Abstract
Herbivore distribution can impact community structure and ecosystem function. On coral reefs, herbivores are thought to play an important role in promoting coral dominance, but how they are distributed relative to algae is not well known. Here, we evaluated whether the distribution, behavior, and condition of herbivorous fishes correlated with algal resource availability at six sites in the back reef environment of Moorea, French Polynesia. Specifically, we tested the hypotheses that increased algal turf availability would coincide with (1) increased biomass, (2) altered foraging behavior, and (3) increased energy reserves of herbivorous fishes. Fish biomass and algal cover were visually estimated along underwater transects; behavior of herbivorous fishes was quantified by observations of focal individuals; fish were collected to assess their condition; and algal turf production rates were measured on standardized tiles. The best predictor of herbivorous fish biomass was algal turf production, with fish biomass increasing with algal production. Biomass of herbivorous fishes was also negatively related to sea urchin density, suggesting competition for limited resources. Regression models including both algal turf production and urchin density explained 94 % of the variation in herbivorous fish biomass among sites spread over ~20 km. Behavioral observations of the parrotfish Chlorurus sordidus revealed that foraging area increased as algal turf cover decreased. Additionally, energy reserves increased with algal turf production, but declined with herbivorous fish density, implying that algal turf is a limited resource for this species. Our findings support the hypothesis that herbivorous fishes can spatially track algal resources on coral reefs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The way in which foraging animals distribute themselves can have important impacts on community structure and ecosystem function (Huntly 1991; Duffy 2002). In the absence of constraints, foraging animals are expected to distribute themselves among habitat patches in proportion to the amount of food contained in them, a concept known as the ideal free distribution (Fretwell and Lucas 1970; Fretwell 1972). Studies in terrestrial systems have documented such spatial tracking of resource availability by herbivores (e.g., McNaughton 1990; Fryxell et al. 2004). Often, however, foraging animals are not distributed in an ideal free fashion because many factors besides resource distribution influence their foraging decisions (reviewed in Tregenza 1995).
The distribution of herbivores can be influenced by several factors, including the ability to detect differences in resource availability or quality among patches (i.e., patch selection), habitat characteristics (Ogutu et al. 2010), interference competition (e.g., Sutherland 1983), social interactions (e.g., Jarman 1974), mobility, and predation risk (e.g., Hebblewhite and Merrill 2009). Of these factors, the most studied models concern ‘interference’, which treats predation or competition as hindrances to foraging behavior (Sutherland and Parker 1992). Herbivore distribution is often the result of trade-offs between resource availability and quality, predation risk, and competition (Fryxell 1991; Brown and Kotler 2004; Burkepile et al. 2013).
Herbivore distribution can influence several aspects of ecosystems. Herbivores can have significant and lasting effects on nutrient cycling and productivity (e.g., McNaughton et al. 1997), plant distribution (e.g., Adler et al. 2001), species diversity (e.g., McNaughton 1985; Huntly 1991), and ecosystem function (e.g., McClanahan and Shafir 1990; Estes et al. 2011). When herbivores are not controlled by predators, they can also exert strong top–down control of primary producers (e.g., Carpenter 1986; Schmitz et al. 2000), sometimes resulting in community phase shifts to alternative ecosystem states (Scheffer et al. 2001). Examples of herbivore-mediated shifts between alternative states have been seen in grasslands (Prins and Vanderjeugd 1993), freshwater lakes (Carpenter et al. 1987), north Pacific kelp beds (Estes et al. 1998), and coral reefs (McClanahan and Shafir 1990).
Compared to some terrestrial systems, relatively little is known about how herbivores on coral reefs distribute themselves relative to their food resources. Coral reefs are diverse and economically, socially, and environmentally important ecosystems, and many are in decline due to increasing anthropogenic and natural disturbances (Hughes 1994; Bellwood et al. 2004), often resulting in benthic community phase shifts (Bellwood et al. 2004). Community shifts on coral reefs are usually from scleractinian coral to macroalgae-dominated communities (e.g., Done 1992; Nyström et al. 2000), resulting in significant alteration of ecosystem function, structure, and diversity (Scheffer et al. 2001; Mumby and Steneck 2008).
While it is well known that herbivorous fishes can reduce the abundance of algae on coral reefs (e.g., Carpenter 1986; Lewis 1986; Morrison 1988), far less is known about how they respond to spatial variation in algal abundance. In the absence of other constraints, the ideal free distribution theory predicts that they should distribute themselves in proportion to food availability (Fretwell and Lucas 1970). Such spatial tracking of algae on reefs would seem to be a prerequisite for control of algal proliferation. In addition, one would expect to see functional (increase in food intake), developmental (increase in size due to higher growth rates), or numerical (increase in abundance) responses by herbivores to increased food availability (Solomon 1949). Five studies have shown that herbivorous fish biomass correlates with algal resource abundance in the absence of disturbance events (Carpenter 1990; Williams and Polunin 2001; Russ 2003; Stockwell et al. 2009; Rasher et al. 2013). Adam et al. (2011) showed evidence of a temporal response to turf algae after disturbance whereby parrotfish biomass quickly increased (within ~1 year) when algal cover increased following extensive coral mortality caused by an outbreak of the corallivorous crown-of-thorns seastar (COTS). Gilmour et al. (2013) documented a similar, dramatic increase in herbivore abundance subsequent to mass mortality of corals in Western Australia, where herbivores maintained the algal community in a cropped algal turf state, facilitating coral repopulation. However, other field evidence of a response by herbivorous fishes to changes in algal abundance is mixed with unclear or no effects of increases in algal abundance on the distribution, density, biomass, and diet composition of some herbivores on the Great Barrier Reef in Australia (Hart et al. 1996a, b).
Relatively few studies have explicitly examined whether herbivorous fishes on coral reefs respond to algal resource availability (e.g., McClanahan 1997; Williams and Polunin 2001), and fewer still have examined the effects of increased (as opposed to decreased) resources (Morrison 1988; Carpenter 1990; Adam et al. 2011; Gilmour et al. 2013). In addition, relatively few studies have incorporated actual estimates of algal production, and those that have compared across different habitat types (Russ 2003; Paddack et al. 2006). Moreover, only one study of herbivore distribution has used algal turf as the primary focus of algal resources despite its relative dominance on many reefs and its role in phase shifts (Russ 2003).
Algal turf is ubiquitous on coral reefs and one of the largest sources of primary production in these systems, and is common in the diets of many herbivorous fishes (Choat et al. 2002; Wilson et al. 2003). Furthermore, algal turf is often among the first functional groups to colonize new substrata (Hay 1981). It can be an intermediate successional stage between bare substrata and macroalgae, and is therefore an important element in phase shifts (Hixon and Brostoff 1996; McClanahan 1997). When considered as the epilithic algal matrix, which includes filamentous algae and accompanying inorganic sediment and detritus (Wilson et al. 2003), algal turf can represent a direct threat to coral by reducing coral recruitment or even overgrowing live coral (Birrell et al. 2008; Vermeij et al. 2010). Thus, algal turf represents a major food item for herbivores, a potential transitional state to macroalgal dominance, as well as a possible suppressor of coral recruitment, growth, and recovery.
Here, we tested the hypothesis that herbivorous fishes respond to variation in algal turf abundance and production among sites spread over tens of kilometres. Natural disturbances (e.g., COTS outbreaks and storms) often generate variation in algal abundance over similar spatial scales (Carpenter 2013). Specifically, we tested three hypotheses: that (1) biomass, (2) behavior (e.g., foraging or movements), and (3) energy reserves of herbivorous fishes would be related to algal resource availability.
Materials and methods
Study area and study sites
This study was performed in the back reef on the north and east shores of Moorea, French Polynesia (17°29′S, 149°50′W) from June to August 2011. The back reef is enclosed by an outer barrier reef and separated from the fringing reef and shore by deep (≥10 m) channels. It is a naturally variable environment in terms of benthic composition but has relatively uniform depth (~1–3 m) and distance from shore. The dominant vertebrate herbivores are fishes in the families Scaridae and Acanthuridae. In addition to these abundant vertebrate herbivores, herbivorous diadematid sea urchins are also present.
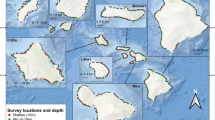
A total of six sites spread over ~20 km were chosen for this study, with four on the north shore (Papetoai, Rotui, Pearl, and Avaiti) and two on the east shore of the island (Temae and Vaiare) (Fig. 1). Sites were selected to span a gradient in algal turf cover, thus allowing for observations of herbivorous fishes in areas with differing algal resource availability. Sites on the eastern shore were included in the sampling design because of their relatively low algal turf cover. Each site was ~300 × 200 m in area. All sites were bordered on one side by the reef crest, and often by a boat channel on the other side. The six sites were all near the more populated coastlines of the island and were known to incur limited artisanal fishing pressure, which appeared to be similar among the sites.
Sampling of fishes, potential competitors, and habitat
To measure the abundance of herbivorous fishes, algal turf, macroalgae, crustose coralline algae (CCA), and live coral, each site was surveyed by snorkeling along six, 50-m-long transects. The six transects were arranged systematically (in two rows of three transects) in order to survey as much of the site as possible. Transects in each row were separated from the next transect by a 50-m gap and the rows were separated by ~75 m. Thus, the six transects spanned an area 250 m long by ~75 m wide.
Benthic surveys were carried out once in June 2011. These were used to select the six sites, which spanned a range of algal turf abundance. Benthic surveys were done only once because we saw little evidence of change in the benthic attributes at the sites over the 2-month duration of the study. Benthic sampling employed a uniform point contact (UPC) method with points at 0.5-m intervals, giving a total of 100 points per transect. UPC data were used to calculate the average percent cover of turfing algae, macroalgae, CCA, and live corals. Algal turf patches were further described as occurring on dead coral rubble (“rubble turf”) and dead coral bommies (“bommie turf”), or inside Stegastes spp. territories because these categories are functionally different for herbivores. Stegastes damselfish territories are highly defended and therefore generally unavailable for foraging by other species (Sammarco and Carleton 1981; Hixon and Brostoff 1983).
Fish surveys were performed twice, in June and August 2011, to estimate the abundance and biomass of herbivorous fishes using underwater visual census along the transects described above. Herbivorous fishes at the study sites were members of the families Acanthuridae, Scaridae, Pomacentridae, and Siganidae. A snorkeler counted all conspicuous herbivorous fishes within 4 m on either side of the transect (i.e., 50 × 8 m transects, 400 m2), from the benthos (which was 1.2–2.0 m deep) to the water surface. The size of each fish was visually estimated to the nearest cm. Although these transects were wider than those used in many studies, other studies have visually estimated fish size over similar distances (e.g., Jouffray et al. 2015). In the shallow, clear water at our study sites, it was not difficult to count and estimate the size of fish from up to 4 m away. Biomass of all herbivorous fish species was calculated using length to weight formulas from Kulbicki et al. (2005), providing an estimate of fish biomass per unit area (g m−2).
To understand potential effects of algal resource availability on herbivorous fishes, it was important to quantify the other major herbivore guild and potential competitor on Moorea, sea urchins. Diadematid echinoids in the genera Diadema and Echinothrix were sampled using 1-m2 quadrats placed at 1-m intervals along the 50-m-long transects. Diadematids were separated from other echinoids (genera Echinometra and Echinostrephus) because they are the largest echinoids on Moorea with similar diets to herbivorous fishes, and are therefore the most likely to compete with herbivorous fishes for algal resources (Hay and Taylor 1985).
To quantify potential shelter habitat for herbivorous fishes at each site, we estimated percent cover of large coral bommies at each site. Point-contact estimates of live coral cover were potentially poor proxies for the abundance of shelter habitat because even completely dead bommies provide shelter habitat for fishes. We used Google Earth satellite images, which clearly showed bommies. For each site, 15 sample areas were selected by randomly projecting points on a photo of the site using Coral Point Count with Excel extensions software (CPCe) (Kohler and Gill 2006). For each sample area, a 20 × 20 m photo was obtained and ten random points were project onto it. We recorded the number of points that fell on bommies to estimate percent cover of bommies within each photo as a replicate (n = 15).
Algal turf production
A critical component of algal resource availability is the rate of algal production, which is not necessary strongly correlated with algal cover. Therefore, it was necessary to establish an estimate of algal turf production at each site. This was accomplished by measuring the change in biomass over time in replicate patches of algal turf where macroherbivores were excluded. An initial algal turf assemblage was allowed to develop on 15 × 15 cm terracotta tiles for 5 weeks in a back-reef rubble area at a depth of 1.5 m (adapted from Russ 2003). Algal turf filaments on the tiles were exposed to herbivory and were therefore intended to be representative of turf communities naturally occurring on the back reef. After 5 weeks, the tiles were placed in sealed plastic bags and transported in a cooler to flow-through seawater tables. The initial biomass of turf algae on the tiles then was estimated from four, randomly-chosen, 1-cm2 subsamples scraped into pre-weighed aluminum foil weighing boats. The turf algae were dried to a constant weight at 65 °C for 24 h and then weighed to ±0.0001 g before and after combustion in a muffle furnace at 550 °C for 6 h. Ash-free dry weight (AFDW) was calculated by subtracting the inorganic mass from the total dry mass before combustion. After the subsamples were taken, the tiles were kept in the seawater tables and returned to the field within 12 h to minimize any effects of the sampling process. Upon redeployment, the tiles (n = 5 per site) were placed in plastic cages (1.5 × 1.5 cm mesh) to exclude herbivores, and affixed to the substratum at 1.3–1.7 m depth. The mesh size of the cages was intended to be small enough to exclude herbivorous fishes and echinoids, but large enough to minimize cage artifacts. Similar size mesh has been used in previous studies (Russ 2003; Paddack et al. 2006; Adam et al. 2011). Seven days later, the tiles were sampled again for algal biomass. This time frame was chosen to obtain a realistic maximum algal production rate immediately following the release of grazing pressure, based on evidence from another study (Adam et al., unpublished data), which showed that primary production rates began to decline after ~8 days without grazing. The production of algal biomass was then calculated as the difference between the final and initial biomass estimates and expressed as AFDW g m−2 day−1. Since herbivores were excluded, this calculation is a measure of the net accumulation rate of algal biomass and includes losses from consumption by microherbivores, senescence, and decomposition. To estimate site-wide algal production, the site-specific rate of biomass production was multiplied by the area occupied by algal turf at each site (using % cover data and the area of the site).
Herbivorous fish behavior
Behavioral observations of two species of herbivorous fishes were made while snorkeling to test the hypothesis that their behavior differs as a function of algal abundance or production. Chlorurus sordidus (Scaridae) and Acanthurus nigrofuscus (Acanthuridae) were chosen because they are two of the most common obligate herbivorous species in Moorea and they feed primarily on the epilithic algal matrix. Both species belong to the ‘scraper’ functional group (Bellwood et al. 2004). This group directly removes algae by cropping the surface of the substratum, which in turn, facilitates settlement, growth and survival of coralline algae and corals (Steneck 1988; Hunte and Wittenberg 1992). As a parrotfish, C. sordidus belongs to an influential functional group in terms of reef resilience (e.g., Hay 1984; Burkepile and Hay 2008; Rasher et al. 2013). Parrotfishes move over relatively large expanses of reef (e.g., 250 m2 8 h−1; Welsh and Bellwood 2012) and excavate or scrape clean relatively more substratum per bite than do acanthurids (Bellwood and Choat 1990).
To evaluate whether behavior of these species changes in response to algal resources, 20 adults of each species were followed for 3- to 5-min periods at all sites. While snorkeling, the observer (JST) recorded the size, sex, foraging rate, linear distance travelled, and area covered by each fish. To determine whether fish foraged over larger areas where algal turf was sparse, the total area covered by each fish and linear distance traveled were calculated with MapSource® software for a handheld GPS unit (GPSMAP® 78) that was attached to the observer. Whenever possible, the observer maintained a position with the same orientation to the focal individual (e.g., directly south) so that their movements accurately traced those of the focal fish without overlap or adding additional distance to GPS measurements. For A. nigrofuscus, a different method was used because the area traveled by this species was too small for the GPS software to resolve. Weighted markers were dropped at the edges of the observed range and area was calculated as a polygon by measuring the distance between points.
Condition and foraging success of herbivorous fishes
To determine if there were physical differences in the condition or foraging success of the two study species among sites that could be related to algal resources, 16–20 adults of each species were collected at each site between 1000 and 1400 hours. Hepatosomatic index (liver mass:total mass; a proxy for energy reserves), gonadosomatic index (gonad mass:total mass; a proxy for reproductive output), Fulton’s condition factor (K = body mass TL−3), and a gut fullness index (gut mass with contents:total body mass; a proxy for foraging success) were calculated from these specimens. Exploratory analysis revealed no effect of time of collection (within the 1000 and 1400 hours period) on gut fullness.
Statistical analyses
To determine whether herbivorous fish biomass and density differed among sites, separate two-way analysis of variance tests (ANOVA) with site and survey period (1st or 2nd) as fixed factors were used. Multivariate analysis of variance (MANOVA) was used to test for differences in benthic composition with site as the factor and cover of coral, macroalgae, CCA, and algal turf as dependent variables. Survey period was not a factor in this model because benthic characteristics were sampled only once. Differences in shelter (bommie cover) among sites were tested with one-way ANOVA. Differences in the rate of algal biomass production among sites were also tested with one-way ANOVA, with tiles as replicates.
Separate, simple linear regressions were used to test relationships between herbivorous fish biomass and benthic percent cover data (except for CCA, where a Pearson’s correlation was used because there is no logical causal effect of CCA on these fishes), site-wide production estimates, and diadematid echinoid density. Sites were treated as replicates, limiting the total number of replicates to only six. Multiple regression using all predictor variables at once was, thus, infeasible. Despite the relatively large number of separate simple linear regression tests, α values were not adjusted in order to preserve the limited statistical power provided by only six replicates (Quinn and Keough 2002). However, multiple regression using only two predictors was used to incorporate the effects of both algal availability (i.e., either % cover or production) and diadematid density on herbivorous fish biomass. These factors were chosen for multiple regression analysis because of the high likelihood that they would affect herbivorous fish biomass (food availability and competitor density). The relationships between diadematid echinoid density and algal turf cover and site-wide production were tested with separate simple linear regressions.
For behavioral analyses, MANOVA was used to test for differences in all of the response variables (e.g., bite rate, distance of movement) among sites. Following significant MANOVA tests, separate univariate ANOVAs were used to interpret the MANOVA results. Bite rate has been shown to be a function of size in fishes, so foraging rate was analyzed separately using analysis of covariance (ANCOVA) with standard length as a covariate. Simple linear regressions were used to test for relationships between behaviors at each site and habitat variables (cover of turf algae, production, and other site characteristics) or density of herbivorous fishes. MANOVA was used to test for differences among study sites in fish condition and foraging indices (Fulton’s K, hepatosomatic index, gonadosomatic index, gut fullness index), followed by ANOVA on each index for interpretation. Simple linear regressions were used to test for effects of all other measured variables of interest (e.g., % cover of algal turf, production, density of other herbivores) on the condition and foraging indices.
For analyses involving algal turf and herbivorous fishes, only ‘usable turf’ was considered. ‘Usable turf’ was defined as turfing algae outside of protected Stegastes spp. territories. In addition, biomass of Stegastes spp. was excluded from the estimate of herbivorous fish biomass.
Site averages of survey data met, or were log transformed to meet, the assumptions of normality and homoscedasticity, but hepatosomatic index data did not, and transformation did not improve the distribution. Parametric analyses on untransformed data were performed, nevertheless, because ANCOVA and ANOVA are generally robust to violations of normality (Ellison and Gotelli 2004).
Results
Habitat variation and algal production
There was substantial variability in the cover of benthic components among the six study sites spread over ~20 km of shoreline (Figs. 1, 2). Benthic surveys showed significant differences in the percent cover of ‘usable’ algal turf, coral, macroalgae, and CCA among sites (Fig. 2a) (MANOVA: site: Pillai’s trace = 1.75, F 20,88 = 3.4, P < 0.001; univariate ANOVA: turf: F 5,22 = 5.6, P = 0.002; coral: F 5,22 = 15.1, P < 0.001; CCA: F 5,22 = 6.6, P = 0.001; macroalgae: F 5,22 = 10.6, P < 0.001). Live coral cover accounted for a higher proportion of benthic cover at eastern shore sites (~20–28 %); whereas sites on the north shore had lower coral cover (~9–15 %) and higher algal turf cover. Shelter habitat (bommie cover) differed significantly among sites (F 5,84 = 3.8, P = 0.004), ranging from 41.5 % (Avaiti) to 56.4 % (Temae).
a Percent cover of the four most prominent benthic groups (algal turf, living coral, CCA, and macroalgae) at six study sites around Moorea, French Polynesia (n = 6 transects per site); b production of algal turf biomass (n = 5 tiles per site); and c biomass of herbivorous fishes (n = 6 transects per site). Bars mean ±1SE
Algal turf production on tiles varied among sites, ranging from 0.8 to 2.0 g AFDW/m2/day (ANOVA: F 5,24 = 9.06, P < 0.001; Fig. 2b). Production and % cover of algal turf were not significantly correlated (Pearson’s correlation: r = 0.68, P = 0.17), indicating that algal abundance and production were not tightly linked.
Herbivorous fish and sea urchin densities
Herbivorous fish biomass density differed among sites (F 5,58 = 7.8, P < 0.001; Fig. 2c). Differences in fish biomass among sites were consistent between the two sampling periods (site × period interaction: F 5,58 = 0.7, P = 0.62), and there was no difference in fish biomass between the two periods (F 1,58 = 1.2, P = 0.29). Similarly, density of herbivorous fishes differed among sites (F 5,58 = 2.5, P = 0.04) and was consistent between the two sampling periods (site × period interaction: F 5,58 = 1.05, P = 0.41). Composition of the herbivorous fish assemblage varied among sites, with scarids accounting for 20.3–52.6 % and acanthurids accounting for 42.1–69.4 % of the total herbivorous fish biomass. Pomacentrids (Stegastes spp.) made up 3.5–17.1 % of total herbivorous fish biomass and correlated with the cover of turf in Stegastes territories (Pearson’s correlation: r = 0.81, P = 0.02). Siganids were a minor proportion of the total fish biomass (0.0–1.1 %).
There was no relationship between total herbivorous fish biomass and algal turf cover (simple linear regression: r 2 = 0.51, P = 0.11, n = 6 sites). There was a negative relationship between herbivorous fish biomass and diadematid sea urchin density (r 2 = 0.61, P = 0.05). When both cover of algal turf and diadematid density were included as predictors in a multiple regression model, the model explained 85 % of the variation in herbivorous fish biomass (r 2 = 0.85, P = 0.03), and here there was a significant positive relationship between biomass of herbivorous fishes and cover of algal turf (P = 0.04, standardized slope = 0.56), as well as a negative relationship with diadematid density (P = 0.03, std. slope = −0.64). Average diadematid density (which ranged from 1.5 to 4.0 m−2 at the six sites) was unrelated to algal turf cover (r 2 = 0.21, P = 0.36).
Herbivorous fish biomass tracked total algal turf production more closely than it tracked turf cover. Total site-wide algal turf production explained 86 % of the variance in herbivorous fish biomass, where higher production corresponded to greater fish biomass (r 2 = 0.86, P = 0.01) (Fig. 3). When diadematid density was incorporated into the regression with site-wide production, the model explained 94 % of the variance in herbivorous fish biomass (r 2 = 0.94; production: P = 0.01, std. slope = 0.73; diadematids: P = 0.07, std. slope = −0.38). Diadematid density was unrelated to algal turf production (r 2 = 0.40, P = 0.18).
Herbivorous fish biomass and the cover of some other benthic categories were tightly related, but herbivorous fish biomass was not related to shelter habitat. As live coral cover declined, herbivorous fish biomass increased (r 2 = 0.72, P = 0.03); whereas CCA cover increased with herbivorous fish biomass (Pearson’s correlation: r = 0.85, P = 0.01). Shelter (bommie) cover, however, did not predict herbivorous fish biomass (r 2 = 0.04, P = 0.71). Herbivorous fish density was unrelated to any of the benthic variables (all P > 0.3).
Behavior
Behavior of Chlorurus sordidus differed among sites (MANOVA: Pillai’s trace = 0.41, F 15,348 = 3.7, P < 0.001). Area traveled per hour differed among sites (ANOVA: F 5,116 = 4.13, P = 0.002), decreasing with increasing cover of algal turf (r 2 = 0.90, P = 0.01; Fig. 4), but being unrelated to density of herbivorous fishes (r 2 = 0.21, P = 0.36). Linear distance traveled differed among sites (ANOVA: F 5,116 = 2.67, P = 0.02), but was unrelated to other individual site variables, including density of herbivorous fishes (P > 0.05). Despite statistically accounting for the significant effect of body size (F 1,116 = 3.5, P = 0.04), there were no differences in foraging rates among sites (F 5,116 = 0.3, P = 0.98). In A. nigrofuscus, there were no detectable differences in behavior among sites (MANOVA: Pillai’s trace = 0.72, F 15,334 = 0.12, P < 0.81).
Herbivorous fish condition
The condition indices of C. sordidus differed among sites (MANOVA: Pillai’s trace = 0.79, F 20,336 = 2.1, P = 0.001). Univariate ANOVA showed there were no significant differences among sites in Fulton’s condition factor (K), gonadosomatic index (GSI), or gut mass:body weight ratio for C. sordidus (ANOVA: P > 0.05); however, the hepatosomatic index (a proxy for energy reserves) differed among sites (univariate ANOVA: F 5,111 = 3.03, P = 0.02). The average hepatosomatic index at each site was negatively related to the total density of herbivorous fishes (Fig. 5a) and positively related to the site-wide algal turf production (Fig. 5b) (multiple regression: r 2 = 0.90; algal production: P = 0.05, std. slope = 0.82; fish density: P = 0.01, std. slope = −1.36; Fig. 5). No differences were observed in any of the condition or foraging indices of A. nigrofuscus (MANOVA: Pillai’s trace = 0.04, F 15,345 = 1.2, P = 0.42).
Relationships between HSI of C. sordidus and a total herbivorous fish density and b algal turf production. Values for HSI represent residuals from regression of a HSI on algal turf production or b HSI on total herbivorous fish density. Each point represents the average for a site (n = 6 sites), based on a sample of n = 16–20 fish per site, n = 6 transects per site to estimate herbivorous fish density (a), or n = 5 tiles per site to estimate algal turf production (b)
Discussion
Our finding that herbivore biomass was tightly related to algal turf production at sites spread along 20 km of coastline on Moorea suggests that turf algae are the primary food of the herbivorous fishes studied, and that their biomass is distributed as predicted by foraging models, i.e., optimal foraging theory (Schoener 1971; Charnov 1976) and the ideal free distribution (Fretwell and Lucas 1970; Fretwell 1972), whereby individual foragers maximize their energy gain and distribute themselves proportionally to resources. This, combined with a negative relationship between herbivorous fish biomass and diadematid urchin density, as well as a negative relationship between herbivore energy reserves and total herbivorous fish density, implies that these fishes are food-limited, resulting in competition as well as density-dependent energy storage.
Our spatial results regarding the distribution of herbivore biomass among sites that differed in turf algal production are similar to the temporal results of studies documenting increases in biomass of herbivorous fishes when the abundance of turf algae increased following major coral die-offs (Adam et al. 2011; Gilmour et al. 2013). In both those studies, the increased abundance of herbivores appeared to keep algal turf in a cropped state, preventing macroalgae from increasing in abundance on the available dead coral substrata, as appears to be the case in the back reef environment we studied, where macroalgae are relatively sparse (<10 % cover) and most herbivores were observed consuming turf algae or detritus. These herbivores likely keep the turf algae in a cropped state that makes it highly productive as a food source (Carpenter 1986). Moreover, the positive correlation between herbivorous fish biomass and crustose coralline algae (CCA) cover supports the hypothesis that herbivorous fishes in our system may also contribute to reef resilience by cropping algal turf, and providing open substrata to the benefit of CCA, which is known to enhance coral recruitment (Morse et al. 1988; Price 2010). The negative relationship we detected between live coral cover and biomass of herbivorous fishes is consistent with a negative effect of these fishes on corals, but we consider this possibility unlikely because we never observed herbivorous fishes consuming live coral during this study.
Why algal turf production differed among sites is unknown, but there was a general trend for algal turf production to decrease with distance away from the center of the more heavily populated north shore, which has higher terrigenous runoff. Thus, the spatial pattern of turf algal production might be caused by eutrophication from terrigenous runoff coming from the numerous pineapple plantations and sewage outlets in the catchment of the streams that feed into the lagoon, in addition to the natural runoff from the two large bays on the north shore. Differences among sites in hydrodynamics might also have contributed in the differences in algal productivity (Carpenter et al. 1991), though our impressions were that the study sites were quite similar in current speeds and wave action. Site placement was chosen to reduce these effects, as water generally flows unidirectionally from the fore reef across the reef crest to the lagoon (Hench et al. 2008). Although, the caging method used here may have underestimated algal production rates (e.g., it did not account for changes in algal biomass caused by micro-grazers, senescence, fragmentation, self-shading, or reproduction), it likely captured the relative differences in algal production among sites. The values obtained were consistent with other shallow coral reef production estimates for algal turf (Polunin and Klumpp 1992; Paddack et al. 2006).
An alternative explanation for the tight relationship between herbivore biomass and turf productivity, and the differences in turf production among sites, is that herbivores stimulated turf production. It is well known that herbivores can stimulate primary productivity through herbivory in some marine (Carpenter et al. 1991) and terrestrial systems (e.g., McNaughton et al. 1997). However, we think it is most likely that herbivores in our study system respond to variation in turf production, rather than create it. For example, in Moorea, Adam et al. (2011) documented a clear temporal lag between the sudden increase in algal turf abundance and the subsequent numerical response of herbivorous fishes. In addition, the lack of a relationship between herbivorous fishes and shelter suggests that herbivores were not disproportionately drawn to sites by factors other than high turf production. It is still possible, however, that herbivory stimulates algal turf production, creating a positive feedback loop whereby algal turf production promotes consumption, which promotes production, thus exaggerating the initial differences in production among sites. Experiments would be needed to determine whether the herbivorous fishes we studied stimulate algal production.
The biomass of herbivorous fishes was negatively related to live coral cover, possibly because sites with more live coral have less food for herbivores due to lower cover of surfaces on which turf algae can grow. This finding supports the hypothesis that abundant live coral is not a prerequisite for large populations of herbivorous fishes, a concept supported by the study of Adam et al. (2011) which revealed large increases in the biomass of herbivorous fishes on the fore reef of Moorea after live coral cover had dropped precipitously as a result of a COTS outbreak and cyclone. This ability of herbivorous fish biomass to track algal production in both space and time despite variations in coral abundance is crucial for herbivore-facilitated recovery of coral reefs.
At first glance the negative correlation between herbivorous fish biomass and live coral cover might be viewed as implying that structural refugia are not important to herbivores. But in our study system, live coral cover was not tightly correlated with the abundance of structural refuge (bommies; r = 0.38, P = 0.46) because much of the coral tissue on the bommies had died during recent mortality events (e.g., a COTS outbreak). The skeletons of these dead corals retain high levels of structural complexity and are common at our study sites. Indeed strong positive associations between fish density and structural complexity are well documented (reviewed in Pratchett et al. 2008; Graham and Nash 2013), confirming that herbivores prosper in areas with high complexity, regardless of whether the corals are alive. We, however, found no relationship between biomass of herbivorous fishes and the abundance of structural refugia. This may be because structural refuges were common at all of our study sites, covering 42–56 % of the bottom. Low abundances of predators may be another cause of the lack of relationship between herbivore biomass and structural refuges because refugia may be less important in reef systems where predators are rare (Jennings and Polunin 1997; Dulvy et al. 2004). In Moorea, large predators (e.g., snappers, jacks, and sharks) are less common than in some other Indo-Pacific reef systems (Sandin et al. 2008; Brooks 2013; M.A.S., personal observations). Due to the correlative, observational nature of our study, we cannot rule out spatial variation in fishing pressure as the cause of differences in biomass of herbivorous fishes among our six study sites, but we view this possibility as unlikely. We observed very little fishing at our study sites, and there were no obvious differences in fishing intensity among sites. Based on our observations (quantified and unquantified), variation in food availability is the most parsimonious explanation for the differences in biomass of herbivorous fishes we found among sites.
In addition to algal turf production, the density of diadematid sea urchins was an important predictor of the distribution of herbivorous fishes, with fish biomass density negatively related to urchin density. This finding suggests that the distribution of herbivorous fishes over scales of several kilometres is constrained by competition with sea urchins for food. Competition between herbivorous fishes and sea urchins has been shown in some studies and hypothesized in several more (e.g., Hay and Taylor 1985; Carpenter 1990; McClanahan et al. 1996), so this finding is not surprising. Avaiti and sites on the East shore (Vaiare and Temae) had notably higher densities of sea urchins than the other North shore sites, a well-established trend in Moorea (Han 2012; Carpenter 2013). This dissimilarity among sites, as well as the fact that diadematid density did not track algal resources as well as did fish biomass, may be due to the limited mobility of echinoids relative to herbivorous fishes, which hinders them from tracking algal resources over large areas as quickly (Jones and Andrew 1990).
It appeared that the parrotfish Chlorurus sordidus was forced to range over larger areas per unit time to maintain food intake rates at sites with relatively low algal resources, as expected based on foraging theory (Pyke et al. 1977; Avgar et al. 2011) and similar to findings of empirical studies in other systems (e.g., Fryxell et al. 2004; Klaassen et al. 2006). Since C. sordidus presumably spent more energy moving over larger areas at sites with fewer resources (both algal abundance and production) while maintaining similar foraging rates, individuals at sites with lower total production may have assimilated less energy. However, if there is heterogeneity in algal production on smaller scales (e.g., within sites), energy uptake could be altered, thus making changes in energy reserves undetectable on the scale of this study. Variation in algal production has been shown to occur on relatively small scales (e.g., within a home range) (Klumpp and McKinnon 1989; Russ 2003).
Most of the variation in energy reserves of C. sordidus (as estimated by the HSI) was explained by algal turf production and the density of herbivorous fishes, suggesting that resource availability and competitor density determine the physiological condition of this species. This result would be expected if grazers not only deplete food resources but also engage in interference competition (e.g., aggressive interactions) (Caughley and Lawton 1981). One might expect that the energy reserves and reproductive effort (estimated by the GSI) would be correlated inversely since they rely on the same energy supply; thus, the lack of a biologically meaningful pattern in the GSI of C. sordidus is somewhat surprising. The HSI, however, varied less among individuals than did the GSI, providing more statistical power to detect differences. Fish at some sites were also in different reproductive stages, suggesting differential reproductive patterns among sites, which in turn would generate high variance in GSI among sites and low statistical power.
The lack of any differences in the condition of A. nigrofuscus may be due to the fact that the differences in resources among sites were not of sufficient magnitude to affect changes in their energy budget, or that real differences did exist, but the physiological indices used were too imprecise to reveal them in this relatively small species. Their behavior also did not differ among sites, which likely occurred for similar reasons. In Moorea, during our relatively short observation periods, A. nigrofuscus had relatively small ranges that were tightly associated with nearby coral structure (e.g., dead massive Porites bommies); however, in other systems, this species is known to travel over longer distances (Robertson and Gaines 1986; Montgomery et al. 1989). A. nigrofuscus may also only occupy space that can be controlled and defended from other species, or preferentially occupy areas where algal resources are clustered, as seen in other systems and closely related surgeonfishes (e.g., Robertson and Polunin 1981; Robertson and Gaines 1986). Even at sites with reduced algal cover, there are still areas where turfing algae grow in relatively large patches. Algal turf patch size and benthic heterogeneity likely affect this fish differently because it does not often travel among patches like parrotfishes do, or even like larger, more mobile surgeonfishes. If A. nigrofuscus does not require a large area of turf-covered reef, then perhaps it can achieve ideal free distribution within sites but not among sites.
Overall, this study shows that biomass of herbivorous fishes closely tracks spatial variation in algal turf production over relatively large scales (>20 km), while being somewhat constrained by densities of potential competitors, diadematid urchins. This finding implies that herbivorous fishes in our study system are food-limited. Spatial tracking of turf algal production combined with temporal increases in herbivorous fish biomass during periods when algae become more abundant (Adam et al. 2011; Gilmour et al. 2013) may allow herbivorous fishes to control algal proliferation and maintain open substrata for coral recruitment, thereby enhancing coral reef resilience.
References
Adam TC, Schmitt RJ, Holbrook SJ, Brooks AJ, Edmunds PJ, Carpenter RC, Bernardi G (2011) Herbivory, connectivity, and ecosystem resilience: response of a coral reef to a large-scale perturbation. PLoS One 6:e23717. doi:10.1371/journal.pone.0023717
Adler PB, Raff DA, Lauenroth WK (2001) The effect of grazing on the spatial heterogeneity of vegetation. Oecologia 128:465–479
Avgar T, Kuefler D, Fryxell M (2011) Linking rates of diffusion and consumption in relation to resources. Am Nat 178:182–190
Bellwood DR, Choat JH (1990) A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. In: Bruton MN (ed) Alternative life-history styles of fishes. Springer, Netherlands, pp 189–214
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833
Birrell CL, McCook LJ, Willis B, Diaz-Pulido GA (2008) Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr Mar Biol 46:25–63
Brooks A (2013) MCR LTER: Coral reef: long-term population and community dynamics: fishes. http://metacat.lternet.edu/knb/metacat/knb-lter-mcr.6.48/lter. Accessed 01 Jan 2013
Brown JS, Kotler BP (2004) Hazardous duty pay and foraging cost of predation. Ecol Lett 7:999–1014
Burkepile DE, Hay ME (2008) Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Natl Acad Sci 105:16201–16206. doi:10.1073/pnas.0801946105
Burkepile DE, Burns CE, Tambling CJ, Amendola E, Buis GM, Govender N, Nelson V, Thompson DI, Zinn AD, Smith MD (2013) Habitat selection by large herbivores in a southern African savanna: the relative roles of bottom-up and top-down forces. Ecosphere 11:139
Carpenter RC (1986) Partitioning herbivory and its effects on coral reef algal communities. Ecol Monogr 56:345–363
Carpenter RC (1990) Mass mortality of Diadema antillarum. Mar Biol 104:79–86
Carpenter RC (2013) MCR LTER: Coral reef: long-term population and community dynamics: other benthic invertebrates. http://metacat.lternet.edu/knb/metacat/knb-lter-mcr.8.26/lter. Accessed 01 Jan 2013
Carpenter SR, Kitchell JF, Hodgson JR, Cochran PA, Elser JJ, Lodge MM, Kretchmer D, He X, Von Ende CN (1987) Regulation of lake primary productivity by food web structure. Ecology 68:1863–1876
Carpenter RC, Hackney JM, Adey WH (1991) Measurements of primary productivity and nitrogenase activity of coral reef algae in a chamber incorporating oscillatory flow. Limnol Oceanogr 36:40–49
Caughley G, Lawton JH (1981) Plant-herbivore systems. In: Caughley G, Lawton JH (eds) Theoretical ecology. Blackwell, Oxford, pp 132–166
Charnov EL (1976) Optimal foraging: the marginal value theorem. Theor Pop Biol 9:129–136
Choat JH, Clements KD, Robbins WD (2002) The trophic status of herbivorous fishes on coral reefs—I: dietary analyses. Mar Biol 140:613–623
Done TJ (1992) Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121–132
Duffy JE (2002) Biodiversity and ecosystem function: the consumer connection. Oikos 99:201–219
Dulvy NK, Polunin NVC, Mill AC, Graham NAJ (2004) Size structural change in lightly exploited coral reef fish communities: evidence for weak ecological release. Can J Fish Aquat Sci 61:466–475
Ellison GN, Gotelli NJ (2004) A primer of ecological statistics. Sinauer, Sunderland
Estes JA, Tinker MT, Williams TM, Doak DF (1998) Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282:473–476
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA (2011) Trophic downgrading of planet earth. Science 333:301–306
Fretwell SD (1972) Populations in a seasonal environment. Princeton University Press, Princeton
Fretwell SD, Lucas HL (1970) On territorial behaviour and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheor 19:16–36
Fryxell JM (1991) Forage quality and aggregation by large herbivores. Am Nat 138:478–498
Fryxell JM, Wilmshurst JF, Sinclair ARE (2004) Predictive models of movement by Serengeti grazers. Ecology 85:2429–2435
Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS (2013) Recovery of an isolated coral reef system following severe disturbance. Science 6128:69–71
Graham NAJ, Nash KL (2013) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32:315–326. doi:10.1007/s00338-012-0984-y
Han X (2012) MCR LTER: Coral reef: diadema predation and recruitment in Moorea, French Polynesia. Retrieved January 01, 2013. knb-lter-mcr.2003.2. http://metacat.lternet.edu/knb/metacat/knb-lter-mcr.2003.2/lter. Accessed 01 Jan 2013
Hart AM, Klumpp DW, Russ GR (1996a) Response of herbivorous fishes to crown-of-thorns starfish Acanthaster planci outbreaks. I. Substratum analysis and feeding ecology of Acanthurus nigrofuscus and Scarus frenatus. Mar Ecol Prog Ser 132:11–19
Hart AM, Klumpp DW, Russ GR (1996b) Response of herbivorous fishes to crown-of-thorns starfish Acanthaster planci outbreaks. II. Density and biomass of selected species of herbivorous fish and fish-habitat correlations. Mar Ecol Prog Ser 132:21–30
Hay ME (1981) The functional morphology of turf-forming seaweeds: persistence in stressful marine habitats. Ecology 62:739–750
Hay ME (1984) Patterns of fish and urchin grazing on Caribbean coral reefs: are previous results typical? Ecology 65:446–454. doi:10.2307/1941407
Hay ME, Taylor PR (1985) Competition between herbivorous fishes and urchins on Caribbean reefs. Oecologia 65:591–598
Hebblewhite M, Merrill EH (2009) Trade-offs between predation risk and forage differ between migrant strategies in a migratory ungulate. Ecology 90:3445–3454. doi:10.1890/08-2090.1
Hench JL, Leichter JJ, Monismith SG (2008) Episodic circulation and exchange in a wave-driven coral reef and lagoon system. Limnol Oceanogr 53:2681–2694
Hixon MA, Brostoff WN (1983) Damselfish as keystone species in reverse: intermediate disturbance and diversity of reef algae. Science 220:511–513
Hixon MA, Brostoff WN (1996) Succession and herbivory: effects of differential fish grazing on Hawaiian coral-reef algae. Ecol Monogr 66:67–90
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Hunte W, Wittenberg M (1992) Effects of eutrophication and sedimentation on juvenile corals. II. Settlement. Mar Biol 114:625–631
Huntly NJ (1991) Herbivores and the dynamics of communities and ecosystems. Annu Rev Ecol Syst 22:477–503
Jarman PJ (1974) The social organization of antelope in relation to their ecology. Behaviour 48:215–266
Jennings S, Polunin NVC (1997) Impacts of predator depletion by fishing on the biomass and diversity of non-target reef fish communities. Coral Reefs 16:71–82
Jones GP, Andrew NL (1990) Herbivory and patch dynamics on rocky reefs in temperate Australasia, the role of fish and sea urchins. Aust J Ecol 15:505–520
Jouffray JB, Nyström M, Norström AV, Williams ID, Wedding LM, Kittinger JN, Williams GJ (2015) Identifying multiple coral reef regimes and their drivers across the Hawaiian archipelago. Phil Trans R Soc B Biol Sci 370:20130268
Klaassen RHG, Nolet BA, Bankert D (2006) Movement of foraging tundra swans explained by spatial pattern in cryptic food densities. Ecology 87:2244–2254
Klumpp DW, McKinnon AD (1989) Temporal and spatial patterns in primary production of a coral reef epilithic algal community. J Exp Mar Biol and Ecol 131:1–22
Kohler KE, Gill SM (2006) Coral Point Count with Excel extensions (CPCe): a Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32(9):1259–1269. doi:10.1016/j.cageo.2005.11.009
Kulbicki N, Guillemot N, Amand M (2005) A general approach to length–weight relationships for New Caledonian Lagoon fishes. Cybium 29:235–252
Lewis SM (1986) The role of herbivorous fishes in the organization of a Caribbean reef community. Ecol Monogr 56:183–200
McClanahan TR (1997) Primary succession of coral-reef algae: differing patterns on fished versus unfished reefs. J Exp Mar Biol Ecol 218:77–102
McClanahan TR, Shafir SH (1990) Causes and consequences of sea urchin abundance and diversity in Kenyan coral. Oecologia 83:362–370
McClanahan TR, Kamukuru AT, Muthiga NA, Gilagabher Yebio M, Obura D (1996) Effect of sea urchin reductions on algae, coral, and fish populations. Conserv Biol 10:136–154
McNaughton SJ (1985) Ecology of a grazing ecosystem: the Serengeti. Ecol Monogr 53:291–320
McNaughton SJ (1990) Mineral nutrition and seasonal movements of African migratory ungulates. Nature 345:613–615
McNaughton SJ, Banyikwa FF, McNaughton MM (1997) Promotion of the cycling of diet-enhancing nutrients by African grazers. Science 278:1798–1800
Montgomery WL, Myrberg AA, Fishelson L (1989) Feeding ecology of surgeonfishes (Acanthuridae) in the northern Red Sea, with particular reference to Acanthurus nigrofuscus (Forsskal). J Exp Mar Biol Ecol 132:179–207
Morrison D (1988) Comparing fish and urchin grazing in shallow and deeper coral reef algal communities. Ecology 69:1367–1382
Morse DE, Hooker N, Morse ANC, Jensen RA (1988) Control of larval metamorphosis and recruitment in sympatric agariciid corals. J Exp Mar Biol Ecol 116:193–217
Mumby PJ, Steneck RS (2008) Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol 23:555–563
Nyström M, Folke C, Moberg F (2000) Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol Evol 15:413–417
Ogutu JO, Piepho HP, Reid RS, Rainy ME, Kruska RL, Worden S, Nyabenge M, Hobbs NT (2010) Large herbivore responses to water and settlements in savannas. Ecol Monogr 80:241–266
Paddack MJ, Cowen RK, Sponaugle S (2006) Grazing pressure of herbivorous coral reef fishes on low coral-cover reefs. Coral Reefs 25:461–472. doi:10.1007/s00338-006-0112-y
Polunin NVC, Klumpp DW (1992) Algal food supply and grazer demand in a very productive coral-reef zone. J Exp Mar Biol Ecol 164:1–15
Pratchett MS, Munday MS, Wilson SK, Graham NAJ, Cinner JE, Bellwood DR, Jones GP, Polunin NVC, McClanahan TR (2008) Effects of climate-induced coral bleaching on coral-reef fishes. Ecological and economic consequences. Oceanogr Mar Biol Annu Rev 46:251–296
Price N (2010) Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163:747–758
Prins HHT, Vanderjeugd HP (1993) Herbivore population crashes and woodland structure in east Africa. J Ecol 81:305–314
Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Quart Rev Biol 52:137–154
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rasher DB, Hoey AS, Hay ME (2013) Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology 94:1347–1358
Robertson DR, Gaines DS (1986) Interference competition structures habitat use in a local assemblage of coral reef surgeonfishes. Ecology 67(1372):1383
Robertson DR, Polunin NVC (1981) Coexistence: symbiotic sharing of feeding territories and algal food by some coral reef fishes from the Western Indian Ocean. Mar Biol 62(185):195
Russ GR (2003) Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral Reefs 22:63–67. doi:10.1007/s00338-003-0286-5
Sammarco PW, Carleton JH (1981) Damselfish territoriality and coral community structure: reduced grazing, coral recruitment, and effects on coral spat. In: Proceedings of the 4th International Coral Reef Symposium, vol 2. pp 524–535
Sandin SA, Smith JE, DeMartini EE, Dinsdale EA, Donner SD, Friedlander AM, Sala E (2008) Baselines and degradation of coral reefs in the northern Line Islands. PLoS One 3:e1548
Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413:591–596
Schmitz OJ, Hambäck PA, Beckerman AP (2000) Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am Nat 155:141–153
Schoener TW (1971) Theory of feeding strategies. Ann Rev Ecol Syst 2:369–404
Solomon ME (1949) The natural control of animal populations. J Anim Ecol 18:1–35
Steneck RS (1988) Herbivory on coral reefs: a synthesis. In: Choat JH et al (eds) Proc 6th Int Coral Reef Symp, vol 1. Townsville, Australia, pp 37–49
Stockwell B, Jadloc CRL, Abesamis RA, Alcala AC, Russ GR (2009) Trophic and benthic responses to no-take marine reserve protection in the Philippines. Mar Ecol Prog Ser 389:1–15
Sutherland WJ (1983) Aggregation and the “ideal free” distribution. J Anim Ecol 52:821–828
Sutherland WJ, Parker GA (1992) The relationship between continuous input and interference models of ideal free distributions with unequal competitors. Anim Behav 44:345–355
Tregenza T (1995) Building on the ideal free distribution. Adv Ecol Res 26:253–307
Vermeij MJA, van Moorselaar I, Engelhard S, Hornlein C, Vonk SM, Visser PM (2010) The effects of nutrient enrichment and herbivore abundance on the ability of turf algae to overgrow coral in the Caribbean. PLoS One 5:e14312. doi:10.1371/journal.pone.0014312
Welsh JQ, Bellwood DR (2012) How far do schools of roving herbivores rove? A case study using Scarus rivulatus. Coral Reefs 31:9911003. doi:10.1007/s00338-012-0922-z
Williams ID, Polunin NVC (2001) Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 19:358–366. doi:10.1007/s003380000121
Wilson SK, Bellwood DR, Choat JH, Furnas MJ (2003) Detritus in the epilithic algal matrix and its use by coral reef fishes. Oceanogr Mar Biol Annu Rev 41:279–309
Acknowledgments
This research was supported the National Science Foundation via the Moorea Coral Reef LTER (OCE 10-26852 to CSUN), the Office of Graduate Studies at California State University Northridge, and CSUN College of Science and Mathematics. P. Edmunds, R. Carpenter, and A. Brooks provided invaluable advice and comments. We appreciate the logistical assistance provided by the staff of the Richard B. Gump South Pacific Research Station. This is contribution number 210 of the Moorea Coral Reef LTER.
Author contribution statement
JST and MAS conceived and designed the study, analyzed the data, and wrote the manuscript. JST conducted fieldwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Deron E. Burkepile.
Rights and permissions
About this article
Cite this article
Tootell, J.S., Steele, M.A. Distribution, behavior, and condition of herbivorous fishes on coral reefs track algal resources. Oecologia 181, 13–24 (2016). https://doi.org/10.1007/s00442-015-3418-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3418-z