Abstract
Herbivorous zooplankton avoid size-selective predation by vertical migration to a deep, cold water refuge. Adaptation to low temperatures in planktonic poikilotherms depends on essential dietary lipids; the availability of these lipids often limits growth and reproduction of zooplankton. We hypothesized that limitation by essential lipids may affect habitat preferences and predator avoidance behavior in planktonic poikilotherms. We used a liposome supplementation technique to enrich the green alga Scenedesmus obliquus and the cyanobacterium Synecchococcus elongatus with the essential lipids, cholesterol and eicosapentaenoic acid (EPA), and an indoor system with a stratified water-column (plankton organ) to test whether the absence of these selected dietary lipids constrains predator avoidance (habitat preferences) in four species of the key-stone pelagic freshwater grazer Daphnia. We found that the capability of avoiding fish predation through habitat shift to the deeper and colder environment was suppressed in Daphnia unless the diet was supplemented with EPA; however, the availability of cholesterol did not affect habitat preferences of the tested taxa. Thus, their ability to access a predator-free refuge and the outcome of predator–prey interactions depends upon food quality (i.e. the availability of an essential fatty acid). Our results suggest that biochemical food quality limitation, a bottom–up factor, may affect the top–down control of herbivorous zooplankton.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an ongoing debate in population ecology about mechanisms that control the strength of interspecific interactions within food webs (Pace et al. 1999; Schmitz et al. 2004; Heath et al. 2014). This is well illustrated by the controversy over the relative importance of resources (bottom–up effects) and predation (top–down effects) for population control. Predation is an important selective force which has been shown to drive the shape of trophic cascades, particularily in aquatic ecosystems, by direct (changes in prey density due to mortality driven by predators) and indirect (changes in prey behavior, morphology, and life history, all of which reduce pressure on the resource density of the prey) interactions (Schmitz et al. 2004; Luttbeg and Kerby 2005). The indirect effects reflect induction of anti-predator defences in prey (reduction of foraging, changes in diet, migration of prey to safer habitats, etc. (Cousyn et al. 2001; Gliwicz 2003).
Diel vertical migration (DVM) of zooplankton is a classical example of a widespread predator avoidance behavior in aquatic ecosystems (Gliwicz 1986; Lampert 1989; Hays 2003; Cohen and Forward 2009). The risk of daytime predation by visually orientated predators, e.g., fish, is reduced when the zooplankton prey reside in the dark, deep layer of the water column. At night, they reappear in the warm, upper water stratum to minimize demographic costs associated with spending time in the cool, food-depleted deep-water refuge (Loose and Dawidowicz 1994). DVM is induced mutually by solar light and by kairomones, i.e. chemicals released by predators (Dodson 1988; Cohen and Forward 2009). As the amplitude of DVM increases with predator abundance, DVM is considered an efficient behavioral defense mechanism which allows optimization of risk management and habitat use (Lampert 1989; Loose and Dawidowicz 1994; Hays 2003).
DVM of zooplankton, with its synchronized ascent and descent of animals in the water column, represents probably the biggest animal migration on the planet (Hays 2003; Reichwaldt and Stibor 2005). Filter-feeding zooplankton represent an aquatic keystone guild, which controls the abundance of primary producers and represents a major food source for higher trophic levels (e.g., fish, birds) in pelagic food webs (Hays 2003; Haupt et al. 2009). Important large-scale phenomena, such as phytoplankton dynamics (clear water stage or bloom), nutrient recycling, and the vertical transport of matter, depend on the taxonomical structure and vertical position of this guild in the water column (Lampert and Taylor 1985; Hays 2003; Reichwaldt and Stibor 2005; Haupt et al. 2009).
It is widely assumed that, in response to kairomones released by fish, zooplankton individuals may easily descend to the deep-water refuge, and that the observed amplitude of DVM only reflects the trade-off between the costs of repressed reproduction in the deep-water refuge and risk of mortality in shallow waters (Loose and Dawidowicz 1994; Hansson and Hylander 2009). Nevertheless, in a typical thermally stratified lake, the temperature difference between the near-surface layer and the deep-water refuge may exceed 10 °C. Individuals performing DVM experience considerably lower mean temperatures (Stich and Lampert 1981; Lampert et al. 2003), which strongly affect the properties of biological membranes in a poikilothermic organism such as Daphnia. Roughly speaking, decreasing temperatures reduce membrane fluidity, while increasing temperatures increase membrane fluidity (Hazel 1995). Poikilotherms try to maintain membrane fluidity through changes in membrane lipid composition (homeoviscous adaptation) (Hazel and Williams 1990; Hazel 1995). This may include an increase in the content of unsaturated fatty acids, which prevents the transition of membranes to the solid phase at lower temperatures (Farkas 1979; Hazel 1995). In line with this, decreases in temperature lead to an increase in the level of polyunsaturated fatty acids (PUFAs) in Daphnia, suggesting a higher PUFA requirement at low temperatures (Schlechtriem et al. 2006; Sperfeld and Wacker 2012). Cholesterol is another indispensable structural component of cell membranes. Due to its multiple effects on membrane fluidity, permeability, and the functioning of membrane proteins, it is also involved in thermal adaptation in poikilotherms (Hazel and Williams 1990; Crockett 1998). The cholesterol content of membranes and animal tissues is often positively correlated with temperature (Crockett 1998), but an increase in the level of cholesterol in cold-adapted poikilotherms has also been reported (Viarengo et al. 1994; Crocket and Hazel 1995).
Some of the PUFAs, i.e. eicosopentaenoic acid (EPA; 20:5ω3), docosahexaenoic acid (DHA; 22:6ω3), arachidonic acid (ARA; 20:4 ω6), α-linolenic acid (ALA; 18:3ω3) and linoleic acid (LIN; 18:2ω6) are described as being essential fatty acids for zooplankton (Wacker and von Elert 2001; Smyntek et al. 2008) in that they cannot be synthesized with an efficiency sufficient to meet the demand (Arts et al. 2001). In addition, cholesterol cannot be synthesized de novo by arthropods (Gurr And Harwood 1991; Von Elert et al. 2003). Growth and reproduction of crustacean zooplankton can be limited by the availability of these resources (Müller-Navarra 1995; Wacker and Von Elert 2001; Martin-Creuzburg et al. 2008); experiments with supplementation of natural seston provide evidence that zooplankton populations are occasinally under bottom–up control due to PUFA limitation (Hartwich et al. 2012). In particular, the prevalence of one of the PUFAs—eicosapentaenoic acid (EPA)—in seston is a powerful predictor of Daphnia growth (Müller-Navarra et al. 2000; Wacker and Von Elert 2001). The content of EPA and of other ω3-PUFAs in phytoplankton decreases with nutrient loading (Müller-Navarra et al. 2004; Persson et al. 2007), while the frequency of cyanobacterial blooms increases (Watson et al. 1997; Jöhnk et al. 2008). The well-known nutritional inadequacy of cyanobacteria for zooplankton is not only due to inhibitory secondary metabolites (Ger et al. 2014) but also to the low content of PUFAs (Cobelas and Lechado 1989) and the absence of phytosterols (Von Elert et al. 2003; Martin-Creuzburg et al. 2008), the latter of which are essential for all arthropods, as they serve as the precursor for the synthesis of cholesterol. The degree of EPA and sterol limitation has been shown to depend on the ambient temperature (Sperfeld and Wacker 2011; Martin-Creuzburg et al. 2012).
As DVM-performing poikilotherms have to face a shift in the ambient temperature, we hypothesized that homeoviscous adaptation in zooplankton is a prerequisite of DVM. We were interested in whether a food-quality limitation (bottom–up effect) may interact with a predator-driven habitat shift (indirect top–down effect). Hence, we expected that the capability of predator avoidance through DVM depends on the availability of dietary lipids, particularly of EPA and cholesterol. We used an indoor setup which simulated the conditions in the open water of a thermally-stratified lake, and applied supplementation of a green alga and a cyanobacterium with EPA and/or cholesterol, to investigate whether availability of these essential lipids constrains the access to the cold-water refuge in fish-threatened Daphnia.
Materials and methods
Organisms
We performed experiments with a clone of the pond-dwelling Daphnia magna and three clones of the lake-dwelling Daphnia longispina complex. The D. magna clone originated from Grosser Binnensee (Lampert 1991), whereas the clones of the D. longispina complex (D. galeata clone, D. hyalina clone and D. hyalina × galeata clone) originating from Lake Roś (northeastern Poland). Daphnids are subjected to fish predation in both lakes. Intra- and interspecific polymorphism with regard to amplitude of habitat change in response to fish threat was reported, and some species of Daphnia are known to be non-migratory or only slightly migratory, while others express pronounced changes in habitat preferences. To account for this polymorphism, different taxa inhabiting two different types of water bodies (shallow lake Grosser Binnensee and deep, dimictic Lake Roś) were chosen. D. hyalina, D. hyalina × galeata and D. magna clones perform DVM, while individuals of selected D. galeata clone do not leave the warm epilimnetic waters.
Daphnia stock cultures were maintained in 1 L of filtered and conditioned tap water, at 21 °C under non-limiting food conditions of Chlamydomonas klinobasis (strain 56, culture collection of the Limnological Institute at the University of Constance).
The food source used in experiments consisted of the cyanobacterium Synechococcus elongatus (strain SAG 89.79), which contains neither cholesterol nor EPA (Von Elert and Wolffrom 2001; Von Elert et al. 2003) or the green alga Scenedesmus obliquus (strain SAG 276-3a), which does not contain EPA (Von Elert 2002; Becker and Boersma 2005) but contains various phytosterols (Martin-Creuzburg et al. 2012). S. elongatus and S. obliquus were cultivated in chemostats (dilution rate 0.2 day−1); the former in Cyano medium (Von Elert and Jüttner 1997) and the latter in WC-medium (Guillard 1975). Cells collected from the chemostats were concentrated by centrifugation (4000 rpm) and resuspended in fresh Cyano medium. The carbon content of the food suspensions was determined by reference to photometric light extinction at 800 nm, along with previously determined carbon-extinction equations (unpublished).
Experimental design
We utilized a plankton organ (Loose and Dawidowicz 1994) for the assessment of the vertical daytime distribution of daphnids. The set-up consisted of plexiglas tubes (1 m long, 1.5 cm in diameter, volume 200 mL) protected with a stopper at the bottom and arranged vertically in a thermally stratified water bath (2.5 × 0.25 × 1.1 m). The tubes were illuminated from the top by a set of halogen lamps (20 W, 12 V), shining through a frosted glass screen. A summer photoperiod (16L:8D) was applied with a daytime light intensity which decreased from 17 µmol × s−1 × cm−2 at the surface to 1.3 µmol × s−1 × cm−2 at the bottom. The temperature in the plankton organ was measured using submerged laboratory thermometer (accuracy ± 0.1 °C), placed from the surface to the bottom at 10 cm intervals. The thermal gradient in the organ (Fig. 1) simulated the conditions in the open water of thermally stratified lakes in summer. The tubes were either filled with water conditioned through the presence of fish or with control water. Fish-conditioned water was obtained by exposing four individuals of daphnid-fed perch (Perca fluviatilis) of 8–10 cm body length in 8 L of tap water for 24 h. Control tap water and fish-conditioned tap water were filtered (0.45 μm pore size) before preparation of the food suspensions. As we focused on the effects of limitation by essential lipids in general and not on interspecific comparisons, a block design of experiments (all treatments checked in one run, only one species tested in each block) was applied.
Food supplementation
Food suspensions were enriched with cholesterol or eicosapentaenoic acid (EPA) by supplementation with liposomes according to Martin-Creuzburg et al. (2008). Supplemented diets were prepared by adding 30 μl of the respective liposome stock suspension per replicate (0.188 mg of essential lipid per 1 mg C in food suspension). A food level of 2 mg C L−1 was kept constant in all treatments. The experimental food treatments were S. elongatus supplemented either with pure liposomes, liposomes containing cholesterol, liposomes containing EPA, or with liposomes containing a mixture of both lipids. Only for D. magna, additionally the habitat preferences were also tested using the green alga S. obliquus as a food source, supplemented either with pure liposomes or with EPA-enriched liposomes.
Experimental procedure
Synchronized cohorts of 3rd clutch neonates raised in filtered (Whatman GF/C) tap water at saturating concentrations of Ch. klinobasis were used to initiate the experiments. When the animals reached the 3rd instar (on days 3–5, depending on the species), they were subjected to the experimental food treatments (see above, with a food concentration of 2 mg C L−1; the medium was exchanged daily). Four replicates were estabilished (0.5 L volume of medium in each) for each treatment, animals were assigned randomly to replicates. The behavioral part of the experiment started with mature, egg-bearing females; the animals were therefore exposed to experimental food treatments only a few days beforehand to minimize size differences of females between treatments. As soon as the eggs were delivered to the brood chambers, the animals were transferred to the tubes of the plankton organ (5 individuals of D. magna, or 8 individuals of either D. galeata, D. hyalina or D. hyalina × galeata per tube) which contained water either with or without fish kairomones and with respective test food. The media were prepared in separate beakers and mixed before being poured into the tubes. Accordingly, all treatments were started with a homogenous distribution of cyanobacterial (or green algae) cells and liposomes. On each consecutive day, animals in the tubes were fed with the respective test food to a concentration of 1 mg C L−1. The fresh food suspensions were prepared in separate beakers, and respective volumes were gently injected into each tube from the top using an automatic 5-ml pipette. Each clone was tested separately. Each treatment consisted of four tubes (n = 4 replicates per treatment for each clone). Since it was not possible to directly measure the temperature at the depth of each animal, the vertical position of the animals in the tubes was measured by visual inspection, with 1 cm accuracy, at noon of the photoperiod time, 2 days after being transferred to the plankton organ. For each tube, a median of the vertical position of all individuals per tube was calculated and used as replicate. The median depth preferred by daphnids was subsequently converted into residence temperature using the thermal gradient in the plankton organ (Fig. 1). The respective temperature relevant to this depth was calculated according to the following depth–temperature equation: (temperature) = 0.00002(depth)3 − 0.0037(depth)2 + 0.0043(depth) + 23.21, R 2 = 0.989. The residence temperature (one value for each replicate) was used for statistical analyses—this is because we were interested in the temperatures preferred by the animals and not in their vertical position in tubes, and the relationship between depth and temperature was curvilinear (see equation above). The values of temperature were log-transformed to meet the assumption of homoscedascity of variances.
Data analysis
A general linear model (GLM) was used to test for the differences between habitat (temperature) preferences. The model included ‘food’ (a four-level factor when S. elongatus was used for preparation of food suspensions: S. elongatus with liposomes, S. elongatus with EPA liposomes, S. elongatus with cholesterol liposomes, S. elongatus with EPA + cholesterol liposomes, or a two-level factor when S. obliquus was used for preparation of food suspensions: S. obliquus with liposomes, S. obliquus with EPA liposomes) and ‘predation’ (a two-level factor: fish kairomones absent or present). Differences between means were tested using Tukey’s test. Analyses were performed using Statistix 7.0 (Analytical Software, USA).
Results
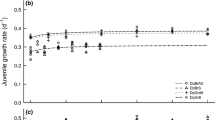
We found that food quality and the presence of kairomones released by fish affected the vertical distribution of daphnids (Tables 1, 2), except that of D. galeata (Table 1). Predator avoidance by D. magna in response to kairomones depended on the diet, irrespective of whether the animals were fed S. elongatus (Table 1, food × predation p < 0.00001) or S. obliquus (Table 2, food × predation p < 0.04). Kairomones failed to induce predator avoidance in Daphnia feeding on the cyanobacterium supplemented with control liposomes (Fig. 2a, c); predator avoidance through DVM was observed only upon EPA-supplementation of the cyanobacterium, as evidenced by different habitat preferences of daphnids (Fig. 2a)—animals subjected to fish kairomones and EPA-supplemented diets stayed in habitat which was, on average, 7.4–8.8 °C colder than animals subjected to fish kairomones and cyanobacteria supplemented with control liposomes (Fig. 2c). Interestingly, although growth and reproduction of Daphnia fed on cyanobacteria can be constrained by the absence of both PUFAs and sterols, only supplementation of EPA but not of cholesterol allowed for this behavioral predator avoidance by Daphnia (Fig. 2a, c).
Habitat preferences of Daphnia magna fed with food of different qualities. a Vertical distribution and c temperature preferences of D. magna after supplementation of the cyanobacterium Synechococcus elongatus with pure liposomes, liposomes enriched with EPA, liposomes enriched with cholesterol, or liposomes enriched with EPA and cholesterol. b Vertical distribution and d temperature preferences of D. magna after supplementation of the green alga Scenedesmus obliquus with pure or EPA-enriched liposomes. Open circles habitat preferences in predator-free treatments, filled circles habitat preferences in treatments with fish kairomones (mean ± SD, n = 4). Identical letters indicate homogeneous groups (Tukey’s post hoc test, p > 0.05). Dashed horizontal lines frame the metalimnion, i.e. the zone with the steepest temperature gradient
When the diet consisted of the green alga S. obliquus, individuals of D. magna subjected to fish kairomones resided in water of lower temperature compared to those in the predator-free environment (Fig. 2b, d). In agreement with a still-prevailing EPA limitation on this green alga, EPA supplementation resulted in daphnids migrating to even lower temperatures in the presence of fish kairomones (Fig. 2d); however, this difference in experienced temperatures (3.4 °C) was only marginally significant (0.04 < p < 0.05).
We found that the response to fish kairomones was affected by lipid supplementation of the cyanobacterium in D. hyalina × galeata (Table 1, food × predation p < 0.02) and D. hyalina (Table 1, food × predation p < 0.005). D. galeata individuals did not change habitat preferences in the presence of fish kairomones, and their preferences were not altered by different food quality (Table 1, predation p = 0.15, food × predation p = 0.54). When feeding on a PUFA- and sterol-free diet, no habitat shift in response to fish kairomones was observed in D. hyalina and D. hyalina × galeata, and supplementation revealed that it was the absence of EPA but not of sterols that suppressed migration to the cold water refuge (Fig. 3a, b). The habitat preferences of the non-migrating D. galeata was affected neither by kairomones nor by food supplementation (Fig. 3c).
Habitat preferences of individuals of the Daphnia longispina complex. Vertical distribution and temperature preferences of a D. hyalina × galeata, b D. hyalina, and c D. galeata fed with the cyanobacterium Synechococcus elongatus supplemented with pure liposomes, liposomes enriched with EPA, liposomes enriched with cholesterol, or liposomes enriched with EPA and cholesterol. Open circles habitat preferences in predator-free treatments, filled circles treatments with fish kairomones (mean ± SD, n = 4). Identical letters indicate homogeneous groups (Tukey’s post hoc test, p > 0.05). Dashed horizontal lines frame the metalimnion, i.e. the zone with the steepest temperature gradient
Neither the presence of EPA nor of cholesterol induced changes in habitat preferences of daphnids per se (Figs. 2, 3).
Discussion
With increased nutrient loading, a decline of particular PUFAs in natural phytoplankton was reported (Müller-Navarra et al. 2004; Persson et al. 2007), and it was shown that growth and reproduction of the keystone pelagic grazer Daphnia are limited by the availability of these essential lipids in natural phytoplankton (Müller-Navarra 1995; Wacker and Von Elert 2001). However, the effects of biochemical food quality limitation on predator avoidance by zooplankton have not been investigated. In this study, EPA supplementation was demonstrated to be a factor which allows predator avoidance: daphnids fed EPA-supplemented food in the presence of fish kairomones started to descend to the colder water refuge, or at least increased the amplitude of their habitat shift compared to non-supplemented conditions. This reaction was observed in species isolated from both a shallow and a deep lake, and was independent of whether the supplementation improved the food quality of cyanobacteria or green algae, suggesting that diet-mediated suppression of DVM may not be restricted to lakes with cyanobacterial blooms. However, it is uncertain whether this enhancement of predator avoidance could be observed when thermal stratification is not estabilished, e.g., in very shallow lakes. Interestingly, there is evidence that the cold-water refuge is not always used by daphnids under natural conditions with predation pressure, despite its potential accessibility (Flik and Ringelberg 1993; Sakwinska and Dawidowicz 2005). Sakwinska and Dawidowicz (2005) have found that fish-threatened daphnids modify their life-histories instead of performing DVM in some lakes, although they have not discovered why one of the defensive strategies is preferred over the other. There are several potential explanations, and one of them could be drawn from our observations: modification of life-history would be the option when the deep-water refuge cannot be accessed due to inadequacy of food quality.
The experimental set-up (plankton organ) was developed to study the effects of temperature on zooplankton performing DVM (Loose and Dawidowicz 1994). Despite the fact that the thermal gradient in the tubes is “compressed” down to only 1 m, it resembles thermal stratification in real lakes (in Lake Roś, the summer temperature in the epilimnion reaches 21–23 °C and 8–10 °C in the hypolimnion; Brzeziński 2010). The fairly “compressed” temperature gradient implies that temperature changes considerably faster with depth than in a stratified lake: in the plankton organ, temperatures drop by 7 °C over a vertical distance of 30 cm, whereas the same absolute change in temperature in Lake Roś occurs within a vertical distance of 6 m. However, the height of the water column is of little importance for migrating Daphnia, since the cost of vertical movement is negligible (Dawidowicz and Loose 1992). Along with the smoother temperature gradient in stratified lakes, we would expect that even small differences in low temperature tolerance would result in pronounced differences in habitat (depth) preferences of animals, whereas the steep temperature gradient in the plankton organ allows only for the differentiation of strong differences with respect to low temperature tolerance.
We suppose that the enhacement of predator avoidance observed after EPA supplementation could be explained by the PUFA-dependent acclimation of poikilothermic animals to the low temperature in the deep-water refuge. Acclimation to a cold environment is achieved by an increase in the proportion of PUFAs within membrane phospholipids, which prevents a transition to a solid phase at lower temperatures and protects the function of membranes (Farkas 1979; Hazel 1995). Altering the cholesterol content of cell membranes is another mechanism by which poikilotherms can acclimate to changing environmental temperatures (Viarengo et al. 1994; Crocket and Hazel 1995). Nevertheless, we have not found evidence that a lack of cholesterol in the diet affects the depth selection behavior of Daphnia. Our findings corroborate the observations that planktonic crustaceans have a higher requirement for PUFAs at lower temperatures (Schlechtriem et al. 2006; Masclaux et al. 2009; Sperfeld and Wacker 2012), and that limitation by dietary sterols is less pronounced at low temperatures (Hassett and Crockett 2009; Sperfeld and Wacker 2009).
It is worth noting that, even without EPA supplementation of Scenedesmus obliquus, individuals of Daphnia magna in the presence of fish kairomones were able to migrate into the cold-water refuge, and EPA supplementation moderately amplified this ability. Since it is known that the strain of S. obliquus used here contains PUFAs other than EPA (Von Elert 2002; Becker and Boersma 2005), this may indicate that PUFAs other than EPA may also be involved in the acclimation to lower ambient temperatures during DVM. This finding is in accordance with Martin-Creuzburg et al. (2012) who showed that growth limitation of Daphnia at low temperatures could be alleviated by supplementation of different PUFAs, suggesting that the effects of EPA on DVM reported here would probably not differ for other PUFAs. Alternatively, our observation may be explained by the finding that Daphnia fed EPA-free S. obliquus were capable of endogenous synthesis of EPA, albeit at rates that are too low to fully alleviate the absence of dietary EPA (Weers et al. 1997; Von Elert 2002).
We have demonstrated that PUFA supplementation allows for DVM, which indicates that without supplementation DVM was suppressed by the absence of PUFAs from the diet. For both test diets used here (S. obliquus, S. elongatus supplemented with cholesterol), it was demonstrated that juvenile growth and reproduction of Daphnia are EPA-limited (Von Elert 2002; Martin-Creuzburg et al. 2009). One may speculate that EPA limitation of somatic growth and reproduction goes along with suppression of DVM; however, we have not determined the effects of EPA supplementation on growth and reproduction, and it remains to be tested whether or not limitations of growth and behavior have similar thresholds of limitation by EPA.
Our finding contradicts the common assumption that zooplankton may easily descend to the deep-water refuge, and that the amplitude of DVM only reflects the evolutionary trade-off between the lower risk of being captured by a predator and the higher demographical costs resulting from staying in a non-optimal habitat (Boriss and Gabriel 1988; Lampert et al. 2003). The access to the deep-water refuge depends on the availability of EPA—daphnids cannot ‘freely’ choose the habitat without potential fish threat. This may also indicate that migration into a deeper and colder habitat without proper adaptations (e.g., with uncompleted homeoviscuous adaptation due to scarcity of PUFAs) has more detrimental effects than staying in upper levels and dealing with the risk of being eaten. In this case, the unavoidable risk of death due to cold shock exceeds the uncertain probability of death by an encounter with a predator. It can be ruled out that the observed effect of EPA on DVM is a food quantity effect, since the experiments were performed at food concentrations that were considerably above the limiting levels and since supplementation of liposomes and liposomes with cholesterol had no effect on DVM. Supplementation provided animals with concentrations of essential lipids higher than levels limiting growth and reproduction, according to Martin-Creuzburg et al. (2009) in the case of cholesterol, and according to Sperfeld and Wacker (2011) in the case of EPA, so that it is reasonable to assume that adaptation of Daphnia to low ambient temperatures after supplementation was no longer constrained by a low availability of the respective essential lipid. Supplementation with EPA (and some other PUFAs) has been shown to improve performance of Dapnia at any temperature between 10 and 25 °C, but the most pronounced effect was observed at 10 °C (Martin-Creuzburg et al. 2012). In our experiment, individuals that changed habitat preferences in response to the presence of fish kairomones stayed near the thermocline at an ambient temperature of less than 21 °C (Figs. 2, 3), which is within the range of temperatures for which an increased demand for PUFAs was found (Martin-Creuzburg et al. 2012; Sperfeld and Wacker 2012). Our results indicate that the effects of food quality on DVM should be taken into consideration in studies that focus on the adaptive value and the evolution of DVM as an anti-predatory mechanism.
When PUFAs are not limiting, species of zooplankton that are able to perform DVM can express adaptation to cold and hide in the deep refuge; thus, direct effects of predators on these species (mortality) can be minimized, whereas indirect effects of the presence of predators (i.e. changes in behavior and life-history, and the demographic costs of staying in the refuge) can be still observed (Fig. 4a). Constraints of DVM driven by a lack of dietary EPA may increase the mortality in zooplankton populations, as a reduced ability to migrate into the predator-free refuge exposes individuals to encounters with predators. Together with direct effects (lowered somatic and population growth rates; Von Elert 2002; Ravet et al. 2003; Becker and Boersma 2005), this indirect effect of PUFA limitation on mortality of Daphnia may increase the strength of top–down control (Fig. 4b). In nutrient-loaded freshwater ecosystems, this may contribute to the observed replacement of large-bodied planktonic filter-feeders (mainly Daphnia) by small-bodied species (Gliwicz 2003) which are less vulnerable to fish predation but also less efficient at grazing on phytoplankton (Gliwicz 1990). Large-bodied herbivorous zooplankton constitute a keystone guild in freshwater ecosystems. When no hypolymnetic refuge is available due to PUFA-poor phytoplankton, large-bodied planktonic herbivores will be exposed to predation (Fig. 4b). As a consequence, they could be replaced by small-bodied species of zooplankton and the grazing on phytoplankton could be reduced (Fig. 4b). The restoration of lakes by biomanipulation is based on a deliberate reduction in planktivory, in order to release this keystone guild from the pressure of zooplanktivores. Availability of antipredatory refuges is important for the success of biomanipulation: The relatively higher success rates of biomanipulations in shallow lakes as opposed to in stratified lakes is frequently attributed to the development of macrophyte beds in shallow lakes; such beds offer a refuge for zooplankton from fish predation and increase the efficiency of piscivorous fish (Perrow et al. 1997; Mehner et al. 2002). We hypothesize that constraints in access to the deep-water refuge due to limitation of zooplankton by essential fatty acids may confine the efficiency of biomanipulation in stratified lakes.
Effects of limitation by polyunsaturated fatty acids (PUFAs) on food-chain topology. Predators affect herbivorous prey directly (causing mortality, broad arrow) and indirectly (by inducing changes in life-history, behavior etc., dashed line). a Under non-limiting conditions (PUFA-rich), the direct effects are mitigated due to a habitat shift of the prey. b When prey is limited by polyunsaturated fatty acids (PUFA-poor), the antipredator refuge cannot be accessed, exposing herbivorous prey the to direct effects of predation. Hence, predator–prey interactions depend on the biochemical quality of phytoplankton
Habitat shifts by herbivorous prey avoiding predators are an important element influencing the effects of these cascades in both aquatic and terrestial ecosystems (e.g., Bernot and Turner 2001; Fortin et al. 2005; Manzur et al. 2014). Habitat shifts are associated with changes in ambient temperature in several terrestrial and aquatic ecosystems. In line with this, it was shown that organisms occupying habitats characterized by different temperatures differ with regard to their PUFA content. For example, copepods overwintering in deep, cold-water strata are characterized by a higher PUFA content than their conspecifics residing in shallow waters (Pond and Tarling 2011). Differences in lipid composition were found between organisms inhabiting different layers of thermally stratified soil: animals found in shallower layers which had been exposed to temperature shifts contained more PUFAs than animals from deeper layers with a more stable temperature (Van Dooremalen et al. 2013). Similarly, soil invertebrates and microbes exposed to low temperatures (0–10 °C) had a lower fatty acid content and a higher degree of desaturation among the fatty acids than conspecifics in moderate (ca. 20 °C) temperatures (Petersen and Klug 1994; Petersen and Holmstrup 2000). In conclusion, occurence of invertebrates in colder terrestrial or aquatic strata goes along with a higher content of unsaturated fatty acids in the animals. It is reasonable to assume that this relationship also reflects a higher PUFA requirement of animals in order to be able to acclimatize their membranes to the cold stratum.
Trait-mediated interactions are considered to be an important factor influencing the strength of effects in trophic cascades (Schmitz et al. 2004). Here, we show that the biochemical supplementation of food influences the habitat use of prey in the presence of kairomones released by predators. The dietary availability of an essential lipid and its indirect effects on the interaction between predator and prey can be a factor moderating bottom–up and top–down effects across the food web. We suggest that a food quality-mediated suppression of predator evasion may contribute to the well-known trophic decoupling, driven by changes in phytoplankton assemblage following eutrophication (Elser et al. 2000). We conclude that the availability of essential PUFAs sets a frame which determines to what degree the zooplankton individuals may realize their potential of behavioral predator avoidance by DVM.
References
Arts MT, Ackman RG, Holub BJ (2001) “Essential fatty acids” in aquatic ecosystems: a crucial link between diet and human health and evolution. Can J Fish Aquat Sci 58:122–137
Becker C, Boersma M (2005) Differential effects of phosphorus and fatty acids on Daphnia magna growth and reproduction. Limnol Oceanogr 50:388–397. doi:10.4319/lo.2005.50.1.0388
Bernot RJ, Turner AM (2001) Predator identity and trait-mediated indirect effects in a littoral food web. Oecologia 129:139–146. doi:10.1007/s004420100705
Boriss H, Gabriel W (1988) Vertical migration in Daphnia: the role of phenotypic plasticity in the migration pattern for competing clones or species. Oikos 83:129–138
Brzeziński T (2010) Ecology of three sympatric Daphnia species and their hybrids. PhD. dissertation, University of Warsaw, Poland (in Polish, with English summary)
Cobelas MA, Lechado JZ (1989) Lipids in microalgae-a review. Biochem Grasas Aceites 40:118–145
Cohen JH, Forward RB (2009) Zooplankton diel vertical migration—A review of proximate control. Oceanogr Mar Biol Annu Rev 47:77–109
Cousyn C, De Meester L, Colbourne JK, Brendonck L, Verschuren D, Volckaert F (2001) Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc Natl Acad Sci USA 98:6256–6260
Crockett EL (1998) Cholesterol function in plasma membranes from ectotherms: membrane specific roles in adaptation to temperature. Am Zool 38:291–304
Crockett EL, Hazel JR (1995) Cholesterol levels explain inverse compensation of membrane order in brush border but not homeoviscuous adaptation in basolateral membranes from intestinal epithelia of rainbow trout. J Exp Biol 198:1105–1113
Dawidowicz P, Loose C (1992) Metabolic costs during predator-induced diel vertical migration of Daphnia. Limnol Oceanogr 37:1589–1595
Dodson SI (1988) The ecological role of chemical stimuli for the zooplankton: predator-avoidance behavior in Daphnia. Limnol Oceanogr 33:1431–1439
Elser JJ, Fagan WF, Denne RF, Dobberfuhl DR, Folarin A, Huberty A, Interlandi S, Kilham S, McCauley E, Schutz KL, Siemann EH, Sterner RW (2000) Nutritional constraints in terrestrial and freshwater food webs. Nature 408:578–580. doi:10.1038/35046058
Farkas T (1979) Adaptation of fatty-acid compositions to temperature-a study on planktonic crustaceans. Comp Biochem Physiol 64B:71–76
Flik B, Ringelberg J (1993) Influence of food availability on the initiation of diel vertical migration (DVM) in Lake Maarseveen. Arch Hydrobiol 39:57–65
Fortin D, Beyer HL, Boyce MS, Smith DW, Duchesne T, Mao JS (2005) Wolves influence elk movements: behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86:1320–1330
Ger KA, Hansson L-A, Lürling M (2014) Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshwat Biol 59:1783–1798. doi:10.1111/fwb.12393
Gliwicz ZM (1986) Predation and the evolution of vertical migration in zooplankton. Nature 320:746–748. doi:10.1038/320746a0
Gliwicz ZM (1990) Food thresholds and body size in cladocerans. Nature 343:638–640. doi:10.1038/343638a0
Gliwicz ZM (2003) Between hazards of starvation and risk of predation: the ecology of offshore animals. In: Kinne O (ed) Excellence of ecology, part 12. International Ecology Institute, Oldendorf
Guillard RR (1975) Cultures of phytoplankton for feeding of marine invertebrates. Culture of marine invertebrate animals. Plenum, New York, pp 29–60
Gurr MP, Harwood JL (1991) Lipid biochemistry—An introduction, 4th edn. Chapman and Hall, London
Hansson LA, Hylander S (2009) Size-structured risk assessments govern Daphnia migration. Proc R Soc Lond B 276:331–336. doi:10.1098/rspb.2008.1088
Hartwich M, Martin-Creuzburg D, Rothhaupt K-O, Wacker A (2012) Oligotrophication of a large, deep lake alters food quantity and quality constraints at the primary producer–consumer interface. Oikos 121:1702–1712
Hassett RP, Crockett EL (2009) Habitat temperature is an important determinant of cholesterol contents in copepods. J Exp Biol 212:71–77
Haupt F, Stockenreiter M, Baumgarten M, Boersma M, Stibor H (2009) Daphnia diel vertical migration: implications beyond zooplankton. J Plankton Res 31:515–524. doi:10.1093/plankt/fbp003
Hays GC (2003) A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 503:163–170. doi:10.1023/B:HYDR.0000008476.23617.b0
Hazel JR (1995) Thermal adaptation in biological membranes—is homeoviscous adaptation the explanation. Annu Rev Physiol 57:19–42. doi:10.1146/annurev.ph.57.030195.000315
Hazel JR, Williams EE (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–227. doi:10.1016/0163-7827(90)90002-3
Heath MR, Speirs DC, Steele JH (2014) Understanding patterns and processes in models of trophic cascades. Ecol Lett 17:101–114
Jöhnk KD, Huisman J, Sharples J, Sommeijer B, Visser PM, Stroom JM (2008) Summer heatwaves promote blooms of harmful cyanobacteria. Glob Change Biol 14:495–512. doi:10.1111/j.1365-2486.2007.01510.x
Lampert W (1989) The adaptive significance of diel vertical migration of zooplankton. Funct Ecol 3:21–27
Lampert W (1991) The dynamics of Daphnia in a shallow lake. Verh Intern Ver Limnol 24:795–798
Lampert W, Taylor BE (1985) Zooplankton grazing in a eutrophic lake: implications of diel vertical migration. Ecology 66:68–82. doi:10.2307/1941307
Lampert W, McCauley E, Manly BFJ (2003) Trade-offs in the vertical distribution of zooplankton: ideal free distribution with costs? Proc R Soc Lond B 270:765–773. doi:10.1098/rspb.2002.2291
Loose CJ, Dawidowicz P (1994) Trade-offs in diel vertical migration by zooplankton: the costs of predator avoidance. Ecology 75:2255–2263. doi:10.2307/1940881
Luttbeg B, Kerby JC (2005) Are scared prey as good as dead? Trends Ecol Evol 20:416–418
Manzur T, Vidal F, Pantoja JF, Fernández M, Navarrete SA (2014) Behavioural and physiological responses of limpet prey to a seastar predator and their transmission to basal trophic levels. J Anim Ecol 83:923–933. doi:10.1111/1365-2656.12199
Martin-Creuzburg D, Von Elert E, Hoffmann KH (2008) Nutritional constraints at the cyanobacteria-Daphnia interface: the role of essential lipids. Limnol Oceanogr 53:456–468
Martin-Creuzburg D, Sperfeld E, Wacker A (2009) Colimitation of a freshwater herbivore by sterols and polyunsaturated fatty acids. Proc R Soc Lond B 276:1805–1814
Martin-Creuzburg D, Wacker A, Ziese C, Kainz MJ (2012) Dietary lipid quality affects temperature-mediated reaction norms of a freshwater key herbivore. Oecologia 168:901–912. doi:10.1007/s00442-011-2155-1
Masclaux H, Bec A, Kainz MJ, Desvilettes Ch, Jouve I, Bourdier G (2009) Combined effects of food quality and temperature on somatic growth and reproduction of two freshwater cladocerans. Limnol Oceanogr 54:1323–1332. doi:10.4319/lo.2009.54.4.1323
Mehner T, Benndorf J, Kasprzak P, Koschel R (2002) Biomanipulation of lake ecosystems: succesful applications and expanding complexity in the underlying science. Freshw Biol 47:2453–2465
Müller-Navarra DC (1995) Evidence that a highly unsaturated fatty acid limits Daphnia growth in nature. Arch Hydrobiol 132:297–307
Müller-Navarra DC, Brett MT, Liston AM, Goldman CR (2000) A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403:74–77. doi:10.1038/47469
Müller-Navarra DC, Brett MT, Park S, Chandra S, Ballantyne AP, Zorita E, Goldman CR (2004) Unsaturated fatty acid content in seston and tropho-dynamic coupling in lakes. Nature 427:69–72. doi:10.1038/nature02210
Pace ML, Cole JJ, Carpenter SR, Kitchell JF (1999) Trophic cascades revealed in diverse ecosystems. Trends Ecol Evol 14:483–488
Perrow MR, Meijer M-L, Dawidowicz P (1997) Biomanipulation in shallow lakes: state of the art. Hydrobiologia 342(343):355–365
Persson J, Brett MT, Vrede T, Ravet JL (2007) Food quantity and quality regulation of trophic transfer between primary producers and a keystone grazer (Daphnia) in pelagic freshwater food webs. Oikos 116:1152–1163. doi:10.1111/j.0030-1299.2007.15639.x
Petersen SO, Holmstrup M (2000) Temperature effects on lipid composition of the earthworms Lumbricus rubellus and Eisenia nordenskioeldi. Soil Biol Biochem 32:1787–1791
Petersen SO, Klug MJ (1994) Effects of sieving, storage, and incubation temperature on the phospholipid fatty acid profile of a soil microbial community. Appl Environ Microbiol 60:242–2430
Pond DW, Tarling GA (2011) Phase transition of wax esters adjust buoyancy in diapausing Calanoides acutus. Limnol Oceanogr 56:1310–1318
Ravet JL, Brett MT, Müller-Navarra DC (2003) A test of the role of polyunsaturated fatty acids in phytoplankton food quality for Daphnia using liposome supplementation. Limnol Oceanogr 48:1938–1947
Reichwaldt ES, Stibor H (2005) The impact of diel vertical migration of Daphnia on phytoplankton dynamics. Oecologia 146:50–56. doi:10.1007/s00442-005-0176-3
Sakwinska O, Dawidowicz P (2005) Life history strategy and depth selection behavior as alternative antipredator defenses among natural Daphnia hyalina populations. Limnol Oceanogr 50:1284–1289. doi:10.4319/lo.2005.50.4.1284
Schlechtriem C, Arts MT, Zellmer ID (2006) Effect of temperature on the fatty acid composition and temporal trajectories of fatty acids in fasting Daphnia pulex (Crustacea, Cladocera). Lipids 41:397–400
Schmitz OJ, Krivan V, Ovadia O (2004) Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol Lett 7:153–156
Smyntek PM, Teece MA, Schulz KL, Storch AJ (2008) Taxonomic differences in the essential fatty acid composition of groups of freshwater zooplankton relate to reproductive demands and generation time. Freshwat Biol 53:1768–1782
Sperfeld E, Wacker A (2009) Effects of temperature and dietary sterol availability on growth and cholesterol allocation of the aquatic keystone species Daphnia. J Exp Biol 212:3051–3059. doi:10.1242/jeb.031401
Sperfeld E, Wacker A (2011) Temperature- and cholesterol-induced changes in eicosapentaenoic acid limitation of Daphnia magna determined by a promising method to estimate growth saturation thresholds. Limnol Oceanogr 56:1273–1284
Sperfeld E, Wacker A (2012) Temperature affects the limitation of Daphnia magna by eicosapentaenoic acid, and the fatty acid composition of body tissue and eggs. Freshwat Biol 57:497–508. doi:10.1111/j.1365-2427.2011.02719.x
Stich HB, Lampert W (1981) Predator evasion as an explanation of diurnal vertical migration by zooplankton. Nature 293:396–398. doi:10.1038/293396a0
Van Dooremalen C, Berg MP, Ellers J (2013) Acclimation responses to temperature vary with vertical stratification: implications for vulnerability of soil-dwelling species to extreme temperature events. Glob Change Biol 19:975–984. doi:10.1111/gcb.12081
Viarengo AR, Accomando R, Roma G, Benati U, Damonte G, Orunesu M (1994) Differences in lipid composition of cell membranes from Antarctic and Mediterranean scallops. Comp Biochem Physiol B 109:579–584
Von Elert E (2002) Determination of limiting polyunsaturated fatty acids in Daphnia galeata using a new method to enrich food algae with single fatty acids. Limnol Oceanogr 47:1764–1773. doi:10.4319/lo.2002.47.6.1764
Von Elert E, Jüttner F (1997) Phosphorus limitation not light controls the exudation of allelopathic compounds by Trichormus doliolum. Limnol Oceanogr 42:1796–1802
Von Elert E, Wolffrom T (2001) Supplementation of cyanobacterial food with polyunsaturated fatty acids does not improve growth of Daphnia. Limnol Oceanogr 46:1552–1558. doi:10.4319/lo.2001.46.6.1552
Von Elert E, Martin-Creuzburg D, Le Coz JR (2003) Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata). Proc R Soc Lond B 270:1209–1214. doi:10.1098/rspb.2003.2357
Wacker A, Von Elert E (2001) Polyunsaturated fatty acids: evidence for non-substitutable biochemical resources in Daphnia galeata. Ecology 82:2507–2520. doi:10.1890/0012-9658(2001)082[2507:PFAEFN]2.0.CO;2
Watson SB, McCauley E, Downing JA (1997) Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol Oceanogr 42:487–495
Weers PM, Siewertsen MK, Gulati RD (1997) Is the fatty acids composition of Daphnia galeata determined by the fatty acid composition of the ingested diet? Freshwat Biol 38:731–738
Winder M, Boersma M, Spaak P (2003) On the cost of vertical migration: are feeding conditions really worse at greater depths? Freshwat Biol 48:383–393
Acknowledgments
This study was supported by a stipend from Deutscher Akademischer Auslandsdienst (DAAD) to T.B. The authors declare no conflict of interests. We thank to Maarten Boersma and anonymous reviewers for their valuable comments, and to Frederic Bartlett for linguistic help.
Author contribution statement
T.B. and E.v.E. worked together to design the experiments. T.B. performed the experiments and analysed the data. T.B. and E.v.E. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Maarten Boersma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brzeziński, T., von Elert, E. Predator evasion in zooplankton is suppressed by polyunsaturated fatty acid limitation. Oecologia 179, 687–697 (2015). https://doi.org/10.1007/s00442-015-3405-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3405-4