Abstract
The last reproductive event of a female is often associated with major changes in terms of both maternal and offspring life-history traits. However, the nature of these changes and the importance of population-specific environmental constraints in shaping their expression are difficult to predict and, as a consequence, poorly understood. Here, we investigated whether and how life-history traits vary between reproductive events and whether this variation is population-dependent in the European earwig Forficula auricularia. In this insect species, females produce up to two clutches during their lifetime and express extensive forms of maternal care. We conducted a common garden experiment, in which we measured 11 life-history traits of the first and second clutches of 132 females originating from three populations. Our results showed that clutch size was higher and the level of care expressed towards juveniles lower in second as compared to the first clutches in all three populations. In contrast, we found a population-specific effect on whether and how the reproductive event shaped juvenile quality and a trade-off between egg developmental time and female weight at hatching. Overall, these findings emphasise that the last reproductive event of a female entails both positive and negative effects on various life-history traits of the female herself and her clutch of juveniles. Moreover, our study stresses the importance of population idiosyncrasies on the expression and nature of such cohort-specific effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life-history traits are crucial phenotypic variants that reflect how an organism allocates time and energy to optimise its development and maximise its reproduction (Roff 1992; Stearns 1992; Flatt and Heyland 2011). These traits can be divided in a great diversity of categories, including the reproductive capability and success of an individual (e.g. age and size at maturity, number and size of offspring), as well as its physiology (e.g. immunocompetence and senescence) and behaviours (e.g. aggressiveness, reproductive tactics and parental care; Southwood 1977; Brommer 2000; Reznick et al. 2000). Studying the determinants of life-history traits is thus a pivot of evolutionary biology and ecology, as it helps to better understand the evolutionary constraints that shape the multiple aspects of an individual’s fitness and, more generally, the factors that drive the evolution of populations and species (Stearns 1992; Schluter 2001; Rundle and Nosil 2005).
Numerous factors are known to either positively or negatively influence an individual’s life-history traits (see examples in Stearns 1992; Nylin and Gotthard 1998; Flatt and Heyland 2011). The nature of the effects of being produced during the late reproductive period of a female (thereafter called ‘cohort’), however, remains controversial. On the one hand, offspring can benefit from being produced in a late cohort. This is mostly because parental effort into reproduction is predicted to increase when the parents’ prospects for survival and future reproduction decline (the ‘terminal investment’ hypothesis; Williams 1966; Clutton-Brock 1984; Javoiš 2013), and this might benefit offspring if the increase is allocated to parental care (rather than to the production of additional offspring). Over the last decades, numerous studies conducted across species and taxa provided empirical support for this prediction (Fox and Czesak 2000; Hasselquist and Nilsson 2009; Royle et al. 2012; Santos and Nakagawa 2012). For instance, females of the North American red squirrels Tamiasciurus hudsonicus and the burying beetle Nicrophorus orbicollis have been shown to produce juveniles of better quality (and/or more juveniles) in late compared to early cohorts (Descamps et al. 2007; Creighton et al. 2009). Similarly, parents showed higher levels of care for late- as compared to early produced juveniles in the collared flycatcher Ficedula albicollis and in N. orbicollis (Part et al. 1992; Creighton et al. 2009).
On the other hand, offspring may also suffer from being produced in a late cohort. This cost often results from physiological constraints that hamper the expression of parental investment and thus ultimately decrease offspring quality (reviewed in Javoiš 2013). Maternal senescence is a well-known physiological constraint that negatively and specifically affects juveniles of late cohorts, as ageing females usually become unable to increase or even maintain their reproductive efforts (McNamara and Houston 1996; Nussey et al. 2013; Kowald and Kirkwood 2015). For instance, in the grey seal Halichoerus grypus, maternal and offspring body mass, weaning mass and the level of lactation have all been shown to decline with age due to physiological degeneration (Bowen et al. 2006).
Importantly, (often population-specific) environmental constraints such as food restriction and pathogen presence can either promote or hamper the expression of a terminal investment and may thus have a substantial influence on the traits of late cohort offspring. For instance, low food intake has been shown to inhibit the expression of terminal investment by females of the Alpine chamois Rupicapra rupicapra (Mason et al. 2011), whereas food restriction and pathogen presence favoured its expression in males of the yellow mealworm beetle Tenebrio molitor (Krams et al. 2015) and of the blue-footed booby Sula nebouxii (Velando et al. 2006), respectively. Although environmental constraints often vary between populations, the occurrence of population-specific differences in the life-history traits expressed by individuals from early and late cohorts remains surprisingly poorly studied (see Mason et al. 2011; Javoiš 2013; Vincze et al. 2013).
In this study, we investigated whether maternal and offspring life-history traits vary between initial and terminal reproductive events in the European earwig Forficula auricularia, and whether the occurrence and nature of this variation are population-specific. In this insect species, females produce up to two clutches during their 1-year lifespan: the initial clutch of eggs is generally produced in early winter and hatches in the following early spring, while the terminal clutch (when present) is produced in mid-spring and hatches in the following early summer (Meunier et al. 2012). From the date of egg laying until several weeks after egg hatching, mothers provide extensive forms of care to their eggs and juveniles (called nymphs) that include the protection against predators and pathogens, as well as the provisioning of nymphs with food (e.g. through regurgitation; Lamb 1976; Kölliker 2007; Boos et al. 2014; Koch and Meunier 2014; Diehl et al. 2015; Kölliker et al. 2015).
In a previous study focusing on differences between semelparous and iteroparous F. auricularia females, Meunier et al. (2012) showed that the second clutches of iteroparous females were smaller and developed faster than their first clutches. However, it remains unknown whether this variation (1) shapes other key life-history traits of earwigs, such as maternal condition and the expression of maternal care and, more importantly, whether it (2) depends on the studied population. Here, we addressed these questions by comparing a total of 11 life-history traits measured in the first and second clutches of 132 F. auricularia females sampled in three distant populations in Europe. These traits encompassed measures of clutch quantity and quality, maternal condition during post-hatching family interactions, as well as the expression of brood defence and food provisioning, two important forms of post-hatching maternal care (Meunier and Kölliker 2012; Thesing et al. 2015).
Materials and methods
Field sampling and laboratory breeding
Our experiment started with 696 F. auricularia individuals sampled in September 2014 in three populations located in Girona (Spain, n = 120 females and 118 males), Montblanc (Spain, n = 118 females and 107 males) and Vincennes (France, n = 119 females and 114 males) (Fig. 1). All these populations belong to the same F. auricularia genetic clade B (Wirth et al. 1998) (M. Veuille and X. Espalader, unpublished data), and are subjected to different environmental conditions in terms of altitude, temperature and precipitation (see details in Fig. 1). Note that neither of these populations corresponded to the population studied in Meunier et al. (2012).
Location and climatic details of the three studied populations of F. auricularia. For each population, we provide the GPS coordinates and altitude, as well as the mean temperatures (T; minimum - maximum) and the mean precipitations (P) recorded in winter and spring over the last 50 years. Information from the Worldclim data base (http://www.worldclim.org/)
Individuals from all three populations were maintained under standard laboratory conditions adapted from Meunier et al. (2012). This allowed the expression of inherited population-specific traits while controlling for plastic responses to the environment. The setup started by haphazardly distributing all field-sampled individuals of each population among large plastic containers to form groups of 58 ± 0.58 (mean ± SE) individuals encompassing a maximum of 30 males and 30 females. These groups were maintained at 20 °C, 60 % humidity and 14:10 h light: dark photoperiod (thereafter called summer conditions) to allow uncontrolled mating (Sandrin et al. 2015). Two months later, a random sample of 237 females (Girona n = 71; Montblanc n = 59; Vincennes n = 107) was isolated to allow egg production. These females were maintained under complete darkness and 60 % humidity, with a temperature of 10 °C for 2 weeks, and then 5 °C for 3 months to mimic winter conditions. Spring was subsequently simulated by first increasing the temperature to 10 °C and 1 week later to 15 °C. When the first nymph hatched, the corresponding family was transferred to and maintained under summer conditions (see above) to favour nymph development. Fourteen days later, each female was isolated to mimic natural family disruption and subsequently maintained under complete darkness to allow second clutch production (Meunier et al. 2012). The laboratory rearing of second clutch eggs and nymphs was similar to the above detailed rearing of the first clutches, except that eggs were maintained under summer temperatures. Among the 209 females that produced first clutch nymphs (28 females did not; Girona n = 9; Montblanc n = 5; Vincennes n = 14), a total of 136 females also produced a second clutch (see “Results”), and were thus used in the present study. For a comparison of the life-history traits expressed by semelparous and iteroparous females, please see Meunier et al. (2012).
All large plastic containers (30 × 17 × 21 cm) contained humid sand as ground material and an egg cardboard as shelter. Isolated females and, upon hatching, their nymphs were set up in Petri dishes (diameters 8.5) containing humid sand as substrate and a plastic tube (cut in half) as shelter. All the tested individuals received an ad libitum amount of standard laboratory food (see composition in Kramer et al. 2015). However, we did not provide food to the isolated females between egg laying and hatching, as they typically stop feeding during this period (Kölliker 2007). All temperature changes were implemented gradually over 4 days. Note that all 14 days old nymphs (of both first and second clutches) were discarded from the experiment.
Measurements of 11 life-history traits
We investigated the effects of population and juvenile cohort on a total of 11 life-history traits reflecting clutch quantity and quality, maternal condition during post-hatching family interactions, and post-hatching maternal care. These measurements started with the (1) egg developmental time, which was defined as the number of days between the first day of egg laying and the first day of egg hatching. We then counted (2) the number of eggs produced within 3 days after the first egg laying, (3) the number of nymphs present 1 day after the first egg hatching and (4) the number of nymphs alive 14 days after hatching. We used different timespans for the first two countings, because F. auricularia females generally need up to 3 days to finish the deposition of their clutch of eggs, whereas egg hatching is well synchronised within each clutch and is generally completed over a single day (Koch and Meunier 2014). We also measured the (5) mean weight of nymphs 1 day and (6) 14 days after egg hatching. To this end, a group of 10 nymphs (or all nymphs if brood size was lower than 10) was haphazardly sampled in each brood and weighed to the nearest 0.01 mg using a microscale (model MYA5; PESCALE, Bisingen, Germany). We then recorded (7) the developmental time of nymphs from the first to the second developmental instar, which was obtained by counting the number of days between egg laying and the emergence of the first second instar nymph in each clutch. This measurement is known to reflect the developmental time of the entire clutch in F. auricularia (Gómez and Kölliker 2013). Finally, changes in maternal condition over the period of post-hatching family interactions were measured by weighing each mother (8) 1 day and (9) 14 days after the first egg hatching.
Post-hatching maternal care was estimated by measuring food provisioning and brood defence. (10) Food provisioning was measured in 102 clutches (a haphazard sample of 34 clutches was omitted from this measurements due to time constraints; see details in Table 1) using a standard method relying on the fact that ingested coloured food is visible through the partially transparent cuticle of first instar nymphs (Staerkle and Kölliker 2008; Kölliker et al. 2015; Kramer et al. 2015). In brief, food was removed from each family on day 5 after hatching. Twenty-four hours later, mothers were isolated and had access to a green pollen pellet (naturally yellow-coloured pollen coloured with blue dye; Hochland Bio-Blütenpollen by Hoyer; Food die by DEKO BACK) for 1 h. Afterwards, mothers were returned to a standardised number of 20 of their own nymphs (or all of their nymphs if the clutch had less than 20 nymphs; mean number of nymphs used in this test ± SE = 18.9 ± 0.21) to allow family interactions. Finally, the number of green-coloured and non-green-coloured nymphs was counted 15 h later using a stereomicroscope (Leica S8 APO, 10×). Note that food provisioning was not measured in clutches with less than five nymphs (n = 5), and that the level of food provisioning was independent of the number of recipient nymphs (Spearman correlation test, ρ = −0.08, S = 23869, P = 0.577). During the food provisioning test, all left-over nymphs were maintained in their original Petri dish and provided with standard laboratory food. To follow the treatments detailed above, all unused families were starved for 24 h on day 5 and fed again on day 6.
Finally, (11) brood defence was assessed using a previously established method (Thesing et al. 2015), in which each female was standardly poked on the pronotum with a glass-capillary (one poke per second) to record the number of pokes necessary to induce her running away beyond a distance of twice the female’s body length. Brood defence was tested at day 4, day 8 and day 12 after hatching, and the average of these three values was used as brood defence. The brood defence test was carried out under red light, as earwigs are nocturnal.
Statistical analyses
We were first interested in testing the effects of population and the juveniles’ cohort on the life-history traits of mothers and offspring. To control for possible non-independence among the 11 measured traits, we first conducted a Principal Component Analysis (PCA) to obtain non-correlated principal components (PCs) reflecting single or combinations of different life-history traits. In this PCA, the values of food provisioning were logit-transformed and the values of brood defence were log-transformed to comply with normal distributions. The PCA was conducted by scaling the data to unit variance, and running a regularised iterative MFA method (with K-fold cross-validation) to handle the few missing values in the dataset (Lê et al. 2008; Husson et al. 2011). The resulting and selected PCs (Table 2) were then analysed separately using Linear Mixed Models (LMMs), in which the population, the juveniles’ cohort and their interaction were entered as fixed factors, and female ID was used as a random factor (Table 3). In case of significant interactions between population and the juveniles’ cohort (see “Results”), pairwise comparisons among each combination were tested using Tukey HSD tests.
To determine the occurrence of an investment trade-off between first and second clutches, we also tested whether the life-history traits measured in second and first clutches were negatively (i.e. reproductive trade-off) or positively (i.e. quality-dependent reproduction) correlated, and whether the occurrence and nature of these correlations were similar across populations. To this end, we conducted a series of five General Linear Models (GLM), in which the second clutch values of each of the five above PCs were used as a response variable, while the corresponding first clutch values, the population and their interaction were entered as fixed variables. All statistical analyses were conducted using R v.3.2.1 (http://www.r-project.org/) loaded with the packages car, FactoMineR, missMDA, lmerTest and lsmeans.
Results
Overall, 52 (83.8 %) of the 62 females from Girona, 39 (72.2 %) of the 54 females from Montblanc and 45 (48.4 %) of the 93 females from Vincennes produced two clutches of nymphs (Pearson’s Chi-squared test, χ 22 = 22.25, P < 0.0001). These proportions were significantly smaller in Vincennes compared to both Girona (χ 21 = 20.00, P value < 0.0001) and Montblanc (χ 21 = 7.93, P value = 0.005), but comparable between the two Spanish populations (χ 21 = 2.32, P = 0.128).
The PCA conducted on the life-history traits measured in the first and second clutches of these 136 females provided 11 orthogonal principal components (PCs), of which we extracted the first five ones (total variance explained = 87.2 %, Table 2). The first component (PC1) was highly and positively loaded with the number of eggs as well as with the number of nymphs at day 1 and day 14, therefore overall positively reflecting clutch size. The second component (PC2) revealed a positive association between the nymphs’ weights on day 1 and day 14, as well as post-hatching developmental speed, which overall reflects nymph quality. Accordingly, high values of PC2 indicate that clutches consisted of heavy nymphs which quickly moulted into the second developmental instar, whereas small values indicate clutches with light nymphs which required more time to develop into the second instar. The third component (PC3) revealed a trade-off between egg developmental time and the mother’s weight at egg hatching. High values of PC3 thus represent clutches in which eggs required a long time to develop and mothers were light at egg hatching (probably because they spent more time caring for the eggs), whereas small values reflect clutches in which eggs developed fast and mothers were heavy at egg hatching. Finally, the fourth (PC4) and fifth (PC5) components were solely and positively loaded with brood defence and food provisioning, respectively.
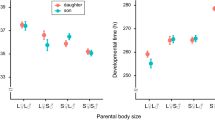
An interaction between population and the juvenile’s cohort significantly shaped PC1, PC2 and PC3 (Table 3). For PC1, pairwise comparisons within the statistical model showed that first clutches were overall larger than second clutches within each population, whereas the interaction emphasised that this difference was smaller in Vincennes as compared to Girona and Montblanc (Fig. 2a; Table S1). In contrast, the interactive effect of population and the juveniles’ cohort on PC2 revealed that first clutch nymphs were of lower quality than second clutch nymphs in Vincennes, but not in Girona and Montblanc (Fig. 2b). Similarly, the significant interaction shaping PC3 showed a slower developmental time of eggs and a lighter weight of females at hatching in the first compared to the second clutches in Girona and Montblanc, but not in Vincennes (Fig. 2c). Note that the raw values of each trait can be found in Table 1.
Interactive effects of population and the juveniles’ cohort on the principal components reflecting clutch size (PC1), nymph quality (PC2) and the trade-off between egg developmental time and the mother’s weight at egg hatching (PC3). Values represent mean ± SEM. Different letters indicate significant differences (see Table S1 for detailed values of pairwise comparisons)
The values of PC4 and PC5, which respectively reflected the level of brood defence and food provisioning, were overall, higher in the second compared to first clutches (Table 3; Fig. 3). However, they were independent of the population or of an interaction between population and the juvenile’s cohort (Table 3).
Finally, we found consistencies (i.e. positive associations) across the two breeding attempts (Table 4) in terms of clutch size (PC1; model estimate ± SE = 0.697 ± 0.039, t = 17.55, P < 0.0001), nymph quality (PC2; estim. = 0.375 ± 0.091, t = 4.13, P < 0.0001), the trade-off between egg developmental time and maternal weight at egg hatching (PC3; estim. = 0.335 ± 0.074, t = 4.55, P < 0.0001) and brood defence (PC4; estim. = 0.469 ± 0.092, t = 5.10, P < 0.0001), but not in terms of food provisioning (PC5; estim. = 0.086 ± 0.083, t = 1.04, P = 0.301; Fig. 4). The occurrence and strength of these associations were independent of the population (interaction in Table 4). These statistical models also confirmed the results of the above analyses by showing that both clutch size and the trade-off between egg developmental time and the mother’s weight at egg hatching observed in the second clutches were population specific (Table 4; Fig. 2).
Discussion
This study overall shows that initial and terminal clutches of the European earwig F. auricularia exhibit differences in life-history traits, and that the occurrence and nature of these differences depend on the trait and/or the studied population. Specifically, we found that nymph quality was higher in the second compared to first clutches of Vincennes females only, and that egg developmental time and female weight at hatching were longer and lighter, respectively, in initial compared to terminal clutches of females from Girona and Montblanc. In contrast, clutch size was lower and maternal care—i.e. brood defence and food provisioning—higher in first as compared to second clutches in all three studied populations. Interestingly, the expression of all traits measured in the first clutches (but food provisioning) was positively associated with the expression of these traits in the second clutches, and the occurrence and nature of these associations were independent of the studied population.
Across species and taxa, the expression of life-history traits often varies among populations. This is the case, for instance, for offspring mass and number in the scorpion Centruroides vittatus (Brown and Formanowicz 1995), for the juveniles’ development and survival in the Mediterranean fruit fly Ceratitis capitata (Diamantidis et al. 2011), and for the level of parental care in the Kentish plover Charadrius alexandrinus and the snowy plover C. nivosus (Vincze et al. 2013). In earwigs, our results do not only demonstrate that each population is characterised by a specific proportion of females producing two clutches under identical (laboratory) conditions (see also Wirth et al. 1998; Meunier et al. 2012), but also that the population determines whether and how certain life-history traits differ between initial and terminal clutches.
Although females from the three populations were maintained under the same laboratory conditions, we found that their population of origin determined the effects of the juveniles’ cohort on nymph quality and the trade-off between egg developmental time and female weight at hatching. This is important, as it reveals that these effects are not determined by the environmental conditions experienced during family life, but instead depend on the conditions experienced during female development and/or on (the conditions that have shaped) the evolutionary history of the population. In line with the first hypothesis, the quality of the environment experienced by F. auricularia juveniles during their development is known to affect the life-history traits of the resulting adults, for instance in terms of investment into second clutch production and maternal care (Wong and Kölliker 2014; Thesing et al. 2015). Conversely, harsh winter conditions have been suggested to select for a delayed production of first clutch eggs (Meunier et al. 2012), which may benefit females by shortening the developmental time of their first clutch eggs and thus allowing the mothers to exhibit a higher condition (i.e. a higher body weight) at hatching. Ultimately, such conditions may prevent differences in the trade-off between egg developmental time/female weight at hatching between first and second clutches (which are always produced in spring, under favourable environmental conditions), and might thus explain its absence in females from Vincennes, the population with the coldest winter (Fig. 1) and the latest production of first clutch eggs (Table 1). Whether our results are the outcome of the interactive or independent effects of these two processes remain to be further studied.
Contrary to the population-specific effects described above, we found that earwig females expressed higher levels of post-hatching care towards second compared to first clutches in all three tested populations. Notably, this effect was present even if the females’ weight at the beginning of family life (i.e. at egg hatching) was comparable between the two clutches (see Table 1). These results are overall in line with a terminal investment of F. auricularia females in terms of maternal care (Williams 1966; Clutton-Brock 1984; Javoiš 2013), as found in the collared flycatcher F. albicollis (Part et al. 1992) and the burying beetle N. orbicollis (Creighton et al. 2009). Furthermore, these findings could indicate that the benefits of maternal care are more important for second than first clutch nymphs and therefore select for higher expression of care towards late clutch nymphs across populations. In line with this idea, spring is often well advanced when second clutch eggs hatch, so that the environmental conditions experienced by the resulting nymphs are likely to increase (as compared to first clutch nymphs) their exposure to pathogens, such as fungi, or their predators, such as other arthropods and even the first clutch nymphs (Dobler and Kölliker 2010). Irrespective of the mechanism mediating the increased level of maternal care towards second clutch nymphs, the absence of a population-specific effect indicates that the environmental conditions experienced by females during first clutch family life (and egg care) are crucial in determining the subsequent expression of maternal care towards their second clutch offspring. This is supported by recent studies showing that maternal condition at egg hatching determines the nature of sibling and mother-offspring interactions in F. auricularia (Wong and Kölliker 2012; Kramer et al. 2015; Kramer and Meunier 2016).
Interestingly, we found a reduction in size of the second as compared to the first clutch in all three studied populations. This reduction is in line with an effect of senescence on female reproduction, but may also reflect an adaptive strategy of females. For example, the uncertainty of surviving until second clutch production could favour a higher investment into first clutch production independent of female age. Such an effect of the perceived risk of death on female investment into reproduction has been nicely demonstrated in the burying beetle Nicrophorus vespilloides, in which an experimental activation of the immune system caused females to switch from reproductive restraint to terminal investment (Cotter et al. 2011). Interestingly, our findings in earwigs reveal that although clutch sizes were different, almost all measurements of life-history traits taken in the first clutches were positively correlated with the corresponding measurements in the second clutches. This highlights that the overall reproduction of a female is tightly linked to her own quality irrespective of whether or not senescence shapes the size of second clutches (Meunier et al. 2012). Further studies should be conducted to experimentally disentangle the (mutually non-exclusive) effects on maternal investment into egg production caused by senescence and/or the perceived risk of death on the one hand, and the effects on maternal care caused by terminal investment and/or reproductive strategy (see above).
To conclude, our study demonstrates that offspring cohort and population-membership interact in determining crucial life-history traits in the European earwig. We showed in a common garden experiment that population-membership affected the expression of two cohort-specific traits only (nymph quality and the trade-off between egg developmental time and female weight at egg hatching), suggesting an important role of past environmental conditions in their expression, but also indicating a limited role of such past conditions for the expression of maternal care. Although our results are overall in line with an effect of terminal investment on maternal care, and of senescence on maternal reproduction, our findings call for further experimental studies deciphering the independent or entangled action of these mechanisms (see e.g. Cotter et al. 2011). Finally, it is important to note that the direction and strength of the cohort-specific effects reported in this study should be interpreted with caution, as our laboratory conditions might have unwillingly favoured individuals from certain populations. Nevertheless, our results demonstrate that even within a single genetic clade (namely the F. auricularia clade B; Wirth et al. 1998), population idiosyncrasies may have major effects on the expression of life-history traits associated with successive reproductive attempts. The question whether these idiosyncrasies reflect the capability of individuals to develop under specific laboratory conditions, the evolutionary history of the population, and/or the biotic/abiotic constraints experienced by the juveniles during their development remains open for further studies.
References
Boos S, Meunier J, Pichon S, Kölliker M (2014) Maternal care provides antifungal protection to eggs in the European earwig. Behav Ecol 25:754–761. doi:10.1093/beheco/aru046
Bowen WD, Iverson SJ, McMillan JI, Boness DJ (2006) Reproductive performance in grey seals: age-related improvement and senescence in a capital breeder. J Anim Ecol 75:1340–1351. doi:10.1111/j.1365-2656.2006.01157.x
Brommer JE (2000) The evolution of fitness in life-history theory. Biol Rev 75:377–404. doi:10.1111/j.1469-185X.2000.tb00049.x
Brown CA, Formanowicz DR (1995) Variation in reproductive investment among and within populations of the scorpion Centruroides vittatus. Oecologia 103:140–147. doi:10.1007/BF00329073
Clutton-Brock TH (1984) Reproductive effort and terminal investment in iteroparous animals. Am Nat 123:212–229
Cotter SC, Ward RJS, Kilner RM (2011) Age-specific reproductive investment in female burying beetles: independent effects of state and risk of death. Funct Ecol 25:652–660. doi:10.1111/j.1365-2435.2010.01819.x
Creighton JC, Heflin ND, Belk MC (2009) Cost of reproduction, resource quality, and terminal investment in a burying beetle. Am Nat 174:673–684. doi:10.1086/605963
Descamps S, Boutin S, Berteaux D, Gaillard J-M (2007) Female red squirrels fit Williams’ hypothesis of increasing reproductive effort with increasing age. J Anim Ecol 76:1192–1201. doi:10.1111/j.1365-2656.2007.01301.x
Diamantidis AD, Carey JR, Nakas CT, Papadopoulos NT (2011) Population-specific demography and invasion potential in medfly. Ecol Evol 1:479–488. doi:10.1002/ece3.33
Diehl JM, Körner M, Pietsch M, Meunier J (2015) Feces production as a form of social immunity in an insect with facultative maternal care. BMC Evol Biol 15(15):40. doi:10.1186/s12862-015-0330-4
Dobler R, Kölliker M (2010) Kin-selected siblicide and cannibalism in the European earwig. Behav Ecol 21:257–263. doi:10.1093/beheco/arp184
Flatt T, Heyland A (2011) Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. Oxford University Press, Oxford
Fox CW, Czesak ME (2000) Evolutionary ecology of progeny size in arthropods. Annu Rev Entomol 45:341–369
Gómez Y, Kölliker M (2013) Maternal care, mother-offspring aggregation and age-dependent coadaptation in the European earwig. J Evol Biol 26:1903–1911. doi:10.1111/jeb.12184
Hasselquist D, Nilsson J-A (2009) Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Philos Trans R Soc Lond B Biol Sci 364:51–60. doi:10.1098/rstb.2008.0137
Husson F, Le S, Pagès J (2011) Exploratory multivariate analysis by example using R. Chapman and Hall/CRC Press, London
Javoiš J (2013) A two-resource model of terminal investment. Theory Biosci 132:123–132. doi:10.1007/s12064-013-0176-5
Koch LK, Meunier J (2014) Mother and offspring fitness in an insect with maternal care: phenotypic trade-offs between egg number, egg mass and egg care. BMC Evol Biol 14:125. doi:10.1186/1471-2148-14-125
Kölliker M (2007) Benefits and costs of earwig (Forficula auricularia) family life. Behav Ecol Sociobiol 61:1489–1497. doi:10.1007/s00265-007-0381-7
Kölliker M, Boos S, Wong JWY et al (2015) Parent-offspring conflict and the genetic trade-offs shaping parental investment. Nat Commun 6:6850. doi:10.1038/ncomms7850
Kowald A, Kirkwood TBL (2015) Evolutionary significance of ageing in the wild. Exp Gerontol 71:89–94. doi:10.1016/j.exger.2015.08.006
Kramer J, Meunier J (2016) Maternal condition determines offspring behavior toward family members in the European earwig. Behav Ecol 27:494–500. doi:10.1093/beheco/arv181
Kramer J, Thesing J, Meunier J (2015) Negative association between parental care and sibling cooperation in earwigs: a new perspective on the early evolution of family life? J Evol Biol 28:1299–1308. doi:10.1111/jeb.12655
Krams IA, Krama T, Moore FR et al (2015) Resource availability as a proxy for terminal investment in a beetle. Oecologia 178:339–345. doi:10.1007/s00442-014-3210-5
Lamb RJ (1976) Parental behavior in the dermaptera with special reference to Forficula auricularia (Dermaptera: Forficulidae). Can J Entomol 108:609–619
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18. doi:10.1016/j.envint.2008.06.007
Mason THE, Chirichella R, Richards SA et al (2011) Contrasting life histories in neighbouring populations of a large mammal. PLoS One 6:e28002. doi:10.1371/journal.pone.0028002
McNamara JM, Houston AI (1996) State-dependent life histories. Nature 380:215–221
Meunier J, Kölliker M (2012) Parental antagonism and parent-offspring co-adaptation interact to shape family life. Proc R Soc B Biol Sci 279:3981–3988. doi:10.1098/rspb.2012.1416
Meunier J, Wong JWY, Gómez Y et al (2012) One clutch or two clutches? Fitness correlates of coexisting alternative female life-histories in the European earwig. Evol Ecol 26:669–682. doi:10.1007/s10682-011-9510-x
Nussey DH, Froy H, Lemaitre J-F et al (2013) Senescence in natural populations of animals: Widespread evidence and its implications for bio-gerontology. Ageing Res Rev 12:214–225. doi:10.1016/j.arr.2012.07.004
Nylin S, Gotthard K (1998) Plasticity in life-history traits. Annu Rev Entomol 43:63–83. doi:10.1146/annurev.ento.43.1.63
Part T, Gustafsson L, Moreno J (1992) Terminal investment and a sexual conflict in the collared flycatcher (Ficedula albicollis). Am Nat 140:868–882
Reznick D, Nunney L, Tessier A (2000) Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol Evol 15:421–425. doi:10.1016/S0169-5347(00)01941-8
Roff DA (1992) The evolution of life histories: theory and analysis. Chapman and Hall, New York
Royle NJ, Smiseth PT, Kölliker M (2012) The evolution of parental care. Oxford University Press, Oxford
Rundle HD, Nosil P (2005) Ecological speciation. Ecol Lett 8:336–352. doi:10.1111/j.1461-0248.2004.00715.x
Sandrin L, Meunier J, Raveh S et al (2015) Multiple paternity and mating group size in the European earwig, Forficula auricularia. Ecol Entomol 40:159–166. doi:10.1111/een.12171
Santos ESA, Nakagawa S (2012) The costs of parental care: a meta-analysis of the trade-off between parental effort and survival in birds. J Evol Biol 25:1911–1917. doi:10.1111/j.1420-9101.2012.02569.x
Schluter D (2001) Ecology and the origin of species. Trends Ecol Evol 16:372–380. doi:10.1016/S0169-5347(01)02198-X
Southwood TRE (1977) Habitat, the templet for ecological strategies? J Anim Ecol 46:337–365. doi:10.2307/3817
Staerkle M, Kölliker M (2008) Maternal food regurgitation to nymphs in earwigs (Forficula auricularia). Ethology 114:844–850. doi:10.1111/j.1439-0310.2008.01526.x
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Thesing J, Kramer J, Koch LK, Meunier J (2015) Short-term benefits, but transgenerational costs of maternal loss in an insect with facultative maternal care. Proc R Soc B Biol Sci 282:20151617. doi:10.1098/rspb.2015.1617
Velando A, Drummond H, Torres R (2006) Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proc R Soc B Biol Sci 273:1443–1448. doi:10.1098/rspb.2006.3480
Vincze O, Székely T, Küpper C et al (2013) Local environment but not genetic differentiation influences biparental care in ten plover populations. PLoS One 8:e60998. doi:10.1371/journal.pone.0060998
Williams GC (1966) Natural selection, the costs of reproduction, and the refinement of Lack’s principle. Am Nat 100:687–690
Wirth T, Le Guellec R, Vancassel M, Veuille M (1998) Molecular and reproductive characterization of sibling species in the European earwig (Forficula auricularia). Evolution 52:260
Wong JWY, Kölliker M (2012) The effect of female condition on maternal care in the European earwig. Ethology 118:450–459. doi:10.1111/j.1439-0310.2012.02030.x
Wong JWY, Kölliker M (2014) Effects of food restriction across stages of juvenile and early adult development on body weight, survival and adult life history. J Evol Biol 27:2420–2430. doi:10.1111/jeb.12484
Acknowledgments
We thank all members of the “Team Earwig” at the University of Mainz for their help in the maintenance of animals in the laboratory. We also thank Arnaud Suwalski from the EPHE for species determination, Xavier Espalader for his help with earwig sampling, Jessica Purcell for her help in obtaining climatic data for the three tested populations and two anonymous reviewers for their comments on a previous version of this manuscript. This research was supported by the German Science Foundation (DFG; ME4179/1-1 to JM) and the ARP-EVOL grant from EPHE obtained by Claudie Doums.
Author contribution statement
TR, JK, MV and JM designed the experiment. MV collected the individuals in the field. TR and JK conducted the experiment. TR and JM analysed the data. JM wrote the first version of the manuscript, which was then commented and corrected by all co-authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Sylvain Pincebourde.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ratz, T., Kramer, J., Veuille, M. et al. The population determines whether and how life-history traits vary between reproductive events in an insect with maternal care. Oecologia 182, 443–452 (2016). https://doi.org/10.1007/s00442-016-3685-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3685-3