species, Prunus grayana. Although this seed-sowing experiment already demonstrated that Prunus seedlings died in a negative distance-dependent (NDD) manner in the first growing season even in gaps, the absolute mortality was remarkably high (55.7%; Bayandala et al. 2016), suggesting that the J–C model would be applicable in the gaps (Nathan and Casagrandi 2004). As expected, a 3-year period of observations demonstrated that NDD seedling mortality was continuously observed not only in FUs but also in gaps, with minor differences in the strength of NDD between the two habitat types (i.e., gaps vs. FUs). This was mainly due to the severe attack of a leaf disease, angular leaf spot, which occurred irrespective of the light conditions beneath conspecific adults.
Beneath Prunus adults, the most prevalent leaf disease, angular leaf spot, caused more severe leaf damage and earlier leaf shedding in the Prunus seedlings relative to that occurring beneath heterospecific adults (Cornus) in both FUs and gaps in the second growing season. This was similar to that already observed in the first growing season (Bayandala et al. 2016), suggesting a continuous strong NDD attack by the leaf disease (Fig. S2). Furthermore, our previous study found host preference of the angular leaf spot, which caused higher seedling mortality in Prunus than in Cornus seedlings, beneath Prunus adults (Bayandala et al. 2016). The leaves infected by angular leaf spot were shed every autumn within a 10- to 15-m radius around the boles of Prunus adults (Yamazaki et al. unpublished data). Hence, Prunus seedlings beneath the adults suffered severe damage annually. Thereafter, following the J–C model, higher seedling mortality would be expected to also be observed in conspecific seedlings throughout the juvenile stage.
Furthermore, our 3-year observation clearly revealed that the leaf disease, angular leaf spot, continuously attacked the conspecific seedlings throughout the juvenile stage irrespective of light conditions, whereas soil-borne damping-off diseases attacked only the current-year seedlings, especially in shaded FUs (Fig. S2; Bayandala et al. 2016). Previous studies have also reported that soil-borne pathogens were less effective under light-abundant conditions compared with shaded forest understories (Augspurger 1983; Hood et al. 2004). In this study, we clearly revealed that the airborne leaf diseases play a more important role compared with that of soil pathogens in facilitating NDD seedling mortality and subsequent the J–C mechanism even in light-abundant gaps.
Although most of the NDD studies to date indicate that NDD is most important in the earlier stages of plant growth, several studies have reported NDD patterns in the recruitment, growth, and survival in the sapling and adult stages (Peters 2003; Stoll and Newbery 2005; Piao et al. 2013; but see Zhu et al. 2015). This study also suggests that the effects of negative-distance dependency (NDD) on plant performance likely persist beyond the seedling stage, when the plants are strongly affected by highly virulent leaf diseases. This suggests that researchers must be mindful that different enemies (microbial, invertebrate, vertebrate) may be important in different systems and that studies that characterize effects over greater periods of time are likely the most relevant for interpreting the factors that regulate forest composition and diversity.
Beneath Cornus adults (i.e., “away”), infection rates of AMF in Prunus seedlings were higher in gaps than in FUs, indicating that abundant light enhances the occurrence of AMF (Gehring and Connell 2006). However, the large seedling mass in gaps may not be simply caused by the increased infection rate of AMF (Fig. S1), because it is possible that antagonists are simply reducing the Prunus seedlings’ performance. Further experimental study such as inoculation experiments in greenhouse should be needed to clarify the effects of AMF on seedling performance.
Recent studies demonstrated that rare species with a small relative abundance showed significantly stronger NDD compared with abundant species within a plant community (Comita et al. 2010; Mangan et al. 2010; Anacker et al. 2014). The species in our study, Prunus, is a rare species within the community of a temperate old-growth forest (relative basal area = 0.30%; Terabaru et al. 2004). The rare abundance is probably due to the strongly NDD seedling mortality caused by the leaf disease, angular leaf spot even in gaps. This indicates that the strength of NDD is significantly affected by the negative effects of accumulating antagonists (e.g., damping-off diseases and leaf diseases).
In conclusion, our study clearly demonstrated that leaf diseases play a crucial role in mediating the J–C mechanism in gaps, because leaf diseases continuously attack the seedlings in a distance-dependent manner regardless of environmental light conditions.
References
Alvare-Loayza, Terborgh J (2011) Fates of seedling carpets in an Amazonian floodplain forest: intra-cohort competition or attack by enemies? J Ecol 99:1045–1054. doi:10.1111/j.1365-2745.2011.01835.x
Anacker BL, Klironomos JN, Maherali H, Reinhart KO, Strauss SY (2014) Phylogenetic conservatism in plant-soil feedback and its implications for plant abundance. Ecol Lett 17:1613–1621. doi:10.1111/ele.12378
Anderson D, Burnham K (2001) Commentary on models in ecology. Bull Ecol Soc Am 82:160–161
Augspurger CK (1983) Seed dispersal by the tropical tree, Platypodium elegans, and the escape of its seedling from fungal pathogens. J Ecol 71:759–771. doi:10.2307/2259591
Augspurger CK (1984) Seedling survival of tropical tree species: interactions of dispersal distance, light-gaps, and pathogens. Ecology 65:1705–1712. doi:10.2307/1937766
Bagchi R, Gallery RE, Gripenberg S, Gurr SJ, Narayan L, Addis CE, Freckleton RP, Lewis OT (2014) Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature 506:85–88. doi:10.1038/nature12911
Bayandala, Fukasawa Y, Seiwa K (2016) Roles of pathogens on replacement of tree seedlings in heterogeneous light environments in a temperate forest: a reciprocal seed sowing experiment. J Ecol 104:765–772. doi:10.1111/1365-2745.12552
Bell T, Freckleton RP, Lewis OT (2006) Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecol Lett 9:569–574. doi:10.1111/j.1461-0248.2006.00905.x
Comita LS, Muller-Landau HC, Aguilar S, Hubbell SP (2010) Asymmetric density dependence shapes species abundances in a tropical tree community. Science 329:330–332. doi:10.1126/science.1190772
Comita LS, Queenborough SA, Murphy SJ, Eck JL, Xu K, Krishnadas M, Beckman N, Zhu Y (2014) Testing predictions of the Janzen-Connell hypothesis: a meta-analysis of experimental evidence for distance and density-dependent seed and seedling survival. J Ecol 102:845–856. doi:10.1111/1365-2745.12232
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: den Boer PJ, Gradwell GR (eds) Dynamics of populations. Centre for Agricultural Publishing and Documentation, Wageningen, pp 298–313
Denslow JS (1987) Tropical rain forest gaps and tree species diversity. Ann Rev Ecol Syst 18:431–451
Dickie IA, Koide RT, Steiner KC (2002) Influence of established trees on mycorrhizas, nutrition, and growth of Quercus rubra seedlings. Ecol Monogr 72:505–521. doi:10.1890/0012-9615(2002)072[0505:IOETOM]2.0.CO;2
Fukasawa Y (2012) Effects of wood decomposer fungi on tree seedling establishment on coarse woody debris. For Ecol Manag 266:232–238. doi:110.1016/j.foreco.2011.11.027
Gehring CA, Connell JH (2006) Arbuscular mycorrhizal fungi in the tree seedlings of two Australian rain forests: occurrence, colonization, and relationships with plant performance. Mycorrhiza 16:89–98. doi:10.1007/s00572-005-0018-5
Hara M, Takehara T, Hirabuki Y (1991) Structure of a Japanese beech forest at Mt. Kurikoma, north-eastern Japan. Saito Ho-on Kai Mus Res Bull 59:43–55
Harms KE, Wright SJ, Calderon O, Hernandez A, Herre EA (2000) Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature 404:493–495. doi:10.1038/35006630
Hood LA, Swaine MD, Mason PA (2004) The influence of spatial patterns of damping-off disease and arbuscular mycorrhizal colonization on tree seedling establishment in Ghanaian tropical forest soil. J Ecol 92:816–823. doi:10.1111/j.0022-0477.2004.00917.x
Hyatt LA, Rosenberg MS, Howard TG, Bole G, Fang W, Anastasia J, Brown K, Grella R, Hinman K, Kurdziel JP, Gurevitch J (2003) The distance dependence prediction of the Janzen-Connell hypothesis: a meta-analysis. Oikos 103:590–602. doi:10.1034/j.1600-0706.2003.12235.x
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Konno M, Iwamoto S, Seiwa K (2011) Specialization of a fungal pathogen on host tree species in a cross-inoculation experiment. J Ecol 99:1394–1401. doi:10.1111/j.1365-2745.2011.01869.x
Kotanen PM (2007) Effects of fungal seed pathogens under conspecific and heterospecific trees in a temperate forest. Can J Bot 85:918–925. doi:10.1139/B07-088
Kulmatiski A, Beard KH, Stevens JR, Cobbold SM (2008) Plant–soil feedbacks: a meta-analytical review. Ecol Lett 11:980–992. doi:10.1111/j.1461-0248.2008.01209.x
Mangan SA, Schnitzer SA, Herre EA, Mack KM, Valencia MC, Sanchez EI, Bever JD (2010) Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466:752–755. doi:10.1038/nature09273
Martin FN, Loper JE (1999) Soilborne plant diseases caused by Pythium spp.: ecology, epidemiology, and prospect for biological control. Crit Rev Plant Sci 18:111–181
Masuya H, Kusunoki M, kosaka H, Aikawa T (2009) Haradamyces foliicola anam. Gen. et sp. Nov., a cause of zonate leaf blight disease in Cornus florida in Japan. Mycol Res 113:173–181. doi:10.1016/j.mycres.2008.10.004
McCarthy-Neumann S, Ibanez I (2013) Plant–soil feedback links negative distance dependence and light gradient partitioning during seedling establishment. Ecology 94:780–786. doi:10.1890/12-1338.1
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. doi:10.1111/j.1469-8137.1990.tb00476.x
Nara K, Hogetsu T (2004) Ectomycorrhizal fungi on established shrubs facilitate subsequent seedling establishment of successional plant species. Ecology 85:1700–1707. doi:10.1890/03-0373
Nathan R, Casagrandi R (2004) A simple mechanic model of seed dispersal; predation and plant establishment: Janzen-Connell and beyond. J Ecol 92:733–746. doi:10.1111/j.0022-0477.2004.00914.x
O’Hanlon-Manners DL, Kotanen PM (2004) Evidence that fungal pathogens inhibit recruitment of a shade-intolerant tree, white birch (Betula papyrifera), in understory habitats. Oecologia 140:650–653. doi:10.1007/s00442-004-1625-0
Packer A, Clay K (2000) Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 404:278–281. doi:10.1038/35005072
Packer A, Clay K (2003) Soil pathogens and Prunus serotina seedling and sapling growth near conspecific trees. Ecology 84:108–119. doi:10.1890/0012-9658(2003)084[0108:SPAPSS]2.0.CO;2
Peters HA (2003) Neighbour-regulated mortality: the influence of positive and negative density dependence on tree populations in species-rich tropical forests. Ecol Lett 6:757–765. doi:10.1046/j.1461-0248.2003.00492.x
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–160
Piao T, Comita LS, Jin G, Kim JH (2013) Density dependence across multiple life stages in a temperate old-growth forest of northeast China. Oecologia 172:207–217. doi:10.1007/s00442-012-2481-y
Sahashi N, Kubono T, Shoji T (1995) Pathogenicity of Colletotrichum dematium isolated from current-year beech seedling exhibiting damping-off. Eur J For Pathol 25:145–151. doi:10.1111/j.1439-0329.1995.tb00329.x
Seiwa K (1998) Advantages of early germination for growth and survival of seedlings of Acer mono under different overstorey phenologies in deciduous broad-leaved forests. J Ecol 86:219–228. doi:10.1046/j.1365-2745.1998.00245.x
Seiwa K, Miwa Y, Sahashi N, Kanno H, Tomita M, Ueno N, Yamazaki M (2008) Pathogen attack and spatial patterns of juvenile mortality and growth in a temperate tree, Prunus grayana. Can J For Res 38:2445–2454. doi:10.1139/X08-084
Stoll P, Newbery DM (2005) Evidence of species-specific neighborhood effects in the dipterocarpaceae of a Bornean rain forest. Ecology 86:3048–3062. doi:10.1890/04-1540
Terabaru M, Yamazaki M, Kano K, Suyama Y, Seiwa K (2004) Influence of topographic positions on tree disribution patterns in a temperate broad-leaved deciduous forest. J Integr Field Sci 20:21–26
Terborgh J (2012) Enemies maintain hyperdiverse tropical forests. Am Nat 179:303–314. doi:10.1086/664183
Tomita M, Hirabuki Y, Seiwa K (2002) Post-dispersal changes in the spatial distribution of Fagus crenata seeds. Ecology 83:1560–1565. doi:10.1890/0012-9658(2002)083[1560:PDCITS]2.0.CO;2
Wright SJ (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130:1–14. doi:10.1007/s004420100809
Yamazaki M, Iwamoto S, Seiwa K (2009) Distance- and density-dependent seedling mortality caused by several diseases in eight tree species co-occurring in a temperate forest. Plant Ecol 201:181–196. doi:10.1007/s11258-008-9531-x
Zhu Y, Comita LS, Hubbell SP, Ma K (2015) Conspecific and phylogenetic density-dependent survival differs across life stages in a tropical forest. J Ecol 103:957–966. doi:10.1111/1365-2745.12414
Acknowledgements
We are very grateful to two anonymous reviewers, who provided useful comments on the article. We thank Tomonori Sasaki, Yu Fukasawa, and members of the Laboratory of Forest Ecology, Tohoku University, for help with the experiments. This research was funded by the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 23380079 to KS).
Author contribution statement
KS conceived and designed the experiments. B and KS performed the experiments. KM analyzed the data. KS and B wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Katherine L. Gross.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bayandala, Masaka, K. & Seiwa, K. Leaf diseases drive the Janzen–Connell mechanism regardless of light conditions: a 3-year field study. Oecologia 183, 191–199 (2017). https://doi.org/10.1007/s00442-016-3757-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3757-4