Abstract
Carotenoid-based ornaments may have evolved as a consequence of their costs of production, which would assure the reliability of the traits as signals of individual quality. Different costs due to carotenoid allocation to the signal have been proposed, considering the scarcity of these pigments at the environment (ecological cost) and their physiological properties that would trade against the maintenance of the organism. Carotenoids of many red ornaments (ketocarotenoids) are often the result of biotransformation of those pigments abundant in the diet (usually lutein and zeaxanthin). Some authors have suggested that such a conversion implies a cost relevant for signaling because it requires high levels of antioxidant vitamins in the tissues where biotransformation takes place. We explore this hypothesis in red-legged partridges (Alectoris rufa) by analyzing ketocarotenoids in the ornaments (bare parts) and carotenoids, vitamin A in different forms (free and esterified) and vitamin E in blood, liver and fat. Ketocarotenoids in ornaments (astaxanthin and papilioerythrinone) were not found in internal tissues, suggesting that they were directly transformed in the bare parts. However, ketocarotenoid levels where positively correlated with the levels of their precursors (zeaxanthin and lutein, respectively) in internal tissues. Interestingly, ketocarotenoid levels in bare parts negatively and positively correlated with vitamin A and E in the liver, respectively, the same links only being positive in blood. Moreover, retinyl and zeaxanthin levels in liver were negatively related. We hypothesize that storing substrate carotenoids in the main storage site (the liver) implies a cost in terms of regulating the level of vitamin A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colored ornaments have attracted the attention of evolutionary ecologists from Darwin (1871) onward. Colored ornaments provide some advantage when competing for a mate with same sex individuals or by being more attractive to the eyes of the choosing sex (Andersson 1994). In many cases, colored traits act as signals informing competitors or potential mates about the quality of the owner. Signaling is maintained when the transmitted information is reliable (non-falsifiable) (Maynard Smith and Harper 2003). To meet this, the expression of a colored signal must proportionally be more costly for low-quality individuals compared to high-quality ones (Zahavi 1975; Grafen 1990), the first being unable to signal or signaling in an inefficient way.
Many colorful traits generated by sexual selection are produced by yellow–orange–red pigments such as carotenoids. Carotenoids produce colors in a plethora of species, adorning plumages in birds and creating conspicuous designs in the skin of amphibians, reptiles and fishes (Goodwin 1984; Britton et al. 2004; McGraw 2006). Since carotenoid ornaments may act as signals of individual quality (Svensson and Wong 2011), different costs have been proposed to explain its evolution. Animals cannot synthesize carotenoids, and, hence, must acquire them with the diet in enough quantity to produce their ornaments (e.g., Britton et al. 2004; McGraw 2006). This implies an ecological cost, as the efficiency of the signal depends on the abundance of specific carotenoids in the environment. Only the better individuals would be able to acquire enough pigments to produce an efficient signal (Endler 1980). This has been rarely demonstrated (Endler 1980; Kodric-Brown 1985; Hill 1990) as carotenoid availability is difficult to estimate. However, this has contributed to the formulation of the idea that carotenoid-based signals are the result of a resource allocation trade-off between investing the scarce resources in sexual signaling (i.e. reproduction) versus self-maintenance (e.g., von Schantz et al. 1999; Simons et al. 2012). In fact, carotenoids are simultaneously required for important physiological functions, such as vision, neutralizing the action of reactive oxygen species (ROS; i.e., as antioxidants), combating pathogens (as immune-boosters) and favoring detoxification (Lozano 1994; McGraw 2006; Pérez-Rodríguez 2009; Simons et al. 2012). To understand the evolution and ecology of carotenoid-based signal, it is hence necessary to understand those proximate mechanisms involved in the trade-off.
Carotenoids are usually divided in two major groups: carotenes and xanthophylls. Those in the first group contain carbon and hydrogen only, whereas those in the second group also contain oxygen (Britton et al. 2004). The most common xanthophylls are in turn subdivided in those carrying a hydroxyl group (hydroxycarotenoids; e.g., lutein and zeaxanthin) or a ketone group (ketocarotenoids or oxocarotenoids; e.g., astaxanthin) as oxygen-containing substituent. The addition of ketone groups leads to a bathochromic shift and redder colors (Britton et al. 2004; McGraw 2006).
Carotenoid-based signaling has mostly been studied in birds. Among the large number of avian species, xanthophylls have been more frequently detected in the ornaments than carotenes (Stradi 1998; McGraw 2006). Xanthophylls allocated to ornaments may be those directly obtained from the diet (e.g., Negro and Garrido-Fernandez 2000), or, instead, be the product of enzymatic transformations from substrate xanthophylls (e.g., Prager et al. 2009; Prum et al. 2012). Interestingly, redder colors of many species are produced by means of ketocarotenoids (e.g., α-doradexanthin and astaxanthin), which are the result of oxidation of yellow hydroxycarotenoids (McGraw 2006). Hill (1996, 2000) suggested that enzymatic transformations would imply some energetic cost that should be summed to those cited above, reinforcing the reliability of the signal, and explaining the high frequency of ketocarotenoid-based ornaments among avian species. However, other non-energetic costs of carotenoid metabolism were subsequently considered (see below).
Carotenoids and, particularly, xanthophylls, are highly lipid-soluble (Britton et al. 2004). Since the liver is considered the most important site for lipid storage in avian species (Blem 2000), this organ has been proposed as a key storage site for xanthophylls (the fat would be other important site; Negro et al. 2001; McGraw 2006; Hill and Johnson 2012). Moreover, some authors have proposed or assumed that biotransformation of hydroxycarotenoids to ketocarotenoids for coloration could take place in the liver too (del Val et al. 2009; Hill and Johnson 2012; Johnson and Hill 2013; but see McGraw 2004, 2009). The liver also contains high levels of vitamin A and E, both of them potent antioxidants linked to carotenoid metabolism (Catoni et al. 2008; Hill and Johnson 2012).
With regard to vitamin E, its antioxidant power may influence the activity of 4-oxidases (ketolases) in producing red ketocarotenoids (see Pérez et al. 2008). Furthermore, carotenoids recycle oxidized vitamin E, which would prevent them from being allocated to pigmentation (e.g., Böhm et al. 1997; Mortensen et al. 2001; Johnson and Hill 2013). Both vitamins A and E may also directly protect carotenoids from being bleached by ROS, which implies that carotenoid-based traits may signal the level of these other non-carotenoid antioxidants in protecting pigments (see Hartley and Kennedy 2004; i.e. the “protection hypothesis”; such as cited in Pérez et al. 2008).
With regard to vitamin A, its antioxidant function is added to other physiological functions. For instance, it plays and important role on gene transcription and favors the epithelial tissue maintenance, nervous system development, vision, immune response, production of sexual steroids and reproduction (e.g., Stahl et al. 2002; Blomhoff and Blomhoff 2006; Noy 2006; McGrane 2007). This vitamin is mostly stored in esterified forms in the liver (retinyls; e.g., Senoo et al. 2010). Hill and Johnson (2012) suggested that vitamin A esterification allows a quick allocation of the bioactive fraction of vitamin A (i.e. retinol) to liver mitochondria in order to maintain its redox balance. A poor capacity to perform this quick allocation would negatively affect the activity of ketolases. Thus, in particular, ketocarotenoid-based ornaments would be revealing the quality of the owner in regulating both vitamin A stores and redox balance into the mitochondria of liver cells (Hill and Johnson 2012; Johnson and Hill 2013). Since the liver is key for metabolizing most compounds (e.g., Blem 2000), ketocarotenoid-based ornaments could thus be signaling an overall quality of this organ.
Lutein and zeaxanthin are the most frequently reported and abundant carotenoids in the diet and blood of birds (McGraw 2006). They are also the most used substrates for enzymes producing those carotenoids giving color to red ornaments, at least among terrestrial species (McGraw 2006). In fact, seabirds and waders feeding on aquatic invertebrates may obtain directly high quantities of ketocarotenoids, whereas in terrestrial birds natural ketocarotenoid dietary sources are mostly unknown (McGraw 2006), being perhaps limited to some insects (e.g., Harashima et al. 1976). In any case, lutein levels always prevails on zeaxanthin levels in both the blood and diet of most avian species, and their concentrations are often reported at approximately a 70:30 rate (lutein:zeaxanthin) (e.g., McGraw et al. 2004), also reflecting their proportions in the diet (McGraw 2006). McGraw et al. (2004) proposed that birds should, however, prioritize the accumulation of zeaxanthin in the body because this pigment would proportionally contribute more in coloring ornaments than lutein. Nonetheless, the relationship between the ratio of these two principal hydroxycarotenoids in the body of the birds and the concentrations and ratios of those pigments deposited in the ornaments has rarely been explored (McGraw and Gregory 2004). Moreover, as far as we know, the relationship between the levels of the antioxidant vitamins A and E in the storage sites and the amount of ketocarotenoids in bare parts of birds has never been studied. We must note that some carotenoids are able to produce two vitamin A molecules after symmetric cleavage (e.g., β-carotene and β-cryptoxanthin; Britton et al. 2004). However, no study has reported this capacity for lutein and zeaxanthin at least among birds or mammals, though it was described for fishes (Goswami and Barua 1981; Schiedt et al. 1985).
To understand how carotenoid availability influences the expression of carotenoid-based signals, the relationship between the amount of those ketocarotenoids giving color to the red ornaments of a bird species (i.e. the red legged-partridge, Alectoris rufa) and the levels of their dietary precursors as well as vitamins A and E in blood and storage sites were explored. The intensity of the red color of bare parts of red-legged partridges influences the reproductive decisions of the mate (Alonso-Alvarez et al. 2012), is sensitive to oxidative stress (Alonso-Alvarez and Galván 2011), and is mostly due to astaxanthin (García-de Blas et al. 2011, 2013). The second most abundant pigment is papilioerythrinone (García-de Blas et al. 2014). Astaxanthin and papilioerytrhinone in ornaments were found in both free and esterified forms (García-de Blas et al. 2011, 2013). Interestingly, papilioerytrhinone would be the product of the most abundant ketocarotenoid substrate (lutein), whereas astaxanthin would be produced from zeaxanthin (García-de Blas et al. 2014). We must note that red-legged partridges feed abundantly on cereals (e.g., Magalhaes et al. 2001; Holland et al. 2006), which are important lutein and zeaxanthin sources (e.g., Panfili et al. 2004; Moore et al. 2005). We predicted that, if the liver is the site where astaxanthin and papilioerythrinone are produced, both pigments should be found there (del Val et al. 2009). The levels of ketocarotenoids giving color to ornaments should also be positively correlated with the levels of each substrate in the internal tissues, and also with the concentrations of antioxidant vitamin A and E, particularly in the liver. The ratio between the two hydroxycarotenoids should be coherently related with the ratio between the two main ketocarotenoids, in any form (free or esterified pigments). Furthermore, we tested the hypothesis suggesting that ketocarotenoid-based ornaments signal the ability to regulate vitamin A levels in the liver during carotenoid biotransformation (Hill and Johnson 2012). We thus analyzed the relationship between the proportion retinol (the bioactive form) versus total vitamin A levels in the liver and the concentration of the pigments giving color to ornaments. A higher capacity to allocate retinol to liver demands (i.e. mitochondria) should positively correlate to a higher efficiency in generating the carotenoid products (Hill and Johnson 2012).
Materials and methods
Eighty adult red-legged partridges were studied. The birds were obtained from our experimental aviaries (Finca Dehesa de Galiana, UCLM; n = 12) and four different commercial facilities (n = 19, 14, 17 and 18 from each one), all of them located in the Castilla-La Mancha region (Central Spain). The red-legged partridge is a game species in Spain and is commercially bred for restocking on wild areas (Díaz-Fernández et al. 2013). Birds were sampled from November 2009 to March 2010, which covers pre-mating and mating periods of this species (Cramp and Simmons 1980). Eighteen birds were sacrificed by intravenous anesthesia (xylazine chloridrate; Rompun; Bayer) plus an intramuscular euthanasia (embutramide, mebezonium iodide and tetracaine hydrochloride; T-61 Intervet; Alcobendas, Spain), under the advice of the veterinarian staff of IREC and ethical guidelines in concert with current Spanish laws. This procedure allowed taking a blood sample (c. 2 mL), which was refrigerated (6 °C), centrifuged within 6 h and then plasma-separated. The other 62 birds were sacrificed by neck dislocation. The red ornaments (bill, eye ring and legs), the liver and subcutaneous fat of the furcula region were extracted. The sex of the bird was established by gonad inspection (36 males and 44 females). In addition, nine wild partridges (five males and four females) were captured during March 2010 in order to validate findings in captive birds. Wild birds were chemically euthanized and their tissues sampled such as described above. The birds of this study were part of a larger sample of individuals where only ornaments were obtained and studied (i.e. García-de Blas et al. 2013; some companies from commercial facilities only allowed taking samples from the ornaments). Samples from different tissues were stored at −80 °C until laboratory analyses.
Carotenoid and vitamin extraction
The procedure for carotenoid extraction from red ornaments was described in García-de Blas et al. (2013). The method for extracting carotenoids and vitamins from the internal tissues was similar to the cited study, only including small modifications (see also Rodríguez-Estival et al. 2010). Briefly, 50 µL of plasma and around 50 mg of liver and subcutaneous fat were weighed into Eppendorf tubes. Thyen, 200 µL of distilled water and 150 µL of ethanol were added. Mixtures were flushed with nitrogen, sonicated for 1 min and vortexed for 5 min. The mixture was then extracted twice with 1 mL of hexane using vortex mixing for 15 min each time. Hexane phases were recovered after centrifuging for 5 min at 2,500g (4 °C). These were combined and evaporated to dryness with a nitrogen flow. Residues were immediately re-dissolved in adequate volume of chromatographic phase A formed by methanol (MeOH): tert-butyl methyl ether (TBME):H2O (81:15:4). For plasma and liver, this volume was 100 µL, and, in the case of subcutaneous fat, this was of 500 µL. Finally, the extract was filtered using nylon filter of 0.2 µm.
Carotenoid and vitamin quantification
Carotenoid quantification in the ornaments was previously described in García-de Blas et al. (2013). Carotenoid and vitamin quantification in the internal tissues (blood, liver and fat) was also carried out as in the ornaments, with minor modifications. Briefly, an Agilent 1100 Series system equipped with a diode array detector (DAD) and a C30 column (YMC Carotenoid 5 µm, 250 × 4.6 mm id) was used. The injection volume was 20 µL. The mobile phase was MeOH:TBME:H2O (81:15:4) (A) and MeOH:TBME (10:90) (B). Samples were initially eluted with 99 % of A and 1 % of B, changing by gradient to 44 % of A and 56 % of B in 39 min, getting final conditions of 100 % of B in 6 min. Then, the system returned to initial conditions in 5 min and these were maintained for 5 min in order to stabilize the column. The flow was 1 mL/min. DAD conditions are specified in García-de Blas et al. (2011). Lutein and zeaxanthin standards were purchased from CaroteneNature (Lupsingen, Switzerland). The standard for free retinol was purchased from Sigma-Aldrich, whereas retynil palmitate was obtained from Fluka. Only the free form of α-tocopherol was detected and quantified, its standard being purchased from Sigma-Aldrich. Concentrations were determined from serial dilutions of standards and linear calibration adjustmentments. Details of the procedure used for detecting and quantifying esterified carotenoids are described in García-de Blas et al. (2011, 2013). The concentration of carotenoids and vitamins in liver and fat was adjustmented to the mass of each sample and expressed in nmol/g (see above). Concentrations in plasma were expressed in nmol/mL. A subsample was assessed twice for repeatability (following Lessells and Boag 1987). All the variables (carotenoids and vitamins) in liver (r range = 0.90–0.99, p < 0.001, n = 10), fat (lutein: r = 0.76–0.99, p < 0.01, n = 12) and plasma (r = 0.80–0.97, p < 0.01, n = 12) were repeatable. Recoveries of samples (n = 3) spiked with carotenoids yielded values (mean ± SD) of 91.0 ± 3.7 % for lutein in liver, 102.2 ± 9.5 % for lutein in plasma, 73.0 ± 3.2 for zeaxanthin in liver and 82.8 ± 8.2 % for zeaxanthin in plasma. Recoveries of vitamin E and A with this method were, in liver, 110.9 ± 5.3 % for free retinol, 83.6 ± 4.4 % for retinyl palmitate and 90.8 ± 1.9 for α-tocopherol (Rodríguez-Estival et al. 2010).
Sample size variability
The head ornaments of six partridges as well as one liver and one fat samples were not assessed due to bad conservation. Results were also explored for outliers. One outlier for the astaxanthin:papilioerythrinone (AP) ratio of free forms in each ornament was detected (different birds) (see also Online Resource, Table 1S). Similarly, one outlier for liver pigments and one outlier for fat pigments were also detected. The analyses were performed with or without outliers, reporting similar findings. Only results obtained from removing the outliers are presented here.
Totals of 58 and 27 birds did not show detectable zeaxanthin and lutein levels in the fat, respectively. Among birds lacking zeaxanthin in fat, 26 birds also lacked detectable lutein. Ratios with a zero value in the numerator or denominator are meaningless and hence were not included in correlation tests (i.e. n = 53). Moreover, 10 birds did not show detectable levels of vitamins in fat (see also Online Resource, Table 2S).
Statistical analyses
Generalized linear mixed models (GLMMs) were used to analyze the relationship between the levels of pigments in the three ornaments (dependent variables) and the levels of carotenoids and vitamins in the liver and fat of the 80 captive partridges (PROC Mixed in SAS software, v.8; SAS Institute 2001; Littell et al. 2006). Wild birds were excluded from main tests due to their low sample size (n = 9), only being compared to those captive birds also sampled in spring (n = 18) by using non-parametric Mann–Whitney U tests due to the low sample size. GLMMs were separately performed for each ornament because of the different nature of each tissue (histological and structural differences or differential exposure to wear and abrasion; see also García-de Blas et al. 2013). GLMMs allowed control for the identity of the provider (population) as a random factor and also for the potential influence of season (November and December data vs. March data) and sex (both fixed factors), also testing their interaction. Factor × covariate interactions were not tested as no a priori prediction was formulated. The possibility of non-linear relationships was also tested. The covariates were alternatively tested for quadratic or inverse relationships. In the first case, the covariate plus its squared value were included in the model. In the second case, the covariate was recalculated by dividing values by one. Only this last case reported some few significant results (three tests) that provided a better adjustmentment than linear (proportional) relationships. The random factor was always maintained in the model, although it was never significant (p range: 0.085–0.568; see Online Resource, Tables 3S–5S). The fixed factors (sex and season) and their interaction were removed when p > 0.10 by following a backward stepwise procedure. In fact, the interaction was always removed. The sex and season were retained by some models (see Tables 1, 2, 3 in “Results”). In such cases, samples from males and those taken in November–December always showed higher pigment concentration in the ornaments than samples from females and those taken in March (see also Online Resource).
In the case of models testing blood variables, since samples from only two captive populations were analyzed, the identity of the provider was tested as a fixed factor. General linear models (GLMs) and type I sum of squares were used, including the identity of the population in a first step. The sex factor and the physiological covariate were subsequently added. The season factor was not included as all the blood samples were taken in spring. Terms were removed at p > 0.10. All the dependent variables and covariates were log-transformed to meet the normality assumption. Nonetheless, fat variables showed a zero-inflated distribution (i.e. non-normal and non-normalizable). To avoid this, we first tested log-transformed fat variables by excluding zeros, which reported normal distributions. Additionally, we also used non-parametric tests including zero values (see below).
Alternative simpler procedures were also explored (see Online Resource, Tables 3S–5S). In this order, Pearson’s and Spearman’s correlation tests were carried out. Non-parametric Spearman’s correlation tests were used when testing fat covariates (above), and also on ratio values. Results from correlation tests provided similar results than GLMMs or GLMs, even providing a higher number of significant tests (Online Resource, Tables 6S–8S).
Results
Descriptive analyses and comparison with wild birds
Astaxanthin was the main carotenoid in the ornaments (mean ± SD from the three traits: 1,123 nmol/g ± 1,046), papilioerythrinone being the second most abundant pigment (52.5 nmol/g ± 48.8; see also Online Resource, Table S1). The AP ratio was very biased to the first compound (mean ± SD from the three traits: 22.8 ± 8.04). The ratio value calculated from the free carotenoids was, nonetheless, lower compared to the ratio obtained from the esterified forms (means 11.2 ± 4.9 vs. 23.6 ± 8.4, respectively; see also Online Resource, Table S1). Neither astaxanthin nor papilioerythrinone were detected in the liver, fat or blood. In contrast to ornaments, no esterified carotenoid was detected in any internal tissue. Lutein and zeaxanthin were the main carotenoids here, the ratio between them (LZ ratio) being biased to the former (mean ± SD for the three tissues: 3.28 ± 1.13; see also Online Resource, Table S2). Among vitamins in internal tissues, only vitamin A was found esterified (i.e. retinyls), and only in the liver (see also Online Resource, Table S2).
Samples from the nine wild individuals allowed to confirm the absence of astaxanthin, papilioerythrinone and esterified carotenoids in any internal tissue, and the presence of esterified vitamin A only in the liver. Higher levels of zeaxanthin in liver, fat and blood (Mann–Whitney tests: all p < 0.007), tocopherol in the liver (p = 0.006), and lutein, retinol and tocopherol in fat (all p values <0.05) were detected in wild birds when compared to captive ones (Online Resource, Table S9).
Associations between carotenoids and vitamins
All the significant tests for the relationship between levels of carotenoids in ornaments and carotenoids in internal tissues reported positive slopes (Table 1). In the case of the legs, no significant association was detected.
With regard to vitamins (Table 1), retinyls in the liver were significantly and negatively related to carotenoid levels in the eye rings. Similarly, when liver retinol and retinyl values were summed and tested, the new variable (vitamin A) showed negative relationships in both the bill (only in papilioerythrinone) and the eye rings, though the tests were not significant (but p values <0.078). Retinol in plasma was also correlated to pigments of the bill and eye rings. However, in contrast to the liver, the relationships were here all positive (Table 1). With regard to tocopherol, the three internal tissues showed positive correlations with pigments in some ornament, including the legs (see tocopherol in fat). In three cases, pigment × vitamin relationships were non-linear, but inversely proportional (Table 1), pigment levels reaching a plateau when decreasing (retinyls) or increasing (tocopherol) vitamin values (Table 1).
With regard to the link between the AP ratios in ornaments and LZ ratios in internal tissues, most part of the tests reported negative relationships (Table 2). Nonetheless, eight models were not significant, six of them including the AP ratio calculated from esterified carotenoids (Table 2).
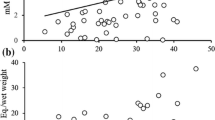
In the internal tissues, retinyl levels in the liver were negatively associated with zeaxanthin values in the same organ (Table 3; see also Fig. 1). In contrast, retinol values were positively linked to lutein and zeaxanthin in blood and to lutein in fat (Table 3). Tocopherol levels exclusively showed positive links with lutein and zeaxanthin in liver and blood (Table 3; Fig. 1).
Discussion
Our results suggest that the production of carotenoid-based signals is not only dependent on the availability of specific carotenoids (Endler 1980; Hill 1990) but also on the individual capacity to regulate carotenoid reserves and biotransformation. Ketocarotenoids giving color to the red-legged partridge ornaments were not present in the storage sites or blood, which suggests that they are produced in situ at the bare parts. This study adds to reports from 11 passerine species (McGraw 2004), but contradicts findings from other 2 others: red crossbills (Loxia curvirostra; del Val et al. 2009) and zebra finches (Taeniopygia guttata; in this case for anhydrolutein only; McGraw and Toomey 2009). Our results would be the first in supporting carotenoid biotransformation at the ornament of a non-passerine avian species. Ketocarotenoid production in the ornament tissue has mostly been suggested for bare parts, the only exception for the plumage probably being the house finch (Carpodacus mexicanus) (McGraw 2004). The variability in the biotransformation site among species could arise from different evolutionary processes. For instance, some species could present high enzymatic requirements in the liver for other purposes such as detoxification for survival (e.g., Rainio et al. 2012), which may compete with reproductive investments such as color expression. This could lead to evolutionary trade-offs (Stearns 1992) promoting honest signaling, but also imply an unsurmountable constraint in some cases. Moreover, some species could not present liver biotransformation due to unknown phylogenetic constraints (e.g., McKitrick 1993).
As predicted, the levels of the two main ketocarotenoids in the ornaments were positively correlated to the levels in the internal tissues of the most abundant carotenoids in the diets of birds: lutein and zeaxanthin (McGraw 2006). The only exception was the leg, where no significant relationship was detected by the mixed models (Table 1). This could perhaps be explained by difficulties in the extraction of carotenoids in this highly keratinized and heterogeneous tissue (García-de Blas et al. 2013). The ratio between carotenoid products (astaxanthin vs. papilioerythrinone) was, in any case, coherently related to the ratio between substrates (lutein vs. zeaxanthin) in any ornament (Table 2), thus supporting the idea of two probably independent metabolic pathways (Stradi 1998; García-de Blas et al. 2014). Nonetheless, Khachik (2003) suggested that lutein could theoretically be transformed to zeaxanthin, though this has not been demonstrated in vivo. The current consensus, however, suggests that astaxanthin would be the result of two oxidations in the 4-position of the β-ionone rings of zeaxanthin, whereas papilioerythrinone would be produced after one 4-oxidation and a subsequent dehydrogenation of lutein (Stradi 1998; McGraw 2006; LaFountain et al. 2013). Intermediate carotenoids proposed for both routes (i.e. adonixanthin and α-doradexanthin, respectively; McGraw 2006, and references therein) were not detected in any tissue. We must anyway consider that enzymes in charge of xanthophyll transformations are still unknown (e.g., Hill and Johnson 2012), and, hence, we may not discard that, at least in some bird species, these reactions may be simultaneously performed, that is, without the accumulation of intermediate compounds.
Nonetheless, a few relationships between the AP and LZ ratios were non-significant. Most of them involved esterified ketocarotenoids (Table 2). Studies in plants suggest that esterification improve pigment stability (Pérez-Gálvez and Mínguez-Mosquera 2005; Rao et al. 2007; Schweiggert et al. 2007) and probably favors carotenoid solubility into the lipidic cell membranes (Pintea et al. 2005; Heras et al. 2007). Accordingly, results suggest that dynamics of free hydroxycarotenoids in internal tissues (lutein and zeaxanthin were never esterified) exert a stronger influence on the variability of levels of those ketocarotenoids recently deposited in pigments (free forms), esterified forms requiring a longer deposition time due to their stability.
The results suggest that ketocarotenoid levels in the ornaments depend on levels of each ketocarotenoid substrate in the storage tissues, but also that biotransformation did not take place at the liver, but at the bare parts. If this is true, a potential oxidative cost associated with biotransformation would be minor, as bare parts are not vital tissues. in the following, we will, however, suggest a new cost unrelated to biotransformation, but derived from storing large levels of ketocarotenoid substrates in their main storage site.
As predicted, the vitamins were correlated to ketocarotenoid levels in all the ornaments, though depending on the type of ornament and vitamin (Table 1). Similarly to carotenoids, the legs reported less significant links, which may again be attributed to population differences and the nature of this trait (see above). The overall picture, nonetheless, suggests a link between bare part pigments and vitamins in all the internal tissues. Interestingly, the sign of the correlations differed. Liver and blood tocopherol levels were positively linked to ketocarotenoid values in the head traits, and fat tocopherol positively correlated with ketocarotenoids in the legs (Table 1). In contrast, esterified vitamin A (retinilys) in the liver showed a negative relationship with ketocarotenoids in the eye ring. Total vitamin A in the same organ also reported negative associations with both ketocarotenoids in eye rings, and with papilioerythrinone in the bill, though these relationships were non-significant (p < 0.078) (see also Online Resource Table 6S). In blood, in contrast, significant correlations were all positive. To better understand this, we went deeper into the results and tested relationships between ketocarotenoid substrates (lutein and zeaxanthin) and vitamins (Table 3). In the liver, carotenoids were positively correlated with tocopherol, but negatively linked to retinoids (Fig. 1). In contrast, in blood, where all vitamin A is present as free retinol, the relationships were positive. Blood positive correlations would discard competitive interactions during intestinal absorption, as hypothesized by some poultry studies (Combs 1976; Sünder et al. 1999; Sünder and Flachowsky 2001). Instead, the negative link between retinyls and xanthophylls in the liver could be revealing some type of regulation.
Individuals allocating more ketocarotenoids to the ornaments would be storing larger amounts of ketocarotenoid precursors and tocopherol at the liver, but also inhibiting retinoid storage in the same organ, and vice versa. To explain this, we must consider the potential pro-oxidative activity of the three types of compounds. In mammals, it has been shown that high doses of carotenoids, retinoids and tocopherols can all lead to oxidative stress by generating pro-oxidative compounds during their cleavage (Palozza et al. 1998; Herrera and Barbas 2001; Amorati et al. 2002; Rietjens et al. 2002; Krinsky and Johnson 2005; Pasquali et al. 2009). From a chemical perspective, however, the three types of molecules differ in their pro-oxidant potential due to differences in their capacity to accept electrons (see Martínez et al. 2008). Xanthophylls would clearly be the most reactive compounds when cleaved, followed by retinoids, and finally, tocopherol (Martínez et al. 2008). In birds, in fact, two recent experimental studies support a pro-oxidative or toxic action of lutein and zeaxanthin at certain doses (Costantini et al. 2007; Huggins et al. 2010). When we take this into account, several explanations to our findings can be formulated. First, we could argue that captive birds received high doses of carotenoids in the diet, which would have negatively affected the liver of some redder birds. However, we must take into account that wild birds showed redder ornaments and higher levels of carotenoids in ornaments than captive partridges in the same sample of birds (i.e. García-de Blas et al. 2013), and wild partridges also showed higher levels of carotenoids in internal tissues than captive birds. Second, we may propose that retinoids were metabolized (exhausted) when combating the pro-oxidative consequences of sustaining high levels of xanthophylls. If this is true, why did tocopherol not show a similar negative association with carotenoids? The answer may be that tocopherol and carotenoids mutually recycle each other (Hickman et al. 1944; Palozza et al. 1998; Mortensen et al. 2001; Catoni et al. 2008; Surai 2012). Third, in the opposite direction, high levels of vitamin A in the liver would be, in some way, particularly toxic for the bird, which would reduce xanthophyll and tocopherol accumulation. In support of this, in poultry, high vitamin A supply in the diet induced lipid peroxidation in the liver (measured as malondialdehyde levels) as well as reduced plasma tocopherol concentration, the authors also detecting a pro-oxidative action of retinoids on tocopherol in the intestines (Sklan and Donoghue 1982; see also Sünder et al. 1999). Unfortunately, the same authors did not test the effect on carotenoids and tocopherol in the liver. A last explanation could be that high levels of xanthophylls impose a challenge to other vital organs, such as the hearth or the brain, retinoids being redistributed to buffer that effect. During heart failure in humans, retinyl levels decrease in the liver and free retinol values increase in the hearth, while liver tocopherol shows no change (Danelisen et al. 2002). Interestingly, the negative relationship between ketocarotenoids and liver retinoids in partridges is only significant in the case of retinyls (i.e. retinoid storage; e.g., Senoo et al. 2010). The proportion of free retinol in the liver may also indicate an oxidative challenge, as suggested for ungulates exposed to metal pollution (Rodríguez-Estival et al. 2011). Accordingly, a positive relationship between zeaxanthin levels and the proportion of free retinol was detected in the liver (Table 3). Nonetheless, a higher proportion of free retinoids may also be explained by a demand for controlling redox balance into the cells (mitochondria) of the same organ, according to Hill and Johnson (2012), thus agreeing with the second explanation above.
We have reported a number of explanations emphasizing the role of oxidative stress. However, we must also mention that all these compounds have also been implicated in many other functions (e.g., Azzi and Stocker 2000; Stahl et al. 2002; Noy 2006; Hill and Johnson 2012 and references therein). Therefore, we cannot discard that the negative link between vitamin A levels and the other molecules in the liver could be explained by factors unrelated to their antioxidant/pro-oxidant properties. One possible explanation could be that these compounds compete directly for metabolism and storage sites in the hepatic tissue (i.e. uptake and processing in hepatocytes and storage at hepatic stellate cells; D’Ambrosio et al. 2011). In that case, the consequences of a reduced capacity for storing vitamin A should surely be relevant to individual homeostasis. For instance, in rats, vitamin A depletion inhibits synthesis of sexual hormones such as testosterone (Appling and Chityl 1981), whereas high testosterone levels contribute to carotenoid absorption and coloration in birds (McGraw and Parker 2006). Hence, the impact of vitamin A depletion should surely be multiple.
Finally, since an important number of tests were performed, we could argue that these findings were, at least partially, due to chance. We explored this possibility by controlling for multiple tests by using the false discovery rate (Benjamini and Hochberg 1995). Following this procedure, significance thresholds moved from 0.05 to 0.032 in Table 1, and to 0.028 in Tables 2 and 3. Only two tests become non-significant in the Table 1, and one test per table in Tables 2 and 3 (Online Resource). We must anyway consider that the establishment of thresholds is basically a convention, the risk of committing type II errors by using p value corrections being largely increased (Stoehr 1999; Moran 2003; Nakagawa 2004). We must also consider that the results of the tests were overall meaningful and coherent among themselves.
In summary, the results suggest that birds expressing highly pigmented ornaments must, in some way, mobilize/regulate retinoid levels, which could imply a cost for the organism, supporting the hypothesis that ketocarotenoid-based ornaments may signal the overall quality of a vital organ, that is, the liver. The results highlight the relevance of retinoids even for non-provitamin A carotenoids such as lutein, zexanthin and their ketocarotenoid products, which also supports the recent attempts to explain the information content of carotenoid-based signaling in terms of efficiency of certain biochemical pathways (i.e. Hill and Johnson 2012; Johnson and Hill 2013). However, our results also support the importance of resource allocation trade-offs between reproductive investments (coloration) and survival (homeostasis) (Edward and Chapman 2011), as individuals should acquire high amounts of carotenoid precursors and vitamins from the diet. Experimental approaches are now needed to confirm these correlative findings. Furthermore, studies on wild birds are also necessary to validate the presence of these mechanisms under natural selection pressures, and examine how environmental variability may affect their evolution.
References
Alonso-Alvarez C, Galván I (2011) Free radical exposure creates paler carotenoid-based ornaments: a possible interaction in the expression of black and red traits. PLoS ONE 6:e19403
Alonso-Alvarez C, Pérez-Rodríguez L, Ferrero ME, García-de Blas E, Casas F, Mougeot F (2012) adjustmentment of female reproductive investment according to male carotenoid-based ornamentation in a gallinaceous bird. Behav Ecol Sociobiol 66:731–742
Amorati R, Ferroni F, Lucarini M, Pedulli GF, Valgimigli L (2002) A quantitative approach to the recycling of alpha-tocopherol by coantioxidants. J Org Chem 67:9295–9303
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Appling DR, Chityl F (1981) Evidence of a role for retinoic acid (vitamin A-acid) in the maintenance of testosterone production in male rats. Endocrinology 108:2120–2123
Azzi A, Stocker A (2000) Vitamin E: non-antioxidant roles. Prog Lip Res 39:231–255
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300
Blem CR (2000) Energy balance. In: Causey Whittow G (ed) Sturkie’s avian physiology, 5th edn. Academic, San Diego, pp 327–341
Blomhoff R, Blomhoff HK (2006) Overview of retinoid metabolism and function. J Neurobiol 66:606–630
Böhm F, Edge R, Land E (1997) Carotenoids enhance vitamin E antioxidant efficiency. J Am Chem Soc 7863:621–622
Britton G, Liaaen-Jensen S, Pfander H (eds) (2004) Carotenoids handbook. Birkhaüser, Basel
Catoni C, Peters A, Martin Schaefer H (2008) Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Anim Behav 76:1107–1119
Combs GF Jr (1976) Differential effects of high dietary levels of vitamin A on the vitamin E-selenium nutrition of young and adult chickens. J Nutr 106:967–975
Costantini D, Coluzza C, Fanfani A, Dell’Omo G (2007) Effects of carotenoid supplementation on colour expression, oxidative stress and body mass in rehabilitated captive adult kestrels (Falco tinnunculus). J Comp Physiol B 177:723–731
Cramp S, Simmons KEL (1980) The birds of the Western Paleartic. Oxford University Press, Oxford
D’Ambrosio DN, Clugston RD, Blaner WS (2011) Vitamin A metabolism: an update. Nutrients 3:63–103
Danelisen I, Palace V, Lou H, Singal PK (2002) Maintenance of myocardial levels of vitamin A in heart failure due to adriamycin. J Mol Cell Cardiol 34:789–795
Darwin C (1871) The descent of man and selection in relation to sex. John Murray, London
Del Val E, Senar JC, Garrido-Fernández J, Jarén M, Borràs A, Cabrera J, Negro JJ (2009) The liver but not the skin is the site for conversion of a red carotenoid in a passerine bird. Naturwissenschaften 96:797–801
Díaz-Fernández S, Arroyo B, Casas F, Viñuela J (2013) Effect of game management on wild red-Legged partridge abundance. Plos ONE 8:e66671
Edward EA, Chapman T (2011) Mechanisms underlaying reproductive trade-offs: costs of reproduction. In: Flatt T, Heyland A (eds) Mechanisms of life history evolution. Oxford University Press, Oxford, pp 137–152
Endler JA (1980) Natural selection on color patterns in Poecilia reticulata. Evolution 34:76–91
García-de Blas E, Mateo R, Viñuela J, Alonso-Alvarez C (2011) Identification of carotenoid pigments and their fatty acid esters in an avian integument combining HPLC-DAD and LC-MS analyses. J Chromatogr B 879:341–348
García-de Blas E, Mateo R, Viñuela J, Pérez-Rodríguez L, Alonso-Alvarez C (2013) Free and esterified carotenoids in ornaments of an avian species: the relationship to color expression and sources of variability. Physiol Biochem Zool 86:483–498
García-de Blas E, Mateo R, Guzmán Bernardo FJ, Martín-Doimeadios RCR, Alonso-Álvarez C (2014) Astaxanthin and papilioerythrinone in the skin of birds: a chromatic convergence of two metabolic routes with different precursors? Naturwissenschaften 101:407–416
Goodwin TW (1984) The biochemistry of the carotenoids, 2nd edn. Vol 2: Animals. Chapman & Hall, London
Goswami BC, Barua AB (1981) Intestinal conversión of lutein into 3-dehydroretinol in H. fossilis and C. striatus. Indian Biochem Biophys 18:88–92
Grafen A (1990) Biological signals as handicaps. J Theor Biol 144:517–546
Harashima K, Nakahara J, Kato G (1976) A new ketocarotenoid in integuments of orange pupae of a swallowtail, Papilio xucthus, and carapaces of a crab, Paralithodes brevipes (Hanasakigani in Japanese). Agric Biol Chem 40:711–717
Hartley RC, Kennedy MW (2004) Are carotenoids a red herring in sexual display? Trends Ecol Evol 19:353–354
Heras H, Dreon MS, Ituarte S, Pollero RJ (2007) Egg carotenoproteins in neotropical Ampullariidae (Gastropoda: Arquitaenioglossa). Comp Biochem Physiol C 146:158–167
Herrera E, Barbas C (2001) Vitamin E: action, metabolism and perspectives. J Physiol Biochem 57:43–56
Hickman KCD, Woodside Kaley M, Harris P (1944) Covitamin studies. J Biol Chem 152:303–311
Hill GE (1990) Female house finches prefer colorful males: sexual selection for a condition-dependent trait. Anim Behav 40:563–570
Hill GE (1996) Redness as a measure of the production cost of ornamental coloration. Ethol Ecol Evol 8:157–175
Hill GE (2000) Energetic constraints on expression of carotenoid-based plumage coloration. J Avian Biol 31:559–566
Hill GE, Johnson JD (2012) The vitamin A-redox hypothesis: a biochemical basis for honest signaling via carotenoid pigmentation. Am Nat 180:E127–E150
Holland JM, Hutchison MAS, Smith B, Aebischer NJ (2006) A review of invertebrates and seed-bearing plants as food for farmland birds in Europe. Ann Appl Biol 148:49–71
Huggins K, Navara KJ, Mendonça MT, Hill GE (2010) Detrimental effects of carotenoid pigments: the dark side of bright coloration. Naturwissenschaften 97:637–644
Johnson JD, Hill GE (2013) Is carotenoid ornamentation linked to the inner mitochondria membrane potential? A hypothesis for the maintenance of signal honesty. Biochimie 95:436–444
Khachik F (2003) An efficient conversion of (3R, 3′R, 6′R)-lutein to (3R, 3′S, 6′R)-lutein (3′-epilutein) and (3R, 3′R)-zeaxanthin. J Nat Prod 66:67–72
Kodric-Brown A (1985) Female preferente and sexual selection for male coloration in the guppy (Poecilia reticulata). Behav Ecol Sociobiol 17:199–205
Krinsky NI, Johnson EJ (2005) Carotenoid actions and their relation to health and disease. Mol Aspects Med 26:459–516
Lafountain AM, Frank HA, Prum RO (2013) Carotenoids from the crimson and maroon plumages of Old World orioles (Oriolidae). Arch Biochem Biophys 539:126–132
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute, Cary
Lozano GA (1994) Carotenoids, parasites, and sexual selection. Oikos 70:309–311
Magalhaes MC, Tavres P, Fontoura AP (2001) Morphometric characters and diet of hunted red-legged partridges (Alectoris rufa) in Portugal. Game Wildl Sci 18:3–4
Martínez A, Rodríguez-Gironés MA, Barbosa A, Costas M (2008) Donator acceptor map for carotenoids, melatonin and vitamins. J Phys Chem A 112:9037–9042
Maynard Smith J, Harper D (2003) Animal signals. Oxford University Press, Oxford
McGrane MM (2007) Vitamin A regulation of gene expression: molecular mechanism of a prototype gene. J Nutr Biochem 18:497–508
McGraw KJ (2004) Colorful songbirds metabolize carotenoids at the integument. J Avian Biol 35:471–476
McGraw KJ (2006) Mechanics of carotenoid-based coloration. In: Hill GE, McGraw KJ (eds) Bird coloration vol 1: mechanisms and measurements. Havard University Press, Cambridge, pp 177–242
McGraw KJ (2009) Identifying anatomical sites of carotenoid metabolism in birds. Naturwissenschaften 96:987–988
McGraw KJ, Gregory A (2004) Carotenoid pigments in male American goldfinches: what is the optimal biochemical strategy for becoming colourful? Biol J Linn Soc 83:273–280
McGraw KJ, Parker RS (2006) A novel lipoprotein-mediated mechanism controlling sexual attractiveness in a colorful songbird. Physiol Behav 87:103–108
McGraw KJ, Toomey MB (2009) Carotenoid accumulation in the tissues of zebra finches: predictors of integumentary pigmentation and implications for carotenoid allocation strategies. Physiol Biochem Zool 83:97–109
McGraw KJ, Hill GE, Navara KJ, Parker RS (2004) Differential accumulation and pigmenting ability of dietary carotenoids in colorful finches. Physiol Biochem Zool 77:484–491
McKitrick MC (1993) Phylogenetic constraint in evolutionary theory: has it any explanatory power? Annu Rev Ecol Syst 24:307–330
Moore J, Hao Z, Zhou K, Luther M, Costa J, Yu L (2005) Carotenoid, tocopherol, phenolic acid, and antioxidant properties of maryland-grown soft wheat. J Agric Food Chem 53:6649–6657
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403–405
Mortensen A, Skibsted LH, Truscott TG (2001) The interaction of dietary carotenoids with radical species. Arch Biochem Biophys 385:13–19
Nakagawa S (2004) A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15:1044–1045
Negro JJ, Garrido-Fernandez J (2000) Astaxanthin is the major carotenoid in tissues of White storks (Ciconia ciconia) feeding on introduced crayfish (Procambarus clarkii). Comp Biochem Physiol B 126:347–352
Negro JJ, Figuerola J, Garrido-Fernández J, Green AJ (2001) Fat stores in birds: an overlooked sink for carotenoid pigments? Funct Ecol 15:297–303
Noy N (2006) Vitamin A. In: Stipanuk MH, Caudill MA (eds) Biochemical, biophysical and molecular aspects of human nutrition, 3rd edn. Elsevier Saunders, St Louis, pp 683–702
Palozza P, Galviello G, Serini S, Moscato P, Bartoli GM (1998) Nutrient requirements and interactions supplementation with canthaxanthin affects plasma and tissue distribution of alpha- and gamma-tocopherols in mice. J Nutr 128:1989–1994
Panfili G, Fratianni A, Irano M (2004) Improved normal-phase high-performance liquid chromatography procedure for the determination of carotenoids in cereals. J Agric Food Chem 52:6373–6377
Pasquali MA, Gelain DP, de Oliveira MR, Behr GA, da Motta LL, da Rocha RF, Klamt F, Moreira JC (2009) Vitamin A supplementation induces oxidative stress and decreases the immunocontent of catalase and superoxide dismutase in rat lungs. Exp Lung Res 35:427–438
Pérez C, Lores M, Velando A (2008) Availability of nonpigmentary antioxidant affects red coloration in gulls. Behav Ecol 19:967–973
Pérez-Gálvez A, Mínguez-Mosquera MI (2005) Esterification of xanthophylls and its effect on chemical behavior and bioavailability of carotenoids in the human. Nutr Res 25:631–640
Pérez-Rodríguez L (2009) Carotenoids in evolutionary ecology: re-evaluating the antioxidant role. BioEssays 31:1116–1126
Pintea A, Diehl H, Momeu C, Aberle L, Socaciu C (2005) Incorporation of carotenoid esters into liposomes. Biophys Chem 118:7–14
Prager M, Johansson EIA, Andersson S (2009) Differential ability of carotenoid C4-oxygenation in yellow and red bishop species (Euplectes spp.). Comp Biochem Physiol B 154:373–380
Prum RO, LaFountain AM, Berro J, Stoddard MC, Frank HA (2012) Molecular diversity, metabolic transformation, and evolution of carotenoid feather pigments in cotingas (Aves: Cotingidae). J Comp Physiol B 182:1095–1116
Rainio MJ, Kanerva M, Wahlberg N, Nikinmaa M, Eeva T (2012) Variation of basal EROD activities in ten passerine bird species: relationships with diet and migration status. PLoS ONE 7:e33926
Rao A, Sarada R, Ravishankar GA (2007) Stabilization of astaxanthin in edible oils and its use as an antioxidant. J Sci Food Agric 87:957–965
Rietjens IMCM, Boersma MG, De Haan L, Spenkelink B, Awad HM, Cnubben NHP, van Zanden JJ, van der Woude H, Alink GM, Koeman JH (2002) The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ Toxicol Pharmacol 11:321–333
Rodríguez-Estival J, Martínez-Haro M, Martín-Hernando MP, Mateo R (2010) Sub-chronic effects of nitrate in drinking water on red-legged partridge (Alectoris rufa): oxidative stress and T-cell mediated immune function. Environ Res 110:469–475
Rodríguez-Estival J, Martinez-Haro M, Monsalve-González L, Mateo R (2011) Interactions between endogenous and dietary antioxidants against Pb-induced oxidative stress in wild ungulates from a Pb polluted mining area. Sci Total Environ 409:2725–2733
SAS Institute (2001) SAS/STAT software: changes and enhancements, version 8.2. SAS, Cary
Schiedt K, Leuenberger FJ, Vecchi M, Glinz E (1985) Absorption, retention and metabolic transformations of carotenoids in rainbow trout, salmon and chicken. Pure Appl Chem 57:685–692
Schweiggert U, Kurz C, Schieber A, Carle R (2007) Effects of processing and storage on the stability of free and esterified carotenoids of red peppers (Capsicum annuum L.) and hot chilli peppers (Capsicum frutescens L.). Eur Food Res Technol 225:261–270
Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y (2010) Hepatic stellate cell (vitamin A-storing cell) and its relative: past, present and future. Cell Biol Intern 34:1247–1272
Simons MJP, Cohen AA, Verhulst S (2012) What does carotenoid-dependent coloration tell? Plasma carotenoid level signals immunocompetence and oxidative stress state in birds: a meta-analysis. Plos One 7(8):e43088
Sklan D, Donoghue S (1982) Vitamin E response to high dietary vitamin A in the chick. J Nutr 112:759–765
Stahl W, Ale-Agha N, Polidori MC (2002) Non-antioxidant properties of carotenoids. Biol Chem 383:553–558
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stoehr AM (1999) Are significance thresholds appropriate for the study of animal behaviour? Anim Behav 57:F22–F25
Stradi R (1998) The colour of flight: carotenoids in bird plumage. Solei, Milan
Sünder A, Flachowsky G (2001) Influence of high vitamin E dosages on retinol and carotinoid concentration in body tissues and eggs of laying hens. Arch Tierernahr 55:43–52
Sünder A, Halle I, Flachowsky G (1999) Vitamin E hypervitaminosis in laying hens. Arch Tierernahr 52:185–194
Surai PF (2012) The antioxidant properties of canthaxanthin and its potential effects in the poultry eggs and on embryonic development of the chick. Part 1. World Poult Sci J 68:465–476
Svensson PA, Wong BBM (2011) Carotenoid-based signals in behavioural ecology: a review. Behaviour 148:131–189
von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc Lond B 266:1–12
Zahavi A (1975) Mate selection: selection for a handicap. J Theor Biol 53:205–214
Acknowledgments
We thank Pablo Camarero, Ester Ferrero and Laura Ramirez for their help with the laboratory work. We also thank the owners and managers of the companies and wild areas that supplied partridges. Particularly, we thank the staff of Finca Dehesa Galiana (UCLM). We thank Sarah Young for her English review of the last version of the text. We acknowledge the suggestions provided by Dr Oliver P. Love and three anonymous referees on a first version of the manuscript. Esther García-de Blas was supported by a pre-doctoral grant (JAE-PRE) from the Consejo Superior de Investigaciones Científicas (CSIC) co-financed by Fondo Social Europeo (EU). This study was funded by Consejería de Educación y Ciencia, Junta de Comunidades de Castilla la Mancha (project ref.: PII1I09-0271-5037) and Ministerio de Economía y Competitividad (CGL2009-10883-C02-02 and CGL2012-40229-C02-01) from the Spanish Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Oliver P. Love.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
García-de Blas, E., Mateo, R. & Alonso-Alvarez, C. Accumulation of dietary carotenoids, retinoids and tocopherol in the internal tissues of a bird: a hypothesis for the cost of producing colored ornaments. Oecologia 177, 259–271 (2015). https://doi.org/10.1007/s00442-014-3163-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3163-8