Abstract
As species become increasingly exposed to novel challenges, it is critical to understand how evolutionary (i.e., generational) and plastic (i.e., within lifetime) responses work together to determine a species’ fate or predict its distribution. The introduction of non-native species imposes novel pressures on the native species that they encounter. Understanding how native species exposed to toxic or distasteful invaders change their feeding behavior can provide insight into their ability to cope with these novel threats as well as broader questions about the evolution of this behavior. We demonstrated that native eastern fence lizards do not avoid consuming invasive fire ants following repeated exposure to this toxic prey. Rather fence lizards increased their consumption of these ants following exposure on three different temporal scales. Lizards ate more fire ants when they were exposed to this toxic prey over successive days. Lizards consumed more fire ants if they had been exposed to fire ants as juveniles 6 months earlier. Finally, lizards from populations exposed to fire ants over multiple generations consumed more fire ants than those from fire ant-free areas. These results suggest that the potentially lethal consumption of fire ants may carry benefits resulting in selection for this behavior, and learning that persists long after initial exposure. Future research on the response of native predators to venomous prey over multiple temporal scales will be valuable in determining the long-term effects of invasion by these novel threats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental change can have both immediate and long-term effects on species whose response is influenced by factors such as the duration, timing (i.e., life stage or time passed), and intensity of exposure to given environmental stimuli (Snyder and Evans 2006; Sax et al. 2007; Sinervo et al. 2010). For instance, organisms alter habitat use based on intensity and duration of predator cues (e.g., Turner 1997), and stimuli experienced early in life can influence how an organism responds to events as an adult (Anisman et al. 1998; McCormick and Green 2013). How these factors dictate a population’s response to environmental change is of fundamental evolutionary significance; however, how these factors work together remains poorly understood.
Organisms can respond to change within their lifetime [plasticity, including learning (West-Eberhard 1989)] and across generations [transgenerational transfer of traits via maternal effects, epigenetics, or genetics (Mousseau and Fox 1998; Jones and Takai 2001)]. For instance, an individual that has previously experienced a novel environment may exhibit a greater response to that stimulus than a naïve individual. This response may be learned from a single exposure or from multiple exposures within the animal’s lifetime (Rogers 1978; Suboski 1992; Chivers and Smith 1994). As opposed to learned responses within an animal’s lifetime, responses may be inherited (genetically or maternally) over multiple generations, resulting in individuals from exposed populations exhibiting a different response from birth than individuals from naïve populations (Sax et al. 2007; Love et al. 2013). Responses within lifetimes and across generations may also interact, with the ability to learn being selected for [i.e., resulting in faster learning (Nussey et al. 2005)]. Here, we examine how the duration and intensity of a novel threat affect an organism’s behavioral response to that threat. We also examine how long this response persists following exposure to the threat. We investigate this using a system of native fence lizards exposed to invasive toxic fire ants that act as novel predator and stressor (Langkilde 2009; Graham et al. 2012).
The red imported fire ant (hereafter, “fire ant”), Solenopsis invicta, is a venomous ant native to central South America (Allen et al. 1974). It has been widely introduced across the globe, and the resulting ecological and economic consequences have been the cause of great concern for the better part of a century (Banks et al. 1990; Callcott and Collins 1996). Fire ants were introduced to the USA in the 1930s via Port Mobile, Alabama, and now occupy 13 states in the southern part of the country. These venomous, predatory invaders often dominate ant communities within their invasive range due to their aggressive foraging behavior (Holway et al. 2002). Mammals, birds, and herpetofauna are all susceptible to negative (potentially population-level) impacts from fire ant invasion (reviewed in Allen et al. 2004). Most research on the impacts of fire ant invasion on native wildlife has focused on the impact of fire ants as novel predators (Tschinkel 2006). Fire ants can swarm comparatively large vertebrates, paralyze, and kill them with venomous stings (Wojcik et al. 2001; Allen et al. 2004; Langkilde 2009). However, fire ants also envenomate and kill native species that attempt to eat them (Webb and Henke 2003; Boronow and Langkilde 2010), but the impact of fire ants as a toxic prey species has been largely ignored (but see Langkilde and Freidenfelds 2010; Robbins and Langkilde 2012).
The eastern fence lizard (hereafter, “fence lizard”), Sceloporus undulatus, occurs both within areas containing fire ants and in areas where fire ants have not yet invaded (Conant and Collins 1998; Regulations CoF 2015). This has facilitated research on the effects of fire ant invasion on this native species. Fire ant-invaded populations of fence lizards appear to have adapted rapidly (within ≈38 generations) to pressure exerted by fire ants, displaying changes in behavior and morphology that decrease the lizards’ vulnerability to fire ant attack (Langkilde 2009). The rapidly adapted response of fence lizards to fire ants is likely the result of novel pressure exerted by fire ants’ highly toxic venom and aggressive behavior (Langkilde 2009). Fence lizards can be envenomated both during predatory attacks by fire ants and while attempting to eat fire ants (Robbins and Langkilde 2012). Both types of interactions can prove lethal for the fence lizards. As few as 12 fire ants can kill adult lizards within a minute (Langkilde 2009), and juveniles succumb to attack by even fewer fire ants and can be killed after eating as few as three fire ants (Langkilde and Freidenfelds 2010). Additionally, encounters with fire ants (both fire ant attack and consumption) that are not immediately lethal can cause delayed mortality of juvenile fence lizards (Langkilde and Freidenfelds 2010).
Considering the significant mortality that fence lizards can suffer following envenomation while consuming fire ants, there should be significant pressure on these lizards to display aversion behavior (learned or inherent) following exposure to fire ants. Interestingly, Robbins et al. (2013) found that the propensity of juvenile fence lizards to consume fire ants increased when lizards were offered a single ant per day over the course of 1 week. However, these results may not be representative of the interactions that occur in the field. Wild lizards living in fire ant-invaded sites likely encounter fire ants far more frequently than once per day, resulting in more stings and a more intense negative stimulus, which may promote aversion behavior. Indeed we have observed fence lizards eating up to six fire ants per encounter in staged semi-natural interactions (Robbins and Langkilde 2012; Robbins et al. 2013), and fence lizards encounter fire ants frequently in the field [every 132 s (±29 SE) (Freidenfelds et al. 2012)]. Here, we explore whether fence lizards display aversion behavior when exposed to ecologically relevant numbers of fire ants. We investigate how differences in stimulus intensity (number of fire ants per exposure) and temporal scales (across generations and following distant and recent past exposure to fire ants) impact this species’ behavioral response to this evolutionarily novel stimulus. We hypothesize that (1) fence lizards will display stronger learned aversion behavior by decreasing their consumption of fire ants over time if exposed to greater, ecologically relevant numbers of ants; and (2) fence lizards with previous exposure to fire ants will be more averse to eating fire ants than relatively naïve lizards. This latter hypothesis should hold true for exposure both across generations and within the lifetime of individual lizards, resulting in lizards from fire ant-invaded sites displaying inherent aversion of fire ants in addition to any behavior learned following exposure within their lifetimes.
Materials and methods
We conducted a feeding experiment to examine the effects of stimulus strength and history of exposure to fire ants on consumption of these toxic prey by fence lizards. Decreased rates of consumption of fire ants with successive exposure would indicate aversion behavior. To test for the effect of stimulus strength, we offered lizards fewer (four) or greater (ten) numbers of fire ants per day. To examine the effects of previous exposure to fire ants, we compared the rates of fire ant consumption by subadult lizards exposed to these toxic ants on different temporal scales: (1) lizards from populations that have coexisted with invasive fire ants for multiple (≈38) generations (multigenerational exposure), (2) lizards that were experimentally exposed to fire ants for 2 weeks as juveniles (distant past exposure within a lifetime), and (3) lizards that had been exposed to fire ants in the preceding days (recent exposure within a lifetime).
Our experimental approach was designed to examine fence lizard foraging behavior on fire ants. Lizards typically encounter fire ants in low numbers in the field (Freidenfelds et al. 2012), and consumption of fire ants under these conditions is consistent with foraging behavior (T. L., unpublished data). However, when lizards encounter large numbers of fire ants in the field (e.g., on mounds or foraging trails), they are quickly attacked by the ants. Lizards may consume fire ants in such situations; however, in this context consumption appears to be a defensive rather than foraging behavior (Robbins and Langkilde 2012). Similar anti-predatory consumption of fire ants is also observed in horned lizards (Webb and Henke 2003). To avoid confounding differences in the consumption of fire ants in these two contexts (Robbins and Langkilde 2012; Robbins et al. 2013), we chose numbers of fire ants that elicited foraging, and not defensive, consumption in fence lizards during trials.
Multigenerational exposure
We tested for the effects of multigenerational exposure to fire ants on the consumption of these toxic prey by fence lizards by comparing lizards from fire ant-invaded and uninvaded populations, and how these were affected by repeated exposure to fire ants. We collected juvenile fence lizards by hand between 3 July 2012 and 9 August 2012 at four sites, two of which had been invaded by fire ants and two of which remain uninvaded, respectively (invaded—Blackwater River State Forest, Santa Rosa County, FL, 30.94°N, 86.82°W, and Geneva State Forest in Geneva Co., AL, 31.12°N, 86.16°W; uninvaded—Edgar Evins State Park, Dekalb County, TN, 36.08°N, 85.83°W, and Standing Stone State Park, Overton County, TN, 36.47°N, 85.42°W).
Distant past exposure
To test whether relatively short-term exposure of juveniles to fire ants would have a lasting effect on their propensity to eat these ants, we exposed half of our individuals to fire ants as juveniles, approximately 6 months prior to the feeding trial (described in the next section). After capture, the lizards were transferred to the Solon Dixon Forestry Education Center in Covington County, Alabama (31.16°N, 86.70°W) and housed individually in tubs [30 × 20 × 25 cm, length × width × height (L × W × H)]. Crickets (Acheta domesticus) and water were provided ad libitum. Tubs were furnished with a refuge, water dish, and paper towels as substrate, and had a heat lamp positioned at one end to create a temperature gradient that allowed lizards to thermoregulate. Beginning on 26 July 2012, groups of ten lizards were placed into one of four 520-m2 outdoor enclosures constructed of aluminum sheeting. Fire ants occurred at normal densities in half of the enclosures, while fire ants were removed from other enclosures daily using a treatment of boiling water to kill fire ant colonies within the enclosures and in surrounding areas (Tschinkel and King 2007). Lizards were recaptured and removed from the enclosures after 2–3 weeks, and the effect of this (distant past) experience on the lizards’ consumption of fire ants 6 months later was quantified.
Feeding trial: recent past exposure and evaluation of fire ant consumption
The feeding experiment represents a recent exposure of fence lizards to fire ants and allowed us to assess the effect of multigenerational, distant and recent past exposure of lizards to fire ants on their consumption of these ants. Following the distant past-exposure treatments, we transported the lizards to our laboratory at the Pennsylvania State University. Here, they were communally housed for approximately 6 months, with two to five lizards per tub (60 × 42 × 30 cm, L × W × H), furnished as described earlier. Crickets and water were provided ad libitum. To commence the feeding experiment lizards were transferred to individual tubs (30 × 20 × 25 cm, L × W × H), furnished as described earlier, and assigned to one of two stimulus treatments, which consisted of us offering either four (low stimulus) fire ants or ten (high stimulus) fire ants per day for 6 days. The lizards were evenly distributed within stimulus treatments with regard to their population of origin, enclosure treatment, size, and sex (Webb and Henke 2003).
Lizards were acclimated to the experimental feeding routine for 6 days prior to the beginning of the trial by feeding them four or eight 0.5-cm crickets, according to their stimulus treatment group, to simulate ant-sized prey (Fig. 1). On day 7, we provided all lizards with twenty 1-cm crickets to ensure that they were satiated before the beginning of the trial period to help offset any effects of the previous differential feeding regime. Prior to feeding on day 7 we recorded the mass and snout-vent length (SVL) of all lizards used in the experiment.
After acclimation, we started the feeding trial by offering lizards four or ten fire ants, according to their stimulus treatment (days 8–13; Fig. 1). After 30 min we recorded the number of fire ants each lizard had consumed. We then removed any remaining fire ants and gave each lizard two 1-cm crickets as a subsistence diet. The 1-cm crickets were left in the tubs overnight, and any that remained the following morning were removed immediately prior to again offering the lizards fire ants to eat. Water dishes and refuges were removed from the tubs during each daily feeding. The paper towel substrate was removed for the duration of the experiment to ensure prey did not hide and to facilitate scoring.
To determine if increasing hunger played a role in the rate of consumption of fire ants across the experiment, on the last day of the feeding experiment (day 13) and after the fire ant feeding was completed, we fed all lizards to satiation by providing them twenty 1-cm crickets. The following day (day 14), we offered all lizards (regardless of treatment group) ten fire ants in order to determine if satiation affected their rate of fire ant consumption (i.e., were any changes in ant consumption over the trial due to increasing hunger).
Data analysis
We tested factors influencing consumption of fire ants by fence lizards during the feeding experiment using repeated-measures ANOVA. We included percent of ants eaten on successive days (days 8–13) as the repeated dependent variable, and fire ant-invasion status of the source populations (multigenerational exposure), exposure of lizards to fire ants as juveniles in enclosures (distant past exposure), and stimulus treatment (low vs. high number of fire ants) during the feeding experiment as factors. Interactions between these main factors were non-significant (P > 0.30) and were thus excluded from the final model. Site (nested within invasion status), enclosure identity, sex, mass, and SVL were included as control variables, but were omitted from the final model as they did not significantly explain variation in consumption behavior (P > 0.31). We analyzed the proportion of available ants consumed (as opposed to number of ants consumed) to facilitate testing of factors within which lizards were offered a different number of ants based on stimulus treatment (i.e., lizards within a single invasion status were fed either four or ten ants).
We tested for effects of hunger on fence lizards’ consumption of fire ants by examining differences between the last day of the feeding experiment (day 13) and the following day (after being fed to satiation, day 14). A significant decrease in fire ant consumption following being fed to satiation would suggest that hunger was driving fire ant consumption. We analyzed hunger effects using repeated-measures ANOVA with number of ants consumed before versus after satiation (day 13 and 14) as the repeated dependent variable, and stimulus treatment (low vs. high stimulus) as the factor. Site (nested within invasion status), enclosure identity, sex, mass, and SVL were included as control variables, but were omitted from the final model as they did not significantly explain variation in consumption behavior (P > 0.09). In the hunger test we analyzed the total number of ants eaten per lizard (as opposed to percentage of offered ants consumed) on the successive days as the dependent variable. The reasoning for this was that individual lizards in the low stimulus treatment were offered a different number of ants before (four ants) and after (ten ants) satiation, making testing changes in proportion of consumed ants uninformative since a lizard that did not change its consumption (i.e., ate the same number of ants on successive days) would eat a different proportion of the ants offered.
Percent of ants eaten was arcsine transformed to meet model assumptions. All statistical analyses were performed using JMP (version 12.1; SAS Institute, Cary, NC) with α = 0.05.
Results
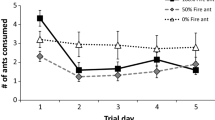
Recent past exposure of lizards to fire ants (previous days) did affect consumption of these toxic prey. However, rather than avoiding eating fire ants with repeated exposure, lizards consumed an increasing proportion of fire ants over the course of the feeding experiment (day of trial, F 5,35 = 3.69, P < 0.01; Fig. 2). Neither multigenerational exposure to fire ants (invasion status of the source population) or strength of the stimulus treatment (four vs. ten fire ants) affected the rate of change in fire ant consumption over time (day of trial × invasion status, F 5,35 = 2.12, P = 0.09; day of trial × stimulus treatment, F 5,35 = 1.22, P = 0.32).
Consumption of fire ants (Solenopsis invicta) by fence lizards (Sceloporus undulatus) during the 6-day feeding experiment. a Lizards originally from fire ant-invaded populations (solid circles) consumed more ants over the course of the experiment than did lizards from uninvaded (open circles) populations (F 1,39 = 7.02, P = 0.01). b Lizards that had been exposed to fire ants as juveniles in outdoor enclosures 6 months prior (solid squares) consumed more fire ants during the experiment than lizards that had not been exposed (open squares; F 1,39 = 4.07, P = 0.05). c Lizards that were presented with a high (ten fire ants per day; open triangles) versus low (four fire ants per day; solid triangles) stimulus intensity did not consume a significantly different proportion of ants (F 1,39 = 1.41, P = 0.24). Points represent mean ± 1 SE
Prior exposure to fire ants (multigenerational and distant past) affected the overall propensity of lizards to eat fire ants (Fig. 2). Lizards originally captured from fire ant-invaded sites, or exposed to fire ants as juveniles in outdoor enclosures 6 months earlier, ate more ants during the feeding experiment than did lizards from uninvaded sites or those placed in fire ant-free enclosures (invasion status, F 1,39 = 7.02, P = 0.01; enclosure treatment, F 1,39 = 4.07, P = 0.05; Fig. 2a, b). Stimulus intensity (number of fire ants offered) did not affect the overall consumption of fire ants by the lizards (F 1,39 = 1.41, P = 0.24; Fig. 2c).
We observed no significant decrease in the number of ants consumed following lizards being fed to satiation (day 13 vs. 14, F 1,41 = 1.09, P = 0.30). Conversely, lizards consumed a greater number of ants following satiation (Fig. 3). However, this is likely due to increased opportunity as lizards in the low stimulus (four ant) treatment were given ten ants after satiation and ate more accordingly, whereas those in the high stimulus treatment (given ten ants before and after satiation) did not change their ant consumption (day 13 vs. 14 × stimulus treatment, F 1,41 = 5.75, P = 0.02; stimulus treatment: F 1,41 = 4.27, P = 0.05; Fig. 3).
Fire ant consumption by lizards presented with low (four fire ants per day) and high (ten fire ants per day) intensity stimuli before (day 13; light bar) and after (day 14; dark bar) being fed crickets to satiation. Neither group of lizards significantly decreased their consumption of fire ants following satiation (F 1,41 = 1.09, P = 0.30). Bars represent mean ±1 SE
Discussion
Lizards did not exhibit aversion learning in response to repeated exposure to fire ants, a toxic prey, even when exposure was high and potentially lethal (Langkilde and Freidenfelds 2010). This species and other lizards can distinguish different ant species (Suarez et al. 2000; Thawley, unpublished data), and are capable of memory retention over 14 weeks (Benes 1969), suggesting that fence lizards should have been able to learn to avoid fire ants. The fact that lizards’ consumption of fire ants increased over multiple temporal scales shows that they were indeed responding to repeated exposure, but surprisingly by increasing their consumption of this toxic prey (Fig. 2). Prior exposure did not, however, affect the rate at which lizards increased their consumption of fire ants with repeated exposure (Fig. 2); that is, lizards with prior exposure to fire ants were not more likely to learn or acquire a taste for fire ants.
Recent past exposure to fire ants (in previous days) increased the propensity of lizards to eat fire ants as they ate successively more fire ants during our 6-day feeding experiment. This was unlikely due to increased hunger during the experiment since feeding the lizards to satiation did not decrease the number of fire ants they consumed. Exposure of juvenile lizards to fire ants in outdoor enclosures increased consumption of fire ants 6 months later (distant past exposure), suggesting that lizards retained memory of this experience (Benes 1969) or that the fire ants had some other lasting physiological effect on the lizards. Finally, lizards from populations that had been exposed to red imported fire ants for multiple (38) generations ate more fire ants than did those from uninvaded populations. This could suggest maternal and/or inherited transfer of this behavior. However, these lizards were collected from the field as juveniles, so invasion status is potentially confounded with early exposure to fire ants (juveniles from fire ant-invaded sites likely encountered these ants prior to being collected, whereas those from uninvaded sites would not have). It should be noted that the “invaded” populations used in this study are within both the invasive range of Solenopsis invicta and the historic distribution of the native fire ants Solenopsis geminata and Solenopsis xyloni (Tschinkel 2006). While S. invicta likely impose a stronger selective pressure than the native fire ants due to their more potent venom (Tschinkel 2006), higher densities (Porter et al. 1988), and more aggressive attack than native fire ant species (Lai et al. 2015), it is possible that the prior exposure to native fire ants contributes to the observed differences between invaded and uninvaded populations.
The results of this study expand upon previous research in this system in several ways:
-
1.
Lizards increase their consumption of fire ants with repeated exposure both when exposure is high and potentially lethal (this study) and when exposure is low and sublethal (as per Robbins et al. 2013). This finding suggests that, in free-ranging lizards, the increased consumption of fire ants, and lack of aversion learning, is likely to occur when ants are at both low densities (e.g., when the first few invading ants arrive at a site) and at higher densities [e.g., once ants have become fully established (Wojcik 1994)].
-
2.
Lifetime (e.g., learning, plasticity) and cross-generational effects (e.g., maternal effects, selection) both contribute to determining consumption of fire ants, the rate of which increases both at the population level [an increasing proportion of lizards in a population consumes fire ants (Robbins et al. 2013)] and at the level of the individual lizard [individual lizards will eat more fire ants (this study)] with repeated exposure to this toxic prey.
-
3.
Effects of exposure to fire ants as juveniles can persist into sub-adulthood. Lizards that were exposed to fire ants for 2 weeks as juveniles (in enclosures) consumed more of these ants as subadults (during the feeding experiment) than did lizards from ant-free enclosures, even after spending 6 months in captivity with no exposure to fire ants. Thus the behavioral changes in fire ant consumption are persistent even after relatively short-term exposures to fire ants early in life.
These behavioral changes in the lizards could have evolutionary significance by changing phenotypic variation within populations, and thus opportunity for selection (Stearns 1992; Roff 2002).
Given the possibly severe consequences of eating fire ants, it is interesting to consider why we saw this increase in fire ant consumption by fence lizards. Several possibilities exist, including:
-
1.
With experience, lizards might become more adept at consuming fire ants without being envenomated and could therefore safely consume more of these ants. Within a lifetime, lizards could learn to modify their feeding behavior in order to prevent fire ant stings. Across generations, lizards could inherit characteristics that could allow them to consume these venomous ants without injury or envenomation (e.g., Sherbrooke and Schwenk 2008; Cushing 2012), for example, by immediately biting and killing ants before they can attach to and sting the inside of the mouth (Robbins et al. 2013).
-
2.
Lizards could also negate the costs of envenomation through resistance to fire ant venom (e.g., Schmidt et al. 1989). Because we observed increases in fire ant consumption both on multigenerational and within-lifetime scales, if toxin resistance is acquired it does not appear to be acquired only across generations. However, there is no evidence of increased resistance to fire ant toxin by fence lizards following fire ant invasion [measured as effects on blood cell lysis and righting ability (Goldy-Brown, unpublished data; Boronow and Langkilde 2010)]. Further research should examine if, with experience, lizards are indeed able to avoid ant stings during feeding or mitigate any negative effects of envenomation through other physiological means.
-
3.
Consuming fire ants could be an anti-predator response (Robbins and Langkilde 2012). Lizards will flee from fire ants when they encounter them at sufficient density, such as on a mound. However, consuming fire ants when they are encountered at low density (such as foraging ants) may prevent further recruitment of additional ants to their location (see Webb and Henke 2003; Freidenfelds et al. 2012).
-
4.
The benefits of fire ants as a novel food resource may outweigh the costs associated with envenomation (Robbins et al. 2013). Where they are present, fire ants are often highly abundant and fundamentally alter the native arthropod communities (Porter and Savignano 1990). The possibility of prey switching by fence lizards, potentially including increased consumption of fire ants, should be investigated.
-
5.
Lizards may actually be developing a preference for fire ants (Robbins et al. 2013). Although rare (Rozin et al. 1979), animals can become “addicted” to unpalatable or irritant substances [e.g., humans exposed to chili pepper (Rozin and Schiller 1980)]. For instance, there is anecdotal evidence that dogs lick toxic invasive cane toads to get high (Hero et al. 2005), and may repeatedly lick cane toads after first exposure (J. Cochran, personal communication). Examining whether fire ants induce a “pleasurable” effect, such as the release of endorphins or dopamine, would be informative in this regard (Sharma and Verma 2014).
-
6.
The toxin of fire ants might be of some anti-predator value to the lizard. Dendrobatid frogs and Asian keelback snakes sequester defensive toxins from ants (Saporito et al. 2004) and toads (Hutchinson et al. 2007), respectively. The possibility that fence lizards sequester fire ant venom to defend themselves against predators would be an interesting avenue of future research (Savitzky et al. 2012).
Understanding the ecological and evolutionary consequences of invasive species, including those that act as novel palatable or toxic prey, is important for predicting and managing this increasing environmental perturbation. Some taxa display innate abilities to avoid toxic prey species with which they have coevolved (Smith 1975), as well as the ability to change feeding behavior when subjected to negative stimuli (Susswein et al. 1986; Crossland 2001; Somaweera et al. 2011). We demonstrate that eastern fence lizards do not avoid consuming fire ants following exposure and potential envenomation. In fact, we found that fence lizards tend to increase their consumption of venomous fire ants with repeated exposure. This trend is demonstrable over multiple temporal scales. Future research on the response of other native predators to venomous prey, over multiple temporal scales, will be valuable in determining the long-term effects of invasion and the evolution of organisms in general.
References
Allen GE, Buren WF, Williams RN, De Menezes M, Whitcomb WH (1974) The red imported fire ant, Solenopsis invicta; distribution and habitat in Mato Grosso, Brazil. Ann Entomol Soc Am 67:43–46
Allen CR, Epperson DM, Garmestani AS (2004) Red imported fire ant impacts on wildlife: a decade of research. Am Midl Nat 152:88–103
Anisman H, Zaharia MD, Meaney MJ, Merali Z (1998) Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci 16:149–164
Banks WA, Adams CT, Lofgren CS, Wojcik DP (1990) Imported fire ant infestation of soybean fields in the southern United States. Fla Entomol 73:503–504
Benes ES (1969) Behavioral evidence for color discrimination by the whiptail lizard, Cnemidophorus tigris. Copeia 1969:707–722
Boronow K, Langkilde T (2010) Sublethal effects of invasive fire ant venom on a native lizard. J Exp Zool A 313A:17–23
Callcott AA, Collins HL (1996) Invasion and range expansion of imported fire ants (Hymenoptera: Formicidae) in North America from 1918–1995. Fla Entomol 79:240–251
Chivers DP, Smith RJF (1994) Fathead minnows, Pimephales promelas, acquire predator recognition when alarm substance is associated with the sight of unfamiliar fish. Anim Behav 48:597–605
Conant R, Collins JT (1998) A field guide to reptiles and amphibians of eastern and central North America, 3rd edn. Houghton Mifflin, Boston
Crossland MR (2001) Ability of predatory native Australian fishes to learn to avoid toxic larvae of the introduced toad Bufo marinus. J Fish Biol 59:319–329
Cushing PE (2012) Spider–ant associations: an updated review of myrmecomorphy, myrmecophily, and myrmecophagy in spiders. Psyche 2012:1–23 (Article ID 151989)
Freidenfelds NA, Robbins TR, Langkilde T (2012) Evading invaders: the effectiveness of a behavioral response acquired through lifetime exposure. Behav Ecol 23:659–664
Graham SP, Freidenfelds NA, McCormick GL, Langkilde T (2012) The impacts of invaders: basal and acute stress glucocorticoid profiles and immune function in native lizards threatened by invasive ants. Gen Comp Endocrinol 176:400–408
Hero J-M et al (2005) Evaluating public cane toad eradication programs. In: Taylor R, Edwards G (eds) A review of the impact and control of cane toads in Australia with recommendations for future research and management approaches. Vertebrate Pests Committee, Canberra
Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ (2002) The causes and consequences of ant invasion. Annu Rev Ecol Syst 33:181–233
Hutchinson DA et al (2007) Dietary sequestration of defensive steroids in nuchal glands of the Asian snake Rhabdophis tigrinus. Proc Natl Acad Sci 104:2265–2270
Jones PA, Takai D (2001) The role of DNA methylation in mammalian epigenetics. Science 293:1068–1070
Lai L-C, Hua K-H, Wu W-J (2015) Intraspecific and interspecific aggressive interactions between two species of fire ants, Solenopsis geminata and S. invicta (Hymenoptera: Formicidae), in Taiwan. J Asia Pac Entomol 18:93–98
Langkilde T (2009) Invasive fire ants alter behavior and morphology of native lizards. Ecology 90:208–217
Langkilde T, Freidenfelds NA (2010) Consequences of envenomation: red imported fire ants have delayed effects on survival but not growth of native fence lizards. Wildl Res 37:566–573
Love OP, McGowan PO, Sheriff MJ (2013) Maternal adversity and ecological stressors in natural populations: the role of stress axis programming in individuals, with implications for populations and communities. Funct Ecol 27:81–92
McCormick CM, Green MR (2013) From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience 249:242–257
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407
Nussey DH, Postma E, Gienapp P, Visser ME (2005) Selection on heritable phenotypic plasticity in a wild bird population. Science 310:304–306
Porter SD, Savignano DA (1990) Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 71:2095–2106
Porter SD, Van Eimeren B, Gilbert LE (1988) Invasion of red imported fire ants (Hymenoptera: Formicidae): microgeography of competitive replacement. Ann Entomol Soc Am 81:913–918
Regulations CoF (2015) Title 7. Agriculture. Part 301.81. Subpart: imported fire ant. http://ecfr.gpoaccess.gov
Robbins TR, Langkilde T (2012) The consequences of lifetime and evolutionary exposure to toxic prey: changes in avoidance behavior through ontogeny. J Evol Biol 25:1937–1946
Robbins T, Freidenfelds N, Langkilde T (2013) Native predator eats invasive toxic prey: evidence for increased incidence of consumption rather than aversion-learning. Biol Invasions 15:407–415
Roff DA (2002) Life history evolution. Sinauer, Sunderland
Rogers JG Jr (1978) Some characteristics of conditioned aversion in red-winged blackbirds. Auk 95:362–369
Rozin P, Schiller D (1980) The nature and acquisition of a preference for chili pepper by humans. Motiv Emot 4:77–101
Rozin PP, Gruss LL, Berk GG (1979) Reversal of innate aversions: attempts to induce a preference for chili peppers in rats. J Comp Physiol Psychol 93:1001–1014
Saporito RA, Garraffo HM, Donnelly MA, Edwards AL, Longino JT, Daly JW (2004) Formicine ants: an arthropod source for the pumiliotoxin alkaloids of dendrobatid poison frogs. Proc Natl Acad Sci USA 101:8045–8050
Savitzky AH, Mori A, Hutchinson DA, Saporito RA, Burghardt GM, Lillywhite HB, Meinwald J (2012) Sequestered defensive toxins in tetrapod vertebrates: principles, patterns, and prospects for future studies. Chemoecology 22:141–158
Sax DF, Stachowicz JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Grosberg RK, Hastings A, Holt RD, Mayfield MM, O’Connor MI, Rice WR (2007) Ecological and evolutionary insights from species invasions. Trends Ecol Evol 22:465–471
Schmidt PJ, Sherbrooke WC, Schmidt JO (1989) The detoxification of ant (Pogonomyrmex) venom by a blood factor in horned lizards (Phrynosoma). Copeia 1989:603–607
Sharma A, Verma D (2014) Endorphins: endogenous opioid in human cells. World J Pharm Pharm Sci 4:357–374
Sherbrooke WC, Schwenk K (2008) Horned lizards (Phrynosoma) incapacitate dangerous ant prey with mucus. J Exp Zool A 309A:447–459
Sinervo B, Méndez-de-la-Cruz F, Miles DB, Heulin B, Bastiaans E, Villagrán-Santa Cruz M, Lara-Resendiz R, Martínez-Méndez N, Calderón-Espinosa ML, Meza-Lázaro RN, Gadsden H, Avila LJ, Morando M, De la Riva IJ, Sepulveda PV, Rocha CFD, Ibargüengoytía N, Puntriano CA, Massot M, Lepetz V, Oksanen TA, Chapple DG, Bauer AM, Branch WR, Clobert J, Sites JW (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328:894–899
Smith SM (1975) Innate recognition of coral snake pattern by a possible avian predator. Science 187:759–760
Snyder WE, Evans EW (2006) Ecological effects of invasive arthropod generalist predators. Annu Rev Ecol Evol Syst 37:95–122
Somaweera R, Webb JK, Brown GP, Shine R (2011) Hatchling Australian freshwater crocodiles rapidly learn to avoid toxic invasive cane toads. Behaviour 148:501–517
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Suarez AV, Richmond JQ, Case TJ (2000) Prey selection in horned lizards following the invasion of Argentine ants in southern California. Ecol Appl 10:711–725
Suboski MD (1992) Releaser-induced recognition learning by amphibians and reptiles. Anim Learn Behav 20:63–82
Susswein A, Schwarz M, Feldman E (1986) Learned changes of feeding behavior in Aplysia in response to edible and inedible foods. J Neurosci 6:1513–1527
Tschinkel WR (2006) The fire ants. Harvard University/Belknap Press, Cambridge
Tschinkel WR, King JR (2007) Targeted removal of ant colonies in ecological experiments, using hot water. J Insect Sci 7:1–12
Turner AM (1997) Contrasting short-term and long-term effects of predation risk on consumer habitat use and resources. Behav Ecol 8:120–125
Webb SL, Henke SE (2003) Defensive strategies of Texas horned lizards (Phrynosoma cornutum) against red imported fire ants. Herpetol Rev 34:327–328
West-Eberhard MJ (1989) Scent-trail diversion, a novel defense against ants by tropical social wasps. Biotropica 21:280–281
Wojcik DP (1994) Impact of the red imported fire ant on native ant species in Florida. In: Williams DF (ed) Exotic ants: biology, impact, and control of introduced species. Westview Press, Oxford, pp 269–281
Wojcik DP, Allen CR, Brenner RJ, Forys EA, Jouvenaz DP, Lutz RS (2001) Red imported fire ants: impact on biodiversity. Am Entomol 47:16–23
Acknowledgments
We thank J. Newman and G. Brooks for help with lizard collection, C. Norjen for help with measuring lizards, E. Baron, A. Crise and M. Goldy-Brown for lizard care, S. Graham and G. McCormick for assistance constructing outdoor enclosures, G. McCormick for help collecting fire ants, and B. Chitterlings for valuable comments on this manuscript. We thank personnel at Standing Stone State Park, Edgar Evins State Park, Geneva State Forest, Blackwater River State Forest, and especially the Solon Dixon Forestry Education Center for logistical support. Animal collection was permitted by the respective states. Funding was provided through an Eberly College of Science Undergraduate Research Grant to M. W. H. and by the National Science Foundation (DEB-0949483 to T. L.).
Author contribution statement
T. R. R. and T. L. originally formulated the idea. M. W. H., A. C., T. R. R., and T. L. designed the experiments. C. J. T., A. C., and M. W. H. conducted field and lab work. M. W. H., T. R. R., C. J. T. and T. L. wrote the manuscript. All authors provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Oliver P. Love.
Rights and permissions
About this article
Cite this article
Herr, M.W., Robbins, T.R., Centi, A. et al. Irresistible ants: exposure to novel toxic prey increases consumption over multiple temporal scales. Oecologia 181, 749–756 (2016). https://doi.org/10.1007/s00442-016-3596-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3596-3