Abstract
Understanding the factors that determine the strength of predator–prey interactions is essential to understanding community structure and stability. Variation in the strength of predator–prey interactions often can be attributed to predator mass and prey mass, or abiotic factors like temperature. However, even when accounting for these factors, there remains a considerable amount of unexplained variation that may be attributed to other traits. We compiled functional response data from the literature to investigate how predator mass, prey mass, prey type (taxonomic identity), temperature, and prey defenses (hard vs soft integument) contributed to the variation found in the predator–prey interactions between freshwater cyclopoid copepods and their prey. Surprisingly, our results indicate that prey identity (taxonomic group) and defenses (hard vs soft integument) are more important for generating variation in interaction strengths than body mass and temperature. This suggests that allometric functions can only take us so far when attempting to better understand variation in individual predator prey interactions, and that we must evaluate how other traits influence interaction strengths. Identifying additional factors such as prey defenses may enable us to better predict potential changes in the structure and function of planktonic and other food webs by better accounting for the variation in the interactions between generalists and their many prey types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An interaction strength is a measure of how important a particular predator–prey interaction is to either the predator or the prey (Novak and Wootton 2010; Gilbert et al. 2014). Interaction strengths strongly influence community structure and stability by determining the potential for predator growth and prey depletion within a community (Paine 1980; Gilbert et al. 2014). Weak predator–prey interactions may be less volatile and more prone to persisting than strong interactions (Rosenzweig 1971; Gilbert et al. 2014), and the distribution of interaction strengths in a food web may be important to overall community stability (McCann et al. 1998).
Although there are many definitions of interaction strength, one way to quantify the strength of a predator–prey interaction is with the functional response, or the relationship between predator consumption rate and prey density (Holling 1959). A typical model illustrating these relationships is the type II (saturating) functional response
where f is the kill rate, N is prey density, a is the area of capture (area or volume cleared of prey per unit time per predator, also known as “attack rate” or “attack efficiency”), and h is the handling time (the time it takes to capture, subdue, consume, and digest a prey item). Because foraging interactions depend strongly on the area of capture and handling time parameters, we take the functional response to be essentially synonymous with interaction strength. The area of capture parameter is set by predator detection distances and encounters between predator and prey, which are influenced by both predator and prey velocities (Aljetlawi et al. 2004), and the handling time parameter sums up all the time costs to the predator of capturing, subduing, and digesting prey and then returning to searching. Both parameters have been shown to depend on predator and prey mass as well as abiotic factors like temperature.
The area of capture parameter typically increases with predator mass over a wide range of body masses (i.e., ~3–8 orders of magnitude) following a power-law relationship (Rall et al. 2012; DeLong and Vasseur 2012a; Pawar et al. 2012). This may be due to the fact that larger predators can cover more ground and encounter more prey per unit time (Brose 2010), but it also may be related to the typical power-law increase in metabolic rate with body size for many types of organisms (Yodzis and Innes 1992). Area of capture may also increase with prey mass because it is easier for a predator to encounter and detect larger prey items in a given area (McCoy et al. 2011; Chang and Hanazato 2011), although a peak in area of capture may occur at intermediate prey body mass in some groups (Vucic-Pestic et al. 2010). In addition to mass, temperature can have a strong influence on the area of capture parameter, at least for ectotherms (Englund et al. 2011; Rall et al. 2012). Ectotherms often move more slowly in colder temperatures because their metabolic processes are reduced, and warming may increase these processes, enabling faster movement of predator and prey and increasing their contacts with each other (Burnside et al. 2014; Dell et al. 2014). With this increase in movement and contacts comes an increase in the area of capture parameter, at least up to a point (Kruse et al. 2008; Englund et al. 2011; Sentis et al. 2012). For these reasons, area of capture is generally thought to be strongly dependent on body mass and environmental temperature.
Handling time often decreases with predator body mass following a power-law relationship, while increasing prey mass may generate an increase in the handling time (Rall et al. 2012). These relationships make sense because larger predators can dispatch and process a given type of prey more quickly, lowering the handling time, while a larger prey item can be harder to process and capture, increasing the handling time (Brose 2010). As with area of capture, an increase in metabolic processes with temperature allows ectotherm predators to process food more quickly, decreasing handling times (Englund et al. 2011). Therefore, handling time is also generally thought to be strongly dependent on body mass and environmental temperature.
Despite the importance of predator mass, prey mass, and temperature in setting functional response parameters, there is still a considerable amount of unexplained variation in the parameters for any given group of predators. For example, there is often 2–3 orders of magnitude of scatter around the power-law relationships between functional response parameters and body size (DeLong and Vasseur 2012a, b; Pawar et al. 2012). It is paramount that we identify the source of this additional variation, because functional response parameters have large effects on the stability of communities and the dynamics of food webs (Kalinkat et al. 2013; Petchey et al. 2008). Some of this variation in interaction strengths may be generated by predator taxonomic identity (Rall et al. 2011), prey traits, or predator traits that are not linked to body size, such as foraging mode or escape tactics (Klecka and Boukal 2013). For example, prey with hard integuments or chemical defenses may reduce the area of capture and increase the handling time of predators attacking them (Plaβmann et al. 1997; Rao and Kumar 2002; Sarma et al. 2013).
Freshwater cyclopoid copepods (hereafter just “copepods”) are generalist predators that feed on a wide variety of planktonic prey and in turn are prey for larger invertebrates and fish, making copepods key players in the transfer of energy up food chains (Sarma et al. 2013). Copepods typically show type II functional responses (Rabette et al. 1998), and thus variation in the strength of interactions between copepods and their myriad prey can be viewed through variation in the area of capture and handling time parameters. Copepod body masses do not vary that much compared with some other taxonomic groups (about tenfold in this study), and therefore much of the variation in functional response parameters for copepods across species may come from sources other than predator body mass. For example, variation in copepod foraging rates may be related to prey attributes such as body mass, shape, taste, hardness, and behavior (Wiujamson 1984; Stemberger 1985; Wickham 1995). As a result, prey type (a taxonomic group or a specific species) may alter the functional response parameters from what may be expected from predator mass, prey mass, or temperature. Identifying those factors is crucial to understanding the role that copepods play in structuring plankton composition and abundance, yet no broad analysis of these factors has been conducted.
In this study, we used a new compilation of functional response data collected from the literature to investigate the factors that influence how strongly copepods interact with their prey. We hypothesized that across studies (1) increasing predator mass would increase area of capture and decrease handling times, (2) increasing prey mass would increase area of capture and increase handling times, (3) increasing temperature would increase area of capture and decrease handling times, (4) functional response parameters would vary with prey type, and (5) the presence of a hard integument that could hinder consumption would influence both handling times and area of capture, regardless of prey type.
Materials and methods
We compiled a new dataset of functional responses for copepods from published sources. We searched multidisciplinary databases such as Academic OneFile, Academic Search Premier, EBSCO, and Google Scholar for terms including combinations of “functional response,” “holling disc,” “cyclopidae,” as well as authors with previous work containing appropriate data, to find papers that contained functional response curves for copepods. Sources must have presented the data in the paper; if they were in a figure, we digitized the figure and extracted the data. In total, there were 13 sources and 50 functional response curves (Table 1).
We compiled body mass data from the original source where possible, but when mass was unreported we searched for alternative sources for that species (Table S1 in the Electronic supplementary material, ESM). Dry weights were converted to wet weights assuming that dry weight was 20 % of wet weight (Burgis 1971). Length was converted to mass using the length-weight regression from Alcaraz and Strickler (1988) [weight (mg) = 0.055 × length (mm)2.73]. Temperature was available for all but one of the studies, and missing values were ignored when conducting the analysis.
We estimated the parameters of the functional response for each experiment using the “fit” routine in Matlab® (nonlinear ordinary least squares regression; Table 1, Table S1 in the ESM). Because in most cases prey were not replenished during the experiments, we used the closed-form version of the Rogers predator equation instead of Eq. 1; i.e.,
where the area of capture and handling time parameters are the same as in Eq. 1, N e is the number eaten, N 0 is the initial number of prey, t is the time elapsed, and W is the Lambert W function (Rogers 1972; Bolker 2011). In one study, the prey was replenished (Roche 1990), so Eq. 1 was used for that study (Table S1 in the ESM).
In several cases, the fitting procedure was unable to estimate the handling time because prey densities were not high enough to detect the saturation of the foraging rate; these handling time estimates were not used in the parameter analysis described below. In most cases, the data were presented only as average foraging rates at a given prey density. As a result, sample sizes were sometimes limited, reducing the ability to provide good confidence intervals on parameters. Nonetheless, the R 2 values for the fits were reasonable (0.38–0.99) and the fits were a good match to the data (Fig. S1 in the ESM), suggesting that our parameter estimates were sufficient for our comparative analysis (Table S1 in the ESM). The parameters were converted from the original units to standard units for area of capture (mL per predator per day) and handling times (days). One of the functional response datasets (dataset 7) did not converge when attempting to fit it to Eq. 2, so Eq. 1 was used for this dataset. The analysis described below was conducted with and without this functional response; since the results were the same, it was included for completeness.
We used general linear models (GLM) to assess the effects of the four explanatory variables (predator mass, prey mass, prey type, and temperature) on the two functional response parameters (area of capture and handling time). We used the natural logs of area of capture, handling time, predator mass, and prey mass, as is standard for allometric analysis, and kept temperature untransformed. We did not use an Arrhenius function for temperature because we were only evaluating an effect of temperature rather than estimating activation energies. We began with a model that contained all main effects, and we removed nonsignificant terms until all terms were significant. We then ranked the models using AICc, selecting the model with the lowest AICc score as the best description of the data. We did not include study as a random effect because the studies were linked to temperature, cyclops mass, and prey type (for example, there is just one study with nematode prey, and different studies that have protist prey also vary in temperature). Thus, trying to pull out a study’s contribution with a random effect would influence the assessment of the other effects associated with that study.
Finally, as prey type was a significant retained factor in the models for area of capture and handling time, we also asked whether this could be accounted for by prey traits. We grouped prey into a simple classification for types with defenses or with hard integuments (insects, cladocerans, copepods, and rotifers) and for types without defenses or with soft integuments (ciliates and the nematode). In this way, we asked if prey traits that inhibit killing and consumption influenced the functional response parameters. We did this by simply replacing the prey type categorical variable with the hard or soft integument categorical variable in the best-ranked model. We also tested whether body mass varied systematically among prey types using a linear model and between hard and soft integument classes using a t-test. A phylogeny for our group was unavailable, so we did not use phylogenetic independent contrasts, and there were not enough species within genera to compare across genera. All analyses were conducted in Matlab.
Results
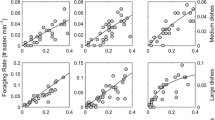
Our compilation of functional response curves for copepods feeding on a variety of prey (n = 50) revealed enormous quantitative variation in both parameters. We found 103-fold variation in area of capture (Fig. 1). The best model for area of capture indicated that predator mass and temperature had positive effects and that copepods varied substantially in area of capture across prey types (Tables 2, 3; Fig. 1), consistent with our hypotheses. The effect of prey type on area of capture was also consistent with our hypothesis that organisms with hard integuments (cladocerans, copepods, insects, and rotifers) would have an area of capture different from those with soft integuments (ciliates and nematodes).
There was 104-fold variation in handling time (Fig. 2). The best model indicated that only prey type was a good predictor of handling time (Tables 1, 2; Fig. 2). Similarly, this was consistent with the presence of prey defense, with handling times higher on prey types with hard integuments than on prey types with soft integuments. Although prey types varied in body mass (F = 17.4, p < 0.001; Fig. 3), there was no overall difference in body mass between prey types with soft versus hard integuments (t = 1.14, df = 47, p = 0.26).
Discussion
Copepods are important intermediate consumers in aquatic food webs (Sarma et al. 2013). As generalist predators, they exploit many other species, and so characterizing and predicting the strength of the interactions between copepods and those other species is essential for understanding the structure and function of the aquatic food webs in which they reside. We investigated variation in the functional responses of copepods to explore the roles of body mass, temperature, prey type, and prey defenses in setting interaction strengths between these generalist predators and their diverse prey.
Typically, predator body mass is strongly related to functional response parameters across a wide range of body masses (Brose 2010; DeLong and Vasseur 2012a, b; Rall et al. 2012; Pawar et al. 2012). For copepods, predator mass was included in the best model for area of capture, although its effect was weak, and it was not included in the best model for handling time. Thus, a simple allometric model may not be the best way to predict the functional responses of copepods, at least over narrow body size ranges. This finding contrasts strongly with previous work and is surprising given the role of body mass in setting metabolic demands. The minimal body mass effect may be related to the fact that predator body mass range was relatively narrow in this study (only approximately tenfold in variation) compared with previous studies on other taxa that had a greater predator body mass range, such as the four orders of magnitude variation seen in the body mass of grazing protists (DeLong and Vasseur 2012a).
Like body mass, temperature usually has a strong effect on the functional response parameters of ectotherms. By increasing encounter rates between prey and predator and increasing biochemical reaction rates, temperature is expected to raise the area of capture and lower the handling time (Englund et al. 2011; Dell et al. 2014). As with predator mass, temperature had a positive effect on the area of capture and no effect on handling time. The effect on area of capture was detectable even though some species of copepods have a sit-and-wait foraging strategy, which may limit the role of temperature in altering their swimming velocities and thus limit the effect of temperature on the area of capture (Novich et al. 2014). Although some copepods may take on a pursuit strategy, the temperature dependence also may arise through the temperature dependence of prey movements. Our data only show a monotonically increasing effect of temperature; it may be that copepods show the peak in area of capture at intermediate temperatures shown by some other species (Englund et al. 2011), but more data must be obtained at higher temperatures to check this possibility.
Despite the generally broad importance of body mass and temperature in setting interaction strengths, we found that prey identity is particularly important for setting copepod functional response parameters. This result complements the observation that predator taxonomic identity may influence interaction strength, even when predator body mass is considered (Rall et al. 2011). Thus, variation in prey type may generate some of the scatter that characterizes the relationships between functional response parameters and body mass and temperature, at least for generalist predators that choose a wide variety of prey. This means that, given a particular predator body mass range, some classes of prey are consumed at a faster rate and others take longer to handle. For example, rotifers and insects are consumed at a high rate, while ciliates require relatively little time for copepods to handle. This variation may be linked to important traits that are facultatively defensive. In particular, hard integuments may make it difficult for copepods to capture or consume food, altering both the handling time directly and, potentially, the willingness of copepods to attack them (Jeschke and Tollrian 2000). Although these prey types may differ in body mass (e.g., ciliates are smaller than insects; Fig. 3), prey body mass was not an important predictor of either area of capture or handling time. Thus, when using allometric models to predict functional response parameters, there may be some utility in considering the identity and defense traits of the prey, in addition to predator mass.
The consequence of all this variation in functional response parameters is that copepods will show a large range of interaction strengths with different species. As generalist predators, they may interact weakly with many species but strongly with many others. Counterintuitively, prey types that have hard integuments and take longer to handle are those that copepods clear out more quickly (i.e., have a higher area of capture). With this higher area of capture, prey with hard integuments (insects, cladocerans, rotifers, and other copepods) may experience more volatile and unstable dynamics due to these predator–prey interactions than species with soft integuments (ciliates and nematodes), influencing the overall structure and stability of these food webs (Rosenzweig 1971; McCann et al. 1998; Kalinkat et al. 2013; Gilbert et al. 2014).
In conclusion, our analysis of functional responses for copepods feeding on a variety of prey revealed limited support for the hypotheses that predator body mass and temperature would influence copepod functional response parameters, but the results did not support a role for prey body mass. Our results also showed that prey type and morphology (hard or soft integument) may play a more important role in setting interaction strengths than body mass or temperature, at least when dealing with this smaller predator mass range. The enormous variation in interaction strengths for this group of generalist predators should have profound effects on the structure and function of aquatic communities by channeling more energy into some parts of a food web than others and creating variation in the stabilities of different interactions. Although this may be particularly important for aquatic food webs, the effect of prey morphology may influence the interaction strength of generalist predators in all food webs. Recognizing and incorporating such variation in functional response parameters should improve our ability to understand and predict the dynamics of food webs.
References
Alcaraz M, Strickler JR (1988) Locomotion in copepods: pattern of movements and energetics of Cyclops. Hydrobiologia 167–168:409–414. doi:10.1007/BF00026333
Aljetlawi AA, Sparrevik E, Leonardsson K (2004) Prey–predator size-dependent functional response: derivation and rescaling to the real world. J Anim Ecol 73:239–252. doi:10.1111/j.0021-8790.2004.00800.x
Bolker BM (2011) Ecological models and data in R. Princeton University, Princeton
Brandl Z (1998) Feeding strategies of planktonic cyclopoids in lacustrine ecosystems. J Mar Syst 15:87–95. doi:10.1016/S0924-7963(97)00042-0
Brose U (2010) Body-mass constraints on foraging behaviour determine population and food-web dynamics. Funct Ecol 24:28–34
Burgis MJ (1971) The ecology and production of copepods, particularly Thermocyclops hyalinus, in the tropical Lake George, Uganda. Freshw Biol 1:169–192. doi:10.1111/j.1365-2427.1971.tb01555.x
Burnside WR, Erhardt EB, Hammond ST, Brown JH (2014) Rates of biotic interactions scale predictably with temperature despite variation. Oikos 123:1449–1456. doi:10.1111/oik.01199
Chang K-H, Hanazato T (2011) The predacious cladoceran Leptodora kindtii as a prey for the cyclopoid copepod Mesocyclops sp.: laboratory observations of predator–prey interaction. J Freshw Ecol 20:655–660
Dell AI, Pawar S, Savage VM (2014) Temperature dependence of trophic interactions are driven by asymmetry of species responses and foraging strategy. J Anim Ecol 83:70–84. doi:10.1111/1365-2656.12081
DeLong JP, Vasseur DA (2012a) Size-density scaling in protists and the links between consumer–resource interaction parameters. J Anim Ecol 81:1193–1201. doi:10.1111/j.1365-2656.2012.02013.x
DeLong JP, Vasseur DA (2012b) A dynamic explanation of size-density scaling in carnivores. Ecology 93:470–476
Englund G, Öhlund G, Hein CL, Diehl S (2011) Temperature dependence of the functional response. Ecol Lett 14:914–921. doi:10.1111/j.1461-0248.2011.01661.x
Enríquez-García C, Nandin S, Sarma SSS (2013) Feeding behaviour of Acanthocyclops americanus (Marsh) (Copepoda: Cyclopoida). J Nat Hist 47:853-862. doi:10.1080/00222933.2012.747637
Gilbert B, Tunney TD, McCann KS et al (2014) A bioenergetic framework for the temperature dependence of trophic interactions. Ecol Lett 17:902–914. doi:10.1111/ele.12307
Holling C (1959) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320
Jeschke JM, Tollrian R (2000) Density-dependent effects of prey defences. Oecologia 123:391–396. doi:10.1007/s004420051026
Kalinkat G, Schneider FD, Digel C et al (2013) Body masses, functional responses and predator–prey stability. Ecol Lett 16:1126–1134. doi:10.1111/ele.12147
Klecka J, Boukal DS (2013) Foraging and vulnerability traits modify predator–prey body mass allometry: freshwater macroinvertebrates as a case study. J Anim Ecol 82:1031–1041. doi:10.1111/1365-2656.12078
Kruse PD, Toft S, Sunderland KD (2008) Temperature and prey capture: opposite relationships in two predator taxa. Ecol Entomol 33:305–312. doi:10.1111/j.1365-2311.2007.00978.x
Krylov PI (1988) Predation of the freshwater cyclopoid copepod Megacyclops gigas on lake zooplankton functional response and prey selection. Arch Für Hydrobiol 113:231–250
Kumar R, Ramakrishna Rao T (2003) Predation on mosquito larvae by Mesocyclops thermocyclopoides (Copepoda: Cyclopoida) in the presence of alternate prey. Int Rev Hydrobiol 88:570–581. doi:10.1002/iroh.200310631
McCann K, Hastings A, Huxel GR (1998) Weak trophic interactions and the balance of nature. Nature 395:794–798. doi:10.1038/27427
McCoy MW, Bolker BM, Warkentin KM, Vonesh JR (2011) Predicting predation through prey ontogeny using size-dependent functional response models. Am Nat 177:752–766. doi:10.1086/659950
Muschiol D, Marković M, Threis I, Traunspurger W (2008) Predator–prey relationship between the cyclopoid copepod Diacyclops bicuspidatus and a free-living bacterivorous nematode. Nematology 10:55–62. doi:10.1163/156854108783360203
Novak M, Wootton JT (2010) Using experimental indices to quantify the strength of species interactions. Oikos 119:1057–1063. doi:10.1111/j.1600-0706.2009.18147.x
Novich RA, Erickson EK, Kalinoski RM, DeLong JP (2014) The temperature independence of interaction strength in a sit-and-wait predator. Ecosphere 5(10):art137. doi:10.1890/ES14-00216.1
Paine RT (1980) Food webs: linkage, interaction strength and community infrastructure. J Anim Ecol 49:666–685. doi:10.2307/4220
Pawar S, Dell AI, Savage VM (2012) Dimensionality of consumer search space drives trophic interaction strengths. Nature 486:485–489. doi:10.1038/nature11131
Petchey O, Beckerman A, Riede J, Warren P (2008) Size, foraging, and food web structure. Proc Natl Acad Sci USA 105:4191–4196
Plaβmann T, Maier G, Stich HB (1997) Predation impact of Cyclops vicinus on the rotifer community in Lake Constance in spring. J Plankton Res 19:1069–1079. doi:10.1093/plankt/19.8.1069
Rabette C, Thouvenot A, Lair N (1998) Laboratory experiments on trophic relationships and remote detection between two ciliates and Cyclops vicinus vicinus. Hydrobiologia 373–374:157–167. doi:10.1023/A:1017001725062
Rall BC, Kalinkat G, Ott D et al (2011) Taxonomic versus allometric constraints on non-linear interaction strengths. Oikos 120:483–492. doi:10.1111/j.1600-0706.2010.18860.x
Rall BC, Brose U, Hartvig M et al (2012) Universal temperature and body-mass scaling of feeding rates. Philos Trans R Soc B Biol Sci 367:2923–2934. doi:10.1098/rstb.2012.0242
Rao TR, Kumar R (2002) Patterns of prey selectivity in the cyclopoid copepod Mesocyclops thermocyclopoides. Aquat Ecol 36:411–424
Roche K (1990) Prey features affecting ingestion rates by Acanthocyclops robustus (Copepoda: Cyclopoida) on zooplankton. Oecologia 83:76–82. doi:10.1007/BF00324637
Rogers D (1972) Random search and insect population models. J Anim Ecol 41:369–383. doi:10.2307/3474
Rosenzweig M (1971) Paradox of enrichment: destabilization of exploitation ecosystems in ecological time. Science 171:385–387. doi:10.1126/science.171.3969.385
Sarma SSS, Jiménez-Contreras J, Fernández R et al (2013) Functional responses and feeding rates of Mesocyclops pehpeiensis Hu (Copepoda) fed different diets (rotifers, cladocerans, alga and cyanobacteria). J Nat Hist 47:841–852. doi:10.1080/00222933.2012.747636
Sentis A, Hemptinne J-L, Brodeur J (2012) Using functional response modeling to investigate the effect of temperature on predator feeding rate and energetic efficiency. Oecologia 169:1117–1125. doi:10.1007/s00442-012-2255-6
Stemberger RS (1985) Prey selection by the copepod Diacyclops thomasi. Oecologia 65:492–497. doi:10.1007/BF00379662
Van den Bosch F, Santer B (1993) Cannibalism in Cyclops abyssorum. Oikos 67:19–28. doi:10.2307/3545091
Vucic-Pestic O, Rall BC, Kalinkat G, Brose U (2010) Allometric functional response model: body masses constrain interaction strengths. J Anim Ecol 79:249–256. doi:10.1111/j.1365-2656.2009.01622.x
Wickham SA (1995) Cyclops predation on ciliates: species.specific differences and functional responses. J Plankton Res 17:1633–1646. doi:10.1093/plankt/17.8.1633
Wiujamson CE (1984) Laboratory and field experiments on the feeding ecology of the cyclopoid copepod, Mesocyclops edax. Freshw Biol 14:575–585. doi:10.1111/j.1365-2427.1984.tb00177.x
Yodzis P, Innes S (1992) Body size and consumer–resource dynamics. Am Nat 139:1151–1175
Acknowledgments
We thank the University of Nebraska–Lincoln Summer Undergraduate Research Program for funding. We thank Emma Erickson for assistance with data collection and several reviewers for helpful suggestions and comments.
Author contribution statement
RMK and JPD conceived and designed the project, conducted the analysis, and wrote the paper; RMK collected the data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Pete Peterson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kalinoski, R.M., DeLong, J.P. Beyond body mass: how prey traits improve predictions of functional response parameters. Oecologia 180, 543–550 (2016). https://doi.org/10.1007/s00442-015-3487-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3487-z