Abstract
Tree species in seasonally dry tropical forests likely vary in their drought-survival mechanisms. Drought-deciduousness, which reduces water loss, and low wood density, which may permit dependence on stored water, are considered key traits. For saplings of six species at two distinct sites, we studied these and two associated traits: the seasonal amount of water released per stem volume (“water released”) and the hydraulic capacitance of the stem (C). Two deciduous species with low stem density, Cavanillesia platanifolia and Bursera simaruba, had high C and high dry-season stem water potential (Ψ stem), but differed in dry-season water released. C. platanifolia did not use stored water during the dry season whereas B. simaruba, in a drier forest, released stored water. In both, water released was highest while flushing leaves, suggesting that stored water supports leaf flushing. In contrast, two deciduous species with intermediate stem density, Annona hayesii and Genipa americana, had intermediate C, low dry-season Ψ stem, and high seasonal change in water released. Meanwhile, two evergreen species with intermediate stem density, Cojoba rufescens and Astronium graveolens, had relatively low C, low dry-season Ψ stem, and intermediate seasonal change in water released. Thus, at least three, distinct stored-water-use strategies were observed. Additionally, bark relative water content (RWC) decreased along with Ψ stem during the dry season while xylem RWC did not change, suggesting that bark-stored water buffers Ψ stem seasonally. Together these results suggest that seasonal use of stored water and change in Ψ stem are associated with functional groups that are characterized by combinations of deciduousness and stem density.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seasonally dry tropical forests (SDTF) occupy areas at the climate transition between moist forest and savanna, making them vulnerable to conversion to savanna if areas become drier (Murphy and Bowman 2012). In fact, aridity is increasing in many regions due to temperature increases associated with climate change (Sherwood and Fu 2014). Furthermore, the extent of SDTF, which may have historically approximated the area of wetter forests, has been inordinately reduced through conversion to agricultural and urban uses, creating a conservation imperative (Murphy and Lugo 1986; Miles et al. 2006). Predicting how the remaining patches of SDTF will respond to drying, including possible expansion of drier habitats (Feng and Fu 2013), requires knowledge of the mechanisms by which tropical trees survive drought.
To avoid dieback and death during droughts, trees must maintain their stem water potential (Ψ stem) above levels that would cause hydraulic failure (Kursar et al. 2009; Urli et al. 2013). Species with low wood density tend to incur hydraulic failure at higher Ψ stem than species with high wood density (Hacke et al. 2001; Markesteijn et al. 2011; Pineda-García et al. 2013). Yet, species with low wood density also maintain higher Ψ stem during droughts than species with high wood density. Consequently, all species tend to maintain a safety margin between the Ψ stem reached during seasonal droughts and the Ψ stem causing hydraulic failure (Choat et al. 2012). Likewise, wood density, which ranges widely among SDTF species (Markesteijn et al. 2011; Pineda-García et al. 2013), is predicted to form an important axis of variation in drought-survival strategies. Specifically, species with low wood density, which are vulnerable to hydraulic failure yet maintain high Ψ stem, survive drought through desiccation avoidance, whereas species with high wood density, which are resistant to hydraulic failure yet reach low Ψ stem, survive drought through desiccation tolerance (Ludlow 1989; Poorter and Markesteijn 2008; Markesteijn and Poorter 2009; Pineda-García et al. 2013).
One mechanism by which desiccation-avoiding species can maintain high Ψ stem during droughts is by accessing moist soil. But, in dry soil, the mechanisms by which desiccation-avoiding species maintain high Ψ stem are unclear. Since water storage capacity (i.e., water stored per volume of wood) and stem capacitance [C (water released per ΔΨ stem); see “Materials and methods”] increase as wood density decreases (Simpson 1993; Meinzer et al. 2008a), desiccation-avoiding species may rely on stored water to maintain high Ψ stem during droughts (Poorter and Markesteijn 2008; Pineda-García et al. 2013). Many SDTF tree species with low-density wood also close their stomata or shed their leaves, traits that help to retain water within stems during droughts (Stratton et al. 2000; Brodribb et al. 2003; Poorter and Markesteijn 2008). Hence, desiccation-avoiding species may minimize the release of stem water and use C to buffer the effects of incidental water loss on Ψ stem (Borchert 1994a). In these species, without access to soil water, stem water release could occur very slowly over the dry season followed by recharge in the wet season, as opposed to the more commonly studied case of daily loss and recharge of stem water (Meinzer et al. 2003; Scholz et al. 2011).
The sapling life stage is highly responsive to reductions in rainfall (Enquist and Enquist 2011) and strongly influences the species composition of forests through habitat filtering (Baldeck et al. 2013). We addressed the issue of divergent mechanisms of drought survival among saplings of SDTF species by comparing two sites that differ in rainfall and by choosing species that likely differ in physiology. Hence, we studied responses of saplings in two SDTF: a dry forest and in a forest that is intermediate between moist and dry forest, hereafter termed “transitional forest.”
Because we are particularly interested in the role of deciduousness, C, and the release of stored water in drought survival, we chose six common species that have a wide range of deciduousness and stem density (analogous to wood density; see Table 1). We measured C and tracked leaf area, Ψ stem, and stem water released during seasonal or monthly intervals. Specifically, our objectives were to determine:
-
1.
The extent to which the seasonal release of stored water and seasonal change in Ψ stem are related to C.
-
2.
How the proportion of bark, xylem and pith tissue in the stem influences C among species.
-
3.
Which stem tissues release water during seasonal droughts.
Materials and methods
Study sites, species, and censuses
Samples were collected in two forests in the Republic of Panama—a dry forest and a transitional forest. The dry forest was the Eugene Eisenmann Reserve in Coronado (8°31′N, 79°53′W), a 43-ha private reserve with mean rainfall of 1592 mm year−1 (measured from 2001 to 2010 by the Empresa de Transmision Electrica in Chame, 8 km west of the study site). The transitional forest was the Parque Natural Metropolitano in Panama City (8°59′N, 79°32′W), a 232-ha protected area with mean rainfall of 1800 mm year−1. Both forests are mature secondary forests and experience an annual dry season from mid-December to May. In each forest, four species were selected for study (Table 1). Two species were shared between the forests, so six species were sampled in total: Astronium graveolens Jacq. (Anacardiaceae); Annona hayesii Saff. in Standl. (Annonaceae); Bursera simaruba (L.) Sarg. (Burseraceae); Cojoba rufescens (Benth.) Britton and Rose (Fabaceae); Cavanillesia platanifolia (Bonpl.) Kunth (Malvaceae); and Genipa americana L. (Rubiaceae). Henceforth, the species will be referred to by genus name only. Since there are few data on leaf phenology, particularly of saplings, we characterized the phenology of saplings in both forests by comparing leaf area during the wet season with four measurements during the 2013 dry season (Electronic supplementary material, Fig. S1).
Sample collection
At each site, we searched an area of ~2 ha and selected healthy looking saplings that were 120–400 cm in height. During the mid-late wet season (August–December 2012) and near the end of the subsequent dry season (March–April 2013), we collected stems from eight saplings (four at predawn, four at midday) of each species in each forest (n = 128 stems). For the three deciduous species (Table 1), which flushed leaves near the onset of the wet season, we also collected stems from eight saplings (four at predawn, four at midday, n = 24 stems) during leaf flush (May 2013; Fig. S1). Species differed in basal diameter and height due to differences in allometry (Table 1). Each stem, at least 110 cm long, was collected by cutting near the base with pruning shears, quickly sealed in opaque plastic bags that were humidified with wet paper towels, and transported to the laboratory to measure water content. An additional set of stems (n = 89) was collected during the 2012 wet season and bench-dried for assessing C; they were removed from their bags in the laboratory to allow them to air dry for 2–120 h and then re-sealed in the bags for 2 h before subsequent measurements.
Stem water potential
In the laboratory, leaf water potential was measured with a Scholander pressure chamber on three leaves per stem and averaged. Leaf water potential was assumed to equal Ψ stem because sealing the stems in opaque bags stopped transpiration. For the stems of deciduous species collected during the dry season, which were leafless, we measured Ψ stem by cutting the stem segment 10 cm from the apical meristem, placing the section with the apical meristem in the pressure chamber, and then pressurizing until equilibrium pressure. We verified the accuracy of these leafless Ψ stem measurements on a subset of samples that was also measured with psychrometers (Fig. S2).
Stem water released
After measuring Ψ stem, each stem was submerged in tap water and a 15-cm-long segment at >50 cm from the cut base was removed for measurement of stem water released. On the distal end, the stem, cambium, and pith diameters were measured with calipers to calculate areas of bark, xylem and pith (Table 1). The segment was blotted dry and measured for fresh mass, then for volume using water displacement on a digital balance. The segments were submerged in distilled water for 26.4 ± 0.7 h (mean ± SE), then removed, blotted dry, and measured for saturated mass. After the first saturated mass measurement, 197 of the 241 samples were submerged again in distilled water and measured again for saturated mass after an additional 35.4 ± 1.8 h. Interpolation was used to calculate the saturated mass of samples at a standardized time of 48 h of submersion. For samples with one measurement, we used the species-specific mean slope of the initial to final saturated mass to calculate the saturated mass at 48 h (Fig. S3). While it is common to measure saturated mass at 24 h, the ideal submersion time is equivocal because over-saturation of capillary spaces may produce artifactual results (Tyree and Yang 1990). We used 48 h because our stem segments were wider and longer than in other studies. Moreover, we verified that the stems were near the theoretical maximum saturation at 48 h and that the values of saturated water content were not affected by the season in which stems were collected (Fig. S4). The segments were oven dried at 60 °C to constant mass to obtain the dry mass. Stem water released was normalized on a stem volume basis by calculating (saturated mass − fresh mass)/stem segment volume.
Hence, all values for water released are relative to saturated stems. Since saturated stems are unlikely to occur in the field, the use of stored water is indicated by differences between sets of water-released measurements. These include dry season minus wet season to infer seasonal use of stored water, midday minus predawn to infer daily use of stored water, or flush minus dry season to infer stored water use during leaf flush at the onset of the wet season.
Relative water content in bark, xylem, and pith
Tissue-level relative water content (RWC) was measured on the stems collected from the dry forest during the wet and dry seasons, and during leaf flush for the deciduous species (n = 80 stems), but not on stems that were bench-dried to calculate C or on stems collected from the transitional forest. A 2-cm segment basal to that used for stem water released was cut under water, then dissected into bark (all tissue radially distal to the xylem, including the cambium), xylem, and pith. To minimize water loss during dissection, we enveloped the stem segments in moist paper towels and completed each dissection in <10 min. We measured the mass of the segment before dissection and then compared it to the sum of the dissected masses of bark, xylem and pith. The sum of the dissected tissue masses was 96.2 ± 0.2 % of the whole-segment mass. Considering that the loss also represents pieces of tissue that were lost, very little or no water was lost during dissection. After each tissue was measured for fresh mass, it was submerged in distilled water for 31.4 ± 1.3 h, and weighed for saturated mass. Samples from 36 of the 80 stems were then submerged in distilled water for an additional 25.6 ± 2.1 h and measured again for saturated mass in order to standardize saturated mass to 48 h of submersion as described above for intact stems (Fig. S3). The tissue samples were then oven dried at 60 °C to constant mass. RWC was calculated as (fresh mass − dry mass)/(saturated mass − dry mass). Two Astronium and six Cojoba had piths that were too small to assess with our dissection method, reducing the sample size for the RWC of pith in these species.
Calculation of C and statistical analyses
All analyses were completed in R version 3.0.1 (R Core Team 2013). Since parameters such as C may vary with plant size, we tested, for each species, whether basal diameter or height differed among seasonal or diurnal samples (two-way ANOVAs, P > 0.1). Since none differed, we did not include diameter or height as explanatory factors for water-relation parameters.
We calculated C for each species in each forest as the slope of the plot of water released as a function of Ψ stem using standardized-major-axis regression in the smatr package (Warton et al. 2006). In order to test whether C values generated from water-release curves of bench-dried stems accurately predict water-release curves of saplings in the field, we used only stems collected during the wet season and either measured these directly or bench-dried them in order to calculate C. Since C decreases as Ψ stem decreases, we fit the regression only on the samples within the Ψ stem range that each species reached in the field (Richards et al. 2014). To our knowledge, assessing C by bench drying long stem segments, each to a single Ψ stem, is a novel technique, so we compared our C values to others that we produced with the common method of sequentially drying short stem segments to obtain repeated measures of water released and psychrometrically determined Ψ stem. For the three species on which we compared the two methods, the C values were either indistinguishable or were higher by 27–79 % using the independent sample/long-stem method (Fig. S5). The difference may be due to artifacts introduced by sequentially drying short stem segments (see “Discussion”; Tyree and Yang 1990).
For each water-relation parameter, a two-way ANOVA was fit for each species, with season, time of day (predawn vs. midday), and their interaction as fixed effects. Response variables were Ψ stem, stem water released, and tissue-level RWC. Linear contrasts were used to test for the a priori hypotheses that the parameters differed between seasons and between times of day within season, correcting for multiple comparisons to α = 0.05 with the false discovery rate method of Benjamini and Hochberg (1995). Finally, to test whether C predicts seasonal changes in Ψ stem and water released among species, we used simple linear regression with C as the independent variable and species’ means of the difference between wet and dry-season Ψ stem and water released as dependent variables, respectively.
Results
Stem capacitance
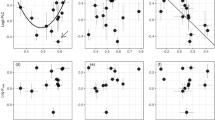
C ranged among species from 36.7 to 140.5 kg m−3 MPa−1 in the transitional forest and 32.3–195.2 kg m−3 MPa−1 in the dry forest (Fig. 1). Among species, C decreased with increasing stem density at a slope and intercept that did not differ between forests (analysis of covariance—stem density, F = 28.4, P = 0.006; forest, F = 4.9, P = 0.09; stem density × forest, F = 0.48, P = 0.53; simple linear regression with all species combined, C = −339 × stem density + 273.4, r 2 = 0.68). For the two species measured in both the transitional forest and the dry forest, C differed between the two forests (Fig. 1e–h). For Astronium, C was 36 % higher in the transitional forest than the dry forest (smatr analysis, P = 0.006). In contrast, for Cojoba, C was 57 % higher in the dry forest than in the transitional forest (smatr analysis, P = 0.053).
The relationship between stem water potential (Ψ stem) and stem water released for four species (Cavanillesia platanifolia, Annona hayesii, Astronium graveolens, Cojoba rufescens) in a transitional forest (a, c, e, g) and four species (Bursera simaruba, Genipa americana, A. graveolens, C. rufescens) in a dry forest (b, d, f, h). Henceforth, the species will be referred to by genus name only. Each symbol represents a separate plant. Open circles are samples within the range of Ψ stem that species reached in the field (see Fig. 2) and were included in the standardized-major-axis regression analysis to compute capacitance (C) as the absolute value of the slope of the dashed line. a–h C (kg m–3 MPa–1) and the r 2 are indicated. Filled circles Stems were bench-dried to lower the Ψ stem relative to that reached in the field and were not included in the calculation of C, solid lines indicate Gompertz functions fit through both open and closed circles
Stem water released
For most species, the amount of stem water released per decrease in Ψ stem during the dry season closely tracked that of the bench-dried stems used to compute C (cf. solid and dashed lines in Fig. 2). However, Annona in the transitional forest and Cojoba in the dry forest had significantly lower water released per decrease in Ψ stem in the field than predicted by C (smatr analysis, P < 0.001).
Daily and seasonal relationship between Ψ stem and stem water released for four species in a transitional forest (a, c, e, g) and four species in a dry forest (b, d, f, h). Each symbol represents a separate plant. Samples collected during the wet season (squares), dry season (circles), leaf flush (triangles), predawn (filled symbols), and midday (open symbols). Dashed lines represent the water-release curves of bench-dried samples used to calculate capacitance (see Fig. 1), solid lines represent standardized-major-axis regressions fit through all points in the scatter plots. a, b Insets have smaller axis ranges to show detail
All species had significantly higher stem water released during the dry season than during the wet season, except for Cavanillesia and Cojoba in the transitional forest (Fig. 3a, b; P-values for all ANOVAs are in Table S1). Since our measure of seasonal use of stored water is the difference in water released between wet and dry seasons, aside from the exceptions noted, all other species used stored water during the dry season. Water released at midday was significantly higher than at predawn during the wet season for Astronium in the transitional forest and for Cojoba in both forests and also during the dry season for Cojoba in the transitional forest (Fig. 3a, b). Annona, Bursera, and Genipa had similar, yet non-significant, trends for higher water released at midday than at predawn during the wet season; however, during the dry season this trend did not occur within most species.
Daily and seasonal stem water released (a, b) and Ψ stem (c, d). Filled bars Predawn measurements, open bars midday measurements (n = 4 per bar). Error bars extend to ±1 SE. Asterisks indicate significant differences between predawn and midday values within seasons (P < 0.05). Seasons that share letters did not differ significantly. Because water released is relative to saturated stems, our measure of stored water use is the difference between two conditions. The key comparisons are predawn vs. midday, wet vs. dry, and dry vs. flush (see “Materials and methods”). Wet Wet season, Dry dry season, Flush leaf flushing at the end of the dry season
While flushing leaves in early May 2013, Cavanillesia and Bursera had significantly higher water released than during the preceding dry season (compare flush vs. dry in Fig. 3a, b; linear contrasts P < 0.001 and P = 0.039, respectively). Hence, stem water was used during leaf flush. In contrast, Genipa had less water released during leaf flush than during the dry season (Fig. 3b; linear contrast P = 0.004). This indicates that during leaf flush, Genipa stems were actually more hydrated than during the dry season (late March–early April). Genipa saplings were likely partially rehydrated by 85 mm of rain that fell in several events in April and May before we conducted our analyses of leaf-flushing stems (Fig. S1b).
Stem water potential
At both sites, all species had significantly lower Ψ stem during the dry season than during the wet season (Fig. 3c, d). Dry-season Ψ stem ranged widely among species, for example, at the transitional forest, from −0.62 ± 0.03 to −3.40 ± 0.036 MPa (mean ± SE, predawn and midday combined). Within seasons, Ψ stem was significantly lower at midday than at predawn for Astronium and Cojoba in the transitional forest during the wet and dry seasons, Bursera during the wet season and during leaf flush, and Cojoba in the dry forest during the wet season (Fig. 3c, d). Trends for Ψ stem to be lower at midday than at predawn were common among the other species, yet they were not significant (Fig. 3c, d).
When Cavanillesia flushed leaves near the onset of the wet season, its Ψ stem was lower than during the dry season (linear contrast P = 0.039), while Bursera Ψ stem did not differ from that of the dry season (linear contrast P = 0.7; Fig. 3c, d). In contrast, while Genipa flushed leaves near the onset of the wet season, it had significantly higher Ψ stem than during the dry season (Fig. 3d). Again, for Genipa, this may reflect stem rehydration from rainfall early in the wet season (Fig. S1b).
Seasonal change in water released and Ψ stem in relation to C
Among all four species in each forest, C was not related to the seasonal change in Ψ stem or the amount of stored water used during the dry season (difference in water released between wet and dry seasons, P > 0.14; Fig. 4). Cojoba and Astronium in the transitional forest were distinct in that they had low C but showed a small change in Ψ stem (Fig. 4a). In both forests, the deciduous species with high C, Cavanillesia and Bursera, also had distinctive responses. These had the lowest seasonal changes in Ψ stem (Fig. 4a, b), yet these species differed in seasonal change in stem water released. In the transitional forest, Cavanillesia had essentially zero change in water released between the wet and dry seasons (Fig. 4c), meaning that its stems did not lose water during the dry season. In the dry forest, Bursera showed an increase in water released between the wet and dry seasons (Fig. 4d), meaning that, in contrast to Cavanillesia, significant water was lost from its stems. Astronium and Cojoba lost similar amounts of water as Bursera, whereas Genipa registered the highest loss of stem water during the dry season for any species (Fig. 4c, d).
The difference between wet and dry seasons for Ψ stem (a, b) and stem water released (c, d) in relation to capacitance. Points represent means for species. Filled circle Cojoba, filled square Astronium, filled triangle Annona, open square Cavanillesia, open triangle Genipa, open circle Bursera. Bars extend to ±1 SE
Water storage in bark, xylem, and pith
Species ranged widely in the proportion of stem composed of bark, xylem, and pith (Table 1), yet C was not correlated with stem-tissue composition (bark proportion vs. C, Pearson’s r = 0.35, P = 0.39; xylem proportion vs. C, r = −0.29, P = 0.49; pith proportion vs. C, r = 0.06, P = 0.88). We measured tissue level RWC in the dry-forest species. Bark RWC was correlated with Ψ stem in all species (Fig. 5a, d, g, j). Bark RWC was significantly lower during the dry season than during the wet season in all species except Bursera, in which it was lower during leaf flush than during the wet and dry seasons. In contrast, xylem RWC was not correlated with Ψ stem and did not differ between wet and dry seasons for any species (Fig. 5b, e, h, k). Pith RWC was correlated with Ψ stem only in Genipa (Fig. 5f). The only significant decrease in RWC from predawn to midday occurred in Bursera bark during leaf flush (Fig. 5a; linear contrast P = 0.016).
Relative water content of bark (a, d, g, j), xylem (b, e, h, k), and pith tissues (c, f, i, l) in relation to Ψ stem and season in the dry forest. Symbols in scatter plots and bar plots are drawn as in Figs. 2 and 3, respectively. For tissues with correlation coefficients with P < 0.05, the standardized-major-axis regression lines and r 2-values are given
Discussion
Variation in C within and among species and ontogenetic stages
In adult trees, the C of sapwood generally decreases among species as sapwood density increases, but the slope of the relationship varies greatly among sites (Meinzer et al. 2008a; Richards et al. 2014). We measured saplings and found that stem C decreased with increasing stem density at a slope that was similar between the transitional and dry forests. Our C values for any given stem density were about 25–230 % lower than values previously reported for the sapwood of adult trees of other species in other forests (Meinzer et al. 2008a) and in adult trees in one of our study sites, Parque Metropolitano [four trees measured had ranges of sapwood density and C of 0.28–0.52 g cm−3 and 80–415 kg m−3 MPa−1, respectively (Meinzer et al. 2003)]. This difference might reflect an ontogenetic shift, as C tends to increase with tree height (Scholz et al. 2011), possibly due to changes in xylem structure (Gartner 1995). Tall trees may benefit more than saplings from high C because they use stored water to overcome the time lag in transporting soil water to the canopy, which increases with tree height (Scholz et al. 2011).
Other factors may have led to lower C in our study than in previous studies. For example, we measured the C of whole stem segments as opposed to the common method of using sapwood cores. Using sapwood cores excludes the bark and pith. Possibly, these tissues have lower C than sapwood (Scholz et al. 2007). Using sapwood cores may also introduce bias because the water column within xylem vessels is broken during core extraction, converting water held tightly within vessels into easily extractable capillary water (Tyree and Yang 1990). Finally, studies vary in how C is calculated. Many have included values of Ψ stem above those experienced in the field, including 0 MPa. Because C commonly declines with declining Ψ stem, this may inflate C values (Richards et al. 2014). While we require more research on how methodology influences the value for C, we posit that bench-drying long, intact stem segments rather than sapwood cores more closely simulates drying in vivo, and therefore likely produces more reliable C measurements (Tyree and Yang 1990).
None of the saplings that we measured in the field reached the Ψ stem at which C decreased (i.e., they did not reach the portion of the water release curves with shallow slopes in Fig. 1). This result coincides with results from adult trees measured by Meinzer et al. (2008b), who predicted that regulation of Ψ stem above the shift to low C is a convergent trait among species that acts to optimize stored water use while preventing hydraulic failure (but see Richards et al. 2014). Although desiccation tolerance is little studied, Cojoba can tolerate desiccation to −8.1 MPa with a 50 % survival rate (Kursar et al. 2009). Hence, both phases of our water release curves that are fit to a Gompertz function may have physiological significance (Fig. 1; solid lines). For Cojoba, the steeper phase down to −3 MPa may indicate the Ψ stem for which water storage is important. In the range from −3 to −8 MPa, with a very low value for C (a shallow slope), desiccation tolerance may be more critical.
The two species measured in both forests had C values that varied between forests; Astronium had higher C in the transitional forest while Cojoba had higher C in the dry forest (Fig. 1e–h). Similarly, Barnard et al. (2011) found plasticity in C among conifers in forests that varied in rainfall, yet both species they studied had higher C at low-rainfall sites. Our result that C shifted to be higher or lower in the dry forest is consistent with either greater desiccation avoidance (higher C) or greater desiccation tolerance (lower C) in drier environments (discussed below).
Comparisons among species and forests of stored water use during the dry season
First, we tested the assumption that most species did not have access to soil water during the dry season. For species with access to soil water, we predicted release of water and decrease in Ψ stem during the daytime (i.e., significant differences between midday and predawn for water released and Ψ stem). Only Cojoba in the transitional forest showed this pattern (Fig. 3), suggesting that Cojoba saplings likely maintained their stems hydrated during the dry season by accessing soil water. In fact, its stems are hydraulically functional at much lower Ψ stem than that experienced in the transitional forest during the dry season; severe wilting associated with loss of hydraulic conductance occurs at −6.9 MPa in this species (Kursar et al. 2009).
By contrast, it appears that the other species and Cojoba in the dry forest did not have access to soil water during the dry season. For these, we predicted that stem water would be released very slowly in the dry season (and recharged during the wet season). Consistent with that, we found that, for most species in both forests, water released in the dry season exceeded water released in the wet season (Fig. 3a, b). This pattern indicates gradual (seasonal) water loss occurred despite shedding leaves or closing stomata. For many species, it seems that it may be impossible for saplings to avoid significant water loss during seasonal droughts. Instead, we suggest that many species rely on the capacitive effect of stored water to buffer Ψ stem against water loss during the dry season (Figs. 2, 3). In other words, although stem water is lost, the rate is low and the stored water buffers Ψ stem in order to provide a margin of safety against hydraulic failure during drought.
In the transitional forest, the species with the highest C, Cavanillesia, did not have higher water released during the dry season than the wet season (Fig. 3a), suggesting that it does not rely on stored water to buffer Ψ stem against seasonal water loss. Cavanillesia may have accessed soil water during the dry season as Cojoba appears to have done, but the water would have to have been from much deeper soil because Cavanillesia maintained much higher Ψ stem than Cojoba did (predawn Ψ stem, −0.60 ± 0.04 vs. −1.65 ± 0.44 MPa). Since tropical deciduous species tend to have shallow roots (Eamus and Prior 2001), it is more likely that dry-season water released was low in Cavanillesia due to deciduousness and high resistance to water loss from the stem. Thus, despite its relatively high C, Cavanillesia appears to rely on water retention to maintain Ψ stem during the dry season. In contrast, the other species with high C, Bursera, appears to rely on capacitive effects to maintain Ψ stem during the dry season; its stems lost a similar amount of water as those of the co-occurring evergreen species, yet its Ψ stem remained near wet-season levels (Fig. 4b, d). This contrast between the two deciduous, high-C species could have resulted because they differ in their water-use strategies; for example, Bursera bark may be more permeable to water than Cavanillesia bark. Alternatively, the drier conditions that Bursera experienced in the dry forest may have caused it to lose more water than Cavanillesia in the transitional forest.
Timing and use of stored water in leaf flush
Cavanillesia and Bursera are similar to three Adansonia species (baobab trees) in Madagascar in that they have higher water released during leaf flush than during the dry season, meaning that they used stored water to flush leaves (Chapotin et al. 2006). This suggests that the use of stored water to flush leaves is common among species with high C. Indeed, among 19 tree species in a Brazilian SDTF, only the six species with the lowest wood density (<0.55 g cm−3) initiated leaf and flower production before the onset of the wet season (Lima and Rodal 2010). Since water from early wet-season rain is inaccessible to plants until soil moisture is recharged to Ψ soil > Ψ stem, and since saplings with high C maintain very high Ψ stem, leaf flushing in these saplings would be greatly delayed if it relied on soil water. Thus high C in these species is likely an adaptation that permits leaf flushing early in the transition to the wet season. Although their stomata likely remain closed until soil water is available, flushing leaves early would extend their productive season (Chapotin et al. 2006), and could facilitate photosynthesis during the early wet season, when understory light levels are higher due to reduced leaf area among canopy trees (Brenes-Arguedas et al. 2011). Also, early leaf flushing could help trees to escape herbivore pressure on susceptible, expanding leaves, which is higher during the wet season (Aide 1988).
In contrast to Cavanillesia and Bursera, Genipa saplings had lower water released during leaf flush than during the preceding dry season, suggesting that they did not rely on stem-stored water to flush leaves. Since Genipa saplings reached relatively low Ψ stem during the dry season (Fig. 3b), early wet-season rains would rehydrate them sooner than Cavanillesia and Bursera Still, Genipa saplings initiated leaf flush before their stems were fully hydrated, evidenced by Ψ stem that was lower during leaf flush than during the wet season (predawn Ψ stem, −1.50 ± 0.07 vs. −0.48 ± 0.06 MPa; Fig. 2d). In contrast to Genipa, adult trees of various deciduous species in a Costa Rican dry forest initiated leaf flush only after their tissues were fully hydrated (Borchert 1994b). Our results suggest that full stem hydration is not a universal prerequisite to flushing leaves. However, Annona, Astronium, and Cojoba saplings flushed leaves only after several wet-season rainfall events (Fig. S1), which is a common observation among trees in SDTF (Daubenmire 1972; Lieberman 1982; Borchert et al. 2002).
The role of pith and bark in buffering xylem tension
Most studies of stored water use in tropical trees have focused on the role of sapwood, which, in adult trees, composes a larger proportion of stem volume than bark and pith (Schulze et al. 1988; Meinzer et al. 2003). However, bark and pith composed 38–67 % of the stem volume of the saplings we studied (Table 1). In other plants that have relatively large pith, such as giant rosettes (Espeletia spp.), daily xylem tension is buffered by water stored within the pith (Goldstein et al. 1984). In the desert shrubs Pittocaulon spp., which also have relatively large bark and pith, a similar role has been hypothesized, whereby water stored within the bark and pith buffers xylem tension seasonally (Olson 2005).
Our results support the hypothesis that water stored in bark helps to buffer xylem tension in SDTF saplings: bark RWC decreased with Ψ stem seasonally while xylem RWC remained constant for all species (Fig. 4). Yet we cannot confirm whether the water released from the bark during the dry season entered the xylem. Alternatively, xylem RWC could be uncorrelated with Ψ stem due to differences among plants in their drought histories and extent of cavitated xylem vessels. If embolisms form and do not refill when Ψ stem rises, then subsequent xylem RWC will be anomalously low. In our study, such a process is unlikely since xylem RWC showed little variability in relation to Ψ stem (Fig. 5b, e, h, k). Similar to our results, sapwood RWC in adult trees of Adansonia species did not vary between wet and dry seasons (Chapotin et al. 2006). However, since the water-use strategies of Adansonia may differ from species with higher wood density (Chapotin et al. 2006), it is not clear that the absence of seasonal change in xylem RWC is applicable more generally to SDTF trees.
Scholz et al. (2007) found that daily changes in bark RWC were less than or the same as in sapwood RWC during the dry season in Brazilian savanna tree species, suggesting that water stored within bark does not buffer xylem tension. However, these results do not directly contrast with ours since Scholz et al. (2007) did not measure seasonal changes in RWC. Dry-forest trees experience relatively large reductions in stem diameter during seasonal droughts (Lieberman 1982; Reich and Borchert 1984), which are caused mostly by changes in water content of the inner bark (De Schepper et al. 2012). It is possible that xylem RWC fluctuates in response to changes in Ψ stem on hourly and daily timescales but remains nearly constant on seasonal timescales through water exchange with pith and bark. Studies that have measured xylem RWC in SDTF trees have generally only made measurements during the dry season, and so do not provide information on seasonal flux. The relatively large seasonal flux in bark RWC (Fig. 5) suggests that bark-stored water is important in the drought response of tropical trees and should be addressed with future studies.
Fitting capacitance and stored water use with plant functional types
Choat et al. (2012) showed with a meta-analysis that a key trait, resistance to cavitation, varies considerably among species from dry biomes. Consistent with this observation, we found considerable variation in traits related to water storage. For example, a simple prediction is that reliance on stored water to buffer Ψ stem during seasonal droughts increases from desiccation-tolerant, evergreen species to desiccation-avoiding, deciduous species. Our results do not support this prediction. Instead, we found two distinct patterns of stored water use among deciduous species, both of which varied from those of evergreen species. Cavanillesia and Bursera had high C (Fig. 1), shed their leaves early in the dry season (Fig. S1), maintained high dry-season Ψ stem, used a moderate amount (Bursera) or no (Cavanillesia) stored water during the dry season (i.e., moderate or no difference in water released between the wet and dry seasons; Fig. 4), and used stored water to flush leaves (i.e., water released during leaf flush > dry-season water released; Fig. 3). In contrast Annona and Genipa had moderate C, shed their leaves later in the dry season, reached low dry-season Ψ stem, had relatively large differences in water released between the wet and dry seasons, and, for Genipa, had lower water released during the dry season than when flushing leaves.
This dichotomy among deciduous species is similar to that described by Borchert (1994a) for adult trees in a Costa Rican dry forest. He found that deciduous species with low-density wood—which he termed “deciduous lightwood” species—maintained high Ψ stem during the dry season and that stem water was depleted during flowering and leaf flush before the onset of the wet season. Others have termed such species “stem-succulent trees” or “caudiciform succulents” (Ogburn and Edwards 2010). Borchert (1994a) also identified “deciduous hardwood” species that reached low Ψ stem, shed leaves gradually, and lost a large fraction of stem water during the dry season. Such species, represented in our study by Annona and Genipa, appear to be highly reliant on stem water during the dry season as they had the highest seasonal change in water released among species in each forest (Fig. 4c, d). Despite their deciduous behavior, these species do not stop water loss during the dry season. Instead their moderate C lessens the impact of water loss on Ψ stem and, presumably, their xylem is resistant to cavitation. Similarly, in a SDTF, even after shedding their leaves during the dry season, the stems of several deciduous species contracted in association with water loss (Daubenmire 1972). These observations contrast with the prediction that deciduous species avoid desiccation and maintain high Ψ stem during droughts through deciduousness (Markesteijn and Poorter 2009). Rather, in terms of Ψ stem, non-stem-succulent deciduous species appear to experience the most desiccation and fit better into a strategy of desiccation tolerance.
Borchert (1994a) proposed that “evergreen softwood” trees that maintain green leaves through the dry season and have moderate wood density, such as the Cojoba and Astronium in our study, depend on soil water throughout the dry season. Our results are partially consistent with this prediction. Cojoba and Astronium had lower dry-season Ψ stem in the dry forest than in the transitional forest, suggesting that Ψ stem tracked Ψ soil. Furthermore, in the transitional forest, Cojoba had a pattern of daily flux in Ψ stem and water released during the dry season (Fig. 3a, c), suggesting that it relied on soil water. However, this was not the case for Astronium in either forest or for Cojoba in the dry forest, where they may not have had access to soil water during the dry season (Fig. 3b, d). Yet, these species also had lower seasonal change in water released than Annona and Genipa despite maintaining leaves through the dry season (Fig. 4). These species may have better access to soil water through deeper roots, a common trait among evergreen, dry-forest species (Markesteijn and Poorter 2009). However, between the two evergreen species that we studied, there was a divergence in water-use patterns between the transitional and dry forests. Cojoba had higher C in the dry forest than in the transitional forest while Astronium had lower C in the dry forest than in the transitional forest (Fig. 1). Also, Cojoba and Astronium had similar seasonal change in Ψ stem and water released in the transitional forest, but in the dry forest Astronium had greater seasonal change in Ψ stem and water released than Cojoba (Fig. 4). Hence, in the drier forest, it appears that Cojoba shifts toward desiccation avoidance while Astronium is more desiccation tolerant. This divergence in strategies is consistent with comparisons of congeneric species in which one species inhabits wetter valleys and the other drier plateaus of Mexican dry forest. Surprisingly, suites of functional traits, including wood density, varied in opposite directions between habitats depending on the species pair (Pineda-García et al. 2011). Taken together, our results suggest that stored water use and seasonal changes in Ψ stem are not directly related to C, but rather to the combination of C and other key traits such as resistance to cavitation, leaf phenology, and rooting depth. Hence, for habitats that experience drought, a better understanding of water storage will be important for understanding species distributions in relation to soil water availability and predicting responses to future drying.
Author contribution satement
B. T. W. and T. A. K. conceived and designed the experiments. B. T. W. performed the experiments and analyzed the data. B. T. W. and T. A. K. wrote the manuscript.
References
Aide TM (1988) Herbivory as a selective agent on the timing of leaf production in a tropical understory community. Nature 336:574–575
Baldeck CA, Harms KE, Yavitt JB, John R, Turner BL, Valencia R, Navarrete H, Bunyavejchewin S, Kiratiprayoon S, Yaacob A et al (2013) Habitat filtering across tree life stages in tropical forest communities. Proc R Soc B 280:20130548
Barnard DM, Meinzer FC, Lachenbruch B, McCulloh KA, Johnson DM, Woodruff DR (2011) Climate-related trends in sapwood biophysical properties in two conifers: avoidance of hydraulic dysfunction through coordinated adjustments in xylem efficiency, safety and capacitance. Plant Cell Environ 34:643–654
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300
Borchert R (1994a) Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 75:1437–1449
Borchert R (1994b) Water status and development of tropical trees during seasonal drought. Trees Struct Funct 8:115–125
Borchert R, Rivera G, Hagnauer W (2002) Modification of vegetative phenology in a tropical semi- deciduous forest by abnormal drought and rain. Biotropica 34:27–39
Brenes-Arguedas T, Roddy AB, Coley PD, Kursar TA (2011) Do differences in understory light contribute to species distributions along a tropical rainfall gradient? Oecologia 166:443–456
Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV (2003) Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ 26:443–450
Chapotin SM, Razanameharizaka JH, Holbrook NM (2006) Baobab trees (Adansonia) in Madagascar use stored water to flush new leaves but not to support stomatal opening before the rainy season. New Phytol 169:549–559
Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Radika B, Bucci S, Feild TS, Gleason SM, Hacke UG et al (2012) Global convergence in the vulnerability of forests to drought. Nature 491:752–755
Daubenmire R (1972) Phenology and other characteristics of tropical semi-deciduous forest in north-western Costa Rica. J Ecology 60:147–170
De Schepper V, van Dusschoten D, Copini P, Jahnke S, Steppe K (2012) MRI links stem water content to stem diameter variations in transpiring trees. J Exp Bot 63:2645–2653
Eamus D, Prior L (2001) Ecophysiology of trees of seasonally dry tropics: comparisons among phenologies. Adv Ecol Res 32:113–197
Enquist BJ, Enquist CAF (2011) Long-term change within a neotropical forest: assessing differential functional and floristic responses to disturbance and drought. Glob Change Biol 17:1408–1424
Feng S, Fu Q (2013) Expansion of global drylands under a warming climate. Atmos Chem Phys 13:10081–10094
Gartner BL (1995) Patterns of xylem variation within a tree and their hydraulic and mechanical consequences. In: Gartner BL (ed) Plant stems: physiology and functional morphology. Academic Press, San Diego, pp 125–149
Goldstein G, Meinzer F, Monasterio M (1984) The role of capacitance in the water balance of Andean giant rosette species. Plant Cell Environ 7:179–186
Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126:457–461
Kursar TA, Engelbrecht BMJ, Burke A, Tyree MT, El Omari B, Giraldo JP (2009) Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Funct Ecol 23:93–102
Lieberman D (1982) Seasonality and phenology in a dry tropical forest in Ghana. J Ecol 70:791–806
Lima ALA, Rodal MJN (2010) Phenology and wood density of plants growing in the semi-arid region of northeastern Brazil. J Arid Environ 74:1363–1373
Ludlow MM (1989) Strategies of response to water stress. In: Kreeb KH, Richter H, Hinckley TM (eds) Structural and functional responses to environmental stresses. SPB Academic, the Hague, pp 269–281
Markesteijn L, Poorter L (2009) Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J Ecol 97:311–325
Markesteijn L, Poorter L, Bongers F, Paz H, Sack L (2011) Hydraulics and life history of tropical dry forest tree species: coordination of species’ drought and shade tolerance. New Phytol 191:480–495
Meinzer FC, James SA, Goldstein G, Woodruff D (2003) Whole-tree water transport scales with sapwood capacitance in tropical forest canopy trees. Plant Cell Environ 26:1147–1155
Meinzer FC, Campanello PI, Domec J-C, Gatti MG, Goldstein G, Villalobos-Vega R, Woodruff D (2008a) Constraints on physiological function associated with branch architecture and wood density in tropical forest trees. Tree Physiol 28:1609–1617
Meinzer FC, Woodruff D, Domec J-C, Goldstein G, Campanello PI, Gatti MG, Villalobos-Vega R (2008b) Coordination of leaf and stem water transport properties in tropical forest trees. Oecologia 156:31–41
Miles L, Newton AC, DeFries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE (2006) A global overview of the conservation status of tropical dry forests. J Biogeogr 33:491–505
Murphy BP, Bowman DMJS (2012) What controls the distribution of tropical forest and savanna? Ecol Lett 15:748–758
Murphy PG, Lugo AE (1986) Ecology of tropical dry forest. Annu Rev Ecol Syst 17:67–88
Ogburn M, Edwards EJ (2010) The ecological water-use strategies of succulent plants. Adv Bot Res 55:179–225
Olson ME (2005) Wood, bark, and pith anatomy in Pittocaulon (~ Senecio, Asteraceae): water storage and systematics. J Torrey Bot Soc 132:173–186
Pineda-García F, Paz H, Tinoco-Ojanguren C (2011) Morphological and physiological differentiation of seedlings between dry and wet habitats in a tropical dry forest. Plant Cell Environ 34:1536–1547
Pineda-García F, Paz H, Meinzer FC (2013) Drought resistance in early and late secondary successional species from a tropical dry forest: the interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant Cell Environ 36:405–418
Poorter L, Markesteijn L (2008) Seedling traits determine drought tolerance of tropical tree species. Biotropica 40:321–331
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reich PB, Borchert R (1984) Water stress and tree phenology in a tropical dry forest in the lowlands of Costa Rica. J Ecol 72:61–74
Richards AE, Wright IJ, Lenz TI, Zanne AE (2014) Sapwood capacitance is greater in evergreen sclerophyll species growing in high compared to low-rainfall environments. Funct Ecol 28:734–744
Scholz FG, Bucci S, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F (2007) Biophysical properties and functional significance of stem water storage tissues in Neotropical savanna trees. Plant Cell Environ 30:236–248
Scholz FG, Phillips NG, Bucci SJ, Meinzer FC, Goldstein G (2011) Hydraulic capacitance: biophysics and functional significance of internal water sources in relation to tree size. In: Meinzer FC, Lachenbruch B, Dawson TE (eds) Size- and age-related changes in tree structure and function. Springer, New York, pp 341–362
Schulze E-D, Mooney HA, Bullock SH, Mendoza A (1988) Water contents of wood of tropical deciduous forest species during the dry season. Bol Soc Bot México 48:113–118
Sherwood S, Fu Q (2014) A drier future? Science 343:737–739
Simpson WT (1993) Specific gravity, moisture content, and density relationship for wood. USDA Forest Service Forest Products Laboratory, Madison
Stratton L, Goldstein G, Meinzer FC (2000) Stem water storage capacity and efficiency of water transport: their functional significance in a Hawaiian dry forest. Plant Cell Environ 23:99–106
Tyree MT, Yang S (1990) Water-storage capacity of Thuja, Tsuga, and Acer stems measured by dehydration isotherms: the contribution of capillary water and cavitation. Planta 182:420–426
Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S (2013) Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol 33:672–683
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line-fitting methods for allometry. Biol Rev 81:259–291
Acknowledgments
B. T. W. was supported by a short-term fellowship from the Smithsonian Tropical Research Institute, an Award in Tropical Botany from the Garden Club of America, a University of Utah Graduate Research Fellowship, and a National Science Foundation GK-12 Program fellowship from the University of Utah’s Think Globally Teach Locally Program. Funding was provided by the National Science Foundation grant DEB-0444590 to T. A. K. We thank Joe Wright for advice and logistical support; Sarah Bruemmer and Mark de Guzman for field and lab assistance; and Duncan Smith, Frederick Meinzer, and two anonymous reviewers for helpful comments on the manuscript. This study was completed with the permission of the Autoridad Nacional del Ambiente de la República de Panamá.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Frederick C. Meinzer.
In this work we offer new insights into the drought responses of trees by focusing on the critical sapling stage, developing a novel technique to measure hydraulic capacitance, and comparing multiple stem tissues for seasonal water use. The results expand our knowledge of the functional traits that influence drought performance in trees and suggest new dimensions for understanding how climate change will potentially drive forest community dynamics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wolfe, B.T., Kursar, T.A. Diverse patterns of stored water use among saplings in seasonally dry tropical forests. Oecologia 179, 925–936 (2015). https://doi.org/10.1007/s00442-015-3329-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3329-z