Abstract
In territorial species, observed density dependence is often manifest in lowered reproductive output at high population density where individuals have fewer resources or are forced to inhabit low-quality territories. The Arctic fox (Vulpes lagopus) in Iceland is territorial throughout the year and feeds mostly on birds, since lemmings are absent from the country. Thus, the population does not exhibit short-term population cycles that are evident in most of the species’ geographical range. The population has, however, gone through a major long-term fluctuation in population size. Because of the stability in hunting effort and reliable hunting records since 1958, the total number of adult foxes killed annually can be used as an index of population size (N t ). An index of carrying capacity (K) from population growth data for five separate time blocks during 1958–2007 revealed considerable variation in K and allowed a novel definition of population density in terms of K, or N t /K. Correlation analysis suggested that the reproductive rate was largely determined by the proportion of territorial foxes in the population. Variation in litter size and cub mortality was, on the other hand, related to climatic variation. Thus, Arctic foxes in Iceland engage in typical contest competition but can adapt their territory sizes in response to both temporal and spatial variation in carrying capacity, resulting in surprisingly little variation in litter size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The analysis of fluctuations in animal populations is central to various studies in ecology, in both theoretical models and in applied topics such as in conservation ecology, pest populations, wildlife management and resource use (e.g., Elton 1924; Andrewartha and Birch 1954; Finerty 1980; Krebs et al. 1995). A general problem when analyzing population fluctuations is to distinguish demographic factors that are related to stochastic events from those that are coupled to biotic interactions (DeAngelis and Waterhouse 1987). Strong stochasticity would, for example, be expected in connection to climate variation. But with strong biotic interactions with frequent feedbacks, stabilizing patterns are expected. The classic attention has been to analyze population number per se and its density dependence (Sinclair 1989). Sinclair (1989) suggested a terminology where “regulation” only referred to density dependence whereas “limitation” should be used in density-independent processes. The principal difference between the two processes would be that regulation could confer a feedback into population density through effects on reproduction and survival whereas limitation would not. However, a measure of population growth rate might better reflect the effects of limiting and regulating factors, and identify which demographic variables are connected to population growth rate (e.g., Hutchinson 1978; Sibly and Hone 2002; Turchin 2003). This can be seen both from a top–down and a bottom–up perspective (Sinclair and Krebs 2002). An obstacle in analyses of population demography has been limitations in empirical data, where both more field experiments and long-term data series are needed (Krebs 2013).
We present here an analysis of the population growth rate and carrying capacity of a unique data series of Icelandic Arctic fox (Vulpes lagopus), spanning over half a century. In comparison to areas where rodents are the main prey of Arctic foxes, the prey base of the Arctic fox in Iceland, mainly birds of various species, has been relatively stable and predictable from year to year (Hersteinsson 1992; Hersteinsson and Macdonald 1996; Pálsson et al. 2016). The Arctic fox population has adapted to this situation by lowered fertility compared to mainland North America and Eurasia, and the population density has probably stayed close to carrying capacity for most of the time since it arrived in the country at the end of the Ice Age (Angerbjörn et al. 2004). The social system is based on the breeding couple occupying and defending a territory where the annual litter is raised each summer (Hersteinsson 1984; Elmhagen et al. 2014). The foxes are therefore subjected to a typical contest competition with limited availability of territories and mates. These resources can vary in time and space in association with food availability. Furthermore, due to the Arctic fox’s dependence on prey derived from both terrestrial and marine origin (Angerbjörn et al. 1994), its population size and dynamics in the long run appears to be affected by both oceanic and terrestrial events (Pálsson et al. 2016). The influence of marine habitat is considered to differ between west and east Iceland (Hersteinsson 1992; Dalerum et al. 2012), with the western part having a far higher proportion of productive seashores (Ingólfsson 1975).
The Arctic fox has been hunted in Iceland ever since the country was first settled by humans around 900 AD. The hunting statistics of Arctic foxes in Iceland, collected by the Environment Agency of Iceland and its predecessor, the Wildlife Management Institute, suggest that the Arctic fox population in Iceland declined from the late 1950s until the 1970s, whereafter the population has been on the increase. The causes of this fluctuation have been related to changes in prey populations as well as climate, but the hunting effort is considered to have been stable during this period (Pálsson et al. 2016). Such factors can directly affect birth and mortality rates and would be of central importance for understanding and managing the Arctic fox population. As mentioned earlier, hunting is believed to be the most important mortality factor in Iceland (Hersteinsson 1992). Other studies have demonstrated that hunting time series can be good proxies for population abundance (e.g., Cattadori et al. 2003). Thus, the main aim of this study was to analyze which proximate causes drive the long-term population changes in the Icelandic Arctic fox population catch, to discover whether the population growth rate could be determined by variation in population parameters, fecundity or fertility rather than by external factors such as weather systems on local or global scales. On the basis of this analysis, it should be possible to understand the forces behind the rise and fall of a carnivore population and thus better predict changes and suggest management action in the Arctic fox population in the near future.
Materials and methods
Changes in Arctic fox population size
An index of changes in population size over time is based on hunting statistics, recorded by the government of Iceland, with hunters required to give a detailed report of the annual catch (Pálsson et al. 2016). Full access to the foxhunting data was granted for the purpose of this study, courtesy of the Environment Agency of Iceland and The Icelandic Ministry for the Environment and Natural Resources.
Fertility
Foxhunters supplied, on a voluntary basis, the lower jaws of killed adult (≥12 months of age) Arctic foxes between 1979 and 1985 (n = 1337) and whole carcasses from 1986 to 2007 (n = 7427). For each fox carcass, the foxhunters filled in a standard form with details on: (1) the location where the fox was killed, (2) date of death of the fox, and (3) whether it was killed as a denner (=reproducing adult killed at a breeding den) or a runner (=adult killed away from a breeding den). In total, 8764 foxes were age-determined by counting annual cementum lines of canine tooth roots (Allen and Melfi 1985). That way, individuals which were old enough to breed could be identified. Placental scars (Allen 1983; Strand et al. 1995) and fetuses were counted in uteri of vixens, ≥12 months of age (n = 3374) as a measure of fertility.
Analysis
The data span was 50 years and the analysis was conducted in the study period in total or in five separate time blocks of 10 years each, i.e., 1958–1967, 1968–1977, 1978–1987, 1988–1997 and 1998–2007. The data were analyzed separately for western and eastern Iceland, geographically divided by municipality borders (Fig. 1). Population growth rate, r t = ln(N t+1/N t ), was calculated for the years 1958–2007, where N t is the total number of adult foxes (denners and runners) in the kill in year t. By definition, all denners recorded in the catch were breeding foxes but not all runners were non-breeding foxes. Although 40 % of runners in western Iceland 1986–1990 had placental scars, they were generally younger and many more of them had failed to breed at a later stage (Hersteinsson 1992). In spite of this shortcoming, the proportion of denners in the total adult catch is regarded to be an index of the proportion of breeding foxes in the catch in individual years.
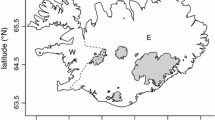
The division into western (W) and eastern (E) Iceland used in the analyses of the present study. While western Iceland, according to this division, is only a quarter of the surface area of eastern Iceland (20,850 vs. 82,150 km2), productive seashores in western Iceland are 1.5 times longer in western than in eastern Iceland (3884 vs. 1985 km). The difference in surface area of productive seashores is much greater, as the productive seashores of western Iceland are 46 times greater than in eastern Iceland (1845 vs. 40 km2). Thus, productive seashores occupy 0.47 % of the surface area of western Iceland compared to only 0.0026 % of surface area of eastern Iceland (Ingólfsson 1975). The locations of meteorological stations used in the analysis of weather variables are indicated by black dots
The carrying capacity for each block b (K b ) was believed to have varied over time during the 50 years. The regression of r t on N t was thus performed in the five separate time blocks of 10 years each, to determine K b from the intersect of the regression of r t on N t (Turchin 2003). Having determined K b for each of the time blocks, the index of population size was from then on defined in terms of K b , i.e. as N t /K b , to make it feasible to use the whole dataset simultaneously in further analyses. The effects of population size in relation to carrying capacity (N t /K b ) on population growth (r t ) was analyzed by linear regression, considering the regional areas of east and west. Partial autocorrelation within the time series of rt was estimated using the pacf function in R (R Core Team 2015). Similarly the variation in proportion of denners was analyzed with regard to N t /K b with linear models, after applying the arcsine transformation (e.g., Sokal and Rohlf 2012). The variation in the population parameters r t and N t /K b were analyzed with respect to the variation in the breeding parameters listed below.
From the hunting data supplied by the Wildlife Management Institute, a litter size index was devised as LSI = C/D, where C is the total number of cubs killed and D is the total number of dens found inhabited by foxes. Litter size index is available for the whole 50-year study period (1958–2007) and was divided into periods when compared to other parameters of interest.
Cub mortality in individual years was calculated as (mean number of placental scars) – (litter size index) for a period of 22 years (1986–2007), as there were data for placental scars only during this period. The number of fetuses in pregnant females was included in the total number of placental scars. The proportion of vixens which had placental scars or fetuses, out of total mature (≥1 year old) females killed was calculated as a measure of fecundity.
Meteorological data
Meteorological data were obtained from the official web site of the Icelandic Meteorological Office (www.vedur.is) for one station in western Iceland (Stykkishólmur) and one station in eastern Iceland (Egilsstaðir) (Fig. 1). Data on mean monthly temperature, total monthly precipitation and mean monthly wind speed were used in a correlation analysis with population parameters (r t , N t /K b ), breeding parameters (placental scar numbers, litter size index) and cub mortality, in order to establish their interrelations. Correlations with the North Atlantic Oscillation (NAO; Hurrel 1995; Jones et al. 1997) winter (December–March) and spring index (April–May) on the same parameters were also conducted. Multiple regression analysis on weather and population parameters was conducted in order to define the main explanatory factors on variables in breeding parameters.
Results
Population index
The number of foxes fell quite sharply from the late 1950s until the early 1970s in both western and eastern Iceland according to the hunting statistics (Fig. 2). This was true of all sections of the catch, i.e., denners, runners and cubs. In western Iceland, the total catch of foxes began to increase again in the late 1970s and rose almost continuously until the end of the century. In eastern Iceland, the increase in catch numbers began approximately a decade later, after 1985, with an almost continuous increase for the next 20 years. Interestingly, cub numbers increased more in the eastern part of the country than in the western part. The population growth rate varied greatly but was lowest at the beginning of the period in both western and eastern Iceland. In western Iceland, it rose to a maximum in the mid- to late 1980s, then declined again, but in eastern Iceland, it may not have reached a maximum during the study period (Fig. 3).
Regression of r on N t (i.e., r = 0) revealed that the intersects varied during the 5 time blocks (Supplementary material, Fig. 1), indicating considerable variation in carrying capacity (K) in both western and eastern Iceland. Furthermore, K differed more over time than between regions (ANOVA: F 4,5 = 42.8, p = 0.0005) and an additive model, including both time and regions, did not improve the fit to the data. A significant difference was observed between the K values for two periods in the western region and all but the second and third periods in eastern Iceland (Fig. 4).
A linear regression of r t on N t /K b showed a negative relationship between growth rate and the ratio of N t /K b (b = −3.47, P = 0.0008) and that the relationship was independent of the region within Iceland (P > 0.32) (Fig. 5).
Litter size
The mean date of foxes killed as denners, and consequently of the death of their cubs, during 1979–2007 was 21 June (SD = 19 days). Litter size index is thus a measure of the mean number of cubs per each denning pair caught aged 5 ± 2.7 weeks, assuming 15 May as the mean date of birth (Hersteinsson 1984). A regression of r t on litter size index revealed no significant relationship, either for the period as a whole or when dividing the whole period into 5 blocks. Litter size index was not correlated with N t /K b and no correlation was found between the mean of the number of placental scars and either r t or N t /K b . The mean number of placental scars per year and the litter size index showed an indication of positive correlation both in west and east Iceland (Fig. 6), but neither were significant (0.12 > P > 0.08).
Cub mortality
No correlation was found between cub mortality and litter size during pregnancy (placental scar number) in western Iceland, but in eastern Iceland cub mortality increased significantly with increasing litter size (Table 1). In eastern Iceland, there was also a significant positive correlation between cub mortality and N t /K b and a negative correlation between cub mortality and mean temperature in May, but not between cub mortality and mean temperature in any other month between January and July in year t (Table 1). Multiple regression of cub mortality against May temperature, N t /K b and mean number of placental scars showed that the mean number of placental scars alone was sufficient to explain over 60 % of cub mortality (R 2 = 0.623; F 1,9 = 14.9; P = 0.004) with neither N t /K b nor May temperature adding significantly to the fit. No correlation was found between cub mortality in western Iceland and any of these parameters. In eastern Iceland, there was also a significant negative correlation between mean temperature in February and mean number of placental scars, but no correlation was found between reproductive parameters and either wind or precipitation in any month (Table 1).
In western Icelandm no correlation was found between mean temperature in any month and reproductive data (Litter size index, placental scar number and cub mortality) but positive correlation was found between wind speed in January, precipitation in January and precipitation in May on the one hand and Litter size index on the other hand. In eastern Iceland there was negative correlation between February temperature and placental scar numbers and between N t /K b and mean January temperature (Table 1). No correlation was found between NAO winter and/or spring indexes and reproductive parameters of the fox data.
Proportion of reproductive foxes
A negative relationship was observed between the proportion of denners in the catch and the index of population size, N t /K b , for the whole period 1958–2007 (b = −0.194, P < 0.001) and a significant difference (P < 0.001) was observed between the eastern (prop. 0.72) and the western region (prop. 0.64). A stronger relationship was in the western part (b = −0.20, P = 0.004) than in the east (b = −0.161, P = 0.089). A further inspection of the relationships for each of the five periods of 10 years in the two regions showed negative relationships for all periods. In western Iceland, all tests were significant (Table 2) except for the latest period which was characterised by high carrying capacity (K). In eastern Iceland only the tests for the first and the fourth period were significant. The proportion of denners followed the changes in population size before and after 1980 and the difference between regions is evident in Fig. 7.
Discussion
Population growth depends on carrying capacity
The relatively pronounced long-term fluctuation (wave length >50 years) is rather surprising in view of the remarkably varied diet of the Arctic fox in Iceland, where it acts as a generalist predator in contrast to its specialist diet in areas where rodents are the mainstay of its diet (Elmhagen et al. 2002). Population fluctuations in Arctic foxes are often explained by variation in litter size in association with cyclic rodent populations (Tannerfeldt and Angerbjörn 1998; Meijer et al. 2013). The long-standing foxhunting intensity with a basis in Icelandic law makes it unlikely that variation in mortality due to foxhunting is the cause of these long-term population fluctuation. In this study, variation in the proportion of the adult population breeding each year appears to play a major role in determining the variation in population growth rates. The overall demographic pattern in our study was that population growth rate in the long term depended on carrying capacity. In years when the populations were below carrying capacity, we observed a positive growth rate, but when population sizes were above carrying capacity, the growth rate was instead negative. Population growth rate is typically divided into birth rate and mortality rate (Hutchinson 1978), where birth rate can be divided into the proportion of females that reproduce and into litter size. In our study, the proportion of foxes that were breeding and defending a territory (measured as the proportion of denners) was indeed related to carrying capacity (N t /K). However, litter size was largely independent of N t /K and instead connected to stochastic climatic changes showing up on a shorter time scale. Neither growth rate rt nor carrying capacity was synchronized between region,s since it varied both in time and space throughout the whole study period.
Arctic fox populations in areas where lemmings and other rodents are their main diet show enormous variation in fecundity, and a fluctuating carrying capacity is intuitively understood with mean litter sizes at weaning varying from near-zero up to 18 (Macpherson 1969; Tannerfeldt and Angerbjörn 1998; Angerbjörn et al. 1999; Meijer et al. 2013). In coastal areas such as Iceland, the population dynamics is mostly described as stabile or at least in equilibrium. However, this study demonstrates that the fox populations vary in both the short term and the long term in which the short stochastic fluctuations were related to climatic variation and the long-term fluctuations to a varying carrying capacity. In the Icelandic population, the litter size at the age of about 5 weeks (den hunting surveys) varied remarkably little with relative population size (N t /K b ) with no association between litter size index and N t /K b . Neither was there any correlation between the mean number of placental scars and N t /K b . Thus, in contrast to the situation in “lemming areas”, the fertility parameters do not play an important role in explaining variability in population size, either in the east or west.
Coastal and inland populations
In eastern Iceland, there was a strong positive correlation between calculated cub mortality and both N t /K b and placental scar numbers on the one hand, and a negative correlation with mean temperature in May on the other hand. Although a multiple correlation of cub mortality on the three variables suggests that placental scar numbers alone are sufficient to explain cub mortality, it is not possible to rule out that mean temperature in May is of some importance. This is the month when litters are born and most of the migrant terrestrial birds begin nesting in Iceland. Cold temperatures at that time and during winter may influence nesting parameters like timing of spring arrival at breeding sites, breeding densities and ultimately breeding success (Alisauskas 2002; Marra et al. 2005; Gunnarsson et al. 2006; Eide et al. 2012), which may in turn impact cub survival negatively in inland habitats. This effect of the conditions during winter seems to have larger effect on the breeding success than the population density. In coastal habitats of the western region, on the other hand, the marine birds most important to the foxes are more affected by marine conditions which are only weakly correlated with ambient temperature and then generally with a long time lag (Fuglei and Ims 2008; Magnusdottir et al. 2014).
The split of Arctic fox in Iceland into western and eastern populations has been supported by other evidence. A clear genetic subdivision was described between the two regions by Norén et al. (2009) and this is further supported by the disease pattern of rabies 1950 (Fooks et al. 2004) and the distribution of ear canker mite (Otodectes cynotis; Gunnarsson et al. 1991). A recent finding also revealed that high levels of mercury are found in the coastal Arctic foxes of the western part but almost absent in the inland foxes of the eastern part of Iceland (Bocharova et al. 2013). The different demographic mode in the western and eastern parts is therefore not surprising. However, taking these differences into account, there were also similarities in demographic response and especially in the carrying capacity. Pálsson et al. (2016) reported changes in many Icelandic bird populations that are included in the Arctic fox diet. So these changes are likely the major component in the carrying capacity for Icelandic Arctic foxes.
Effect of territorial and breeding behaviour
Breeding Arctic foxes defend territories throughout the year (Hersteinsson and Macdonald 1982; Strand et al. 2000). While territory borders may remain stable from year to year when maintained by the same foxes, a high turnover in territorial foxes, such as occurs in Iceland with the heavy hunting pressure on them during the breeding season, may result in shifts in territorial borders (Hersteinsson and Macdonald 1982; Hersteinsson 1999) and may even result in drifting territories as seen in urban red fox Vulpes vulpes populations with a high rate of turnover (Doncaster and Macdonald 1991). The proportion of denners in the catch is a measure of the proportion of foxes that was successful in obtaining or holding on to a territory. The strong correlation between the proportion of denners in the catch and N t /K b in both eastern and western Iceland and the lack of correlation between the mean number of placental scars and litter size index on the one hand and N t /K b on the other hand strongly suggest that it is mainly through a change in the proportion of foxes able to obtain or hold onto a territory that the population reacts to changes in carrying capacity. Heavy hunting pressure would thus promote a fast response to carrying capacity. It appears that the buffer effect, where individuals concentrate in the best sites during low density but at higher densities they are more widely distributed (Brown 1969; Sutherland 1998), is either weak or absent among the Arctic foxes of Iceland. This suggests that, during periods of lower carrying capacity, the foxes compensate for lower availability of food by expanding their territories and reduce them during conditions of high food availability. Indeed, territory sizes are dynamic and would vary temporally in response to changes in carrying capacity, and spatially in response to the productivity of the habitat (Macdonald 1983; Jepsen et al. 2002), thus compensating for both temporal and spatial variation in productivity.
Population fluctuations
During the study period, the population size has fluctuated around the carrying capacity, which has been increasing during the last decade possibly due favourable conditions both in temperature and food resources (Pálsson et al. 2016). In years when the size has become larger than the carrying capacity, the population growth has generally been negative. The Arctic foxes in Iceland engage in typical contest competition, as they seem to have the capacity to adapt their territory sizes in response to both temporal and spatial variation in carrying capacity. In contrast to Arctic foxes in lemming areas, fertility per female in Iceland appears to be fixed, as the increase in population growth rate is not explained by larger litter sizes but by a higher proportion of mature vixens taking part in breeding, as well as changes in the size or number of available territories. This behavior may be one of the most important differences between the Arctic fox population in Iceland and populations inhibiting areas where cyclic rodents are the main prey source (e.g., Meijer et al. 2013). In this study, the reproduction was mainly limited by density-independent factors (e.g., Sinclair 1989) and winter and spring weather, whereas the population growth rate was regulated through density dependence and fluctuations in carrying capacity. This study has revealed atypical responses to varying factors acting on carrying capacity and new opportunities to sustain a viable carnivore population in a changing environment. This suggests that management actions on the proportion of breeding females would be less efficient as long as there are surplus non-breeding females. However, a change in carrying capacity would affect the population growth rate and thus the population size.
Conclusion
Our analyses of Arctic foxes in Iceland demonstrate several things. First, we can see a strong and steady population increase over many years, due to an increase in carrying capacity. Secondly, the stabilizing factors in the population were through social factors acting on the proportion of breeding animals and territoriality. And thirdly, there are also strong stochastic factors where climate variation has effects on other demographic parameters such as litter size and cub mortality.
References
Alisauskas RT (2002) Arctic climate, spring nutrition, and recruitment in mid-continent lesser snow geese. J Wildl Manag 56:43–54
Allen SH (1983) Comparison of red fox litter sizes determined from counts of embryos and placental scars. J Wildl Manag 47:860–863
Allen DH, Melfi RC (1985) Improvements in techniques for ageing mammals by dental cementum annuli. Proc Iowa Acad Sci 92:100–102
Andrewartha HG, Birch LC (1954) The distribution and abundance of animals, 1st edn. University of Chicago Press, Chicago
Angerbjörn A, Hersteinsson P, Liden K, Nelson E (1994) Dietary variation in arctic foxes (Alopex lagopus)—an analysis of stable isotopes. Oecologia 99:226–232
Angerbjörn A, Tannerfeldt M, Erlinge S (1999) Predator-prey relations: arctic foxes and lemmings. J Anim Ecol 68:34–49
Angerbjörn A, Hersteinsson P, Tannerfeldt M (2004) Arctic foxes: Consequences of resource predictability in the Arctic fox—two life history strategies. In: Macdonald DW, Sillero-Zubiri C (eds) Biology and Conservation of Wild Canids. Oxford University Press, Oxford, pp 163–172
Bocharova N, Treu G, Czirják GÁ, Krone O, Stefanski V, Wibbelt G, Unnsteinsdóttir ER, Hersteinsson P, Schares G, Doronina L, Goltsman M, Greenwood AD (2013) Correlates between feeding ecology and mercury levels in historical and modern Arctic foxes (Vulpes lagopus). PLoS ONE 8(5):e60879
Brown JL (1969) The buffer effect and productivity in tit populations. Am Nat 103:347–354
Cattadori IM, Haydon DT, Thirgood SJ et al (2003) Are indirect measures of abundance a useful index of population density? The case of red grouse harvesting. Oikos 100:439–446
Dalerum F, Perbro A, Magnusdottir R, Hersteinsson P, Angerbjörn A (2012) The influence of coastal access on isotope variation in Icelandic arctic foxes. PLoS ONE 7(3):e32071
DeAngelis DL, Waterhouse JC (1987) Equilibrium and nonequilibrium concepts in ecological models. Ecol Monogr 57:1–21
Doncaster CP, Macdonald DW (1991) Drifting territoriality in the red fox Vulpes vulpes. J Anim Ecol 60:423–439
Eide NE, Stien A, Prestrud P, Yoccoz NG, Fuglei E (2012) Reproductive responses to spatial and temporal prey availability in a coastal Arctic fox population. J Anim Ecol 81:640–648
Elmhagen B, Tannerfeldt M, Angerbjörn A (2002) Food niche overlap between arctic and red foxes. Can J Zool 80:1274–1285
Elmhagen B, Hersteinsson P, Norén K, Unnsteinsdottir ER, Angerbjörn A (2014) From breeding pairs to fox towns: the social organisation of arctic fox populations with stable and fluctuating availability of food. Pol Biol 37:111–122
Elton C (1924) Periodic fluctuations in the numbers of animals: their causes and effects. Brit. J Exp Biol Edinb 2:119–163
Finerty JP (1980) The population ecology of cycles in small mammals: mathematical theory and biological fact, 1st edn. Yale University Press, New Haven
Fooks AR, Roberts DH, Lynch M, Hersteinsson P, Runolfsson H (2004) Rabies in the United Kingdom, Ireland and Iceland. In: King AA, Fooks AR, Aubert M, Wandeler AI (eds) Historical perspective of rabies in Europe and the Mediterranean Basin: a testament to rabies by Dr Arthur A. King. World Organization for Animal Health, Paris, pp 25–32
Fuglei E, Ims RA (2008) Global warming and effects on the arctic fox. Sci Prog 91:175–191
Gunnarsson E, Hersteinsson P, Adalsteinsson S (1991) Prevalence and geographical distribution of the ear canker mite (Otodectes cynotis) among arctic foxes (Alopex lagopus) in Iceland. J Wildl Dis 27:105–109
Gunnarsson TG, Gill JA, Atkinson PW, Gélinaud G, Potts PM, Croger RE, Gudmundsson GA, Appleton GF, Sutherland WJ (2006) Population-scale drivers of individual arrival times in migratory birds. J Anim Ecol 75:1119–1127
Hersteinsson P (1984) The behavioural ecology of the Arctic fox (Alopex lagopus) in Iceland. PhD dissertation, University of Oxford, Oxford
Hersteinsson P (1992) Demography of the arctic fox (Alopex lagopus) population in Iceland. In: McCullough DR, Barrett RH (eds) Wildlife 2001: populations. Elsevier, London, pp 954–964
Hersteinsson P (1999) The Arctic foxes of Hornstrandir. Ritverk sf, Reykjavik
Hersteinsson P, Macdonald DW (1982) Some comparisons between red and Arctic foxes, Vulpes vulpes and Alopex lagopus, as revealed by radio-tracking. Symp Zool Soc Lond 49:259–289
Hersteinsson P, Macdonald DW (1996) Diet of Arctic foxes (Alopex lagopus) in Iceland. J Zool Lond 240:457–474
Hurrell JW (1995) Decadal trends in the North Atlantic Oscillation and relationships to regional temperature and precipitation. Science 269:676–679
Hutchinson GE (1978) An introduction to population ecology. Yale University Press, New Haven
Ingólfsson A (1975) Life on seashores. Rit Landverndar 4:61–99 (In Icelandic with English summary)
Jepsen JU, Eide NE, Prestrud P, Jacobsen LB (2002) The importance of prey distribution in habitat use by arctic foxes (Alopex lagopus). Can J Zool 80:418–429
Jones PD, Jónsson T, Wheeler D (1997) Extension to the North Atlantic oscillation using early instrumental pressure observations from Gibraltar and South-West Iceland. Int J Climatol 17:1433–1450
Krebs CJ (2013) Population fluctuations in rodents. University of Chicago Press, Chicago
Krebs CJ, Boutin S, Boonstra R, Sinclair AR, Smith JN, Dale MR, Martin K, Turkington R (1995) Impact of food and predation on the snowshoe hare cycle. Science 269:1112–1115
Macdonald DW (1983) The ecology of carnivore social behaviour. Nature 301:379–384
Macpherson AH (1969) The dynamics of Canadian arctic fox populations. Can Wildl Serv Rep Ser 8:1–49
Magnusdottir R, von Schmalensee M, Stefansson RA, Macdonald DW, Hersteinsson P (2014) A foe in woe: American mink (Neovison vison) diet changes during a population decrease. Mammal Biol-Zeit Säug 79:58–63
Marra PP, Francis CM, Mulvihill RS, Moore FR (2005) The influence of climate on the timing and rate of spring bird migration. Oecologia 142:307–315
Meijer T, Elmhagen B, Eide NE, Angerbjörn A (2013) Life history traits in a cyclic ecosystem—a field experiment of the Arctic fox. Oecologia 173:439–447
Norén K, Angerbjörn A, Hersteinsson P (2009) Population structure in an isolated Arctic fox, Vulpes lagopus, population: the impact of geographical barriers. Biol J Linn Soc 97:18–26
Pálsson S, Hersteinsson P, Unnsteinsdóttir ER, Nielsen ÓK (2016) Population limitation in a non-cyclic arctic fox population in a changing climate. Oecologia 180:1147–1157
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Sibly RM, Hone J (2002) Population growth rate and its determinants: an overview. Philos Trans R Soc Lond B 357:1153–1170
Sinclair ARE (1989) Population regulation in animals. In: Cherrett JM, Bradshaw AE. Ecological concepts: contributions of ecology to an understanding of the world. 29th Symposium of the British Ecological Society, University College, London, 12–13 April 1988. Blackwell, Boston, pp 197–241
Sinclair ARE, Krebs CJ (2002) Complex numerical responses to top–down and bottom–up processes in vertebrate populations. Philos Trans R Soc Lond B 357:1221–1231
Sokal RR, Rohlf, FJ (2012) Biometry: the principles and practice of statistics in biological research (4th edn). Freeman, New York
Strand O, Skogland T, Kvam T (1995) Placental scars and estimation of litter size—an experimental test in the arctic fox. J Mammal 76:1220–1225
Strand O, Landa A, Linnell JDC, Zimmermann B, Skogland T (2000) Social organization and parental behavior in the arctic fox. J Mammal 81:223–233
Sutherland WJ (1998) The importance of behavioural studies in conservation biology. Anim Behav 56:801–809
Tannerfeldt M, Angerbjörn A (1998) Fluctuating resources and the evolution of litter size in the arctic fox. Oikos 83:545–559
Turchin P (2003) Complex population dynamics: a theoretical/empirical synthesis. Monographs in population biology, vol 35. Princeton University Press, Princeton
Acknowledgments
This paper is dedicated to its main author, Professor Pall Hersteinsson, who was actively working on this manuscript when he sadly passed away in October 2011. The Arctic fox was his main interest and he spent his entire scientific life in studying and understanding the ecology of the species. This study is based on his work and the long-term dataset he achieved. The study was partly funded by the Science Research Fund of Iceland and the Ministry of the Environment, Iceland. Thanks are due to all the foxhunters who supplied material for the study in spite of no financial compensation. A. Gardarsson and O.K. Nielsen kindly read and criticized an early draft of this manuscript.
Author contribution statement
PH conceived and designed the initial study but sadly passed away before this work was finished. PH, ERU and SP analyzed the data. ERU, PH, SP and AA wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ilpo Kojola.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Unnsteinsdottir, E.R., Hersteinsson, P., Pálsson, S. et al. The fall and rise of the Icelandic Arctic fox (Vulpes lagopus): a 50-year demographic study on a non-cyclic Arctic fox population. Oecologia 181, 1129–1138 (2016). https://doi.org/10.1007/s00442-016-3635-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3635-0