Abstract
Insect carnivores frequently use olfactory cues from plants to find prey or hosts. For plants, the benefits of attracting parasitoids have been controversial, partly because parasitoids often do not kill their host insect immediately. Furthermore, most research has focused on the effects of solitary parasitoids on growth and feeding of hosts, even though many parasitoids are gregarious (multiple siblings inhabit the same host). Here, we examine how a gregarious parasitoid, the tachinid fly Drino rhoeo, uses olfactory cues from the host plant Datura wrightii to find the sphingid herbivore Manduca sexta, and how parasitism affects growth and feeding of host larvae. In behavioral trials using a Y-olfactometer, female flies were attracted to olfactory cues emitted by attacked plants and by cues emitted from the frass produced by larval Manduca sexta. M. sexta caterpillars that were parasitized by D. rhoeo grew to lower maximum weights, grew more slowly, and ate less of their host plant. We also present an analytical model to predict how tri-trophic interactions change with varying herbivory levels, parasitization rates and plant sizes. This model predicted that smaller plants gain a relatively greater benefit compared to large plants in attracting D. rhoeo. By assessing the behavior, the effects of host performance, and the variation in ecological parameters of the system, we can better understand the complex interactions between herbivorous insects, the plants they live on and the third trophic level members that attack them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When attacked by herbivores, many plants synthesize and release herbivory-induced plant volatiles (HIPVs) (van Loon et al. 2000; Heil 2008; Kessler and Heil 2011). In turn, insect carnivores (predators and parasitoids) use the information encoded in these olfactory cues to find suitable prey and hosts. Although cues can provide simple information about the presence of herbivores, they can also give insect carnivores more elaborate information about the species of herbivores present (DeMoraes et al. 1998), the developmental stage and density of the herbivore (Yoneya et al. 2009; Girling et al. 2011) the status of any infections (Rostás et al. 2006) and the parasitism state of herbivores (Poelman et al. 2012). In short, the potential information available in plant-generated HIPVs can be large, and carnivores may benefit by using it.
More controversial has been whether plants themselves derive any benefits from attracting insect carnivores—in particular, whether or not there has been selection on HIPVs because of fitness benefits provided to plants by the removal of insect herbivores by insect carnivores (van der Meijden and Klinkhamer 2000; Allison and Hare 2009; Kessler and Heil 2011). This debate can be summarized by two main points: first, in natural systems, fitness benefits to plants that are derived from attracting insect carnivores have only been shown in a few cases, and second, many examples of insect carnivores that use HIPVs as host-finding cues are parasitoids and not predators (Ode 2006). There are a number of examples where production of HIPVs can depress levels of herbivory (DeMoraes et al. 1998; Kessler and Baldwin 2001), but this may not always translate into changes in plant fitness (Maron and Crone 2006; Kessler and Heil 2011). A few recent examples show that plants have higher fitness when attracting parasitoids and predators via olfactory cues (Schuman et al. 2012; Gols et al. 2015), but it is unclear how frequent these scenarios are.
From a plant’s perspective, parasitoids are not necessarily beneficial (van Loon et al. 2000). First, unlike predators, many parasitoids are koinobionts and do not immediately kill their host herbivore. Rather, they continue to develop inside the host as it feeds and grows on the plant. Selection acts on parasitoids to maximize their own success within a developing host, which may not necessarily translate into reduced herbivory. Indeed, parasitism may increase herbivory (Guillot and Vinson 1973; Slansky 1978; Brewer and King 1980; Powell 1989; Coleman et al. 1999; Shi et al. 2002; Hasan and Ansari 2012) or decrease it (Jones and Lewis 1971; Gómez and Zamora 1994; van Loon et al. 2000; Hoballah and Turlings 2001).
The situation is further complicated by the division among parasitoids into gregarious and solitary species. Solitary parasitoids develop through immature stages alone in a single host and do not compete with others within their hosts, meaning that each individual can consume part or all of the host as needed to complete its own development. In contrast, gregarious parasitoids develop in a group within a single host, and compete with each other for resources and space. Thus, gregarious parasitoids may benefit by manipulating hosts to eat more and grow longer so that the parasitoids themselves have more resources. In fact, most solitary parasitoids decrease feeding by hosts (Jones and Lewis 1971; Gómez and Zamora 1994; van Loon et al. 2000; Hoballah and Turlings 2001). The effects of gregarious parasitoids are mixed, with some examples of parasitism increasing host feeding (Slansky 1978; Brewer and King 1980; Slansky 1986; Coleman et al. 1999; Hasan and Ansari 2012) and others decreasing feeding (Führer and Keja 1976; Karowe and Schoonhoven 1992; Harvey 2000; Smallegange et al. 2008). The mixed results are likely a function of the number of parasitoids within a given host, where superparasitism results in increased feeding rates of hosts (Smallegange et al. 2008). Furthermore, most of the studies above have focused on hymenopteran parasitoids, while other major parasitoid groups have been ignored.
Tachinid flies (Diptera: Tachinidae) are one such group. Tachinids are internal parasitoids of other arthropods, mostly attacking larval stages of herbivorous insects. Unlike hymenopterans, most tachinids lack piercing ovipositors and must therefore lay eggs on or near their hosts. Also unlike hymenopterans, many tachinids are thought to be generalists (Stireman et al. 2006). This predisposition may make them more desirable defenders for plants attacked by multiple species of herbivores. With approximately 10,000 described species that span the range of terrestrial biomes, tachinids can have powerful roles in shaping ecological communities and have been used successfully in biocontrol (Stireman et al. 2006). However, the details of tri-trophic interactions between tachinids, hosts and plants, as well as the behavioral mechanisms by which tachinids find hosts, have been relatively unexplored. Furthermore, relatively few studies have examined the effects on host-feeding after parasitism by tachinids (Brewer and King 1980) and none, to our knowledge, in a natural system.
The tachinid Drino rhoeo attacks caterpillars of the hawkmoth Manduca sexta (Bernays and Woods 2000; Mira and Bernays 2002). At our field site in southeastern Arizona, we often see female flies battling fourth- and fifth-instar caterpillars on Datura wrightii (Solanaceae) plants. Though flies target later instars, which have already done some damage to the plant, parasitism may still be beneficial because the majority of food consumption by caterpillars takes place during the last larval instar (Goodman et al. 1985). Females lay eggs on the surface of the caterpillar and in < 20 min, first-instar fly larvae hatch and burrow into the hemocoel of the caterpillar (personal observation), where they begin to feed and grow. Flies develop inside the caterpillar for the duration of the caterpillar’s final instar. At the wandering stage, the caterpillar descends from the plant and buries itself underground, where the fly larvae finally kill and consume it, pupating in the surrounding soil and emerging as adults. Death from parasitism does occasionally occur before wandering, which likely happens when a caterpillar is parasitized earlier than usual (at the end of the fourth instar) or when it supports a large number of parasitoids.
Here, we assess (1) whether D. rhoeo uses plant HIPVs to find caterpillars of M. sexta, (2) what effects parasitism has on the growth and feeding of M. sexta, and (3) how natural variation in ecological parameters like plant size, parasitism rate and herbivore density affects the outcomes of tri-trophic interactions. To our knowledge, this study is one of few that address these questions using a gregarious parasitoid in a natural system under field conditions.
Materials and methods
Collection and rearing of caterpillars
Forty-five fifth-instar (final instar) M. sexta larvae were collected from D. wrightii at our long-term field site near Portal, Arizona, from August 7 to 16, 2014. Caterpillars were returned to the Southwestern Research Station and placed temporarily in a communal rearing bin with cuttings from local D. wrightii. In the evening, caterpillars were weighed (Mettler P10) and placed into individual plastic containers (13 cm × 12 cm × 14 cm). Caterpillars were kept at ambient laboratory temperature, humidity and lighting conditions. Temperatures in the laboratory typically ranged between 19 and 27 ºC (H.A. Woods, unpublished data) and the average relative humidity was 58 % during the experiment at the local C-Bar Ranch Weather Station. The light cycle in the laboratory varied, but was approximately 16:8 h light:dark. D. wrightii leaves were changed twice daily to ensure that caterpillars had a continuous supply of fresh food. All parasitized caterpillars were attacked in the field prior to collection, as parasitoids present in the laboratory were kept caged throughout the project. At the onset of the wandering phase, at the end of the last instar, caterpillars were placed in 270-mL plastic drinking cups filled with local soil and a lid was secured to trap the caterpillar. In the cups, caterpillars buried themselves, built pupal chambers (Sprague and Woods 2015), and metamorphosed if they were healthy and unparasitized.
Caterpillars were weighed twice daily (morning and evening), and frass from individuals was collected every evening from the previous 24-h period. Frass was dried in an oven (TPS Lunaire Model CE210) for 24 h at 50º C and weighed. Here, we used frass production as a measurement of feeding rate. Measuring feeding rate on multiple caterpillars can be labor intensive and time consuming. Because we had values for information on assimilation efficiencies of caterpillars in the field on wild D. wrightii plants (Woods et al. 2002), we used an approach that extrapolated feeding rates from frass production and allowed us to increase our sample size.
Parasitism
Of the 45 caterpillars collected, 2 were parasitized by another common parasitoid in the area, the ichneumonid wasp Rhyncophion flammipennis, and 2 others were infected with unknown pathogens and died before wandering. Four caterpillars disappeared from their containers, and another 3 were found dead and partially consumed by rodents in the laboratory. Here, we present data on the 34 remaining caterpillars that were intact or parasitized by tachinid flies. We found an overall rate of parasitism of 44%, in accordance with previous field estimates (Woods and Wilson, unpublished data).
Caterpillar pupae were brought back from Arizona to our laboratory in Missoula, Montana, where they were scored for parasitism. M. sexta showed signs of parasitism at different stages of development, which is likely related both to the stage at which they were parasitized and to the number of larval parasitoids they contained. Some caterpillars died before wandering, while others became pupae before their parasitoids killed them. In all cases, the presence or absence of parasitism was noted, and the number of fly pupae counted. Fly pupae were allowed to emerge as adults, and were identified to species by Dr. James O’Hara with the Canadian National Collection of Insects (specimens are vouchered under the lot number 2015-063). All parasitism in this project was by D. rhoeo, though in other years we have observed parasitism in the field by other species (Woods and Wilson, unpublished). Here, we consider caterpillars to be unparasitized if no fly larvae had emerged by September 9, 2014. One such pupa was subsequently found to be parasitized after this date—the possible identity of this individual is shown in Fig. 2.
Behavioral responses of parasitoid flies to caterpillar frass
Adult flies (D. rhoeo) were captured with hand nets near Portal, Arizona at sites with M. sexta on D. wrightii plants, placed into plastic collection containers, and returned to the Southwestern Research Station. Flies were fed daily with a 1:10 solution of honey water soaked into a cotton ball.
In testing tachinid preference for olfactory volatiles in the laboratory, 15 flies (3 males and 12 females) were used. Individual flies were tested in a custom-made glass Y-olfactometer (each of the three sides was 18 cm long with 84º between the two arms) that was inclined approximately 30º. Individuals were placed in the laboratory refrigerator for 2 min to slow them down, so that their chances of escape during handling were reduced. Flies were then placed in the base of the Y-olfactometer, the bottom end was stoppered, and a pump was turned on to pull air down the apparatus at a flow rate of approximately 50 mL/min per arm. Both arms of the olfactometer were attached to plastic cups via 40 cm of 1/8 in. ID polyvinyl tubing. In a given run, one cup was empty (control) and the other had 20 g of fresh caterpillar frass collected from fifth-instar M. sexta on the morning of the experiment. The side with the stimulus (frass) was switched randomly to control for sensory bias in choices made by flies. Furthermore, analysis showed that there was no preference for the stimulus towards the left or right (χ 2 = 0.013, df = 1, p = 0.9093). Once the pump was switched on, a large piece of cardboard was placed over the apparatus, which encouraged the flies to move up the inclined tube toward the light at the top and also minimized directional bias from laboratory lighting. Flies were scored as making a decision when they reached the top 1 cm of tubing exposed above the cardboard. Subsequently, flies were placed in a plastic cup and returned to the refrigerator for another 2 min before being tested again. Flies were tested up to 5 times, but some were tested fewer times because they did not move up the Y-maze even after extended periods of time (~30–40 min). To minimize scent contamination between one run and the next, the Y-maze was rinsed with water and dried in a drying oven at 40 °C between trials on different flies. After choice trials, flies were returned to plastic cups and used in other experiments, and once flies died, we determined their sex by examining their genitalia under a dissecting microscope.
Behavioral responses of parasitoid flies to Datura leaves in the field
In testing fly preference for D. wrightii volatiles in the field, 16 flies were used (5 males and 11 females). Each was tested multiple times (3–5). These experiments used the same Y-olfactometer described above. Trials were performed between August 8 and 14 (2014) during daytime (between 0930 and 1535 hours) at 4 different D. wrightii plants from a long-term study site near Portal, Arizona. Plants had been treated with three 5th-instar M. sexta larvae 48 h earlier to induce HIPV production. Intact, non-eaten leaves were used in all Y-olfactometer trials. The glass Y-olfactometer was set up on a metal folding table with the terminal ends (arms) elevated approximately 30º, each portion of the apparatus was connected to a plastic cup with 200 cm of 1/8 inch ID polyvinyl tubing, enclosing either an intact D. wrightii leaf or nothing (background olfactory stimulus used as a control). Flies were fed in the morning before trials and then brought to the field in a small styrofoam cooler in plastic cups and kept cool via an ice pack in the bottom of the cooler. Flies were introduced into the base of the olfactometer, the end of the tube was stoppered and the pump turned on. Air was pulled down the Y-olfactometer at a flow rate of 50 mL/min down each arm. The Y-olfactometer was immediately covered with aluminum foil to prevent overheating and to darken the Y-olfactometer and coax the flies to the ends. As in the frass trials, a choice was determined by the first appearance of the fly in the top 1 cm of either side of the Y. Once a fly made a choice, it was captured and returned to the cooler for 2 min before being tested again. During the test, the Y-olfactometer was pointed away from the sun to minimize light biases. Additionally, the stimulus side (D. wrightii leaf) was switched randomly during the experiment to account for any directional bias. The Y-olfactometer was rinsed with water and dried with paper towels between testing different individuals.
Y-olfactometers
Y-olfactometers are used frequently to assess insect behavior under controlled laboratory conditions, though they have come under some recent criticism for their inability to accurately predict ecological scenarios (Ballhorn and Kautz 2013). The main criticism is that Y-olfactometer experiments often do not account for ecological parameters that might modify the outcome of an observed behavior in a Y-olfactometer. In contrast to many laboratory studies, our work addresses many of these parameters: (1) sex of the insects, (2) use of olfactory sources from the field, and (3) controlling herbivory levels to help standardize plant induction levels. Using field-collected insects in Y-olfactometers entails a set of trade-offs. On the one hand, the behavior of field-collected insects incorporates a scope of ecological variation that may change the results compared to lab-raised insects (Ballhorn and Kautz 2013). On the other hand, our flies were at different stages and of unknown gravidity (Mondor and Roland 1997), and they had different and unknown histories of contact with hosts and plants. Some tachinid species appear to learn host cues (Stireman 2002a), and prior learning may lead to differences in individual preference in the Y-olfactometer. Testing how life-stage and experience affect behavior would be challenging in this system, as we have yet to successfully raise D. rhoeo in the laboratory, a scenario that is common in tachinids (Stireman et al. 2006). However, given that female flies are attracted to host and host-plant odors in the face of this potential variation demonstrates that the behavior is strong and ecologically relevant in our system.
Analytical model
We developed a simple model for predicting the magnitude of herbivory on plants of varying sizes with varying numbers of caterpillars under varying rates of parasitism. A list of definitions and symbols in the model can be found in Table 1 and model analysis was done in R (v.3.1.1., www.R-project.org). We began by estimating leaf area (D T (r)) of plants of different sizes (as defined by its radius, r) by the equation:
which describes the surface area of a hemisphere with a radius of a given length (r). There are many methods for estimating leaf area on plants (Peper and McPherson 1998); here, we use a simple equation that models the allometric relationship between increases in volume and surface area. We derived a few initial relationships from our experiments with caterpillar growth and feeding described above. First, we describe the reduction in herbivory due to parasitism (δ) by the equation:
where A NP is the leaf area consumed by a non-parasitized caterpillar, and A P is the leaf area consumed by a parasitized caterpillar. This reduction is shown to be approximately 493 cm2 based on experimental measurements described above (see “Results”). The total number of caterpillars on a plant (N T) is:
where N P is the number of parasitized caterpillars on an individual plant and N NP is the number of non-parasitized caterpillars. We also describe the proportion of caterpillars parasitized (P P) as:
Furthermore, the fraction of leaf material eaten (F) is:
where C T is the total consumption of leaf area by all caterpillars on a single plant. C T can be described in terms of the number of caterpillars (N T), the reduction in herbivory due to parasitism (δ), the number of non-parasitized caterpillars (A NP) and the proportion of parasitism (P P):
Substituting Eq. 6 into Eq. 5 gives an equation that describes the fraction of material eaten as a function of the number of caterpillars (N T ), the reduction in herbivory due to parasitism (δ), the number of non-parasitized caterpillars (A NP) and the proportion of parasitism (P P):
Solving Eq. 7 gives the total number of caterpillars at defoliation (when F = 1):
Assimilation efficiencies and conversions
We estimated the leaf area consumed by caterpillars by using frass production as a proxy. We converted this weight difference into differences in dry leaf material using known dry-weight assimilation efficiencies (Woods et al. 2002). We accounted for any potential changes in assimilation efficiency due to parasitism by examining a range of efficiencies from 30 to 50 % (the reported value in Woods et al. 2002 is 40.4 %). We then measured the area of 15 differently sized D. wrightii leaves using ImageJ (v.1.48, www.rsb.info.nih.gov) and compared this to dry weights of leaves obtained by placing leaves in a drying oven at 60º for 24 h. Leaf dry mass and area were strongly correlated (F 1,13 = 880.4, R 2 = 0.98, p < 0.0001). We used this relationship to convert the difference in dry leaf material consumed into a difference in leaf area consumed.
Data analysis
All statistical analyses were performed in R (v.3.1.1, www.R-project.org). Linear mixed-effects models (nlme package in R v.3.1-117) were used for the analysis of caterpillar growth rates as a function of parasitism. Model comparison using AIC scores showed that the best fit model was one that included time and parasitism as fixed effects, and took into account the slope and intercept of individual caterpillars (Table 2). Simple linear regressions were used to analyze caterpillar development time (Table 3) and frass production (Table 4) as a function of parasitism status. In Tables 2–4, models outlined in bold were those we deemed the best models, and reported in further results and analyses. Tachinid preference was tested using repeated G-tests of goodness-of-fit against an expected ratio of 0.5 (McDonald 2014). These types of tests allow for accounting of multiple trials within an individual and avoid pseudo-replication by binning all trials together.
Results
Tachinid Y-olfactometer experiments
Female D. rhoeo flies (n = 8) preferentially moved toward olfactory stimuli from D. wrightii plants that had been fed on by M. sexta caterpillars (Fig. 1). Females moved towards plant odors [volatile organic compounds (VOCs)] on average 69 % of the time. A G test of independence showed that data among flies could be pooled (G = 3.015, df = 6, p = 0.807). These pooled behavioral responses showed that, overall, females moved towards leaf odors significantly more than 50 % of the time (Fig. 1; G = 5.263, df = 1, p = 0.011). We used G tests of goodness of fit from each individual to determine whether, in general, all flies deviated from the expected 0.5 proportion. Although the overall trend in our data showed that females were attracted to VOCs produced by D. wrightii, there were differences among females, and some individuals conformed to the 0.5 proportion (Fig. 1; G = 8.275, df = 7, p = 0.309).
Drino rhoeo behavioral Y-olfactometer assays in field Datura wrightii trials and laboratory Manduca sexta frass trials. Each open square represents the average proportion moving towards a given olfactory stimulus of a single fly tested multiple times. Closed circles are average group proportions. Asterisks beside sex denotations indicate significance difference from 0.5 (p < 0.05) based on repeated measures G test goodness-of-fit analysis. The top two panels show field trials using leaves and the bottom two panels caterpillar frass trials. Points are jittered vertically to allow easier reading of data of similar or equal values
Male flies (n = 5) showed no preference for olfactory stimuli from D. wrightii leaves (Fig. 1). Male flies moved toward the olfactory stimulus on average 47 % of the time. A G test of independence showed that choice among individuals could be pooled (G = 1.253, df = 2, p = 0.535). Pooled behavioral responses showed that overall, male responses did not differ from 0.5 (Fig. 1; G = 0.091, df = 1, p = 0.381). The sample size for male flies was low, so there may not have been enough power to detect differences even if they existed.
Female D. rhoeo flies (n = 12) also preferentially moved towards odors given off by fresh caterpillar frass (Fig. 1). Females moved towards frass VOCs on average 72 % of the time. A G test of independence showed that choice among individual females could be pooled (G = 9.507, df = 11, p value = 0.575). Pooled behavioral responses showed that overall, females moved towards frass odors significantly more than 50 % of the time (Fig. 1; G = 9.723, df = 1, p = 0.0009). As in leaf trials, G-scores for individuals were summed and we concluded that although the overall trend in the data suggest that females are attracted to VOCs produced by M. sexta frass, there are differences among females, and some individuals do in fact conform to the expected 0.5 proportion (Fig. 1; G = 19.226, df = 12, p = 0.0832).
Male flies (n = 3) showed no preference for olfactory stimuli from M. sexta frass (Fig. 1). Male flies moved towards the olfactory stimulus on average 44 % of the time (Fig. 1). A G test of independence showed that choice among individuals could be pooled (G = 1.726, df = 2, p = 0.422). Pooled behavioral responses showed that overall, male responses did not differ from 0.5 (Fig. 1; G = 0.505, df = 1, p = 0.239). Again, sample size for males was small, resulting in low power to detect a preference or non-preference for odors (Fig. 2).
Growth rates of unparasitized and parasitized M. sexta caterpillars. For each caterpillar, day 0 is the day it was collected in the field. Gray lines represent individual growth trajectories of individuals that were either unparasitized (solid lines) or parasitized (dashed). Dark black lines in the foreground are trend lines for each group (parasitized and unparasitized) based on a best fit linear-mixed effects model that included individual caterpillar as a random effect (marginal R 2 = 0.396, conditional R 2 = 0.966, p < 0.0001). The thicker solid gray line is the growth trajectory for what is likely the single mislabeled caterpillar described in “Materials and methods”
Effects of fly parasitism on M. sexta larval growth
Parasitism affected larval growth rates (Fig. 3; supplementary material Fig. 1). Because caterpillars were collected from the field as fifth instars, without regard to size, caterpillar weights were tracked in relation to how many days they had been in the laboratory (first day in the laboratory = day 0). The best-fit linear mixed-effects model (Table 2) showed that parasitized caterpillars grew 0.835 g less per day than unparasitized caterpillars (p < 0.0001). The model suggests that initial weights between groups were not significantly different from each other (p = 0.0676), but that parasitized caterpillars trended towards having higher initial weights (1.56 ± 0.826 g) than unparasitized caterpillars.
Days to the onset of wandering as a function of the initial weight of parasitized (open triangles) and unparasitized (crosses) M. sexta larvae. Trendlines from each group (parasitized or unparasitized) are from the best fit linear model (F 1,30 = 23.42, R 2 = 0.6709, p < 0.0001) where dashed lines are from parasitized caterpillars, solid lines from unparasitized
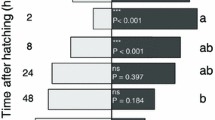
We also found that the development time of caterpillars depended on their initial weight when we started observing them (Fig. 3). We used AIC values to select among different models that included log-transformed data and whether parasitism status was included in the model. The best model (Table 3) showed no difference between parasitized and unparasitized caterpillars, but showed a significant negative correlation between a caterpillar’s weight at the beginning trial and the number of days it took to wander (Fig. 4).
Total frass produced by parasitized (open triangles) and unparasitized (crosses) M. sexta caterpillars in the laboratory over the collection time as a function of the number of days until the onset of wandering. Trendlines for each group are from the best fit linear model, which has no significant difference in the slope between the two groups, but a difference in the intercept (F 2,31 = 36.61, R 2 = 0.68, p < 0.0001). The dashed line is the parasitized group and the solid line unparasitized
Effects of parasitism on feeding rate
We measured the effects of parasitism on caterpillar feeding rate by examining frass production (until wandering or death). The best fit linear model demonstrated a positive relationship between frass production and caterpillar age (days to wander) (F 2,31 = 36.61, R 2 = 0.68, p < 0.0001). This model also demonstrated no difference in the slopes between groups, but a difference in the intercept (p < 0.0001). Parasitized fifth-instar caterpillars produced 1.04 g (dry) less frass over the course of their development than did unparasitized caterpillars (Fig. 5). Based on our estimates of leaf consumption from frass data, parasitized caterpillars ate between 408 and 570 cm2 less leaf area than unparasitized caterpillars. This is a reduction of 22–41 % in total leaf area consumed, based on field estimates of caterpillar feeding (Heinrich 1971; Casey 1976) and varying assimilation efficiencies between 30 and 50 %.
Proportion of plant leaf area eaten as a function of plant size, total number of caterpillars on the plant and parasitism rate. Different total numbers of caterpillars are represented by each pair of curves and caterpillar number is listed at the top. Dotted lines are curves where 90 % of caterpillars are parasitized. Gray bars show proportional decreases in herbivory due to parasitism at two plant sizes—one with a radius of 1 m, and the other with a radius of 0.5 m
Effects of parasitoid load on rates of growth and feeding
The total number of parasitoid larvae present (mean 28.25, range 8–50) in a parasitized caterpillar had no significant effects on either growth rate (F 1,10 = 0.1459, R 2 = 0.01, p = 0.710; supplementary Fig. 2) or frass production (F 1,12 = 0.0058, R 2 = 0.005, p = 0.9408; supplementary Fig. 3) based on best fit linear models.
Analytical model
The model (Eqs. 1, 2, 3, 4, 5, 6, 7, 8) estimates the effects of parasitism and herbivory given variation in herbivore density, plant size, and parasitism rate. First, it predicts that even a limited number of caterpillars (<8) can easily defoliate a medium-sized plant (Fig. 5), which we frequently see in the field. Second, compared to larger plants, smaller plants may benefit more from increasing rates of parasitism (Fig. 5) because small plants gain a larger proportional decrease in leaf area consumed. However, large plants can support a greater absolute number of caterpillars when parasitism rates are high (Fig. 6).
The number of caterpillars giving total defoliation as a function of plant size and parasitism rate. Each solid line represents the number of caterpillars present on an individual plant that achieve total defoliation at 0, 50 and 90 % parasitism rates. Gray boxes represent gains in caterpillar numbers at defoliation due to a 90 % parasitism rate for plants with a radius of 1.0 and of 0.5 m
Discussion
This study is the first to examine whether tachinid parasitoids defend plants effectively. We assess two key elements: whether flies use olfactory cues from plants and hosts and whether flies reduce herbivory. Parasitoids can affect the ecology of natural systems both in terms of populations (Bonsall and Hassell 1997) and communities (Muller et al. 1999). However, most of what we know about how parasitoids find hosts comes from work on hymenopteran parasitoids. Other groups, including tachinids, may use different strategies (Feener and Brown 1997; Stireman 2002b). Understanding the host-finding strategies of tachinids is important because they are abundant and diverse, and may strongly influence interactions between plants and herbivores (Stireman et al. 2006).
In our experiments, female (but not male) flies were attracted to HIPVs from attacked plants and to odors from the frass produced by caterpillars. This attraction suggests that females use olfaction to find hosts in the field. Tachinid host-finding has been examined infrequently (Stireman et al. 2006), and different species of flies appear to use different sensory modalities. Some rely on olfaction (Nettles and Burks 1975; Martin et al. 1990; Roland et al. 1995; Mondor and Roland 1997; Kainoh et al. 1999) while others use visual cues (Stireman 2002b). Though we did not test visual stimuli here, female flies of some species rely on movement (Stireman 2002a) by hosts. Movement by feeding caterpillars has been implicated as a potentially dangerous necessity (Bernays 1997) and, in fact, one defensive behavior of M. sexta caterpillars is to stop moving and slightly curl their head towards the body, which may function as visual camouflage. This characteristic behavior mimics the pose of the Egyptian sphinx, and earned the entire family of sphinx moths (Sphingidae) their name by Linnaeus in 1778 (Messenger 1997).
Parasitoids are thought to face a detectability–reliability problem when searching for hosts (Vet et al. 1991)—host olfactory cues are reliable indicators of host-presence, but can be masked by hosts and may be relatively limited (poor detectability). In contrast, plants have an evolutionary interest in attracting parasitoids and predators to attack herbivores, and are also capable of producing far larger signals than a host (Vet et al. 1991). However, if plants benefit from HIPV release, selection may favor the over-production of olfactory cues, though both allocation costs (Hoballah et al. 2004) and ecological costs (Heil 2002) may place an upper limit on production, to the point where they are dishonest signals. This would lead to the exploitation of parasitoids (low reliability but high detectability). Frass odors occupy a middle ground because they are derived from both the host and the plant: plants have control over the chemistry of the tissue that herbivores eat, but this material is processed by caterpillars before being excreted. Furthermore, frass odors may complement the wealth of information contained in plant HIPVs (Wilson et al. 2015), as they likely provide species-specific cues about the location of the host and how recently it has been active.
In fact, plant chemistry can affect frass odors, which in turn targets caterpillars for predation (Weinhold and Baldwin 2011). Frass odors strongly attract natural enemies, to the extent that some caterpillar species have evolved behaviors to fling frass far from the area surrounding them (Weiss 2003). We often observe M. sexta frass from multiple caterpillars accumulating at the base of D.wrightii plants in the field, creating large olfactory signals for parasitoids. Though M. sexta do not fling their frass like some species of caterpillars, these types of behaviors in other species highlight the importance of olfactory cues from frass in shaping tri-trophic interactions.
Female D. rhoeo flies were attracted to host and host-plant odors, but males were not. Differential odor use between male and female insects is common (e.g., Faucher et al. 2006; Ballhorn and Kautz 2013), and whereas female flies benefit from finding hosts for their offspring, males gain no immediate benefit from finding caterpillars. However, sample sizes for males in our experiments were likely too low to have enough power to detect whether they were attracted to VOCs. All flies in the experiment were field-collected, and males were less abundant, probably because we searched for flies near D. wrightii plants, where females were present because they were searching for hosts. The behavior and natural history of D. rhoeo is unstudied—in many tachinid species, individuals come together at aggregation sites for mating (often hilltop or tree trunks; Stireman et al. 2006). Males may not frequent D. wrightii plants, and so were underrepresented in our collections.
Overall, unparasitized caterpillars had higher growth rates than parasitized caterpillars, and though model prediction lines in Fig. 2 intersect each other, there was no significant difference in the intercept between the two groups in the best fit model. As described in “Materials and methods”, one caterpillar was found to be parasitized after it was scored as unparasitized. The possible identity of this individual is shown in Fig. 2—a caterpillar that had a long growth period (and presumably was parasitized early), but with a maximum weight that is well below the group average. The reason for this individual’s delayed death is unclear, but the timing of fly development and death of a caterpillar is a function of a variety of factors: the time parasitism, the physiology of the host, and the number of internal parasitoids.
The number of developing larvae inside a host can modify effects on host growth and feeding in complex ways (Smallegange et al. 2008). For example, Pieris brassicae caterpillars that were parasitized by a single brood of the gregarious Cotesia glomerata ate less than unparasitized caterpillars, but once brood size increased, this effect disappeared (Smallegange et al. 2008). Moreover, scramble competition occurred among developing parasitoids inside the host, indicating that increased feeding by caterpillars with more wasp larvae inside them might be the result of selection for parasitoids manipulating host behavior to benefit wasp larvae developing at high densities. Interestingly, we found no relationship between brood size and growth rate or frass production. Whether this pattern represents a major difference between gregarious braconid and gregarious tachinid parasitoids is unclear, because so little work has been done on tachinids. However, if so, it would result in differential benefits to plants (plants would gain more of a benefit from attracting tachinids than braconids).
Parasitized caterpillars produced less frass across all initial weights, meaning that the time at which a caterpillar was parasitized during the fifth instar had no effect on the change in frass production (i.e., there was no difference in the slopes of the models for each group in Fig. 4). This result is striking because our prediction was that caterpillars that were parasitized early would produce less total frass than caterpillars that were parasitized late, as there are more cumulative effects of parasitism over the course of a caterpillar’s development. However, we think this pattern is offset by the fact that most growth and feeding occurs late in the fifth instar (Goodman et al. 1985), so any alteration to frass production during this time will have larger consequences on overall frass production than earlier in the fifth instar.
We used a range of assimilation efficiencies in our estimates of feeding rates for two reasons. First, to account for individual variation in assimilation efficiencies (Woods et al. 2002), and second, because parasitism can affect assimilation efficiency of the host (Cloutier and Mackauer 1979). In principle, parasitized caterpillars produce less frass for one of two reasons: (1) they eat at lower rates or (2) they assimilate more of what they eat. We reject this second mechanism because parasitized insects frequently show reductions in assimilation efficiencies (Cloutier and Mackauer 1979) or no change (Duodu and Davis 1974; Slansky 1978). Nonetheless, we include a range of assimilation efficiencies (10 % in either direction around the reported values from Woods et al. 2002) in our estimates to account for any changes parasitism might have.
Many of the ecological and evolutionary dynamics of plant HIPV release are still unclear. Under what conditions do plants benefit by releasing HIPVs? How much of a benefit do third trophic level members provide? How do different ecological parameters affect tri-trophic systems? The model we propose addresses some of these questions in our system. The model allows us to vary a few key parameters of the system (plant size, herbivore density, parasitism rate) and to use results from our feeding experiments to predict outcomes in the natural environment. Though D. rhoeo can significantly depress herbivory by individual caterpillars, our model suggests that these impacts may be less potent when scaled up to herbivory on whole plants. One of the main arguments against the effectiveness of some parasitoids as defenders is that, unlike predators, they do not remove the herbivore from the plant (van Loon et al. 2000). Our model suggests tachinid parasitoids may be ineffective defenders in our system—even at high parasitism rates (90%), plants across most sizes can become totally defoliated by a moderate number of caterpillars. Furthermore, the type of benefit derived from attracting parasitoids depends on whether a plant is small or large. Small plants gain more in terms of the percentage in feeding reduction (Fig. 5), but large plants gain an overall advantage in the number of caterpillars they can tolerate before complete defoliation (Fig. 6). This may translate into greater benefits on average for small plants that attract tachinids, but with high variance that depends on rates of oviposition by female M. sexta and fractional survival of M. sexta during the egg and early larval stages. For example, a small plant with a 0.5-m radius can easily be defoliated by 8 caterpillars (Fig. 5), but the addition or reduction of only a single fifth-instar caterpillar can have large effects on the resulting level of defoliation, whereas the same addition or reduction would have little consequence on a plant with a 1-m radius. One of the interesting results in work on HIPVs over the last 20 years has been that individuals within populations of plants often demonstrate significant variation in the quality and quantity of HIPVs they produce (Dicke and Baldwin 2010; Wason and Hunter 2013). If HIPVs functioned as effective indirect defenses, then what maintains variation in these traits within populations? Our model provides one hypothesis—that selection on these traits is life-stage (size)-dependent and that in some life-history stages (small plants) this selection may be extremely variable.
One simple question is why do plants emit VOCs into the environment? The answer is probably context-dependent: intra-plant communication, recruitment of defenders and abiotic stress are just a few mechanisms that have support. Ultimately, assessing a defense-recruitment hypothesis in a tri-trophic system requires measuring the fitness consequences of herbivory. Many plants are quite tolerant of herbivory (Strauss and Agrawal 1999) and D. wrightii may be one such plant. D. wrightii have substantial tap-roots (Potter et al. 2009), which hold carbon stores that could be re-allocated to above-ground tissue after herbivory. Additionally, there are many types of parasitoids and predators that attack M. sexta at our field site (Mira and Bernays 2002) which may benefit D. wrightii plants by using plant VOCs. Plant fitness can be difficult to measure, particularly in perennial plants like D. wrightii, and measuring the fitness effects of herbivory across multiple growing seasons represents a challenging and worthwhile next step in exploring this system. In this study, we focus on the first steps in describing this interaction—how insect parasitoids use olfactory cues, the impact that the parasitoids have on host growth and performance, and how variation in different ecological parameters shape the outcomes of tri-trophic interactions.
Conclusions
By integrating across fields and combining behavioral assays with physiological measurements of growth and feeding and an analytical modeling approach, we gain deeper insight into how interactions play out in the complex natural world. If we had stopped at simply describing D. rhoeo behavior and effects on growth and feeding, our conclusion would have been that parasitoids can strongly reduce herbivory by hosts, and are potentially effective plant defenders. However, by incorporating important ecological parameters that vary in our system, we come to a different conclusion—that parasitism may not be effective on a whole-plant level at reducing defoliation, and that the benefit depends on plant size. We suggest that examining tri-trophic interactions using a multi-disciplinary approach is important in determining outcomes in natural systems.
References
Allison JD, Hare JD (2009) Learned and naive natural enemy responses and the interpretation of volatile organic compounds as cues or signals. New Phytol 184:768–782. doi:10.1111/j.1469-8137.2009.03046.x
Ballhorn DJ, Kautz S (2013) How useful are olfactometer experiments in chemical ecology research? Commun Integr Biol 6:1–3. doi:10.4161/cib.24787
Bernays EA (1997) Feeding by lepidopteran larvae is dangerous. Ecol Entomol 22:121–123. doi:10.1046/j.1365-2311.1997.00042.x
Bernays EA, Woods HA (2000) Foraging in nature by larvae of Manduca sexta—influenced by an endogenous oscillation. J Insect Physiol 46:825–836. doi:10.1016/S0022-1910(99)00172-9
Bonsall MB, Hassell MP (1997) Apparent competition structures ecological assemblages. Nature 388:371–373. doi:10.1038/41084
Brewer FD, King EG (1980) Consumption and utilization of a soyflour–wheat germ diet by larvae of the tobacco budworm parasitized by the tachinid Eucelatoria sp. Entomophaga 25:95–101. doi:10.1007/BF02377527
Casey TM (1976) Activity patterns, body temperature and thermal ecology in two desert caterpillars (Lepidoptera: Sphingidae). Ecology 57:485. doi:10.2307/1936433
Cloutier C, Mackauer M (1979) The effect of parasitism by Aphidius smithi (Hymenoptera: Aphididae) on reproduction and population growth of the pea aphid (Homoptera: Aphididae). Can Entomol 57:919–926. doi:10.1139/z79-210
Coleman RA, Barker AM, Fenner M (1999) Parasitism of the herbivore Pieris brassicae L. (Lep., Pieridae) by Cotesia glomerata L. (Hymenoptera, Braconidae) does not benefit the host plant by reduction of herbivory. J Appl Entomol 123:171–177. doi:10.1046/j.1439-0418.1999.00334.x
DeMoraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573. doi:10.1038/31219
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the “cry for help”. Trends Plant Sci 15:167–175. doi:10.1016/j.tplants.2009.12.002
Duodu YA, Davis DW (1974) A comparison of growth, food consumption, and food utilization between unparasitized alfalfa weevil larvae and those parasitized by Bathyplectes curculionis (Thomson). Environ Entomol 3:705–710. doi:10.1093/ee/3.4.705
Faucher C, Forstreuter M, Hilker M, de Bruyne M (2006) Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol 209:2739–2748. doi:10.1242/jeb.02297
Feener DH, Brown BV (1997) Diptera as parasitoids. Annu Rev Entomol 42:73–97. doi:10.1146/annurev.ento.42.1.73
Führer E, Keja TD (1976) Physiological interrelationships between Pieris brassicae and its endoparasite, Apanteles glomeratus-effect of parasitism on growth and body weight of the host. Entomol Exp Appl 19:287–300. doi:10.1111/j.1570-7458.1976.tb02608.x
Girling RD, Stewart-Jones A, Dherbecourt J, Stayley JT, Wright DJ, Poppy GM (2011) Parasitoids select plants more heavily infested with their caterpillar hosts: a new approach to aid interpretation of plant headspace volatiles. Proc R Soc Lond B 278:2646–2653. doi:10.1098/rspb.2010.2725
Gols R, Wagenaar R, Poelman EH, Kruidhof M, van Loon JJA, Harvey JA (2015) Fitness consequences of indirect plant defence in the annual weed, Sinapis arvensis. Funct Ecol. doi: 10.1111/1365-2435.12415
Gómez JM, Zamora R (1994) Top-down effects in a tritrophic system: parasitoids enhance plant fitness. Ecology 75:1023–1030. doi:10.2307/1939426
Goodman W, Carlson RO, Nelson KL (1985) Analysis of larval and pupal development in the tobacco hornworm (Lepidoptera: Sphingidae), Manduca sexta. Ann Entomol Soc Am 78:70–80. doi: 10.1093/aesa/78.1.70
Guillot FS, Vinson BS (1973) Effect of parasitism by Cardiochiles nigriceps on food consumption and utilization by Heliothis virescens. J Insect Physiol 19:2073–2082. doi:10.1016/0022-1910(73)90200-X
Harvey JA (2000) Dynamic effects of parasitism by an endoparasitoid wasp on the development of two host species: implications for host quality and parasitoid fitness. Ecol Entomol 25:267–278. doi:10.1046/j.1365-2311.2000.00265.x
Hasan F, Ansari MS (2012) Superparasitism in Cotesia glomerata does not benefit the host plant by reduction of herbivory caused by Pieris brassicae. Saudi J Biol Sci 19:65–71. doi:10.1016/j.sjbs.2010.11.002
Heil M (2002) Ecological costs of induced resistance. Curr Opin Plant Biol 5:345–350. doi:10.1016/S1369-5266(02)00267-4
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178:41–61. doi:10.1111/j.1469-8137.2007.02330.x
Heinrich B (1971) The effect of leaf geometry on the feeding behaviour of the caterpillar of Manduca sexta (Sphingidae). Anim Behav 19:119–124. doi:10.1016/S0003-3472(71)80145-8
Hoballah MEF, Turlings TCJ (2001) Experimental evidence that plants under caterpillar attack may benefit from attracting parasitoids. Evol Ecol Res 3:553–565
Hoballah ME, Köllner TG, Degenhardt J, Turlings TCJ (2004) Costs of induced volatile production in maize. Oikos 1:168–180
Jones L, Lewis WJ (1971) Physiology of the host–parasite relationship between Heliothis zea and Microplitis croceipes. J Insect Physiol 17(5):921–927. doi:10.1016/0022-1910(71)90108-9
Kainoh Y, Tanaka C, Nakamura S (1999) Odor from herbivore-damaged plant attracts the parasitoid fly Exorista japonica Townsend (Diptera: Tachinidae). Appl Entomol Zool 34:463–467. doi:10.1248/cpb.37.3229
Karowe DN, Schoonhoven LM (1992) Interactions among three trophic levels: the influence of host plant on performance of Pieris brassicae and its parasitoid, Cotesia glomerata. Entomol Exp Appl 62:241. doi:10.1007/BF00353443
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144. doi:10.1126/science.291.5511.2141
Kessler A, Heil M (2011) The multiple faces of indirect defences and their agents of natural selection. Funct Ecol 25:348–357. doi:10.1111/j.1365-2435.2010.01818.x
Maron JL, Crone E (2006) Herbivory: effects on plant abundance, distribution and population growth. Proc R Soc Lond B 273:2575–2584. doi:10.1098/rspb.2006.3587
Martin WR, Nordlund DA, Nettles WC (1990) Response of parasitoid Eucelatoria bryani to selected plant material in an olfactometer. J Chem Ecol 16:499–508. doi:10.1007/BF01021781
McDonald J (2014) Handbook of biological statistics, 3rd edn. Sparky House, Baltimore
Messenger C (1997) The sphinx moths (Lepidoptera : Sphingidae) of Nebraska. Trans Nebraska Acad Sci 24:89–141
Mira A, Bernays EA (2002) Trade-offs in host use by Manduca sexta: plant characters vs natural enemies. Oikos 97:387–397. doi:10.1034/j.1600-0706.2002.970309.x
Mondor EB, Roland J (1997) Host locating behaviour of Leschenaultia exul and Patelloa pachypyga: two tachinid parasitoids of the forest tent caterpillar, Malacosoma disstria. Entomol Exp Appl 85:161–168. doi:10.1023/A:1003178423250
Muller CB, Adriaanse CT, Belshaw R, Godfray HCJ (1999) The structure of an aphid–parasitoid community. Anim Ecol 68:346–370. doi:10.1046/j.1365-2656.1999.00288.x
Nettles WC, Burks ML (1975) A substance from Heliothis virescens larvae stimulating larviposition by females of the tachinid, Archytas marmoratus. J. Insect Physiol 21:965–978. doi:10.1016/0022-1910(75)90108-0
Ode PJ (2006) Plant chemistry and natural enemy fitness: effects on herbivore and natural enemy interactions. Annu Rev Entomol 51:163–185. doi:10.1146/annurev.ento.51.110104.151110
Peper PJ, McPherson EG (1998) Comparison of five methods for estimating leaf area index of open-grown deciduous trees. J Arboric 24:98–111
Poelman EH, Bruinsma M, Zhu F, Weldegergis BT, Boursault AE, Jongema Y, van Loon JJA, Vet LEM, Harvey JA, Dicke M (2012) Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host. PLoS Biol. doi:10.1371/journal.pbio.1001435
Potter K, Davidowitz G, Woods HA (2009) Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J Exp Biol 212:3448–3454. doi:10.1242/jeb.033365
Powell JE (1989) Food consumption by tobacco budworm (Lepidoptera: Noctuidae) larvae reduced after parasitization by Microplitis demolitor or M. croceipes (Braconidae). J Econ Entomol 82:408–411. doi:10.1093/jee/82.2.408
Roland J, Denford KE, Jimenez L (1995) Borneol as an attractant for Cyzenis albicans, a tachinid parasitoid of the winter moth, Operophtera brumata L. (Lepidoptera: Geometridae). Can Entomol 127:413–421. doi:10.4039/Ent127413-3
Rostás M, Ton J, Mauch-Mani B, Turlings TCJ (2006) Fungal infection reduces herbivore-induced plant volatiles of maize but does not affect naïve parasitoids. J Chem Ecol 32:1897–1909. doi:10.1007/s10886-006-9147-3
Schuman MC, Barthel K, Baldwin IT (2012) Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. Elife 2012:1–29. doi:10.7554/eLife.00007
Shi Z, Liu S, Li Y (2002) Cotesia plutellae parasitizing Plutella xylostella: host-age dependent parasitism and its effect on host development and food consumption. Biocontrol 47:499–511. doi:10.1023/A:1016577406820
Slansky F (1978) Utilization of energy and nitrogen by larvae of the imported cabbageworm, Pieris rapae, as affected by parasitism by Apanteles glomeratus. Environ Entomol 7:179–185. doi:10.1093/ee/7.2.179
Slansky F (1986) Nutritional ecology of endoparasitic insects and their hosts: an overview. J Insect Physiol 32:255–261. doi:10.1016/0022-1910(86)90036-3
Smallegange RC, van Loon JJA, Blatt SE, Harvey JA, Dicke M (2008) Parasitoid load affects plant fitness in a tritrophic system. Entomol Exp Appl 128:172–183. doi:10.1111/j.1570-7458.2008.00693.x
Sprague JC, Woods HA (2015) Costs and benefits of underground pupal chambers constructed by insects: a test using Manduca sexta. Physiol Biochem Zool 88:521–534. doi:10.1086/682251
Stireman JO (2002a) Learning in the generalist tachinid parasitoid Exorista mella walker (Diptera: Tachinidae). J Insect Behav 15:689–706. doi:10.1023/A:1020752024329
Stireman JO (2002b) Host location and selection cues in a generalist tachinid parasitoid. Entomol Exp Appl 103:23–34. doi:10.1046/j.1570-7458.2002.00958.x
Stireman JO, O’Hara JE, Wood DM (2006) Tachinidae: evolution, behavior, and ecology. Annu Rev Entomol 51:525–555. doi:10.1146/annurev.ento.51.110104.151133
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185. doi:10.1016/S0169-5347(98)01576-6
van der Meijden E, Klinkhamer PGL (2000) Conflicting interests of plants and the natural enemies of herbivores. Oikos 89:202–208. doi: 10.1034/j.1600-0706.2000.890123.x
van Loon JJA, De Boer JG, Dicke M (2000) Parasitoid-plant mutualism: parasitoid attack of herbivore increases plant reproduction. Entomol Exp Appl 97:219–227. doi:10.1023/A:1004032225239
Vet LEM, Wäckers FL, Dicke M (1991) How to hunt for hiding hosts: the reliability–detectability problem in foraging parasitoids. Netherlands J Zool 41:202–213. doi:10.1163/156854291x00144
Wason EL, Hunter MD (2013) Genetic variation in plant volatile emission does not result in differential attraction of natural enemies in the field. Oecologia. doi:10.1007/s00442-013-2787-4
Weinhold A, Baldwin IT (2011) Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc Natl Acad Sci USA 108:7855–7859. doi:10.1073/pnas.1101306108
Weiss MR (2003) Good housekeeping: why do shelter-dwelling caterpillars fling their frass? Ecol Lett 6:361–370. doi:10.1046/j.1461-0248.2003.00442.x
Wilson JK, Kessler A, Woods HA (2015) Noisy communication via airborne infochemicals. Bioscience 65:667–677. doi:10.1093/biosci/biv062
Woods HA, Perkins MC, Elser JJ, Harrison JF (2002) Absorption and storage of phosphorus by larval Manduca sexta. J Insect Physiol 48:555–564. doi:10.1016/S0022-1910(02)00060-4
Yoneya K, Kugimiya S, Takabayashi J (2009) Can herbivore-induced plant volatiles inform predatory insect about the most suitable stage of its prey? Physiol Entomol 34:379–386. doi:10.1111/j.1365-3032.2009.00701.x
Acknowledgments
Thank you to Steve Lane, Nikita Cooley, Daniel Olson, Lauren Smith and two anonymous reviewers for comments on the manuscript. Thanks also to James O’Hara for identifying the tachinids we used and to Mike Singer for discussing early stages of this work and for suggesting the frass experiments. Finally, thanks to the director and staff at the Southwestern Research Station and to Erin McCullough, Emily Ding, Amanda Carrasco, Antoine Boussard and Valentina Giombini for help in the field. Thank you to Anna Sala for initial discussions and suggestions regarding the analytical model. This work was supported by the National Science Foundation (IOS 0844916) to H.A.W. and the University of Montana.
Author contribution statement
JKW and HAW conceived and designed experiments. JKW carried out field work, JKW and HAW wrote the manuscript jointly.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Colin Mark Orians.
Electronic supplementary material
Below is the link to the electronic supplementary material.
442_2015_3419_MOESM1_ESM.pdf
Supplemental Fig. 1 Maximum weight of unparasitized and parasitized fifth-instar M. sexta caterpillars plotted against their initial weight. Open triangles represent parasitized caterpillars and crosses represent unparasitized individuals. Trendlines for each group are from the best fit linear model (F 3,31 = 7.291, R 2 = 0.3638, p = 0.00082) dashed lines are from parasitized caterpillars, solid lines from unparasitized. (PDF 102 kb)
442_2015_3419_MOESM2_ESM.pdf
Supplemental Fig. 2 Modeled growth rate of parasitized caterpillars as a function of their parasitoid load. Growth rate was modeled by calculating the slope of line that best fit individual caterpillars’ growth curves (Fig. 2). There was no significant relationship between growth rate and the number of parasitoids (F 1,10 = 0.1459, R 2 = 0.01, p = 0.710). Fewer caterpillars are examined here than in other datasets because some caterpillars had fewer measurements of growth over time that gave erroneous values of modeled growth rates. These individuals were excluded from the analysis (PDF 83 kb)
442_2015_3419_MOESM3_ESM.pdf
Supplemental Fig. 3 Total frass production of parasitized caterpillars as a function of their parasitoid load. There was no significant relationship between growth rate and the number of parasitoids (F 1,12 = 0.0058, R 2 = 0.005, p = 0.9408).(PDF 91 kb)
Rights and permissions
About this article
Cite this article
Wilson, J.K., Woods, H.A. Protection via parasitism: Datura odors attract parasitoid flies, which inhibit Manduca larvae from feeding and growing but may not help plants. Oecologia 179, 1159–1171 (2015). https://doi.org/10.1007/s00442-015-3419-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3419-y