Abstract
Predicting demographic consequences of climate change for plant communities requires understanding which factors influence seed set, and how climate change may alter those factors. To determine the effects of pollen availability, temperature, and pollinators on seed production in the alpine, we combined pollen-manipulation experiments with measurements of variation in temperature, and abundance and diversity of potential pollinators along a 400-m elevation gradient. We did this for seven dominant species of flowering plants in the Coast Range Mountains, British Columbia, Canada. The number of viable seeds set by plants was influenced by pollen limitation (quantity of pollen received), mate limitation (quality of pollen), temperature, abundance of potential pollinators, seed predation, and combinations of these factors. Early flowering species (n = 3) had higher seed set at high elevation and late-flowering species (n = 4) had higher seed set at low elevation. Degree-days >15 °C were good predictors of seed set, particularly in bee-pollinated species, but had inconsistent effects among species. Seed production in one species, Arnica latifolia, was negatively affected by seed-predators (Tephritidae) at mid elevation, where there were fewer frost-hours during the flowering season. Anemone occidentalis, a fly-pollinated, self-compatible species had high seed set at all elevations, likely due to abundant potential pollinators. Simultaneously measuring multiple factors affecting reproductive success of flowering plants helped identify which factors were most important, providing focus for future studies. Our work suggests that responses of plant communities to climate change may be mediated by flowering time, pollination syndrome, and susceptibility to seed predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
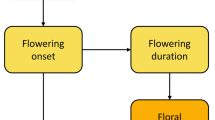
Predicting demographic consequences of climate change for flowering plants is one of the most difficult and important questions faced by pollination ecologists (Hegland et al. 2009; Forrest 2011; Willmer 2012; Straka and Starzomski 2014). Accurately predicting seed set under varying conditions involves simultaneously studying multiple processes affecting plant physiology, pollination, and pollen limitation. Many factors influence seed set (e.g. Lee and Bazzaz 1982; Ehrlén 1992; Totland 1997), and the ways in which climate change might affect them can be complex (Fig. 1). Meta-analyses have shown that sexual reproduction of most flowering plants is pollen limited (Larson and Barrett 2000; Knight et al. 2005), which runs contrary to the prediction that the number of ovules plants produce should be optimized to match availability of pollination and abiotic resources (Haig and Westoby 1988).
Despite a predominance of pollen limitation, plants are not always pollen limited because factors driving pollen limitation vary in space and time (Ehrlén 1992; Totland 2001; García-Camacho and Totland 2009) and are confounded with other limitations on reproduction (Knight et al. 2005). Self-pollinating species, for example, tend not to be pollen limited (Knight et al. 2005), but often benefit from outcrossing (Darwin 1862). The number and quality of seeds that plants produce may be limited by lack of resources (e.g. water, nutrients, light), unpredictability of resource availability (Lee and Bazzaz 1982; Ehrlén 1992; Totland 1997), failure to attract pollinators (Burd 1994), and spatial or temporal variation in pollinator preferences (e.g. Forrest and Thomson 2009), abundance (Totland 2001), or effectiveness (Rafferty and Ives 2012). After fertilization occurs, seeds can still be lost to seed predators (e.g. Lee and Bazzaz 1982), or fail to mature during short seasons (Thórhallsdóttir 1998; Cooper et al. 2011).
Climate can drive seed set in the alpine in a number of ways (Fig. 1). Snow cover and atmospheric or soil degree-days (DD) affect seed set by influencing pollinator activity and abundance (Schemske 1977; McCall and Primack 1992; Totland 1994) or by inducing plant–pollinator mismatch through altered phenology (e.g. Inouye et al. 2000, 2002; Kudo et al. 2004; Hegland et al. 2009). Temperature interacts with plant physiology to reduce reproductive output when temperatures are too low or seasons too short for seeds to mature (Galen and Stanton 1993; Burd 1994), with frost leading to aborted ovules before or after fertilization (Inouye et al. 2000; Kudo and Hirao 2006; Inouye 2008).
Pollen-manipulation experiments can show how pollen limitation interacts with climate to influence patterns of seed set (Thórhallsdóttir 1998; Fabbro and Körner 2004). Pollen manipulation has been used to study breeding systems and pollen limitation for decades (e.g. Kearns and Inouye 1993; Dafni et al. 2005), but most knowledge about factors driving pollen limitation comes from meta-analyses of data collected in various years and locations (Ashman et al. 2004; Knight et al. 2005), or from studies focusing on single species (e.g. Schemske 1977; Totland 1997; Kudo and Hirao 2006). Many studies make assumptions about spatial variation in abundance of potential pollinators without measuring it directly (e.g. Fabbro and Körner 2004; though see Brosi and Briggs 2013), or use temperature data from few weather stations to predict local phenology, which can poorly represent conditions within field sites (Forrest 2011). Few studies have simultaneously manipulated availability of pollen for multiple species making up a flowering plant community (e.g. Motten 1986) in an alpine environment, across a measured gradient in abundance of potential pollinators and temperature conditions. Combining experiments with measured gradients at a field site could provide insight into which abiotic and biotic variables affect seed set in an alpine plant community.
We performed community-level pollen limitation experiments in a series of alpine meadows (Marriott Basin) in the Coast Range Mountains of British Columbia, Canada. ‘Pollen limitation’ refers to reduction of seed set when ovules fail to receive sufficient quantities of pollen for fertilization. Pollen limitation can be estimated by comparing pollen-supplemented plants to ‘open’ (or control) plants experiencing natural levels of pollination: higher seed set of pollen-supplemented plants relative to controls suggests that plants are pollen limited (Kearns and Inouye 1993; Dafni et al. 2005).
The objectives of our experiments were to: (1) verify the breeding systems of the most abundant flowering plants at Marriott Basin, and (2) determine the major drivers of variation in reproductive output (seed set). If access to potential pollinators limits reproduction of plants, we predicted that pollen-supplemented plants would have the highest seed set, open plants with natural pollination would have lower seed set, and plants with pollinators excluded would have the lowest seed set. To determine the drivers of reproductive output in alpine plants, we evaluated evidence for six non-mutually exclusive hypotheses to explain reproductive output: pollen limitation (pollen quantity), abiotic limitation, biotic limitation, combined abiotic and biotic limitation, mate limitation (pollen quality) and limitation by seed predation. To test this set of hypotheses, we combined pollen manipulation with measurements of natural variation in abiotic and biotic factors that could limit seed set, across an elevation gradient.
Materials and methods
Location and study species
Our experiments and observations took place over the 2011 growing season (Online Resource 1) at Marriott Basin, in the Coast Range Mountains of British Columbia, Canada (50.429 N, −122.459 W). The site includes a series of alpine meadows ranging in elevation from 1,700 to 2,140 m a.s.l. We estimated relative abundance of species at our study site using a point-intercept method (from Barnett and Stohlgren 2003) in twenty-four, 1 × 1-m plots, distributed across our field site at Marriott Basin (K. Baldwin-Corriveau, unpublished data). The most abundant flowering plants (hereafter referred to by genus) were Erythronium grandiflorum (Liliaceae), Anemone occidentalis (Ranunculaceae), Claytonia lanceolata (Portulacaceae), Caltha leptosepala (Liliaceae), Valeriana sitchensis (Valerianaceae), Arnica latifolia (Asteraceae), Lupinus arcticus (Fabaceae), Sorbus sitchensis (Rosaceae), and Vaccinium membranaceum (Ericaceae). These species are perennials that vary markedly in flowering time, sexual compatibility, and floral display (Online Resource 2; Pojar 1974). All nine species were included in pollen-manipulation experiments, but Valeriana and Sorbus failed to produce seeds in 2011, preventing analysis. We report on the seven remaining species, though Anemone and Caltha were only available in sufficient numbers from high and low elevation.
All of the insects we refer to as ‘potential pollinators’ were observed visiting our study species of flowers at least once during the field season, and were visibly carrying pollen when observed at flowers, or caught in pan traps. We observed floral visitors as part of another (unpublished) study, in which we watched flowers for 10-min periods, with 80 h of observation in total. The most abundant floral visitors in 2011 were several species of Brachycera (including Muscidae and Anthomyiidae) that we could not reliably distinguish on the wing and lumped as one ‘black fly’ morphospecies, Bombus spp., a diverse assemblage of Syrphidae, and sawflies (Tenthredinidae).
Estimating pollen limitation
We established nine sites over a total area of 1.2 km2, stratified into three low-elevation (~1,706–1,772 m), three mid elevation (~1,841–1,870 m), and three high-elevation (~2,010–2,090 m) sites. At each site, we located and marked 36 flowers or inflorescences, each from separate plants, of each of the seven species before flowers or inflorescences opened (see Online Resource 1 for floral display descriptions). We randomly assigned one-third of the plants at each site to one of two treatments: pollen-supplemented (12 plants per site) and bagged (12 plants per site); or a control—open (12 plants per site). Plants designated as pollen supplemented or bagged were covered with 10 × 12-cm transparent organza cloth bags, which have little or no effect on floral development (Kearns and Inouye 1993). We returned every 2 days to watch for the onset of flowering. Extreme weather (hail, heavy rainfall), or grazing animals (marmots and grouse) destroyed approximately 10 % of marked plants. We replaced destroyed plants by assigning the nearest plant to the appropriate treatment. Replacement plants flowered at about the same time as the original plants because we replaced them with plants at the same stage, and each treatment had similar proportions of plants destroyed.
As flowers opened, we applied pollen-supplementation treatments, brushing stigmas using cotton swabs with pollen brushed directly from anthers of at least three separate ‘donor’ individuals (≥10 m from treated plants). We then allowed plants to set seed, re-visiting every 2–4 days to ensure bags remained in place and plants were intact. Once seeds were sufficiently developed to count (but before dispersal), we collected seed heads from all marked plants, and counted viable and non-viable seeds per seed head or fruit. We assessed viability based on size, shape, and colour. For plants with one flower (Anemone, Erythronium) or inflorescence (Arnica), we recorded seed set as the number of viable seeds per plant. For plants with more than one flower within a bag (Vaccinium, Lupinus, Claytonia, Caltha), we treated flowers or seed heads as sub-replicates, averaging the number of viable seeds for flowers within the same bag, and expressed this as the mean number of viable seeds per flower for a plant.
Measuring temperature
We recorded hourly air temperature using four Thermochron iButton temperature loggers (Maxim Integrated Products, USA) per site, taped 10 cm above ground on wooden stakes. Summarized measures included likelihood ratio tests (LRT), average temperatures, minimum and maximum temperatures, and total hours below freezing. We mounted time-lapse cameras to the wooden stakes to take photos every 3 h during daylight. The photos were used to assess weather and estimate the phenological stage of plants at our sites on each day.
Trapping potential pollinators
We trapped potential pollinators to determine whether relative abundance of potential pollinators and seed predators varied across the 400-m elevation gradient. We set pan traps >20 m from the study sites to minimize interference with our pollination experiment. We set a line of 45 traps perpendicular to the slope, spaced at 3-m intervals, at each of the three elevations. The 45 traps included 15 traps of each of three colours: yellow, blue, and white. The colours used were standard for the Canadian Pollination Initiative and United States Department of Agriculture, selected to broadly sample pollinator abundance and diversity (Droege et al. 2010). Traps were set before the slope received full sunlight (~8 a.m., before most activity of potential pollinators), and collected around dusk (~8 p.m.), well after the daily peak in activity. Colours were pooled per collection day for analysis. To include variation among sampling dates due to weather and emergence phenology of insects, we trapped on 3 days: 18, 23, and 27 August 2011. We used keys to identify each individual to family or genus (unpublished keys to bees by C. Sheffield and J. Gibbs; Syrphidae by S. Marshall; Triplehorn et al. 2005; Marshall 2006), and used distinct morphological characteristics to separate them into morphospecies (Online Resource 3).
Statistical analyses: pollen limitations and breeding system
Because of the large number of zero values (fruit with no viable seeds), data could not be transformed or analysed with statistics assuming a normal distribution. We therefore used Kruskal–Wallis and Mann–Whitney U-tests with Dunn’s multiple comparisons in GraphPad Prism version 5.01 (GraphPad Software, USA, 2007) to determine whether the number of viable seeds on bagged, pollen-supplemented, and open plants were significantly different among elevations. We calculated effect sizes as log response ratios of seed set to bagging [ln(X B)–ln(X O)] and pollen-supplementation [ln(X PS)–ln(X O)], following Hedges et al. (1999), pooling samples across the elevation gradient to focus on the effect of our treatments.
Statistical analyses: factors limiting seed set
We performed multiple regressions in R, version 2.15.0 (R Development Core Team 2012), using the zeroinfl() function in the pscl package (Zeileis et al. 2008). We assessed the significance of models by comparing models that included predictors of seed set to a null model (a model including only the intercept) to test whether adding predictors improved the model’s ability to describe our observed patterns in seed set. We assessed models using LRT implemented by the lrtest() function in the package lmtest (Hothorn et al. 2012).
Temperature
We tested for differences in temperature between high and low elevations (the two extremes, for which there was a complete temperature record) using repeated-measures ANOVA in GraphPad Prism, with each hourly temperature as a repeated measure. There was an interaction between temperature and elevation over time, so summarizing temperature differences among the three elevations was not as straightforward as calculating mean values. For multiple regressions we therefore used several predictors of seed set based on temperature (Online Resource 4). The predictors were average temperature, maximum temperature, minimum temperature, frost-hours (total hours below 0 °C), maximum number of continuous frost-hours, and DD. We converted temperatures to DD by counting each hour during the recording period for which the temperature exceeded a given threshold. The thresholds were DD >0 °C, DD >5 °C, DD >10 °C, DD >15 °C (DD15), and DD >20 °C.
DD thresholds for development are often higher for insects than plants, but vary among species (e.g. Hülber et al. 2010; Forrest and Thomson 2011). To estimate which temperature variables were the best predictors of seed set to include in regression models, we constructed a series of models that varied only in the temperature variable used as a predictor of seed set. We compared these models using log-likelihoods and Akaike’s information criteria (AIC). We used the temperature variable that was included in the best-supported model as a predictor of seed set in the final regression model.
Diversity and abundance of potential pollinators
To test whether there were differences in the number of potential pollinators per trap at each elevation, we used repeated-measures ANOVA with elevation as a factor having three levels and each of the three sampling days treated as a repeated measure. Treating date as a repeated measure made our results conservative by controlling for the effect of re-sampling the same locations. We characterized differences in diversity, abundance, and community composition of potential pollinators among the three elevations and three sampling dates using Bray–Curtis dissimilarity (Bray and Curtis 1957). To test for differences in the composition of communities of potential pollinators at low, mid, and high elevation we used analysis of similarity and similarity percentages (SIMPER) in PRIMER-E, version 6.1.13 (Primer-E, UK; Clarke 1993). SIMPER revealed that similarity among elevations was driven by the ‘black fly’ morphospecies (by far the most abundant flower visitor we observed), which included a mixture of Muscidae, and Anthomyiidae. Relative abundance of bees, Syrphidae, Tenthredinidae, and Lepidoptera across the elevation gradient showed the same pattern as relative abundance of black flies. We therefore used the total number of potential pollinators trapped as a predictor of seed set in multiple regression models. For a very conservative measurement of abundance of potential pollinators, we repeated the analyses using Syrphidae, Tenthredinidae, bees (Andrena, Bombus, and Lasiglossum), and adult Lepidoptera. Using the full set of potential pollinators including black flies did not change the results of the model selection, so we present the results using all potential pollinators (i.e. flower visitors). We used the log-likelihood-based procedure described above for temperature variables to assess the quality of predictors of seed set that were based on abundance of potential pollinators captured in traps, which included total number of potential pollinators collected at each site, and average number of potential pollinators collected per trap at each site.
Seed predators
After collecting seeds, we inspected seed pods or inflorescences for signs of seed predation, recording the number of seed predators per flower when they were present. Seed predation was rare for most species, in both bagged and unbagged flowers. In Erythronium and Caltha, seed predators removed the entire flower head, so these species were not analysed. In Lupinus, seed predation was relatively rare. In Arnica, 199 plants were bagged serendipitously after eggs of Tephritidae (fruit flies) were already present. Bagging could increase predation rates if predators of seed predators were excluded from the bags. However, we found no difference in rates of seed predation between bagged and unbagged flowers (t = 0.54, df = 181, p = 0.2900). Larvae developed on the inflorescences throughout the season and had pupated when we collected the seeds. When the seeds were collected, we placed the inflorescences in cloth bags, and adult flies emerged in the bags. We counted emerged adult flies per inflorescence and inspected inflorescences for pupal cases to confirm the number of flies per inflorescence. We tested the effect of seed predators on the number of viable seeds using Mann–Whitney U-tests, comparing bagged flowers with seed predators to bagged flowers without. We tested for differences in abundance of seed predators among the three elevations using Kruskal–Wallis tests with Dunn’s multiple comparisons. For Arnica, we used the number of seed predators as a predictor of seed set in the multiple regression with number of viable seeds per inflorescence as the response, and number of larvae per flower as a single predictor.
Predicting limitations to seed set
To evaluate temperature and abundance of potential pollinators as predictors of seed set, we used multiple regression with the number of viable seeds per flower as a response variable, and a combination of predictors based on temperature and abundance of potential pollinators. Having examined effects of pollen supplementation separately, we only used data from control plants for the multiple regressions. We ran models separately for each species because grouping species for analysis would cause variation in life history traits among the small number of species to inflate type II error, obscuring any important signals of factors influencing seed set.
Before performing regressions, we centred and scaled all variables. For each model, we chose one temperature variable as a predictor of seed set and one measurement of abundance of potential pollinators as a second predictor of seed set. We chose temperature variables based on developmental thresholds found to be important for alpine plants and bees at Rocky Mountain Biological Laboratory, Colorado (Forrest and Thomson 2011). For Arnica (the only species with abundant seed predators), we incorporated number of seed predators per flower as a third predictor. We performed a sensitivity analysis by creating a series of models using each of the predictors of seed set in turn, which we compared using log likelihoods and AIC.
To account for non-normality, overdispersion, and a large number of zeros in the response variable (seed set), we used zero-inflated count models based on a negative binomial distribution (Zeileis et al. 2008). We verified the validity of this approach using Vuong tests (Vuong 1989), comparing zero-inflated models to generalized linear models following negative binomial or Poisson distributions. We began by using ‘saturated’ models with both temperature and abundance of potential pollinators as predictors of seed set, then simplified models using a backward-forward model selection implemented by stepAIC in R. We verified results of the model selection by directly comparing AIC scores, dropping predictors to simplify the model when removing them did not reduce the AIC score by more than 2. Only the best-supported models are reported.
Results
Pollen limitation and breeding system
Rates of self-fertilization were low for most species, regardless of elevation (compare pollen-supplemented to bagged and open treatments in Fig. 2). Pollen supplementation increased seed set over bagged plants in five of seven species, by an average of 60 % (Fig. 3a). Plants left open to natural pollination (control plants) set an average of 54 % more seeds than pollen-supplemented plants in four of seven species (Fig. 3b).
Effects of pollen-manipulation treatments at three elevations on seed set of seven species of alpine plants at Marriott Basin, BC. a–d Early flowering species, e–g late-flowering species. Different letters indicate statistically significant differences as calculated using Kruskal–Wallis and Mann–Whitney U-tests (p < 0.05). Statistical tests were done as planned comparisons between seed set at different elevations, indicated by letters of the same case (e.g. a vs. a, A vs. A, or a′ vs. b′). Bars are means + SE; n = number of replicate plants/treatment per elevation
Comparisons of seed set in response to different experimental pollination treatments. Lines represent a 1:1 relationship on log-linear axes; deviations from the line represent effect sizes. Statistically significant departures from the 1:1 line are marked with an asterisk. ANEM Anemone occidentalis, ARNI Arnica latifolia, CALT Caltha leptosepala, CLAY Claytonia lanceolata, ERYT Erythronium grandiflorum, LUPI Lupinus arcticus, VACC Vaccinium membranaceum
Effects of elevation on seed set
Seed set generally did not differ among plants from three elevations (Fig. 2). This was due to small effect sizes and large variance (lack of biologically significant differences), rather than inability to detect differences with small sample sizes. Early flowering species generally produced more seed at high elevation, while late-flowering species produced more seed at low elevation (Fig. 2). Among pollen-supplemented individuals, plants at high elevation set more seed than those at low elevation for early flowering Erythronium (H = 9.46, df = 2, p < 0.0088) and Claytonia (H = 5.96, df = 2, p < 0.05) (Fig. 2c, d). Open (control) plants at high elevation also set more seed than those at low elevation for Claytonia (H = 7.19, df = 2, p < 0.0274) and early flowering Caltha (U = 27.00, df = 1, p = 0.0302) (Fig. 2b, c).
Vaccinium, a late-flowering species, had greater seed set at low elevation than at high elevation for pollen-supplemented (H = 8.12, df = 2, p < 0.0172) and open plants (H = 8.58, df = 2, p < 0.0137; Fig. 2g). For Arnica, a late-flowering species, mid-elevation plants had lower seed set than low-elevation and high-elevation plants for both pollen-supplemented (H = 19.05, df = 2, p < 0.0001) and open (H = 15.73, df = 2, p = 0.0004; Fig. 2e) plants.
Temperature
Average temperature was 9 % higher at high than at low elevation (F 1,1284 = 6.96, p = 0.0167; Online Resource 4). Maximum temperatures, DD15, and frost-hours were significant predictors of seed set for Erythronium, Lupinus, Caltha, and Vaccinium (Online Resource 5).
Relative abundance and diversity of potential pollinators
We identified 170 morphospecies from 5,902 specimens trapped at Marriott Basin. We considered 3,729 to be potential pollinators (Online Resource 3). Communities of potential pollinators differed among the three elevations (R = 0.173, p = 0.016, though note low R-value), as did average abundance of potential pollinators (F = 4.06, dfn = 2, dfd = 316, p = 0.0182). Community composition of potential pollinators differed between low and mid elevation (R = 0.261, p = 0.007), and between mid and high elevation (R = 0.204, p = 0.028), but not between low and high elevation (R = 0.062, p = 0.141). Differences among sites were primarily driven by abundance of the black fly morphospecies (Online Resource 6). Bees, Syrphidae, Tenthredinidae, and Lepidoptera contributed less than 2 % of dissimilarity between sites (Online Resource 6). Important functional differences among communities at the three elevations were therefore better captured by considering abundance of the black fly morphospecies.
There was an interaction between trapping date and elevation with regard to abundance of potential pollinators (F = 4.42, dfn = 4, dfd = 42, p = 0.0045). Abundance peaked at low elevations on 23 August 2011, when the number of potential pollinators was 50 % lower at mid and high elevations. Images from time-lapse cameras and recorded temperatures indicated weather on this date was cloudy at high elevation with more variable temperatures across the gradient (6.891 °C ± 3.84 SD for 18 August, 7.99 °C ± 8.24 for 23 August, and 12.75 °C ± 7.79 for 27 August).
Effect of seed predators
Arnica was affected by larval Tephritidae most frequently at mid elevations (H = 49.81, df = 2, p < 0.001). The number of seed predators was a significant predictor of seed set (zero-inflated negative binomial regression, df = 5, p < 0.0001). There were 3 % fewer viable seeds in flowers with seed predators than in flowers without seed predators (U = 5030, df = 1, p < 0.0001).
Combinations of factors limiting seed set
A combination of temperature and abundance of potential pollinators predicted seed set for the bee-pollinated species Erythronium (LRT, p = 0.0003) and Vaccinium (LRT, p < 0.0001). For Erythronium, DD15 were positively associated with instances of zero seed set, and total number of potential pollinators captured was positively associated with the number of seeds. For Vaccinium, DD15 were negatively associated with instances of zero seed set and number of seeds, and total number of potential pollinators captured had a positive relationship with number of seeds. For Caltha, which was pollinated by bees, flies, and butterflies, DD15 were a significant predictor of seed set (LRT, p < 0.0001), having a negative relationship with the number of seeds. For bee-pollinated Lupinus, DD15 were a weakly significant predictor of seed set, but the overall model was not significant (LRT, p = 0.0636).
Maximum temperature was a weakly significant predictor of seed set for Claytonia, which is pollinated by both bees and flies, but the overall model was not significant (LRT, p = 0.148). There was little variation in seed set of the fly-pollinated, self-compatible species Anemone between high and low elevations, and neither temperature nor abundance of potential pollinators were significant predictors of seed set (LRT, p = 0.1414). Seed set of fly-pollinated Arnica was best predicted by abundance of seed predators and frost-hours (LRT, p < 0.0001). For Arnica, total frost-hours had a positive relationship with seed set, while number of seed predators had a negative relationship with seed set.
Discussion
Several processes limit reproduction of flowering plants at Marriott Basin. These include biotic factors (limited availability or quality of pollen in most species, and seed predation in one species) and climatic factors (DD, maximum temperatures, and frost). Quantity and quality of pollen are important: most species had higher seed set in control (open) plants than in bagged or pollen-supplemented plants. Pollen supplementation significantly increased seed set over bagged plants by an average of 60 % across all species, except Anemone and Erythronium, which are self-compatible (see Online Resource 2). In Anemone and Erythronium, pollen supplementation increased seed set by 14 % on average but the effect was not statistically significant. In general, outcrossing increases seed production over selfing. Advantages of outcrossing (number of pollen donors, quality of pollen, repeated visitation, and mechanically appropriate dispersal of pollen across stigmas) can be greater in insect-pollinated than in hand-pollinated plants, particularly when visitation rates are high, and pollinators have constancy (Motten 1986; Dafni et al. 2005). Further studies on pollinator foraging behaviour and stigma receptivity (see Dafni et al. 2005) could determine whether lack of pollen quality or quantity contributed to reduced seed set in hand-pollinated plants.
Elevation, flowering time and seed set
Early flowering and late-flowering species responded differently to manipulations of pollen availability across the 400-m elevation gradient. The early flowering species Erythronium, Caltha, and Claytonia all set about 54 % more seed at high elevation than at low elevation in open and pollen-supplemented plants, though the differences were not always statistically significant due to high variance. The early flowering, fly-pollinated species Anemone did not follow this pattern; it had no variation in seed production across the elevation gradient, and showed little evidence of reproductive limitation. Anemone has high selfing rates (Pojar 1974), confirmed by seed set in bagged plants. Uniformly high seed set across elevation may also have resulted from Anemone attracting many pollinators, and receiving ample pollen regardless of location. Anemone flowers were frequently covered with black flies (also noted by Pojar 1974). Black flies were the most abundant morphospecies in our pan traps, and the most frequent floral visitor observed at our field site. Although bees are often more effective pollinators per visit, flies can compensate for pollination deficits and cause high seed set through sheer abundance (Kearns and Inouye 1994).
Effects of temperature and elevation on seed set
Seed set of early flowering Erythronium, Claytonia, and Caltha was related to temperature, but inconsistently, and temperature differences among elevations were small (Online Resource 4). Counterintuitively, we found that high-elevation sites were warmer on average, which was likely the result of cold air sinking into the valley at night, causing a temperature inversion. Morning shading of lower elevations by nearby peaks also played a role. Maximum temperatures (8 % higher at high elevation) were positively associated with seed production in Claytonia and Caltha, which could be explained by the influence of maximum temperatures on snowmelt, or by increased pollinator activity on hot, sunny days in the alpine (McCall and Primack 1992; Totland 1994). The link between seed set of Erythronium and temperature is difficult to explain. Cumulative DD15 during flowering and seed development were positively correlated with instances of zero seed set. This pattern may correspond with an unmeasured variable, or indicate something particular to Erythronium but not other early flowering species. Perhaps DD15 were associated with growth of tall, late-blooming plants (e.g. Chamerion, Thalictrum, and Veratrum), which shaded shorter Erythronium to a degree that prevented fruit set (Schemske 1977). If shading affected seed set, we would expect DD15 to be linked to seed set for other low-lying, early blooming species, but this was not the case for Claytonia or Caltha.
Vaccinium and Lupinus, both late-flowering species, showed opposite patterns from early flowering species, with higher seed set at lower elevations for open and pollen-supplemented plants, though the differences were not statistically significant for Lupinus. Temperature was important for Vaccinium, which had lower seed set in both pollen-supplemented and open plants at high elevation. In Vaccinium, cumulative DD15 were negatively associated with instances of zero seed set. Abundance of potential pollinators was positively associated with seed set. Low temperatures toward the end of the flowering season may restrict the period for fertilization and seed development of late-flowering plants (Thórhallsdóttir 1998), but this likely did not affect our results because temperatures had not begun to drop by the end of our study. Further experiments would be necessary to separate effects of pollination from physiological limitations to seed set (Totland 1997; Dafni et al. 2005). Monitoring visitation rate or number of pollen donors could indicate how much pollen is needed to set seed. Manipulating pollen availability and climatic conditions (warming, shading, watering), or stripping leaves could help separate the effects of climate and within-season resource availability for plants (e.g. Totland 1997).
The patterns in seed set across elevation in both open and pollen-supplemented plants suggest an interaction between pollen receipt and plant physiology, which varied with flowering time. This is reasonable given that flowering time of late-flowering species is driven primarily by cumulative DD rather than snowmelt (e.g. Dunne et al. 2003). Seed set can also depend on the physiological state of plants, which is affected by conditions up to 2 years prior (Krebs et al. 2009). Despite Vaccinium and Erythronium showing opposite trends in seed set with respect to elevation, the best predictors of seed set for both were DD15 and abundance of potential pollinators. Both Vaccinium and Erythronium are pollinated almost exclusively by Bombus (Vander Kloet 1988; Thomson 2010). The relationship between temperature and seed set for these species may reflect the influence of temperature on phenology of shared pollinators. The same temperature threshold (DD15) is a predictor of seed set for bee-pollinated Lupinus, but the relationship between seed set and elevation is weak for Lupinus due to variable seed set.
Seed predation
Arnica was the only species strongly affected by seed predators. Unlike other late-blooming species, it had highest seed set at low and high elevation, probably because of seed predation at mid elevation. Arnica hosts several species of specialist seed-eating Tephritidae (Scheidel et al. 2003). Populations of Tephritidae can vary over small elevation gradients, but the cause of variation is uncertain (Scheidel et al. 2003; Hodkinson 2005). For Arnica, there was a positive relationship between number of frost-hours and seed set, which might have reflected a negative relationship between frost-hours and abundance of seed predators [some Tephritidae are known to be limited by frost (Gutierrez et al. 2008)]. There were 42–50 % fewer hours below freezing and fewer hours of continuous frost at middle elevations, where seed predators were most abundant (Online Resource 4). Lower seed set of Arnica at mid elevation could also be explained by lower abundance of potential pollinators at mid elevation. This unexpected pattern challenges the assumption that abundance of potential pollinators (and thus pollination) should decline as elevation increases (Fabbro and Körner 2004).
Resilience of alpine plants to climate change
We found complex responses to variable climate conditions across an elevation gradient in our alpine plant community. There was little direct evidence of pollen limitation in early or late-flowering species, mainly because effects of pollen supplementation were obscured by high rates of outcrossing in open (control) plants. However, there was evidence that seed set (and thus, reproduction) could be limited by seed predators (in Arnica), and that DD during the flowering season could be a useful predictor of seed set (in Erythronium, Lupinus, and Vaccinium). For Arnica, frost-hours were a positive predictor of seed set, perhaps because of a link to development or survival of seed predators, which typically require higher temperatures to break dormancy and develop, and can be killed by late-season frost (Hodkinson 2005; Gutierrez et al. 2008). Climate warming could decrease frost-hours during the flowering season, increasing abundance of seed predators at all elevations. However, predicting future patterns of abundance of alpine herbivores is difficult given that variation across elevation gradients seems to be best explained by factors such as local topography and aspect, and interactions with hosts, predators, and competitors (Hodkinson 2005).
Warmer temperatures might interact with plant physiology to increase seed set in some cases. We found indirect evidence of physiological limitations to seed set in Vaccinium, Claytonia, and Erythronium, where seed set varied with elevation despite plants receiving similar amounts of pollen through hand-pollination. Warmer temperatures might ensure faster floral development and extension of the season for ripening seeds (Thórhallsdóttir 1998). Earlier snowmelt might create more snow-free days (Price and Waser 1998), but make early flowering species vulnerable to frost (e.g. Inouye et al. 2002; Inouye 2008), or extreme weather events such as hail storms (Fig. 2). Increased late-spring snow accumulation could delay flowering times and truncate flowering seasons (Inouye et al. 2002; Kudo and Hirao 2006), which can negatively affect seed set (Cooper et al. 2011). Ultimately, the demographic consequences of climate change for alpine flowering plants may depend on longevity of individual plants, and recruitment from seed banks. Over many seasons, reproductive success of perennials in ‘good’ years might compensate for failure in ‘bad’ years. Further work examining the impacts of increased variance in climate parameters would be valuable.
Conclusions and future directions
There was little evidence for pollen limitation of seed set, with only two of seven species having lower seed set in open plants than in hand-pollinated plants. Five of seven species produced more viable seed when exposed to natural levels of pollination. We found that biotic and abiotic factors both limit seed set in the alpine, and the most important factors for predicting seed set vary among species. While this makes it difficult to predict community-wide demographic responses to climate change, several patterns emerge:
-
1.
Early flowering species appear to respond differently to pollen manipulation over elevation gradients than late-flowering species. The reason is unclear, but seed production may be affected by different developmental thresholds for early versus late-flowering species, and conditions for development can vary with elevation.
-
2.
Different factors may limit reproduction in bee versus fly-pollinated plants, suggesting plants with different pollinators may respond divergently to climate change. Bee-pollinated plants risk pollen limitation when flowering time is poorly synchronized with emerging queen bees in early spring, or workers later in the season. Fly-pollinated plants can sometimes set seed regardless of flowering time, particularly when they are also self-compatible, like Anemone.
-
3.
Several temperature variables are linked with seed production. DD15 were predictors of seed production for several bee-pollinated species, suggesting a link between this threshold and phenology of Bombus. Frost-hours were a predictor of seed set in Arnica, which was negative impacted by seed predators (frost-sensitive Tephritidae) at mid elevation.
-
4.
Seed predators had a strong, negative effect on seed set of Arnica, which swamped weaker effects of pollination and interactions between physiology and climate. While phenological cues for plants are fairly well known, little work has been done to determine how the same cues affect pollinators and seed predators (Forrest 2011; Rafferty et al. 2013).
This study highlights the challenges, but also the importance, of measuring multiple factors contributing to reproductive success of flowering plants in the alpine. Further studies combining observations with experiments over natural gradients may help demonstrate which factors are most important, and how changes to biotic and abiotic conditions might interact to predict the demographic consequences of climate change for flowering plants.
References
Ashman T-L, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell DR, Dudash MR, Johnston MO, Mazer SJ, Mitchell RJ, Morgan MT, Wilson WG (2004) Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421. doi:10.1890/03-8024
Barnett DT, Stohlgren TJ (2003) A nested-intensity design for surveying plant diversity. Biodivers Conserv 12:255–278. doi:10.1023/A:1021939010065
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr 27:325–349. doi:10.2307/1942268
Brosi BJ, Briggs HM (2013) Single pollinator species losses reduce floral fidelity and plant reproductive function. Proc Natl Acad Sci 110(13044):13048. doi:10.1073/pnas.1307438110
Burd M (1994) Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot Rev 60:83–139. doi:10.1007/BF02856594
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. doi:10.1111/j.1442-9993.1993.tb00438.x
Cooper EJ, Dullinger S, Semenchuk P (2011) Late snowmelt delays plant development and results in lower reproductive success in the high Arctic. Plant Sci 180:157–167. doi:10.1016/j.plantsci.2010.09.005
Dafni A, Kevan PG, Husband BC (2005) Practical pollination biology. Enviroquest, Cambridge
Darwin C (1862) On the various contrivances by which British and foreign orchids are fertilised by insects: and on the good effects of intercrossing. Murray, London
Droege S, Tepedino VJ, Lebuhn G, Link W, Minckley RL, Chen Q, Conrad C (2010) Spatial patterns of bee captures in North American bowl trapping surveys. Insect Conserv Divers 3:15–23. doi:10.1111/j.1752-4598.2009.00074.x
Dunne JA, Harte J, Taylor KJ (2003) Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecol Monogr 73:69–86. doi:10.1890/0012-9615(2003)073%5B0069:SMFPRT%5D2.0.CO;2
Ehrlén J (1992) Proximate limits to seed production in a herbaceous perennial legume, Lathyrus vernus. Ecology 73:1820–1831. doi:10.2307/1940033
Fabbro T, Körner C (2004) Altitudinal differences in flower traits and reproductive allocation. Flora Morphol Distrib Funct Ecol Plants 199:70–81. doi:10.1078/0367-2530-00128
Forrest J (2011) Plant-pollinator interactions in a changing climate. PhD dissertation, Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, Ontario, Canada
Forrest J, Thomson JD (2009) Pollinator experience, neophobia and the evolution of flowering time. Proc R Soc B 276:935–943. doi:10.1098/rspb.2008.1434
Forrest J, Thomson JD (2011) An examination of synchrony between insect emergence and flowering in Rocky Mountain meadows. Ecol Monogr 81:469–491. doi:10.1890/10-1885.1
Galen C, Stanton ML (1993) Short-term responses of alpine buttercups to experimental manipulations of growing season length. Ecology 74(4):1052–1058 doi:10.2307/1940475
García-Camacho R, Totland Ø (2009) Pollen limitation in the alpine: a meta-analysis. Arct Antarct Alp Res 41:103–111. doi:10.1657/1523-0430-41.1.103
Gutierrez A, Ponti L, d’Oultremont T, Ellis C (2008) Climate change effects on poikilotherm tritrophic interactions. Clim Change 87:167–192. doi:10.1007/s10584-007-9379-4
Haig D, Westoby M (1988) On limits to seed production. Am Nat 131:757–759. Article Stable: http://www.jstor.org/stable/2461676
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156. doi:10.1890/00129658(1999)080%5B1150:TMAORR%5D2.0.CO2
Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland Ø (2009) How does climate warming affect plant–pollinator interactions? Ecol Lett 12:184–195. doi:10.1111/j.14610248.2008.01269.x
Hodkinson ID (2005) Terrestrial insects along elevation gradients: species and community responses to altitude. Biol Rev 80:489–513. doi:10.1017/S1464793105006767
Hothorn T, Zeileis A, Millo G, Mitchell D (2012) lmtest: testing linear regression models. R project.org
Hülber K, Winkler M, Grabherr G (2010) Intraseasonal climate and habitat-specific variability controls the flowering phenology of high alpine plant species. Funct Ecol 24:245–252. doi:10.1111/j.1365-2435.2009.01645.x
Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89:353–362. doi:10.1890/06-2128.1
Inouye DW, Barr B, Armitage KB, Inouye BD (2000) Climate change is affecting altitudinal migrants and hibernating species. PNAS 97:1630–1633. doi:10.1073/pnas.97.4.1630
Inouye D, Morales M, Dodge G (2002) Variation in timing and abundance of flowering by Delphinium barbeyi; Huth (Ranunculaceae): the roles of snowpack, frost, and La Niña, in the context of climate change. Oecologia 130:543–550. doi:10.1007/s00442-001-0835-y
Kearns CA, Inouye DW (1993) Techniques for pollination biologists. University Press of Colorado, Niwot
Kearns CA, Inouye DW (1994) Fly pollination of Linum lewisii (Linaceae). Am J Bot 81:1091–1095. doi:10.2307/2445470
Knight TM, Steets JA, Vamosi JC, et al. (2005) Pollen limitation of plant reproduction: pattern and process. Annu Rev Ecol Evol Syst 36:467–497. doi:10.1146/annurev.ecolsys.36.102403.115320
Krebs CJ, Boonstra R, Cowcill K, Kenney AJ (2009) Climatic determinants of berry crops in the boreal forest of the southwestern Yukon. Botany 87:401–408. doi:10.1139/B09-013
Kudo G, Hirao A (2006) Habitat-specific responses in the flowering phenology and seed set of alpine plants to climate variation: implications for global-change impacts. Popul Ecol 48:49–58. doi:10.1007/s10144-005-0242-z
Kudo G, Nishikawa Y, Kasagi T, Kosuge S (2004) Does seed production of spring ephemerals decrease when spring comes early? Ecol Res 19:255–259. doi:10.1111/j.14401703.2003.00630.x
Larson BMH, Barrett SCH (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linn Soc 69:503–520. doi:10.1111/j.10958312.2000.tb01221.x
Lee TD, Bazzaz FA (1982) Regulation of fruit and seed production in an annual legume, Cassia fasciculata. Ecology 63:1363–1373. doi:10.2307/1938864
Marshall SA (2006) Insects: their natural history and diversity: with a photographic guide to insects of eastern North America. Firefly, Buffalo
McCall C, Primack RB (1992) Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. Am J Bot 79:434–442. doi:10.2307/2445156
Motten AF (1986) Pollination ecology of the spring wildflower community of a temperate deciduous forest. Ecol Monogr 56:21–42. doi:10.2307/2937269
Pojar J (1974) Reproductive dynamics of four plant communities of southwestern British Columbia. Can J Bot 52:1819–1834. doi:10.1139/b74-234
Price MV, Waser NM (1998) Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology 79:1261–1271. doi:10.2307/176741
Rafferty NE, Ives AR (2012) Pollinator effectiveness varies with experimental shifts in flowering time. Ecology 93:803–814. doi:10.1890/11-0967.1
Rafferty NE, CaraDonna PJ, Burkle LA, et al. (2013) Phenological overlap of interacting species in a changing climate: an assessment of available approaches. Ecol Evol 3:3183–3193. doi:10.1002/ece3.668
Scheidel U, Röhl S, Bruelheide H (2003) Altitudinal gradients of generalist and specialist herbivory on three montane Asteraceae. Acta Oecol 24:275–283. doi:10.1016/j.actao.2003.09.004
Schemske DW (1977) Flowering phenology and seed set in Claytonia virginica (Portulacaceae). Bull Torrey Bot Club 104:254–263. doi:10.2307/2484307
Straka JR, Starzomski BM (2014) Humming along or buzzing off? The elusive consequences of plant-pollinator mismatches. J Pollinat Ecol [Suppll] 13, Available at: http://www.pollinationecology.org/index.php?journal=jpe&page=article&op=view&pah%5B%5D=221
Thomson JD (2010) Flowering phenology, fruiting success and progressive deterioration of pollination in an early-flowering geophyte. Philos Trans R Soc B Biol Sci 365:3187–3199. doi:10.1098/rstb.2010.0115
Thórhallsdóttir TE (1998) Flowering phenology in the central highland of Iceland and implications for climatic warming in the Arctic. Oecologia 114:43–49. doi:10.1007/s004420050418
Totland Ø (1994) Influence of climate, time of day and season, and flower density on insect flower visitation in alpine Norway. Arct Alp Res 26:66–71. doi:10.2307/1551879
Totland Ø (1997) Limitations on reproduction in alpine Ranunculus acris. Can J Bot 75:137–144. doi:10.1139/b97-016
Totland Ø (2001) Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82:2233–2244. doi:10.2307/2680228
Triplehorn CA, Johnson NF, Borror DJ (2005) Borror and DeLong’s introduction to the study of insects. Thompson Brooks/Cole, Belmont
Vander Kloet SP (1988) The genus Vaccinium in North America. Research Branch Agriculture Canada, Ottawa
Vuong QH (1989) Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica 57:307–333. doi:10.2307/1912557
Willmer P (2012) Ecology: pollinator–plant synchrony tested by climate change. Curr Biol 22:R131–R132. doi:10.1016/j.cub.2012.01.009
Zeileis A, Kleiber C, Jackman S (2008) Regression models for count data in R. J Stat Softw 27:1–25 http://www.jstatsoft.org/v27/i08
Acknowledgments
Funding was provided by the Natural Sciences and Engineering Research Council of Canada, the Pacific Institute for Climate Solutions, and the University of Victoria. Two anonymous reviewers provided comments that greatly improved the manuscript. We thank Luise Hermanutz, Andrew Trant, Kimberly Carlson, Kira Hoffman, and Katharine Baldwin-Corriveau for comments on earlier versions. Andrew Sheriff and Erika Dort were instrumental in completing the field work. The experiments comply with the current laws of the country (Canada) in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by John Thomas Lill.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Straka, J.R., Starzomski, B.M. Fruitful factors: what limits seed production of flowering plants in the alpine?. Oecologia 178, 249–260 (2015). https://doi.org/10.1007/s00442-014-3169-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3169-2