Abstract
Increases in terrestrial primary productivity across the Arctic and northern alpine ecosystems are leading to altered vegetation composition and stature. Changes in vegetation stature may affect predator–prey interactions via changes in the prey’s ability to detect predators, changes in predation pressure, predator identity and predator foraging strategy. Changes in productivity and vegetation composition may also affect herbivores via effects on forage availability and quality. We investigated if height-dependent effects of forage and non-forage vegetation determine burrowing extent and activity of arctic ground squirrels (Urocitellus parryii). We collected data on burrow networks and activity of arctic ground squirrels across long-term vegetation monitoring sites in Denali National Park and Preserve, Alaska. The implications of height-specific cover of potential forage and non-forage vegetation on burrowing behaviour and habitat suitability for arctic ground squirrels were investigated using hierarchical Bayesian modelling. Increased cover of forbs was associated with more burrows and burrow systems, and higher activity of systems, for all forb heights. No other potential forage functional group was related to burrow distribution and activity. In contrast, height-dependent negative effects of non-forage vegetation were observed, with cover over 50-cm height negatively affecting the number of burrows, systems and system activity. Our results demonstrate that increases in vegetation productivity have dual, potentially counteracting effects on arctic ground squirrels via changes in forage and vegetation stature. Importantly, increases in tall-growing woody vegetation (shrubs and trees) have clear negative effects, whereas increases in forb should benefit arctic ground squirrels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changing climate is leading to substantial vegetation changes in many arctic and northern alpine ecosystems. Responses of arctic vertebrates to recent climate warming have been difficult to identify, and the influence of indirect responses has been stressed (Gauthier et al. 2013). For arctic herbivores, climate can have effects on populations via altered forage dynamics (Post and Forchhammer 2008). However, interactions with forage can be further modified by other components of environmental change such as forage accessibility [e.g. through icing events (Hansen et al. 2011)], and interactions with pests and predators (Sharma et al. 2009). Physical vegetation structure can have major effects on predator–prey interactions (Kauffman et al. 2007). These effects could all lead to changes in herbivore abundance, distribution, and further associated ecological impacts with changing arctic and northern alpine vegetation.
Widespread increases in primary terrestrial productivity are occurring across the Arctic and northern latitudes, associated with rapid warming (Jia et al. 2003), and further increases in primary productivity are projected (Epstein et al. 2004). Increasing primary terrestrial productivity in tundra habitats is associated with changes in plant stature and community composition (Henry et al. 2012). In particular, local expansion of shrub cover has been observed (Sturm et al. 2001; Myers-Smith et al. 2011) and northward expansion of shrub cover is projected in many locations (Pearson et al. 2013). Large herbivores can alter the trajectories of vegetation communities under change (Blinnikov et al. 2011; Willerslev et al. 2014). In the Arctic, two different trajectories are observed. In some ecosystems, shrub expansion occurs and above-ground biomass increases, whereas in others herbivory moderates biomass increases and shrub expansion (Post and Pedersen 2008; Olofsson et al. 2009; Zamin and Grogan 2013; Ravolainen et al. 2014). The ability of vertebrate herbivores to reduce local biomass and suppress canopy-forming shrubs may depend a number of interlinked factors. These include the productivity of the environment and any allochthonous inputs to the system, the dynamics and interactions between predator and prey populations, and the level of herbivore regulation by predators (Oksanen 1983; Batzli et al. 1980; Aunapuu et al. 2008; Gauthier et al. 2011; Hoset et al. 2014). As semi-domesticated reindeer can suppress shrub (Olofsson et al. 2009), humans may therefore also influence herbivory processes through herding practices. These processes will contribute to ecosystem trajectories and future dynamics in above-ground biomass, plant species composition and canopy-forming shrub cover.
Which of these two trajectories occurs could have major impacts on the entire community. Expansion of woody species can alter the composition of vertebrate fauna, from assemblages more associated with open habitats to assemblages typical of boreal forest (Sokolov et al. 2012). Alpine and arctic small herbivores may be negatively affected by changes in vegetation stature associated with warming-driven productivity increases via effects on predation. Vegetation change may alter predator–prey interactions via a number of mechanisms. Firstly, some prey species rely on high visibility for predator detection (Blumstein et al. 2006) and open escape routes (Schooley et al. 1996). Secondly vegetation stature can affect the type of predator, predator foraging strategy (Gillis et al. 2005a), or predator density. Tall vegetation may, however, also have positive effects by providing cover for foraging herbivores (Hannon et al. 2006).
Small herbivores may benefit from increased productivity if forage quality or quantity is improved (Bennett 1999; Byrom et al. 2000), or experience negative effects if favoured forage species are less abundant under community changes. Changes in potential forage functional groups may occur both with warming and associated with changes in shrub cover. Trends in cover of herbaceous plants appear highly contingent on local factors (Elmendorf et al. 2012; Gough et al. 2012). This variety of vegetation effects may have complex interplays. In Uinta ground squirrels (Urocitellus armatus, Kennicott 1863), shrubs may have antagonistic effects as both visual obstructions and as beneficial cover, with the result that effects on forage are likely to determine the net effect on the squirrels (Hannon et al. 2006). Understanding the contributions of these different roles of vegetation stature and forage to the distribution and local abundance of alpine and arctic small herbivores is key to understanding the likely impacts of future vegetation change.
Arctic ground squirrels (Urocitellus parryii, Richardson 1825) are distributed broadly across arctic and northern alpine areas. Their densities and distribution may be substantially affected by changes in forage and vegetation stature. Changes to their density may result in feedbacks to the ecosystem state, due to the ecosystem effects they impose via extensive burrowing and as prey to many avian and terrestrial predators (Wheeler and Hik 2013). Comparative survival and density trends at boreal, shrub-dominated and more open alpine tundra sites show characteristics of source-sink dynamics, and elevated predation risk in sites with taller vegetation may underlie these site differences (Gillis et al. 2005a; Donker and Krebs 2011, 2012; Wheeler 2012). However, experimental food enrichment and subsequent population trends after cessation of enrichment suggest that forage may also play an important role in population dynamics (Byrom et al. 2000; Karels and Boonstra 2000). Previous studies suggest that both food and predators limit population sizes in boreal forest habitat, whereas studies in alpine tundra have highlighted food and territoriality as most important for population size (Batzli et al. 1980; Byrom et al. 2000; Karels and Boonstra 2000; Karels et al. 2000). At the ecosystem level, support for the ecosystem exploitation hypothesis has been found in northern habitats (Aunapuu et al. 2008). This hypothesis suggests that the role of predator regulation should increase with increases in productivity. By assessing the relative role of vegetation as potential forage and vegetation structure via its effects on visibility and consequently predation risk, we explore the role of these processes in determining habitat quality.
In this study, the effects of vegetation composition and stature upon the burrowing activity of arctic ground squirrels were investigated in an area undergoing vegetation change. In Denali National Park and Preserve, the upslope advance of tree line by 150-m elevation was documented between 1953 and 2005, accompanied by local increases in tree density (Stueve et al. 2010), indicating the movement of lower elevation vegetation communities to higher elevations. Our objectives were to:
-
1.
Identify key plant functional groups that constitute important forage by evaluating if burrowing was likely to be more extensively associated with cover of potential forage functional groups (graminoids, forbs, dwarf shrub and shrub).
-
2.
Assess the effect of non-forage and forage vegetation on burrow distribution and activity. In particular, we predicted more extensive burrowing and greater activity in areas of higher cover of preferred forage. We predicted that the effect of non-forage cover would depend on the relative role of cover in providing shelter and safety versus obscuring visibility. We predicted that greater non-forage cover would be associated with increased burrowing and activity if cover provided shelter and safety, but decreased burrowing and activity if non-forage cover increased predation risk, or that there might be trade-offs between these two opposing effects.
-
3.
Investigate how vertical vegetation height affects the influence of forage and non-forage vegetation upon burrow distribution and activity; in particular whether negative effects of cover were greater when vegetation was taller. This will improve our understanding of the pressures that will influence landscape response of this species to vegetation changes. Specifically, the response of burrowing distribution and activity to vegetation are documented as an indicator of occupancy, habitat quality and the extent of the ecosystem role of arctic ground squirrels as bioturbators.
Materials and methods
Study species
Arctic ground squirrels are a colonial burrowing rodent, distributed across northern Canada, Alaska and eastern Siberia. Squirrels are active from April/May to until August/September and hibernate for the rest of the year (Buck and Barnes 1999a). Squirrels can reach up to 1.1 kg in mass (Morrison and Galster 1975) and mass varies considerably throughout the active season (Buck and Barnes 1999). Arctic ground squirrels occur in alpine and arctic tundra, shrub-tundra, shrub, boreal forest and meadow habitats; reported densities vary from 0.1 to 16 squirrels per hectare (Wheeler and Hik 2013). Although broadly generalist in their diet, evidence from caching behaviour and stomach contents suggests there is a degree of selectivity in foraging, with particular avoidance of evergreen shrub and lichen (Batzli and Sobaski 1980; Gillis et al. 2005b). Diet at one site varied seasonally, with a preference for forbs in the middle of the active season and more varied diet at the start and end. This broad diet at the start and end of the active season may reflect changing forage availability in the short active season, driven by snow cover and senescence (Batzli and Sobaski 1980).
Arctic ground squirrels are prey to a wide range of both terrestrial and avian predators including arctic foxes (Vulpes lagopus), red foxes (Vulpes vulpes), coyotes (Canis latrans), lynx (Lynx canadensis), grizzly bears (Ursus arctos), golden eagles (Aquila chrysaetos), goshawks (Accipiter gentilis), great horned owls (Bubo virginianus) and gyrfalcons (Falco rusticolus) (Wheeler and Hik 2013). Predation can contribute substantially to active season mortality [e.g. of juveniles (Byrom and Krebs 1999)] and differences in predation risk may explain some of the temporal and spatial variation in active season survival and reproduction (Gillis et al. 2005a; Donker and Krebs 2011). Winter survival has been found to vary between boreal forest and alpine tundra sites (Gillis et al. 2005a), but did not differ between sites across a shrub to alpine tundra ecotone (Wheeler 2012). In winter, snow accumulation appears important for insulation of hibernacula (winter hibernation burrows) and arctic ground squirrel survival as ground squirrels select areas with warmer soil temperatures (Buck and Barnes 1999b).
Study site
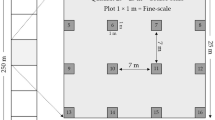
Sampling was conducted in the northeastern region of Denali National Park and Preserve, south-central Alaska, USA (63°41′N, 150°25′W), co-located with long-term vegetation monitoring plots (details in Roland et al. 2012) sampled between mid June and early August 2006–2012. Within this design, a systematic grid with random start spans a 12,800-km2 area of the park, with nodes spaced every 20 km and additional nodes added at 10-km intervals in certain cases. At each node, a nested sampling grid is located with sampling stations in a five by five arrangement and each station separated by 500 m. Selection of stations for this study was determined by hiking accessibility due to the remote nature of the park, and encompassed 34 sampling stations across six different grids with 5.7 ± 1.3 stations sampled per grid (Supplementary material S1). All burrow recordings were made between 13 and 29 June 2013. Although the vegetated areas of the park cover a wide range of habitat types, from boreal forest at lowest elevations to high tundra at higher elevations, only sampling stations in shrub tundra and alpine tundra habitats were used in this study. These stations were located between 903 and 1,454-m elevation.
Vegetation cover and abiotic data
Plot slope, plot equivalent latitude and soil depth were measured at every sampling station as part of long-term monitoring (Roland et al. 2004). Plot slope was measured as the slope across the 16-m diameter of the plot. Plot equivalent latitude (θ′) was used as a measure of potential solar isolation to the land surface, calculated from a combination of latitude (θ), slope (k) and aspect (h) using the following formula (Dingman and Koutz 1974; Densmore 2008):
Plot soil depth was the mean of 16 soil-depth measurements derived by insertion of a steel tile probe into the ground surface.
Vegetation data
Percent cover of vegetation functional groups in 0–30-, 30–50-cm and greater than 50-cm above-ground vertical height strata were calculated. Vegetation sampling was conducted along two perpendicular 16-m transects running east–west and north–south across the centre of the sampling station. Along each transect, vegetation cover was assessed every 50 cm (Roland et al. 2004). Cover in 0–10, 10–20, 20–30, 30–50, 50-cm to 1, 1–1.5, 1.5–2, 2–3 m. 3–4-m and over 4-m above-ground height categories were recorded. In order to estimate cover, a pin was dropped from 1.5 m to the ground surface, and vegetation was viewed through a point densitometer above this level. Proportional plant cover for each height stratum was derived by dividing the number of vegetation ‘hits’ in a stratum by the number of transect stations (with the result expressed as percentage cover by stratum). Species were combined by functional groups to obtain cover at this level.
Cover which did not contribute to vertical structure, such as moss and lichen, was excluded from the non-forage category. As standing dead material can also contribute to cover, this was included in the non-forage category. The final groups included in the non-forage category (excluding forb, graminoid or shrub and dwarf shrub, which were only included in models where they were not included as forage) were: fern, horsetail, lycophyte, tree, dead forb, dead tree, woody debris and dead shrub.
Burrowing metrics
Three burrowing metrics were estimated at each sampling station to reflect the local burrow networks and activity of arctic ground squirrels at each location. The total number of burrow entrances within 30 m of the plot centre was recorded. Next, the total number of burrow systems was recorded; this was estimated as the number of collections of burrow entrances connected by burrows that had no more than 3 m between them. A distance of 3 m between burrows was considered appropriate based on descriptions of arctic ground squirrel behaviour and apparent female rejection of more distant burrows (Carl 1971) and mapping of underground connections for arctic ground squirrels (Batzli and Sobaski 1980) and similar species [e.g. Richardson’s ground squirrel, Urocitellus richarsonii (Ovens 2011)]. Both metrics were recorded independently by two observers. One observer also recorded the number of burrows in each system and the activity status of each system. Burrow systems were recorded as ‘active’ if arctic ground squirrel faeces were present or there was evidence of fresh squirrel digging.
Modelling of burrow networks and activity
This study aims to analyse the three response variables: the number of burrows, the number of systems, and the number of active systems. These variables are not independent from each other: the total number of burrows at a site is related to the number of systems and the mean number of burrows per system and the total number of active burrows are, in part, determined by the total number of burrows at a site. This dependence between the variables describing the burrowing characteristics of the ground squirrels obscures the amount of inference that can be drawn from many standard forms of statistical analysis. For example, the observation that there are more burrows in one location over another could be explained by an increase in the number of systems or by there being more burrowing in each system. To facilitate the disentangling of the separate environmental effects on each of these variables we developed a hierarchical model (see Fig. 1 for a graphical overview) linking each of the response variables of interest to the environmental and floral characteristics of the area. This hierarchical model had three submodel components: the system density and burrow density submodels, and the burrow activity submodel.
Directed acyclic graph detailing the structure of the modelling approach used to analyse the relationship between environmental and vegetation covariates of burrowing density and activity. Elliptical nodes represent stochastic nodes (nodes related to other nodes by a probability distribution) and rectangular nodes represent logical nodes (nodes related to other nodes by arithmetic functions). Grey nodes denote nodes for which we have data directly available, such that the white nodes represent those nodes with values for which we wish to draw an inference. Multiple copies of a set of nodes (such as nodes that have a different value at each site) are denoted by a bounding box with a stacked border and the index for each copy is given by the variable in the top-right corner of the bounding box
System density submodel
The following linear submodel was constructed to relate the mean number of systems at a site to an n × d s matrix (where the mean number of systems present at site i as is defined as \(m_{{{\text{s}}_{i} }}\) n represents the number of sites, and d s represents the number of covariates for the system density submodel) of environmental and vegetation covariates, C s, and the column vector (of length d s) of model coefficients, β s:
Here, the covariate matrix C s contains one row for each of the n sites with each column containing the value of the relevant covariate. This covariate matrix can contain columns of polynomial terms of the covariates and also included an intercept term through the use of a ‘dummy’ column containing only the value 1 at all sites.
The mean system density at a site was related to the counts of systems made by two observers at that site, \(o_{{{\text{s}}_{1i} }}\) and \(o_{{{\text{s}}_{2i} }}\) for each observer respectively, by using a standard Poisson error model such that:
where the * notation is a convenience to denote that both observer counts follow the same error model.
Burrow density submodel
Similarly to the system density submodel described above, an n × d b covariate matrix was defined for the burrow density submodel (where d b represents the number of covariates used in the burrow density submodel) as C b and the coefficient vector (of length d b) as β b. The mean number of burrows per system at site i, \(m_{{{\text{b}}_{i} }}\), was then related to the covariate matrix using the linear submodel:
The total number of burrows expected at any given site, \(t_{{{\text{b}}_{i} }}\), is then the product of the number of burrows per system at the site (\(m_{{{\text{b}}_{i} }}\)) and the number of systems at the site (\(m_{{{\text{s}}_{i} }}\)). The total number of burrows counted by two observers at site i, \(o_{{{\text{b}}_{1i} }}\) and \(o_{{{\text{b}}_{2i} }}\) for each observer respectively, was therefore be related to \(t_{{{\text{b}}_{i} }}\) using a similar Poisson error model to that applied in the system density submodel:
System activity submodel
In addition to a count of the number of systems at a given site, there were also data available for the second observer on the number of active systems at that site, \(o_{{{\text{a}}_{2i} }}\). In a similar fashion to the other submodels, an n × d a matrix of covariates was constructed (where d a represents the number of covariates used in the system activity submodel), C a, and a vector of corresponding coefficients β a (of length d a). However, unlike the other submodels, a logistic regression submodel was created for the variable ρ i , the probability of a system being active at site i, such that:
The number of systems that were observed as active at site i, \(o_{{{\text{a}}_{2i} }}\), was then related to the number of systems recorded by the relevant observer (here denoted as observer 2, \(o_{{{\text{s}}_{2i} }}\)) and the probability of activity (\(\rho_{i}\)) with the binomial error model:
Model parameterisation and inference
A formulation of the model was implemented in the Bayesian analysis package JAGS (Plummer 2003) that could be called from the R statistical platform using the package rjags. A variety of different specifications for the number and types of covariates for the linear submodels were analysed. Non-informative priors were chosen for the coefficients of the linear submodels using an approximation to the improper unbounded-flat distribution [corresponding to the relevant Jeffrey’s prior for Bayesian linear regression (Ibrahim and Laud 1991)].
In all analyses, 1,010,000 Markov chain Monte Carlo iterations were performed, discarding the first 10,000 iterations, for each of four chains. Chains were thinned so that only every 10th iteration was retained for final analysis due to computer memory constraints. For each tested model, convergence was assessed through the calculation of the multivariate and univariate Gelman–Rubin convergence diagnostics (Gelman and Rubin 1992; Brooks and Gelman 1998), ensuring that all models with highest (top 50) Bayes factor weights values were close to one (defined here as less than 1.05). Convergence diagnostics for models and plots over time for the best model are supplied in Supplementary materials S2 and S4.
Model testing and selection
Associations between environmental and vegetation characteristics, and burrow distribution and activity, were evaluated in alternate models. Three environmental variables were considered in candidate models. Equivalent latitude was used as an index of the climatic conditions of the sampling station, where included, a quadratic term was included, reflecting an optimum climate with less optimal conditions at high and low extremes. Slope is likely to have effects on local drainage and was therefore also included as a possible covariate (linear on the link scale). Finally, a link-linear term for soil depth was considered in candidate models given that a sufficient depth of soil is likely to be required for burrowing.
Models with vegetation covariates were considered in three vertical strata; 0–30, 30–50 and 50 m and over. In any given model, vegetation was either attributed to forage or non-forage. Attribution to either class was specific to each model; if potential forage functional groups were not being considered as forage in a given model they would contribute to cover of the non-forage category for that model. Dwarf shrub, forb, graminoid and shrub were evaluated as candidate forage functional groups with possible linear relationships to burrowing metrics. Non-forage was any vegetation cover in a vertical stratum not considered forage in that model. Models for both linear and quadratic relationships between non-forage species and burrow metrics were considered as candidate models to reflect possible positive and negative effects of non-forage cover and where a cover-dependent trade-off between the two might be found.
Given there were no a priori reasons to believe certain predictor variables would act in concert or exclude others, we considered the combinations of predictor variables detailed in Supplementary material S2. This reflected all possible combinations of predictor variables, but modelled each response variable (number of burrows, number of systems and proportion of systems active) with the same predictors. Subsequently, effect sizes of our best model were evaluated to assess which predictors were important to each specific response variable to be evaluated. The most parsimonious model given the amount of variation explained was selected using comparison of Bayes factors.
Results
The best model explaining number of burrows, number of burrow systems and proportion of systems active contained linear effects of slope and forb cover as well as of non-forage vegetation. Positive effects of forb cover on burrow distribution and activity were found, whereas dominant effects of non-forage were negative. There was strong support for this model over other candidate models (posterior weight > 0.9999). The multivariate Gelman–Rubin metric of this model was calculated to be <1.005, ensuring that convergence was reached. Greater numbers of burrows and greater numbers of systems were associated with steeper slopes (Table 1; Fig. 2). The positive association between slope and proportion of burrows active had a particularly large effect size. Neither soil depth nor equivalent latitude was contained in the best model to explain burrowing metrics (Appendix S1).
Response curve showing contributions of plot slope to arctic ground squirrel burrowing metrics and activity from the best model explaining these responses selected using Bayes factors. The effect of slope on a number of burrows, b number of systems and c probability of arctic ground squirrel activity as measured by the multiplicative effect on respective burrowing metrics estimated in a hierarchical Bayesian framework. The y-axis is therefore the expected number of burrows, number of systems, and the probability of system activity, respectively, in the absence of all other covariates (except the intercept term). Solid line shows the mean of the submodel responses calculated from the sampled covariate coefficients, whereas the dashed line represents the median of these responses. Grey area depicts 95 % credible interval of the calculated responses
Of our potential forage functional groups, only forbs were included in our best model. Increased cover of forbs was associated with more burrow entrances, more burrow systems and greater system activity (Table 1; Supplementary material S3). The effects of forb cover on total number of burrows, number of system and the probability of system activity were greater for forb in the 30–50-cm than the 0–30-cm height stratum (Table 1; Fig. 3; Supplementary material S3). However, variation in forb cover at the 30–50-cm height stratum was relatively low, reaching 1.7 % cover at its maximum, compared to 10.6 % in the 0–30-cm height stratum. The 95 % credible intervals for forb cover effects on burrowing metrics did not encompass a coefficient value of zero, except for the effect of forb in the 30–50-cm strata on proportion of burrows active, which, despite a potentially large effect size, had high associated uncertainty.
Response curve showing contributions of forb cover to arctic ground squirrel burrowing metrics and activity from the best model explaining these responses selected using Bayes factors. Effects of forb cover in two vertical strata are shown. The effect of forb cover from 0- to 30-cm height on a number of burrows, b number of systems and c probability of arctic ground squirrel activity as measured by the multiplicative effect on respective burrowing metrics estimated in a hierarchical Bayesian framework; d–f shows equivalent effects of forb cover in the 30–50-cm height stratum. The y-axis is therefore the expected number of burrows, number of systems, and the probability of system activity, respectively, in the absence of all other covariates (except the intercept term). Solid line shows the mean of the submodel responses calculated from the sampled covariate coefficients, whilst the dashed line represents the median of these responses. Grey area depicts 95 % credible interval of the calculated responses
Effects of vertical structure of non-forage vegetation were also observed; non-forage vegetation showed height-dependent effects on all burrowing metrics (Fig. 4). There were consistent strong negative effects of increasing non-forage vegetation cover at the 50-cm and higher stratum on all burrowing metrics (Table 1; Fig. 3; Supplementary material S3). Effects of non-forage cover in the 0–30- and 30–50-cm height strata were strongest on the number of burrow systems, with positive effects of cover on the number of systems in the 0–30-cm strata and negative effect of cover on the number of systems in the 30–50-cm strata, and 95 % credible interval of the estimates not spanning zero. Effects of non-forage cover in the 0–30- and 30-50-cm strata had only weak effects on the total number of burrows and probability of systems being active with credible intervals of all estimates spanning zero. The greatest negative effect of cover in the 50-cm and higher strata was on activity of systems (Table 1; Fig. 3; Supplementary material S3). In general, the variable with greatest effect on burrowing metrics was non-forage cover in the 50-cm and higher stratum followed by effects of forb cover.
Response curve showing contributions of non-forage cover to arctic ground squirrel burrowing metrics and activity from the best model explaining these responses selected using Bayes factors. Effects of forb cover in two vertical strata are shown. The effect of forb cover from 0- to 30-cm height on a number of burrows, b number of systems and c probability of arctic ground squirrel activity as measured by the multiplicative effect on respective burrowing metrics estimated in a hierarchical Bayesian framework; d–f shows equivalent effects of non-forage cover in the 30–50-cm height stratum and g–i in the height stratum containing vegetation over 50 cm. The y-axis is therefore the expected number of burrows, number of systems, and the probability of system activity, respectively, in the absence of all other covariates (except the intercept term). Solid line shows the mean of the submodel responses calculated from the sampled covariate coefficients, whilst the dashed line represents the median of these responses. Grey area depicts 95 % credible interval of the calculated responses
Shrub was the dominant plant functional group contributing to non-forage vegetation cover in all height strata (Fig. 5). In the 0–30-cm stratum, dead shrub and graminoids were also considerable components of cover. In the 30–50-cm height stratum, the dominance of living shrub cover was greatest and dead shrub and graminoid also provided a little cover. In the 50-cm and higher stratum, cover additional to that created by living shrub was from tree and dead shrub. Given alternative models allowing both positive and negative effects of potential forage species and shrub were included in the modelling, results indicated that variation in total non-forage cover across all functional groups has a stronger association with burrowing metrics than any effects of shrubs alone.
Discussion
Arctic vegetation is changing under climate change, with increasing productivity and expansions of woody plants (Sturm et al. 2001; Myers-Smith et al. 2011; Henry et al. 2012). This study shows that these vegetation changes are likely to have impacts on an important prey species and bioturbator, the arctic ground squirrel. Consistent positive effects of forb cover on the number of arctic ground squirrel burrows and systems, and the activity of those systems, suggest that more favourable habitat is associated with high forb cover. Similarly, there were consistent negative effects of cover over 50 cm in height of all non-forage functional groups. This suggests that tall vertical structures result in less favourable habitat, most likely as a result of reduced visibility and possibly changes in associated predator communities. Changes in characteristics of burrow networks with increased cover of low-stature (below 50 cm tall) vegetation suggest that high cover in lower vertical strata may affect the type of burrow systems with respect to their likelihood of long-term and consistent occupancy. Steeper slopes had higher numbers of burrows and systems. Steep slopes may aid drainage, reducing waterlogging and saturation of soils, which may increase burrowing activity. Another possibility is that steeper slopes are associated with better visibility and thereby create higher quality habitat (Barker and Derocher 2010).
Our results indicate that forbs are likely to be important forage for arctic ground squirrels. This is supported by prior evidence from analysis of stomach contents, seasonal diet and cheek pouch contents (Batzli and Sobaski 1980; McLean 1985; Gillis et al. 2005b). There was no evidence that other functional groups were routinely important to the same extent, although single species within other functional groups may be individually influential. A dietary preference for forbs has been found in a number of sciuirids, including arctic ground squirrels, and may be related to greater digestibility and higher nutrient concentrations relative to other available forage functional groups (Batzli and Sobaski 1980; Carey 1985; Garin et al. 2008). In addition, forbs and particularly their flowers and seeds are rich in fatty acids, a core source of energy during hibernation (Hill and Florant 1999; Lyman 2013). Recent evidence also suggests the Pleistocene steppe-tundra landscape, under which most of the evolutionary history of arctic ground squirrels occurred, was more forb dominated than previously recognised (Willerslev et al. 2014). We found no evidence that the benefit of taller forbs was reduced due to their dual role as forage and visual obstruction. This may be partly due to a relatively low level of cover of taller forbs.
Effects of arctic ground squirrels on vegetation through burrowing and foraging could also contribute to associations with biotic variables; however, at the scale in question, most evidence suggests the positive association with forbs is more likely to reflect habitat selection than effects of arctic ground squirrels on vegetation. Small mammal central-place foragers can have substantial effects on vegetation community structure (Huntly 1987; Gálvez-Bravo et al. 2011; Davidson, Detling and Brown 2012). Arctic ground squirrels alter habitats via extensive burrowing behaviour and potentially through localised fertilisation of soils from faeces and selective foraging (Price 1971; McKendrick et al. 1980). Associations with vegetation might represent the effects of these species via mechanisms of disturbance (through burrowing behaviour), fertilization or selective or more generalist foraging. For example, low-stature forbs or species with adaptations to regrow after foraging may gain a competitive advantage under high herbivory (Evju et al. 2010), while dicot herbs may gain an advantage under grazing in low-productivity habitats (Hawkes and Sullivan 2001). However, most arctic studies to date focus on large grazing herbivores (e.g. Post and Pedersen 2008). Unlike large browsing herbivores, burrowing rodents may have access to below-ground plant material and are also observed to pull up roots and tubers in addition to clipping above-ground biomass. Despite elevated local nitrogen and phosphorus in the immediate vicinity of arctic ground squirrel burrow systems, no local increase in forbs has been reported, rather an increase in graminoids was observed (McKendrick et al. 1980). In addition, a decreased abundance of leguminous forbs has been observed directly surrounding burrow systems, possibly reflecting the effects of very localised foraging and depletions of preferred forage (McKendrick et al. 1980). Given this information, it seems likely that the scale of vegetation measurements within this study reflects the broader habitat selection of this species, rather than more localised effects on vegetation.
The implications of the strong association between burrowing, activity and forb cover for future distributions of arctic ground squirrels will depend on future trends in forb abundance and distribution. Likely future trends in forb cover are less clear than for shrubs and graminoids (Elmendorf et al. 2012). Trends in forb cover at the level of the functional group have shown no major change with experimental warming either in wet or dry sites (Wahren et al. 2005) and no consistent temporal trends in a large-scale analysis of tundra responses to warming (Elmendorf et al. 2012). This study suggests the contingencies which determine success of forbs under changing ecological structure of communities are likely to strongly influence arctic ground squirrel success. Such contingencies include the presence of large grazing herbivores (Post and Pedersen 2008), nitrogen and phosphorus fertilisation, and the initial community composition and soil conditions (Gough et al. 2012). Local heterogeneity in these characteristics may substantially influence source–sink dynamics and regional distribution of this species.
Our results indicate a negative effect of high-stature vegetation on arctic ground squirrel burrowing and activity. Negative effects were stronger when all non-forage species were combined rather than occurring for any single functional group, suggesting negative effects are stature related. High-stature vegetation has a negative effect on a number of ground-dwelling sciuirid species (Blumstein et al. 2006; Ordenana et al. 2012). One effect of increased stature is visual obstruction, which may create poorer arctic ground squirrel habitat (Karels and Boonstra 1999; Wheeler 2012). Increased vegetation stature can cause a change in predator assemblage and thereby predator foraging strategy (Gillis et al. 2005a), or in direct predation pressure (van Vuren 2001; Aaltonen et al. 2009; Lehrer et al. 2011). Indirect effects of predator–prey interactions via changes in predator sensitivity may also affect these species (Creel and Christianson 2008). In arctic ground squirrels, increased predator-sensitive behaviour has been observed to be associated with spatial transitions from open tundra to shrub habitat (Wheeler and Hik 2014). Shrub and tree line advance may reduce both the suitability of habitats for arctic ground squirrels and the ecosystem role of both their burrowing and activity; however, increases in vegetation stature, associated with increased productivity, that do not exceed approximately 50 cm in height may exert less influence.
In addition to documenting decreased burrowing in tall vegetation cover, this study indicates a more subtle change in the nature of burrowing behaviour associated with non-forage cover at lower heights. From heights of 0–30 cm, increases in the number of burrow systems are observed without concomitant increases in the total number of burrows in the area, such that burrow systems comprised fewer burrows. Such systems, defined as ‘duck holes’, may represent more transient colonies in marginal habitat or burrowing activity associated with territorial disputes or interactions with predators (Carl 1971). The potential for source-sink dynamics has been suggested for arctic ground squirrels (Gillis et al. 2005a; Donker and Krebs 2012). Burrowing activity with such characteristics may be indicative of sink-like populations or transient habitat associations. Conversely, a decrease in the number of systems without a decline in the number of burrows was observed associated with increasing non-forage cover in the 30–50-cm strata. This would suggest a transition to more permanent burrow systems with greater intermediate-height cover. For this reason, increases in vegetation cover at heights under 50 cm may also affect population processes in arctic ground squirrels.
Our study uses a space-for-time substitution to assess the likely effects of vegetation change on arctic ground squirrels. Here, we consider some of the uncertainties associated with this approach. Perhaps the most important of these is how changing climate will affect the large-scale distribution of shrub across the landscape. When predicting the impacts of vegetation at larger scales, regional processes may be important. In this study, the effect of vegetation cover on burrowing and activity was estimated in a landscape with high heterogeneity with respect to vegetation stature, with more open alpine tundra habitats generally at higher elevations. However, if encroachment of woody species is substantial, open areas could become fewer and more isolated. Indications of source-sink dynamics have been identified in arctic ground squirrels (Donker and Krebs 2012) and allow this species to occur in marginal habitat. Habitats of marginal quality may become less frequently occupied if high-quality (presumably forb-rich and lacking in dense high-stature vegetation) habitat becomes rarer. If this is the case, stronger relationships between habitat quality-related variables and occurrence and activity should be observed where local heterogeneity in these quality-related variables in reduced and may cause an underestimation of effects of future change.
Other concerns relating to space-for-time substitutions include the concern that spatial and temporal transitions may not produce comparable habitats if, for example the processes driving temporal transitions are not equivalent to those driving spatial variation. For example, warming might create non-analogue vegetation communities, which differ from those observed across space today. One benefit of working in an area undergoing substantial current vegetation change, with a altitudinal increase in the tree line averaging approximately 3 m year−1 from 1953 to 2005 (Stueve et al. 2010), is that current vegetation observed may actually reflect recent temporal vegetation transitions. As much of our interest relates to temperature-related variation in vegetation, similarities might be expected in the temperature-driven vegetation transitions observed with elevation in the study area and those driven by altitudinal variation in temperature, and therefore montane landscapes such as these are a good candidate for space-for-time substitutions. Given the relative proximity of sites, we also minimise confounding factors such as variation in the species pool of predators. However, shrub expansion is not driven by solely temperature. In the arctic, the three core factors affecting shrub expansion are temperature, disturbance and herbivory (Myers-Smith et al. 2011) and these may change if warming induces geomorphic processes, e.g. through melting of permafrost and thermokarst formation or alters the browsing herbivore community, e.g. through changing forage. Despite this, our study broadly implies that where processes (e.g. herbivory) suppress tall vegetation there is a greater likelihood of high-quality arctic ground squirrel habitat, and where processes promote taller vegetation, habitat quality might decline. In addition, processes that favour high cover of forbs should promote more extensive arctic ground squirrel communities.
The processes that regulate herbivores across productivity gradients have received considerable attention both in arctic ecosystems (e.g. Oksanen 1983; Aunapuu et al. 2008; Gauthier et al. 2011) and for the specific case of arctic ground squirrels (Batzli et al. 1980; Byrom et al. 2000; Karels and Boonstra 2000; Karels et al. 2000). We found a positive association of burrowing metrics with forb cover and a negative association with shrub, suggesting that the joint effect of predation risk and food may extend beyond the boreal forest, where they have been previously described (Karels and Boonstra 2000), into shrub and potentially alpine habitats. Two potential mechanisms which could create predation costs for arctic ground squirrels under increased vegetation productivity are: increased predator abundance, and increased perceived or realised predation risk under changes in visibility. Increased predation pressure could occur under increases in productivity. It has been suggested that arctic food webs along productivity gradients follow the predictions of the ecosystem exploitation hypothesis (e.g. Aunapuu et al. 2008). This would result in more productive habitats under warming also supporting a larger predator community. This should increase both direct and indirect effects of predators on prey and reduce habitat suitability for arctic ground squirrel. Changes in visibility could reduce the ability of squirrels to detect predators and subsequent direct mortality related to increased risk or indirect fitness effects associated with an increased need for investment in predator-sensitive behaviour. Monitoring predator and prey populations across spatial gradients from shrub to tundra and over time as these transitions occur will be key to separating these processes and ecosystem shifts under ongoing future warming.
Vegetation in arctic habitats is transitioning (Myers-Smith et al. 2011; Normand et al. 2013; Pearson et al. 2013) and associated with these changes, alterations to fauna are expected (Sokolov et al. 2012). Changes in productivity associated with warming may enrich food supplies for herbivores with more forage; however, changes in vegetation stature may also alter a suite of biotic interactions. Here, height-dependent relationships between cover of non-forage and arctic ground squirrel burrowing and activity suggest that the nature of vegetation transitions with respect to vegetation stature may be core to the landscape response of predator-sensitive herbivores. In particular, encroachment of taller canopy-forming vegetation such as shrub and trees may greatly alter the landscape dynamics of arctic species adapted to open landscapes. However, projections of these effects must be balanced against changes in forage associated with changes in community composition under warming, likely to be contingent upon local and landscape-scale conditions.
Author contribution statement
HCW designed the study and conducted the fieldwork. CR designed the monitoring and contributed vegetation data. JDC and HW analysed the data. HW, JDC and JCS wrote the manuscript. CR provided editorial advice.
References
Aaltonen K, Bryant AA, Hostetler JA, Oli MK (2009) Reintroducing endangered Vancouver Island marmots: survival and cause-specific mortality rates of captive-born versus wild-born individuals. Biol Conserv 142:2181–2190. doi:10.1016/j.biocon.2009.04.019
Aunapuu M, Dahlgren J, Oksanen T, Grellmann D, Oksanen L, Olofsson J, Rammul U, Schneider M, Johansen B, Hygen HO (2008) Spatial patterns and dynamic responses of arctic food webs corroborate the exploitation ecosystems hypothesis (EEH). Am Nat 171:249–262
Barker OE, Derocher AE (2010) Habitat selection by arctic ground squirrels (Spermophilus parryii). J Mamm 91:1251–1260. doi:10.1644/10-mamm-a-030.1
Batzli GO, Sobaski ST (1980) Distribution, abundance, and foraging patterns of ground squirrels near Atkasook. Alaska Arct Antarct Alp Res 12:501–510. doi:10.2307/1550497
Batzli GO, White RG, MacLean SF, Pitelka FA, Collier BD (1980) The herbivore-based trophic system. In: Brown J, Miller PC, Tieszen LL, Bunnell FL (eds). An Arctic ecosystem: the coastal tundra at barrow, Alaska. Dowden, Hutchinson & Ross, Stroudburg
Bennett RP (1999) Effects of food quality on growth and survival of juvenile Columbian ground squirrels (Spermophilus columbianus). Can J Zool 77:1555–1561. doi:10.1139/z99-144
Blinnikov MS, Gaglioti BV, Walker DA, Wooller MJ, Zazula GD (2011) Pleistocene graminoid-dominated ecosystems in the Arctic. Quat Sci Rev 30:2906–2929. doi:10.1016/j.quascirev.2011.07.002
Blumstein DT, Ozgul A, Yovovich V, van Vuren DH, Armitage KB (2006) Effect of predation risk on the presence and persistence of yellow-bellied marmot (Marmota flaviventris) colonies. J Zool 270:132–138. doi:10.1111/j.1469-7998.2006.00098.x
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. J Comput Gr Stat 7:434–455. doi:10.1080/10618600.1998.10474787
Buck CL, Barnes BM (1999a) Annual cycle of body composition and hibernation in free-living arctic ground squirrels. J Mamm 80:430–442. doi:10.2307/1383291
Buck CL, Barnes BM (1999b) Temperatures of hibernacula and changes in composition of arcitic ground squirrels over winter. J Mamm 80:1264–1276
Byrom AE, Krebs CJ (1999) Natal dispersal of juvenile arctic ground squirrels in the boreal forest. Can J Zool 77:1048–1059
Byrom AE, Karels TJ, Krebs CJ, Boonstra R (2000) Experimental manipulation of predation and food supply of arctic ground squirrels in the boreal forest. Can J Zool 78:1309–1319. doi:10.1139/cjz-78-8-1309
Carey HV (1985) Nutritional ecology of yellow-bellied marmots in the white mountains of California. Ecography 8:259–264
Carl EA (1971) Population control in arctic ground squirrels. Ecology 52:395–413. doi:10.2307/1937623
Creel S, Christianson D (2008) Relationships between direct predation and risk effects. Trends Ecol Evol 23:194–201. doi:10.1016/j.tree.2007.12.004
Davidson AD, Detling JK, Brown JH (2012) Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands. Front Ecol Environ 10:477–486. doi:10.1890/110054
Densmore RV (2008) Evidence for higher soil temperature and potassium promoting invasion of the Common Dandelion, Taraxacum officinale, in Denali National Park and Preserve Alaska. Can Field Nat 112:67–69
Dingman SL, Koutz FR (1974) Relations among vegetation, permafrost, and potential insolation in Central Alaska. Arct Antarct Alp Res 6:37–47
Donker SA, Krebs CJ (2011) Habitat-specific distribution and abundance of arctic ground squirrels (Urocitellus parryii plesius) in southwest Yukon. Can J Zool 89:570–576. doi:10.1139/z11-041
Donker SA, Krebs CJ (2012) Evidence for source-sink dynamics in a regional population of arctic ground squirrels (Urocitellus parryii plesius). Wildl Res 39:163–170. doi:10.1071/wr11167
Elmendorf SC, Henry GHR, Hollister RD, Bjork RG, Bjorkman AD, Callaghan TV, Collier LS, Cooper EJ, Cornelissen JHC, Day TA, Fosaa AM, Gould WA, Gretarsdottir J, Harte J, Hermanutz L, Hik DS, Hofgaard A, Jarrad F, Jonsdottir IS, Keuper F, Klanderud K, Klein JA, Koh S, Kudo G, Lang SI, Loewen V, May JL, Mercado J, Michelsen A, Molau U, Myers-Smith IH, Oberbauer SF, Pieper S, Post E, Rixen C, Robinson CH, Schmidt NM, Shaver GR, Stenstrom A, Tolvanen A, Totland O, Troxler T, Wahren CH, Webber PJ, Welker JM, Wookey PA (2012) Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol Lett 15:164–175. doi:10.1111/j.1461-0248.2011.01716.x
Epstein HE, Beringer J, Gould WA, Lloyd AH, Thompson CD, Chapin FS, Michaelson GJ, Ping CL, Rupp TS, Walker DA (2004) The nature of spatial transitions in the Arctic. J Biogeogr 31:1917–1933. doi:10.1111/j.1365-2699.2004.01140.x
Evju M, Halvorsen R, Rydgren K, Austrheim G, Mysterud A (2010) Interactions between local climate and grazing determine the population dynamics of the small herb Viola biflora. Oecologia 163:921–933. doi:10.1007/s00442-010-1637-x
Gálvez-Bravo L, López-Pintor A, Rebollo S, Gómez-Sal A (2011) European rabbit (Oryctolagus cuniculus) engineering effects promote plant heterogeneity in Mediterranean dehesa pastures. J Arid Environ 75:779–786. doi:10.1016/j.jaridenv.2011.03.015
Garin I, Aldezabal A, Herrero J, Garcia-Serrano A, Remón (2008) Diet selection of the Alpine marmot (Marmota m. marmota L.) in the Pyrenees. Rev Ecol 63:383–390
Gauthier G, Berteaus D, Bȇty J, Arnaud T, Therrien J, McKinnon L, Legagneux P, Cadieux M (2011) The tundra food web of Bylot Island in a changing climate and the role of exchanges between ecolsystems. Ecoscience 18:223–235
Gauthier G, Bêty J, Cadieux M-C, Legagneux P, Doiron M, Chevallier C, Lai S, Tarroux A, Berteaux D (2013) Long-term monitoring at multiple trophic levels suggests heterogeneity in responses to climate change in the Canadian Arctic tundra. Philos Trans R Soc B Biol Sci 368:20120482. doi:10.1098/rstb.2012.0482
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472. doi:10.1214/ss/1177011136
Gillis EA, Hik DS, Boonstra R, Karels TJ, Krebs CJ (2005a) Being high is better: effects of elevation and habitat on arctic ground squirrel demography. Oikos 108:231–240. doi:10.1111/j.0030-1299.2005.13535.x
Gillis EA, Morrison SF, Zazula GD, Hik DS (2005b) Evidence for selective caching by arctic ground squirrels living in alpine meadows in the Yukon. Arctic 58:354–360. doi:10.14430/arctic449
Gough L, Moore JC, Shaver GR, Simpson RT, Johnson DR (2012) Above- and belowground responses of arctic tundra ecosystems to altered soil nutrients and mammalian herbivory. Ecology 93:1683–1694. doi:10.1890/11-1631.1
Hannon MJ, Jenkins SH, Crabtree RL, Swanson AK (2006) Visibility and vigilance: behavior and population ecology of Uinta ground squirrels (Spermophilus armatus) in different habitats. J Mamm 87:287–295. doi:10.1644/05-MAMM-A-081R2.1
Hansen BB, Aanes R, Herfindal I, Kohler J, Sæther B-E (2011) Climate, icing, and wild arctic reindeer: past relationships and future prospects. Ecology 92:1917–1923. doi:10.1890/11-0095.1
Hawkes CV, Sullivan JJ (2001) The impact of herbivory on plants in different resource conditions: a meta-analysis. Ecology 82:2045–2058. doi:10.1890/0012-9658(2001)082[2045:tiohop]2.0.co;2
Henry GR, Harper K, Chen W, Deslippe J, Grant R, Lafleur P, Lévesque E, Siciliano S, Simard S (2012) Effects of observed and experimental climate change on terrestrial ecosystems in northern Canada: results from the Canadian IPY program. Clim Change 115:207–234. doi:10.1007/s10584-012-0587-1
Hill VL, Florant GL (1999) Patterns of fatty acid composition in free-ranging yellow-bellied marmots (Marmota flaviventris) and their diet. Can J Zool 77:1494–1503
Hoset KS, Kyrö K, Oksanen T, Oksanen L, Olofsson J (2014) Spatial variation in vegetation damage relative to primary productivity, small rodent abundance and predation. Ecography 37:894–901. doi:10.1111/ecog.00791
Huntly NJ (1987) Influence of refuging consumers (Pikas: Ochotona princeps) on subalpine meadow vegetation. Ecology 68:274–283. doi:10.2307/1939258
Ibrahim JG, Laud PW (1991) On Bayesian analysis of generalized linear models using Jeffreys’s prior. J Am Stat Assoc 86:981–986. doi:10.2307/2290514
Jia GJ, Epstein HE, Walker DA (2003) Greening of arctic Alaska, 1981–2001. Geophys Res Lett 30:2067. doi:10.1029/2003gl018268
Karels TJ, Boonstra R (1999) The impact of predation on burrow use by Arctic ground squirrels in the boreal forest. Proc R Soc B Biol Sci 266:2117–2123. doi:10.1098/rspb.1999.0896
Karels TJ, Boonstra R (2000) Concurrent density dependence and independence in populations of arctic ground squirrels. Nature 408:460–463. doi:10.1038/35044064
Karels TJ, Byrom AE, Boonstra R, Krebs CJ (2000) The interactive effects of food and predators on reproduction and overwinter survival of arctic ground squirrels. J Anim Ecol 69:235–247
Kauffman MJ, Varley N, Smith DW, Stahler DR, MacNulty DR, Boyce MS (2007) Landscape heterogeneity shapes predation in a newly restored predator–prey system. Ecol Lett 10:690–700. doi:10.1111/j.1461-0248.2007.01059.x
Lehrer EW, Schooley RL, Whittington JK (2011) Survival and antipredator behavior of woodchucks (Marmota monax) along an urban–agricultural gradient. Can J Zool 90:12–21. doi:10.1139/z11-107
Lyman C (2013) Hibernation and torpor in mammals and birds. Elsevier (eBook)
McKendrick JD, Batzli GO, Everett KR, Swanson JC (1980) Some effects of mammalian herbivores and fertilization on tundra soils and vegetation. Arct Antact Alp Res 12:565–578. doi:10.2307/1550501
McLean IG (1985) Seasonal patterns and sexual differences in the feeding ecology of arctic ground squirrels (Spermophilus parryii plesius). Can J Zool 63:1298–1301. doi:10.1139/z85-195
Morrison P, Galster W (1975) Patterns of hibernation in the arctic ground squirrel. Can J Zool 53:1345–1355. doi:10.1139/z75-160
Myers-Smith IH, Forbes BC, Wilmking M, Hallinger M, Lantz T, Blok D, Tape KD, Macias-Fauria M, Sass-Klaassen U, Levesque E, Boudreau S, Ropars P, Hermanutz L, Trant A, Collier LS, Weijers S, Rozema J, Rayback SA, Schmidt NM, Schaepman-Strub G, Wipf S, Rixen C, Menard CB, Venn S, Goetz S, Andreu-Hayles L, Elmendorf S, Ravolainen V, Welker J, Grogan P, Epstein HE, Hik DS (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett 6:045509. doi:10.1088/1748-9326/6/4/045509
Normand S, Randin C, Ohlemüller R, Bay C, Høye TT, Kjær ED, Körner C, Lischke H, Maiorano L, Paulsen J, Pearman PB, Psomas A, Treier UA, Zimmermann NE, Svenning J-C (2013) A greener greenland? Climatic potential and long-term constraints on future expansions of trees and shrubs. Philos Trans R Soc B Biol Sci 368:20120479. doi:10.1098/rstb.2012.0479
Oksanen L (1983) Trophic exploitation and arctic phytomass patterns. Am Nat 122:45–52
Olofsson J, Oksanen L, Callaghan T, Hulme PE, Oksanen T, Suominen O (2009) Herbivores inhibit climate-driven shrub expansion on the tundra. Glob Change Biol 15:2681–2693. doi:10.1111/j.1365-2486.2009.01935.x
Ordenana MA, van Vuren DH, Draper JP (2012) Habitat associations of California ground squirrels and Botta’s pocket gophers on levees in California. J Wildl Manage 76:1712–1717. doi:10.1002/jwmg.402
Ovens C (2011) Kinship and use of underground space by adult female Richardson’s ground squirrels (Urocitellus richarsonii). Master’s thesis, University of Lethbridge, Canada
Pearson RG, Phillips SJ, Loranty MM, Beck PSA, Damoulas T, Knight SJ, Goetz SJ (2013) Shifts in Arctic vegetation and associated feedbacks under climate change. Nat Clim Change 3:673–677. doi:10.1038/nclimate1858
Plummer M (2003) JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. In: Hornik K, Leisch F, Zeileis A (eds) Proceedings of the 3rd International Workshop on Distributed Statistical Computing, Vienna
Post E, Forchhammer MC (2008) Climate change reduces reproductive success of an arctic herbivore through trophic mismatch. Philos Trans R Soc B Biol Sci 363:2367–2373. doi:10.1098/rstb.2007.2207
Post E, Pedersen C (2008) Opposing plant community responses to warming with and without herbivores. Proc Natl Acad Sci 105:12353–12358. doi:10.1073/pnas.0802421105
Price LW (1971) Geomorphic effect of the arctic ground squirrel in an alpine environment. Geogr Ann Ser A Phys Geogr 53:100–106. doi:10.2307/520669
Ravolainen VT, Bråthen KA, Yoccoz NG, Nguyen JK, Ims RA (2014) Complementary impacts of small rodents and semi-domesticated ungulates limit tall shrub expansion in the tundra. J Appl Ecol 51:234–241. doi:10.1111/1365-2664.12180
Roland CA, Oakley K, Debevec EM, Loomis T (2004) Monitoring vegetation structure and composition at multiple scales in the Central Alaska Network. NPS technical report CAKN-001. National Park Service, Fairbanks. http://science.nature.nps.gov/im-monitor/protocols/CAKN_Vegetation.zip. Accessed 28 November 2011
Roland CA, Schmidt JH, Nicklen EF (2012) Landscape-scale patterns in tree occupancy and abundance in subarctic Alaska. Ecol Monogr 83:19–48. doi:10.1890/11-2136.1
Schooley RL, Sharpe PB, van Horne B (1996) Can shrub cover increase predation risk for a desert rodent? Canadian. J Zool 74:157–163. doi:10.1139/z96-020
Sharma S, Couturier S, Côté SD (2009) Impacts of climate change on the seasonal distribution of migratory caribou. Glob Change Biol 15:2549–2562. doi:10.1111/j.1365-2486.2009.01945.x
Sokolov V, Ehrich D, Yoccoz NG, Sokolov A, Lecomte N (2012) Bird communities of the arctic shrub tundra of Yamal: habitat specialists and generalists. PLoS ONE 7:e50335. doi:10.1371/journal.pone.0050335
Stueve KM, Isaacs RE, Tyrrell LE, Densmore RV (2010) Spatial variability of biotic and abiotic tree establishment constraints across a treeline ecotone in the Alaska range. Ecology 92:496–506. doi:10.1890/09-1725.1
Sturm M, Racine C, Tape K (2001) Climate change: increasing shrub abundance in the Arctic. Nature 411:546–547. doi:10.1038/35079180
van Vuren DH (2001) Predation on yellow-bellied marmots (Marmota flaviventris). Am Midl Nat 145:94–100. doi:10.1674/0003-0031(2001)145[0094:poybmm]2.0.co;2
Wahren CHA, Walker MD, Bret-Harte MS (2005) Vegetation responses in Alaskan arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Glob Change Biol 11:537–552. doi:10.1111/j.1365-2486.2005.00927.x
Wheeler HC (2012) Population, individual and behavioural approaches to understanding the implications of habitat change for arctic ground squirrels. University of Alberta, Alberta
Wheeler HC, Hik DS (2013) Arctic ground squirrels Urocitellus parryii as drivers and indicators of change in northern ecosystems. Mamm Rev 43:238–255. doi:10.1111/j.1365-2907.2012.00220.x
Wheeler HC, Hik DS (2014) Giving-up densities and foraging behaviour indicate potential effect of shrub encroachment on arctic ground squirrels. Anim Behav 95:1–8
Willerslev E, Davison J, Moora M, Zobel M, Coissac E, Edwards ME, Lorenzen ED, Vestergard M, Gussarova G, Haile J, Craine J, Gielly L, Boessenkool S, Epp LS, Pearman PB, Cheddadi R, Murray D, Brathen KA, Yoccoz N, Binney H, Cruaud C, Wincker P, Goslar T, Alsos IG, Bellemain E, Brysting AK, Elven R, Sonstebo JH, Murton J, Sher A, Rasmussen M, Ronn R, Mourier T, Cooper A, Austin J, Moller P, Froese D, Zazula G, Pompanon F, Rioux D, Niderkorn V, Tikhonov A, Savvinov G, Roberts RG, MacPhee RDE, Gilbert MTP, Kjaer KH, Orlando L, Brochmann C, Taberlet P (2014) Fifty thousand years of Arctic vegetation and megafaunal diet. Nature 506:47–51. doi:10.1038/nature12921
Zamin TJ, Grogan P (2013) Caribou exclusion during a population low increases deciduous and evergreen shrub species biomass and nitrogen pools in low Arctic tundra. J Ecol 101:671–683. doi:10.1111/1365-2745.12082
Acknowledgments
This article is a contribution by the Center for Informatics Research on Complexity in Ecology (CIRCE), funded by the Aarhus University Research Foundation under the AU Ideas program (H. W., J. C. S.), a Murie Science and Learning Research Fellowship from Alaska Geographic (H. W.) and by the Central Alaska Network of the National Park Service (C. R.). J. D. C. was supported by a grant from the Rhineland-Palatinate Ministry of Education, Science, Youth and Culture (The consequences of global change on biological resources, legislation and setting of standards), and by the NoAClim project grant (number 225005) of the Norge Forskningsråd. We thank T. Karels and two anonymous reviewers for helpful comments on the manuscript. We thank L. C. Dempsey, C. Gibson, R. Noel, A. Carlyle and M. Richard for assistance with fieldwork. We thank C. McIntyre and L. Tyrrell for helpful discussions and logistical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Janne Sundell.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wheeler, H.C., Chipperfield, J.D., Roland, C. et al. How will the greening of the Arctic affect an important prey species and disturbance agent? Vegetation effects on arctic ground squirrels. Oecologia 178, 915–929 (2015). https://doi.org/10.1007/s00442-015-3240-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3240-7