Abstract
In the eastern United States, winter temperature has been increasing nearly twice as fast as summer temperature, but studies of warming effects on plants have focused on species that are photosynthetically active in summer. The terrestrial orchid Tipularia discolor is leafless in summer and acquires C primarily in winter. The optimum temperature for photosynthesis in T. discolor is higher than the maximum temperature throughout most of its growing season, and therefore growth can be expected to increase with warming. Contrary to this hypothesis, experimental warming negatively affected reproductive fitness (number of flowering stalks, flowers, fruits) and growth (change in leaf area from 2010 to 2012) in T. discolor. Temperature in June–July was critical for flowering, and mean July temperature greater than 29 °C (i.e., 2.5 °C above ambient) eliminated reproduction. Warming of 1.2 °C delayed flowering by an average of 10 days and fruiting by an average of 5 days. Warming of 4.4 °C reduced relative growth rates by about 60 %, which may have been partially caused by the direct effects of temperature on photosynthesis and respiration. Warming indirectly increased vapor pressure deficit (VPD) by 0.2–0.5 kPa, and leaf-to-air VPD over 1.3 kPa restricted stomatal conductance of T. discolor to 10–40 % of maximum conductance. These results highlight the need to account for changes in VPD when estimating temperature responses of plant species under future warming scenarios. Increasing temperature in the future will likely be an important limiting factor to the distribution of T. discolor, especially along the southern edge of its range.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mean temperature in the United States is expected to increase by 2–6 °C by 2100 (Karl et al. 2009). Plant responses to warming are highly variable, with the direction and magnitude of responses depending on species and initial environmental conditions (Arft et al. 1999; Rustad et al. 2001; Wu et al. 2011). Current temperatures in the native environment of many plant species are below their growth optimum, particularly in temperate and boreal regions (Way and Oren 2010), so future warming is expected to increase growth for many plant species. This expectation, however, is complicated by future changes to atmospheric vapor pressure deficit (VPD), which has strong effects on plant physiology that vary from species to species. Higher temperatures increase the water-holding capacity of the atmosphere, and if relative humidity remains fairly constant in the future (e.g., Trenberth et al. 2005), global warming will cause VPD and the evaporative demand of the atmosphere to rise.
While species success ultimately requires net C gain, it also depends on successful reproduction, which may be influenced by climate in ways distinct from the influence of climate on growth. Higher temperatures often increase plant reproduction (Dormann and Woodin 2002; Pfeifer et al. 2006; De Frenne et al. 2011; Klady et al. 2011), but there are exceptions (Totland and Alatalo 2002) and just as for all responses to temperature, optima beyond which performance declines. Warming effects on reproduction are more difficult to generalize than are effects on growth. Reproduction is influenced by plant investment strategies and, in many species, by interactions with pollinators (which may in turn be influenced by climate). In cool temperate regions, increases in temperature influence the timing of flowering and fruiting in plants (Went 1953; Rathcke and Lacey 1985), with warming typically shifting reproductive phenology to earlier flowering dates (Fitter and Fitter 2002; Dunne et al. 2003; Parmesan and Yohe 2003; Sherry et al. 2007). Whether the same is true in warm temperate regions is less clear.
Studies of understory species in forests are particularly lacking. A unique feature of some understory plant species is that they are winter active and hence potentially influenced both by summer temperatures and by winter temperatures. The effects of winter warming might be very different than those of summer warming, as winter temperatures in the eastern US have been increasing nearly twice as fast as the annual average (Karl et al. 2009). Mean winter temperature in the eastern US has increased by 1.5–2.2 °C since 1970 (Karl et al. 2009), and the recent winter of 2012 was the fourth warmest on record in the contiguous US (NOAA 2012). In one of the few cases where a winter-active species has been studied, warming was reported to increase population growth of the orchid Himantoglossum hircinum (Pfeifer et al. 2006).
This study examines the effect of experimental warming on the winter-active terrestrial orchid, Tipularia discolor (Pursh) Nutt., in the understory of a temperate forest (Duke Forest, NC). T. discolor has a unique wintergreen phenological pattern, where leaves emerge in autumn and senesce in the spring. Due to its phenology, plants are photosynthetically active during periods of low temperature, low VPD, and relatively high light due to a leafless overstory canopy. Previous work has found that the optimal temperature for photosynthesis (T opt) of T. discolor is 26 °C, which is over 10 °C higher than maximum temperatures throughout much of its growing season (Tissue et al. 1995). Here, we test the hypothesis that experimental warming of the forest understory will increase growth and reproduction in T. discolor. This work was performed by actively heating open-top chambers to 1.2–5 °C above ambient temperature. As a consequence of heating, mean VPD increased by 0.18–0.53 kPa inside the chambers. To understand how the temperature response of T. discolor might be confounded by simultaneous changes in VPD, we also quantified the effect of VPD on orchid physiological responses in the field and under controlled laboratory conditions. Terrestrial orchids are often the first organisms to disappear from disturbed ecosystems and thus serve as bioindicators and early warning signs of problems for ecosystem health (Swarts and Dixon 2009). Understanding the physiological limits to plant growth and reproduction is important for developing conservation strategies for species susceptible to population declines as a result of climate change.
Materials and methods
Study species
The crane-fly orchid, Tipularia discolor (Epidendroideae, Calypsoae), is a terrestrial orchid native to eastern North America. It occurs from Massachusetts west to Michigan and south to Texas and Florida (Brown 1997). The species is summer deciduous with leaves emerging in the fall (October–November) and senescing in the spring (late April). Individual plants typically consist of a single leaf and produce a corm at its base, which persists for several years (Snow and Whigham 1989). Leaves are commonly found in dense clusters, and seemingly distinct plants may be genetic clones (Frye 1993). Different genotypes have been identified within a single cluster, however, indicating genets may intermingle within dense leaf clusters (Smith et al. 2002). Flowering occurs when plants are leafless in late July–August, and fruits mature in September–October (Whigham and McWethy 1980). Only a single pollinator species, a nocturnal moth (Pseudaletia unipuncta, armyworm), has been identified for T. discolor (Whigham and McWethy 1980).
Experimental study site
This study was conducted at an ongoing, long-term warming experiment in a ca. 80-year-old oak-hickory forest stand of Duke Forest (36°2′11″N, 79°4′39″W, 130 m a.s.l.), in the piedmont region near Hillsborough, North Carolina (Lynch 2006). The mean annual temperature at Duke Forest is 15.5 °C, and the mean annual precipitation is 1,140 mm. Winter precipitation in North Carolina has increased by approximately 0.5 mm year−1 over the past 50 years (Boyles and Raman 2003). Climate data for the site are available from a nearby weather station (Duke Forest Remote Automatic Weather Station, Orange County, NC).
The experimental warming site consists of 15 plots in the forest understory: nine are heated, three are unheated chamber controls, and three are control plots that lack chambers but are equal in surface area to the chambers. The octagonal, open-top chambers are 21.7 m3 in volume: 5 m in diameter with eight walls that are 1.9 m wide by 1.2 m tall. The chambers are heated by forced air blown over hydronic radiators fed by a closed-loop mixture of hot water and propylene glycol (antifreeze). The heated air is blown into the chambers through 15-cm-diameter plastic plena which hang 45 cm above the ground and run in two concentric rings, one 0.8 and the other 1.7 m from the chamber walls. Air enters the chambers via two rows of 2-cm-diameter holes separated by 20 cm along the bottom of the plena. Heat delivery to the chambers began in January 2010, and chambers are constantly heated year-round, both day and night. The experiment uses a regression design of chamber heating, where each chamber is heated to a target of 1.5–5.5 °C above ambient temperature with 0.5 °C increments between chambers. Maintaining precise target air temperatures over long time periods is difficult, and the assigned treatment levels varied by a small amount over time. Despite this imperfection, the use of a regression design is useful for revealing potential nonlinearities and threshold effects in plant temperature responses. We sampled naturally occurring plants that were present at the site before the chambers were installed. Specifically, the study species was present in nine of the 15 experimental plots (0, 0, 0, 1.5, 2, 2.5, 3, 4, 4.5 °C).
Air temperature (two temperature probes per chamber at 22 cm above ground level), relative humidity (HS-2000V capacitive polymer sensor; Precon, Memphis, TN), soil moisture (model CS616 TDR probes; Campbell Scientific, Logan, UT), and photosynthetically active radiation (PAR; model SQ110; Apogee Instruments, Logan, UTA) are measured inside each experimental chamber every minute and recorded as hourly means by automated data loggers (CR1000; Campbell Scientific). To determine how environmental conditions varied among experimental chambers, mean daily air temperature, VPD, and relative extractable soil water content (REW) were calculated for each chamber. REW was calculated according to the equation:
where \(\theta\) is the hourly soil water content, \(\theta_{\hbox{min} }\) is minimum soil water content, and \(\theta_{\hbox{max} }\) is the mean maximum volumetric soil water content over the study period. Readings from three or four saturating rainfall events per year were averaged to determine \(\theta_{\hbox{max} }\). To account for soil macropore drainage and thus avoid over-estimating \(\theta_{\hbox{max} }\), volumetric soil water content from 2 h after the peak \(\theta\) of each saturating rainfall event was used. Further details of the warming experiment can be found in Pelini et al. (2011).
Growth, reproduction, and phenology
At the beginning of the warming experiment in 2010, there were 99 ramets of T. discolor distributed across nine of the 15 study plots. To increase the sample size of leaves in the chamberless control plots (n = 5), an additional 16 leaves were measured outside the experimental plots. All leaves (n = 115) were numbered and mapped for relocation in subsequent years. Leaf area (A; cm2) of these orchids was estimated annually from 2010 to 2012 by measuring leaf length (L; mm) and width (W; mm) and using the allometric equation: \(A = 1.23 - 0.037L - 0.026W + 0.008LW\) (r 2 = 0.998, n = 28). Changes in leaf area within chambers over time were estimated as relative growth rates as described by Hoffmann and Poorter (2002).
For 3 years (2010, 2011, 2012) from late July up to and including September, the experimental site was visited weekly to determine the reproductive phenological stage of all orchids. The number of flowering stalks, flower number per stalk, and fruit number per stalk was monitored for each experimental chamber. Flowering and fruiting date were determined for each flowering stalk in 2010 and 2011. Because orchid reproduction was low in experimental chambers, orchid flowering and fruiting data from all years were combined to increase the sample size for analyses. Mean July temperature (for each study year) inside the chamber was used to compare different flowering responses, because the onset of flowering is correlated to mean temperature during the month of flowering for many plants (Sparks et al. 2000; Menzel et al. 2006).
Gas exchange physiology
To determine the effect of temperature on biophysical and biochemical photosynthetic parameters in T. discolor, the response of net CO2 assimilation (A n) to varying concentrations of intercellular CO2 (C i) was measured from 1100 to 1530 hours on 11 March 2011. These A n/C i curves were measured on a total of five leaves in control plots (i.e., inside two control chambers or the chamberless control plot) and a total of five leaves in the warmest chambers (i.e., inside +4- or +4.5 °C chambers). Photosynthesis was measured using a portable infrared gas analyzer equipped with a red–blue light source (LI-6400; LI-COR, Lincoln, NE) at chamber temperature (10.2–15.4 °C) and VPD (0.48–1 kPa), under saturating PAR (500 μmol m−2 s−1). The ambient CO2 (C a) was lowered stepwise from 400 to 50 p.p.m. and then increased from 400 to 1,000 p.p.m. with a total of 11 points per A n/C i curve. Rates of photosynthesis were recorded after 2 min at each C a. Values of A n were corrected for CO2 leakage by subtracting ‘apparent’ photosynthesis quantified with photosynthetically inactive leaves (n = 5), following Flexas et al. (2007). These leaves were thermally killed by immersion in boiling water for 2 min, after which no variable fluorescence was detected by a PAM-2100 fluorometer (Heinz Walz, Effeltrich, Germany). The parameters maximum carboxylation rate (V cmax), photosynthetic electron transport rate (J), triose phosphate use (TPU), daytime respiration (R d), and mesophyll conductance (g m) were estimated at leaf temperature for each curve using the Farquhar et al. (1980) model of C3 photosynthesis, as described by Sharkey et al. (2007). A n under saturating CO2 (A max) was estimated at a CO2 concentration of 550–1,000 p.p.m.
A n was measured under local environmental conditions in seven experimental chambers using the LI-6400 on 8 days throughout 2010–2012 (Table S1). For all measurements, temperature inside the cuvette was set to match the chamber treatment, the relative humidity was held ±10 % of ambient conditions, and the CO2 concentration was set to 400 p.p.m.. Leaves (n = 5 per chamber) were measured under a PAR of 100 μmol m−2 s−1 on 12 November 2010, 10 December 2010, 20 January 2011, and 7 March 2011 (Table S1). This level of PAR corresponds to measured light intensity in November, when the overstory is not yet leafless, and is representative of approximately 30 % of daily irradiance conditions in the understory from November to March. Leaves were also measured under saturating PAR (450–500 μmol m−2 s−1) on 18 February 2011, 24 March 2011, 6 March 2012, and 13 December 2012 (Table S1). All measurements were made on clear days between 1000 and 1530 hours, and chamber sampling order was randomized across days. Temperature changes throughout the day affected the maximum and minimum achievable leaf temperature during measurement, so the daily range of temperatures varied across sampling dates (Table S1). Gas exchange was recorded for a period of 4 min after conditions inside the cuvette stabilized (usually 1–2 min) and then averaged to determine A n for each leaf. Because open-top chambers can cause microclimate changes that might affect gas exchange physiology in T. discolor, we tested for a chamber effect on mean A n and g s. We found no difference in mean A n (t 11 = 1.976, P = 0.074) or g s (t 11 = 1.626, P = 0.132) of T. discolor in control chambers, relative to orchids in chamberless experimental plots (Fig. S1).
The relationship between VPD and stomatal conductance (g s) was determined under a series of leaf-to-air VPD values (VPDleaf; 0.4–2.6 kPa) for six orchid leaves using the LI-6400 at saturating photosynthetic photon flux density (500 μmol m−2 s−1) and C a (400 p.p.m.). In February–March 2011, three g s–VPDleaf curves were measured on leaves in control chambers (T leaf = 12–18 °C), and three curves were measured on leaves in heated chambers (T leaf = 19–22 °C). The VPD inside the cuvette was varied by adjusting the flow rate and proportion of air passing through a desiccant (calcium sulfate). The VPDleaf was increased stepwise from the lowest value obtainable under field conditions, 0.4–1.1 kPa, to a maximum VPDleaf of 1.2–2.6 kPa, with a total of 6–8 points per g s–VPDleaf curve. Rates of g s were recorded after 5–10 min at each VPDleaf. The stomatal sensitivity (m) of T. discolor was calculated using the approach described by Oren et al. (1999):
where m is equal to the slope of the relationship between g s and ln(VPDleaf) and b is a reference conductance at VPDleaf = 1 kPa.
Temperature curves
A laboratory experiment was conducted on 14–21 January 2013 to isolate the effect of temperature from the effect of VPD on photosynthesis. Plants (n = 5) were excavated from the study site with roots undisturbed in native soil and transported to the laboratory for gas exchange measurements, which were completed within a week of excavation. Plants were maintained under fluorescent light in the laboratory at 25 °C and may have partially acclimated to this higher temperature (Berry and Björkman 1980; Yamasaki et al. 2002). Our primary goal was to not to determine T opt, however, but to compare the A n–T response under conditions of constant VPDleaf to the previously reported A n–T response when VPDleaf was allowed to covary with temperature (Tissue et al. 1995). The temperature dependence of A n was analyzed under a series of leaf temperatures (10–30 °C) at C a (400 p.p.m.), under both low light (PAR = 100 μmol m−2 s−1) and saturating PAR (500 μmol m−2 s−1). Measurements were made using the LI-6400 equipped with an expanded temperature control kit (6400-88; LI-COR), which consists of two metal blocks with circulating water channels connected to a water bath to heat or cool the cuvette. The VPD inside the cuvette was held constant (1.0–1.3 kPa) during the temperature curves by adjusting the flow rate and proportion of air passing through a desiccant. Steady-state rates of photosynthesis were reached after 20–30 min at each temperature. Photosynthesis–temperature response curves were fitted to determine T opt using a quadratic polynomial equation.
Foliar C isotope ratios
C isotope ratios (δ13C) of plant leaves provide a time-integrated measure of the ratio of C i to atmospheric CO2 concentration (c i/c a), which is dependent on seasonal regulation of stomata and photosynthetic demand for CO2 (Farquhar et al. 1989; Ehleringer 1991). Leaves (n = 5–6) of T. discolor were collected from the experimental chambers on 24 March 2011 and 3 April 2012 for C isotope analyses. Leaves were oven-dried at 70 °C and ground to a fine powder in liquid N. When necessary, two leaves were pooled to ensure adequate tissue was available for analysis. The δ13C of foliar tissue was analyzed with an elemental analyzer (model 1110; Carla Erba, Milan) coupled to a Thermo-Finnigan Delta Plus gas isotope mass spectrometer (Bremen, Germany) at the Stable Isotope Mass Spectrometry Laboratory (Kansas State University, Manhattan, KS). Values of δ13C were calculated according to standard δ notation:
where R is the ratio of the heavy isotope (13C) to the lighter isotope (12C). The standard was belemnite carbonate from the Pee Dee formation, South Carolina, and the precision of the δ13C measurements was ±0.15 ‰.
Statistical analyses
Seasonal differences in VPD among the experimental chambers were analyzed with two-way ANOVAs. The effects of warming on environmental conditions in the experimental chambers (VPD, REW), relative growth rate, foliar δ13C, and orchid reproductive fitness (total flowering stalks per leaf, flowers per stalk, fruits per stalk) were analyzed with least squares regression, as appropriate for the experimental design. Differences in biophysical and biochemical photosynthetic parameters between heated and control chambers were determined using a Student’s t-test. One-way ANOVAs were used to determine if there were differences in flowering and fruiting phenology between heated and control plots. A generalized linear model was used to test for the effect of July temperature on total flowering stalks per leaf. All analyses were performed using JMP 9.0 (SAS Institute, Cary, NC).
Results
The temperate forest understory inside experimental open-top chambers in Duke Forest was warmed by a mean of 1.2–5 °C, relative to control chambers, during the study period (2010–2012). This level of warming corresponds well to expectations of temperature increases for the US over the next century, which ranges from 2 to 6 °C (Karl et al. 2009). Heating did not significantly affect the REW of soil in the experimental chambers over the study period (F 1,11 = 3.19, P = 0.104).
As a direct effect of temperature manipulation, VPD was also significantly higher inside the heated chambers and ranged from a mean of 0.18–0.53 kPa above controls (F 1,11 = 40.79, P < 0.0001). This effect closely matched the increase expected due to heating of ambient air without any addition of water vapor (Fig. S2a). The effect of warming on VPD was greater in summer (June–August) than in winter (December–February) both years (F 1,11 ≥ 100.55, P < 0.0001; Fig. S2b), as expected from the curvilinear relationship between temperature and the saturated vapor pressure of air (e sat). Mean daily summer VPD was 0.85 ± 0.03 kPa above controls in the hottest chamber, compared to an increase of 0.26 ± 0.02 kPa in mean daily winter VPD. This difference mirrors the climatic difference in the temperature–VPD relationship between summer and winter seasons in Duke Forest over the last 12 years (Fig. S2c), where the range in mean monthly VPD is larger in the summer than the winter. Mean daytime VPD in Duke Forest in winter was rarely higher than 1.5 kPa throughout the study period, whereas mean daytime VPD is frequently higher in summer and can reach values over 2.5 kPa (data not shown).
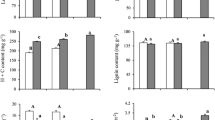
Temperature had a significant negative effect on the number of flowering stalks produced per individual (r 2 = 0.34, P = 0.024), the number of flowers per stalk (r 2 = 0.76, P < 0.001), and the number of fruits per stalk (r 2 = 0.66, P = 0.027) in 2010–2012 (Fig. 1). Mean July temperature had a significant negative effect on the proportion of individuals producing flowering stalks (\(\chi_{1}^{2}\) = 6.72, P = 0.010). Only individuals at +0 and +1.2 °C produced flowers that developed into fruits in 2010–2011, whereas flowering stalks aborted before any flowers or fruits were produced for orchids growing in chambers heated above +3.0 °C. In the +2.5 °C chamber, flowers were produced on one flowering stalk in 2010 and 2011, but the flowers died without producing fruits in both years. Only one individual flowered in the heated chambers in 2010, so it was not possible to analyze the effect of temperature on flowering time in that year. In 2011, warming of 1.2 °C significantly delayed the onset of flowering in T. discolor by an average of 10 days (F 1,15 = 39.97, P < 0.0001) and fruiting by an average of 5 days (F 1,10 = 5.73, P = 0.040, Fig. S4). A power failure caused chamber heating to fail from 11 to 24 July 2012, which restricted warming to a maximum of +2.1 °C and provided the opportunity to better understand the importance of July temperature on flowering in T. discolor. Loss of heating at this critical time for flowering doubled the number of flowering stalks in the chambers (24 in 2012 vs. 12 in 2010 and 2011) and increased the mean number of flowers per stalk (26 in 2012 vs. 11 in 2010, 12 in 2011; F 1,47 = 7.92, P = 0.001).
The effect of experimental warming on a the number of flowering stalks per leaf (y = −0.047x + 1.45, r 2 = 0.34, P = 0.024), b mean flowers per stalk (y = −8.39x + 248.38, r 2 = 0.76, P < 0.001), and c mean fruits per stalk [ln(y) = −1.59x + 42.84, r 2 = 0.66, P = 0.027] for each experimental chamber containing reproductive individuals (n = 1–8) of Tipularia discolor in 2010–2012. A power failure prevented chamber heating from 11 to 24 July 2012, so mean July temperatures in 2012 are lower than in 2010 and 2011. Relationships are fit using linear regression, except for c which was fit using a two-parameter exponential function. In c, the linear regression was also significant (y = −2.32x + 66.73, r 2 = 0.54, P = 0.006)
Increases in total leaf area of T. discolor were observed in all chambers from 2010 to 2012, due to increases in leaf number and/or leaf size (depending on the chamber). Contrary to our hypothesis, chamber temperature was negatively correlated to relative growth rate of T. discolor (r 2 = 0.51, P = 0.031; Fig. 2). Warming of 4.4 °C reduced relative growth rates by about 60 %. There was no difference between relative growth rates in the two control chambers and chamberless experimental plots (0.41 and 0.34 vs. 0.40 cm2 cm−2 yr−1).
Relationship between mean air temperature and relative growth rate of chamber leaf area [RGR LA; cm2 cm−2 yr−1] of T. discolor from 2010 to 2012 (y = −0.058x + 1.18, r 2 = 0.51, P = 0.031). Values are means of two to 44 leaves per chamber. Black circles control chambers, gray circles heated chambers
Experimental warming significantly increased biochemical photosynthetic rates in T. discolor (Table 1; Fig. S3). Higher growth temperatures resulted in a 1.4- to 1.7-fold increase in mean V cmax (t 9 = 3.96, P = 0.004), J (t 9 = 5.49, P = 0.001), and TPU (t 9 = 10.77, P < 0.0001), but did not affect R d (t 9 = 0.64, P = 0.541) or g m (t 9 = 1.27, P = 0.240; Table S2). As a result, mean A max was significantly higher in the heated chambers than in the control chambers (10.8 vs. 7.4 μmol m−2 s−1, respectively, t 9 = 6.39, P = 0.0002; Table 1). Maximum in situ A n was 4.3–5.6 μmol CO2 m−2 s−1 and occurred at a temperature of 12 °C under low light conditions (Fig. 3a). Under saturating light, maximum in situ A n was 8.2–11.0 μmol CO2 m−2 s−1 and occurred at a temperature of 16.9 °C (Fig. 3a). While the peak temperature for A n differed by about 5 °C between high and low irradiance levels under field conditions, the decline in A n at high temperatures was observed at similar values of VPDleaf (1.3 kPa). When VPDleaf was held constant in laboratory measurements, there was a decline in A n above the T opt of 19.1 °C under low light and 19.8 °C under saturating light (Fig. 3b). Irradiance did not significantly affect T opt in T. discolor under laboratory conditions (t 9 = 0.73, P = 0.489).
a Relationship between leaf temperature (°C) and mean in situ net CO2 assimilation (A n) of T. discolor leaves (n = 5) in experimental chambers from 2010 to 2012. Low light [photosynthetically active radiation (PAR) = 100 μmol m−2 s−1] is shown as circles (r 2 = 0.49, P = 0.001), and saturating light (PAR = 450–500 μmol m−2 s−1) as inverted triangles (r 2 = 0.30, P = 0.021). Symbols are open when VPDleaf > 1.3 kPa. b Relationship between leaf temperature and A n of orchid leaves measured in the laboratory under constant VPDleaf (1.0–1.3 kPa). Low light (PAR = 100 μmol m−2 s−1) is shown as circles (r 2 = 0.57, P < 0.001), and saturating light (PAR = 500 μmol m−2 s−1) is shown as inverted triangles (r 2 = 0.75, P < 0.001)
The effect of experimental warming on in situ rates of photosynthesis was dependent on VPDleaf. When leaves were measured under constant temperature but varying VPDleaf, A n was negatively correlated with g s in five of six leaves (r 2 ≥ 0.81, P ≤ 0.006; Fig. 4). In T. discolor, g s was highly sensitive to VPDleaf (r 2 = 0.63, P < 0.0001; Fig. 5a). In situ g s varied significantly with sampling date (F 8,256 = 37.24, P < 0.0001), and the range of g s was lower for orchids in heated chambers than for those in control chambers (1–198 vs. 13–240 mmol m−2 s−1; Fig. 5b). The critical threshold for g s in T. discolor is 1.3 kPa, because g s was restricted to 10–40 % of maximum conductance at VPDleaf greater than 1.3 kPa (Fig. 5b). Over the study period of 2010–2012, there was a positive correlation between mean daily VPD and the C isotope ratios of T. discolor leaves (r 2 = 0.40, P = 0.037, Fig. 6). Differences in mean δ13C ranged from −30.4 ‰ in a control chamber to −27.6 ‰ in a chamber maintained at 3.9 °C above ambient.
Relationship between stomatal conductance (g s) and A n of T. discolor leaves in Duke Forest. Leaves were measured under constant temperatures inside control (black symbols) and heated (gray symbols) chambers. Significant linear regressions are indicated by solid lines (r 2 ≥ 0.81, P ≤ 0.006). Note log scale used for g s
The relationship between leaf-to-air vapor pressure deficit (VPDleaf) and g s of T. discolor leaves in Duke Forest under a saturating light (500 μmol m−2 s−1) and b low light (100 μmol m−2 s−1) for control (black circles) and heated (gray circles) chambers. The dotted line at 1.3 kPa represents the VPDleaf at which stomatal closure restricted g s. In a, each g s–VPDleaf curve (n = 3 per treatment) was obtained at a temperature of 12–18 °C (control) or 19–22 °C (heated) and ambient CO2 concentration of 400 p.p.m. in February–March 2011. The mean relationship between ln(VPDleaf) and g s is plotted [y = −118 ln(x) + 124, r 2 = 0.63, P < 0.001]. In b, each value is the g s of an individual leaf (n = 161) measured under chamber conditions during winter of 2010–2011
Discussion
Experimentally increased temperature had a negative effect on reproduction (Fig. 1) and growth (Fig. 2) of the temperate terrestrial orchid, T. discolor. Our results show that reproductive failure is likely to strongly limit the success of this species as climate warms in the future. Experimental warming of 2.5 °C resulted in a complete failure to produce fruits (Fig. 1c). The decline in growth rate of orchids with increasing temperature (Fig. 2) will likely have a smaller effect on species success, considering that growth rates were positive under all treatments. It is important to note that the experimental chambers excluded large herbivores (i.e., deer), however. Artificial defoliation has been shown to have negative effects on carbohydrate reserves in T. discolor (Zimmerman and Whigham 1992), indicating that exclusion of herbivory contributed to the positive growth rates in our study. Disproportionate effects of warming on reproduction, relative to growth, are consistent with the finding that artificial reduction of orchid storage organs reduces flowering more than growth (Zimmerman 1990). Persistence of T. discolor will become increasingly dependent on vegetative growth of established clones, rather than sexual reproduction, especially along the southern edge of its range.
Although photosynthesis of T. discolor occurs only in winter, flowering occurs in late summer when plants are leafless. At ambient temperatures this species produced an average of 30.8 ± 1.2 flowers and 10.7 ± 1.0 fruits per plant (n = 24), similar to observations by Snow and Whigham (1989). With warming of 1.2–2.6 °C, reproduction was drastically reduced to a mean of 11.5–20 flowers per plant and from zero to 0.3 fruits per plant (Fig. 1). We observed the formation of flowering stalks in chambers warmed by >3 °C, but these elongating stalks and floral buds quickly senesced and died before any flowers were produced. The cost of fruit production is double the cost of producing an inflorescence in T. discolor (Snow and Whigham 1989), so the larger reduction in fruit production in this experiment may be indicative of C limitation. Carbohydrate content of corms is an important determinant of flowering in T. discolor (Zimmerman and Whigham 1992), but temperature can also directly affect flowering in orchids (Blanchard and Runkle 2006). Exposure to 29 °C for 8 h or longer inhibits flowering in several Phaleonopsis hybrids (Newton and Runkle 2009). Thus, the elimination of reproduction in this experiment could be caused directly by a temperature effect on signaling pathways (Posé et al. 2013) or indirectly by a reduction in carbohydrate storage (Zimmerman and Whigham 1992) or both.
This response of orchid reproduction to a few degrees of warming is consistent with the current distribution of T. discolor in the eastern US. The southern limit of the species distribution is northern Florida (FL3 climate division), where mean July temperature is 2.3 °C warmer than the study site [NC4 climate division (Lawrimore et al. 2011)]. T. discolor is currently listed as threatened in Florida (Coile and Garland 2003), and the species will become increasingly rare as reproduction becomes inhibited by warming within this century. It has been recognized that conservation of orchid species may require translocation of propagated individuals to favorable sites (Swarts and Dixon 2009). Our results indicate that locations for successful reproduction of T. discolor must have a mean July temperature below 29 °C (Fig. 1). It is likely that June temperature also affects flowering in T. discolor, since the flowering response to warming differed between years with similar mean July temperatures (2010 and 2011; Fig. 1).
Warming effects on reproductive phenology
Experimental warming of 1.2 °C delayed the onset of flowering in T. discolor by 10 days and delayed fruiting by 5 days (Fig. S4). While many species flower earlier with warming (Fitter and Fitter 2002; Dunne et al. 2003; Parmesan and Yohe 2003), including most terrestrial orchids (Molnar et al. 2012), other species show no response to warming (Bradley et al. 1999) or delays in flowering (Hollister et al. 2005; Sherry et al. 2007; Yu et al. 2010; Dorji et al. 2013). Delayed phenology may be related to timing of reproduction in species that flower after the peak summer temperature (Sherry et al. 2007), as is the case for T. discolor. While warming in the spring increases the developmental rates of plants, warming in mid-summer can exceed optimal temperatures for reproduction and suspend reproductive development (Sherry et al. 2007), as observed here.
Speculations on possible disruptions of plant–pollinator interactions due to climate change are frequent in the literature (Parmesan 2006; Hegland et al. 2009). Could delayed flowering of T. discolor in the future cause a temporal mismatch with its pollinator, P. unipuncta? Pollination of T. discolor is important for maintaining the orchid population, as selfing is thought to be rare in this species (Whigham and McWethy 1980; Snow and Whigham 1989). It is not likely that T. discolor could easily switch from one mutualist partner to another, because there are few pollinator species active while this orchid is flowering (Whigham and McWethy 1980). The upper temperature limit for survival of P. unipuncta is 31 °C (Guppy 1969), and high temperatures are thought to limit populations of P. unipuncta in the southern US (Callahan and Chapin 1960; McLaughlin 1962). Since the upper temperature limit for flowering in T. discolor is 29 °C (Fig. 1), global warming can be predicted to restrict T. discolor before it restricts P. unipuncta. No mismatch in the plant–pollinator relationship should occur as long as the phenological responses to warming in both species shift linearly and at parallel magnitudes (Hegland et al. 2009). Further research is required to determine the likelihood of a future plant–pollinator mismatch between T. discolor and P. unipuncta.
Warming effects on the C balance of winter-active species
Warming caused a decline in relative growth rate of T. discolor (Fig. 2), which is contrary to the temperature response observed in most species (Arft et al. 1999; Rustad et al. 2001; Wu et al. 2011) and is particularly surprising considering that this species is photosynthetically active only in winter, when temperatures are usually well below the optimum for photosynthesis. Specifically, maximum A n was observed at 26 °C in a previous study (Tissue et al. 1995) and at 19–20 °C in this study (Fig. 3b), both of which are higher than maximum daytime air temperatures on 130 of the 180 days from November 2010 to April 2011. If we consider only the direct effects of temperature on assimilation, warming should substantially enhance C gain in T. discolor, since orchids in heated chambers had higher biochemical rates of photosynthesis (e.g., V cmax, J, TPU) than in control chambers (Table 1; Fig. S3). Clearly, the optimum temperature for photosynthesis was higher than the optimum temperature for growth of T. discolor in our study. This decoupling has not been well recognized, although it has also been found in other C3 species with low growth temperatures (Yamori et al. 2014).
Most evergreen and summer-active species, including deciduous trees, coniferous trees, shrubs, and herbaceous plants, acclimate to maintain high C uptake across a range of growth temperatures (Kattge and Knorr 2007; Smith and Dukes 2013). The resulting shift in the T opt is generally less than one half of the shift in growth temperature (Berry and Björkman 1980; Skillman 1994), though at least one winter-active species, the orchid Aplectrum hyemale, exhibits a large shift in T opt of about 15 °C throughout its growing season (Adams 1970). Unlike these species, T. discolor had no detectable acclimation of photosynthesis to seasonal fluctuations in temperature in an earlier study (Tissue et al. 1995). Other species with little or no temperature acclimation tend to be spring ephemeral species (Lapointe 2001), which can have optimal growth rates at cold, rather than warm temperatures (Lapointe and Lerat 2006; Badri et al. 2007; Bernatchez and Lapointe 2012). The inability of such cold-adapted species to balance the ratio of net C assimilation to dark respiration (A n/R) across different growth temperatures may prevent maintenance of C homeostasis as climate warms in the future (Atkin et al. 2006; Way and Sage 2008; Gandin et al. 2011).
Changes in photosynthesis, respiration, stomatal processes, and carbohydrate storage with warming are central to understanding why temperature decreased growth of T. discolor in heated chambers (Fig. 2). Respiration generally doubles for every 10 °C increase in temperature (i.e., Q 10 ≈ 2.0) in the absence of acclimation (Atkin and Tjoelker 2003), which would have negative effects on C balance. We found no increase in R d of heated leaves relative to control leaves (Table 1), suggesting acclimation of respiration, which commonly occurs and leads to homeostasis of plant C balance (Atkin and Tjoelker 2003). Admittedly, the temperature response of R d may not be equivalent to that of nighttime respiration (Atkin et al. 2000; Way and Sage 2008) or to respiration of corms, so warming could have increased respiration of orchids in this experiment.
Although warmer temperatures had positive direct effects on C assimilation of T. discolor (Table 1; Fig. S3), this is counteracted by the response of stomata to atmospheric VPD (Figs. 4, 5, 6), which reduces or negates these benefits. It is well established that g s decreases exponentially with increasing VPD (Monteith 1995; Oren et al. 1999), and reductions in g s as a function of higher VPD at higher temperature are expected (Duursma et al. 2013). Here, we found that the stomata of T. discolor were more sensitive to VPD than the stomata of most mesic species (Oren et al. 1999). The slope of the g s–VPD relationship for T. discolor was −0.95 (Fig. 5a), whereas the mean slope of this relationship is consistently −0.6 for mesic forest species (Oren et al. 1999). This is particularly relevant to this study because VPD was significantly elevated by the warming treatments (Fig. S2a), causing sufficient declines in g s to substantially reduce A n (Figs. 3a, 4) and cause a downward shift in the T opt of T. discolor, at least under some conditions. The greater stomatal limitation of photosynthesis in heated chambers was further supported by the higher δ13C of leaves, compared to orchids in control chambers (Fig. 6).
Although stomatal response to VPD contributed a negative effect on plant C balance in the heated chambers, other factors may also have contributed to the decline in growth of T. discolor with warming (Fig. 2). Work with spring ephemeral species indicates that reduced growth under warmer temperatures can be controlled by source–sink imbalances, where faster starch accumulation and smaller sink capacity leads to early saturation of the storage organ (i.e., bulb), causing soluble sugars to accumulate in the leaf and induce leaf senescence before the bulb has grown to full size (Gandin et al. 2011). As in spring ephemerals, the majority of the biomass in T. discolor is allocated to below-ground storage in corms (Whigham 1984) that provide carbohydrates to support leaf formation and reproduction (Zimmerman and Whigham 1992; Tissue et al. 1995). If carbohydrate accumulation increased in orchid corms with warming, a similar mechanism may have contributed to our observations. High carbohydrate accumulation is not likely, however, given the negative effect of stomatal responses (Figs. 4, 5, 6) and possibly increased respiration on plant C balance. We also observed that leaf senescence occurred later in heated leaves than in control leaves (Marchin, unpublished data). Rather, the reduced growth of orchids observed here suggests that higher temperature and VPD resulted in lower carbohydrate accumulation in corms. Carbohydrate reserves in corms are known to affect the size of T. discolor leaves formed in autumn, where experimental reductions in corm mass by about 30 % significantly decreased leaf size relative to unmanipulated plants (Zimmerman and Whigham 1992).
Growth of terrestrial orchids is sensitive to changes in soil moisture (Liu et al. 2010), but it appears that this did not substantially contribute to the warming effects we observed. Higher temperature and VPD results in greater evaporation of water from soils, but there was no detectable effect of warming on REW in this study. We did not measure predawn leaf water potential of any T. discolor leaves, but measurements of predawn leaf water potential of tree seedlings did not reveal any difference in soil water potential among chambers in the summer (Marchin 2013). These results are not surprising, because soil evaporation accounts for only 10 % of water losses in this system (Oishi et al. 2008). The water budget is dominated by transpiration of the canopy, which was not exposed to the warming treatment.
Increases in VPD tend to accompany increases in temperature (Day 2000; Will et al. 2013), so the two parameters are often confounded in natural and experimental systems. For example, warming of 1.2–5 °C in this experiment was accompanied by a concurrent increase in VPD of 0.18–0.53 kPa, which closely followed expected vapor pressure changes due to heating based on the relationship between temperature and e sat (Fig. S2a). Our results emphasize the importance of explicitly accounting for changes in VPD when estimating temperature responses of plant species under future warming scenarios. If relative humidity remains fairly constant in the future (e.g., Trenberth et al. 2005), atmospheric VPD in mesic forests will increase with global warming. In such a scenario or in any scenario in which precipitation decreases, climate change will likely have a negative impact on this species, and perhaps other winter-active species where the T opt of photosynthesis is not a good predictor of plant responses to warming. Increased atmospheric CO2 concentrations may at least partially offset growth reductions in T. discolor, as photosynthesis of T. discolor is not CO2 saturated at current atmospheric CO2 concentrations (Fig. S3).
References
Adams MS (1970) Adaptations of Aplectrum hyemale to environment: effects of preconditioning temperature on net photosynthesis. Bull Torrey Bot Club 97:219–224
Arft AM, Walker MD, Gurevitch J, Alatalo JM, Bret-Harte MS, Dale M, Diemer M, Gugerli F, Henry GHR, Jones MH, Hollister RD, Jonsdottir IS, Laine K, Levesque E, Marion GM, Molau U, Molgaard P, Nordenhall U, Raszhivin V, Robinson CH, Starr G, Stenstrom A, Stenstrom M, Totland O, Turner PL, Walker LJ, Webber PJ, Welker JM, Wookey PA (1999) Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecol Monogr 69:491–511
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351
Atkin OK, Holly C, Ball MC (2000) Acclimation of snow gum (Eucalyptus pauciflora) leaf respiration to seasonal and diurnal variations in temperature: the importance of changes in the capacity and temperature sensitivity of respiration. Plant Cell Environ 23:15–26
Atkin OK, Scheurwater I, Pons TL (2006) High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob Change Biol 12:500–515
Badri MA, Minchin PEH, Lapointe L (2007) Effects of temperature on the growth of spring ephemerals: crocus vernus. Physiol Plant 130:67–76
Bernatchez A, Lapointe L (2012) Cooler temperatures favour growth of wild leek (Allium tricoccum), a deciduous forest spring ephemeral. Botany 90:1125–1132
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol Plant Mol Biol 31:491–543
Blanchard MG, Runkle ES (2006) Temperature during the day, but not during the night, controls flowering of Phalaenopsis orchids. J Exp Bot 57:4043–4049
Boyles RP, Raman S (2003) Analysis of climate trends in North Carolina (1949-1998). Environ Int 29:263–275
Bradley NL, Leopold AC, Ross J, Huffaker W (1999) Phenological changes reflect climate change in Wisconsin. Proc Natl Acad Sci USA 96:9701–9704
Brown PM (1997) Wild orchids of the Northeastern United States. Cornell University Press, Ithaca
Callahan P, Chapin JB (1960) Morphology of the reproductive systems and mating in two representative members of the family Noctuidae, Pseudaletia unipuncta and Peridroma margaritosa, with comparison to Heliothis zea. Ann Entomol Soc Am 53:763–782
Coile NC, Garland MA (2003) Notes on Florida’s endangered and threatened plants, botany contribution no 38, 4th edn. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville
Day ME (2000) Influence of temperature and leaf-to-air vapor pressure deficit on net photosynthesis and stomatal conductance in red spruce (Picea rubens). Tree Physiol 20:57–63
De Frenne P, Brunet J, Shevtsova A, Kolb A, Graae BJ, Chabrerie O, Cousins SA, Decocq G, De Schrijver A, Diekmann M, Gruwez R, Heinken T, Hermy M, Nilsson C, Stanton S, Tack W, Willaert J, Verheyen K (2011) Temperature effects on forest herbs assessed by warming and transplant experiments along a latitudinal gradient. Glob Change Biol 17:3240–3253
Dorji T, Totland Ã, Moe SR, Hopping KA, Pan J, Klein JA (2013) Plant functional traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet. Glob Change Biol 19:459–472
Dormann CF, Woodin SJ (2002) Climate change in the Arctic: using plant functional types in a meta-analysis of field experiments. Funct Ecol 16:4–17
Dunne JA, Harte J, Taylor KJ (2003) Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecol Monogr 73:69–86
Duursma RA, Payton P, Bange MP, Broughton KJ, Smith RA, Medlyn BE, Tissue DT (2013) Near-optimal response of instantaneous transpiration efficiency to vapour pressure deficit, temperature and CO2 in cotton (Gossypium hirsutum L.). Agric For Meteorol 168:168–176
Ehleringer JR (1991) 13C/12C fractionation and its utility in terrestrial plant studies. In: Coleman D, Fry B (eds) Carbon isotope techniques. Chapman and Hall, New York, pp 187–200
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Fitter AH, Fitter RSR (2002) Rapid changes in flowering time in British plants. Science 296:1689–1691
Flexas J, Díaz-Espejo A, Berry JA, Cifre J, Galmes J, Kaidenhoff R, Medrano H, Ribas-Carbo M (2007) Analysis of leakage in IRGA’s leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. J Exp Bot 58:1533–1543
Frye CT (1993) Population genetics and ecology of the clonal orchid Tipularia discolor (Pursh) Nutt. (Orchidaceae). Master’s thesis, Wake Forest University, Winston-Salem
Gandin A, Gutjahr S, Dizengremel P, Lapointe L (2011) Source-sink imbalance increases with growth temperature in the spring geophyte Erythronium americanum. J Exp Bot 62:3467–3479
Guppy JC (1969) Some effects of temperature on immature stages of armyworm, Pseudaletia unipuncta (Lepidoptera–Noctuidae), under controlled conditions. Can Entomol 101:1320–1327
Hegland SJ, Nielsen A, Lazaro A, Bjerknes AL, Totland O (2009) How does climate warming affect plant–pollinator interactions? Ecol Lett 12:184–195
Hoffmann WA, Poorter H (2002) Avoiding bias in calculations of relative growth rate. Ann Bot 90:37–42
Hollister RD, Webber PJ, Bay C (2005) Plant response to temperature in northern Alaska: implications for predicting vegetation change. Ecology 86:1562–1570
Karl TR, Melillo JM, Peterson TC (2009) Global climate change impacts in the United States. US Global change research program. Cambridge University Press, New York
Kattge J, Knorr W (2007) Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant Cell Environ 30:1176–1190
Klady RA, Henry GHR, Lemay V (2011) Changes in high arctic tundra plant reproduction in response to long-term experimental warming. Glob Change Biol 17:1611–1624
Lapointe L (2001) How phenology influences physiology in deciduous forest spring ephemerals. Physiol Plant 113:151–157
Lapointe L, Lerat S (2006) Annual growth of the spring ephemeral Erythronium americanum as a function of temperature and mycorrhizal status. Can J Bot 84:39–48
Lawrimore JH, Menne MJ, Gleason BE, Williams CN, Wuertz DB, Vose RS, Rennie J (2011) An overview of the global historical climatology network monthly mean temperature data set, version 3. J Geophys Res Atmos 116
Liu H, Feng CL, Luo YB, Chen BS, Wang ZS, Gu HY (2010) Potential challenges of climate change to orchid conservation in a wild orchid hotspot in southwestern China. Bot Rev 76:174–192
Lynch IP (2006) The Duke Forest at 75: a resource for all seasons. Office of the Duke Forest, Durham
Marchin RM (2013) Using a physiological approach to improve predictions of climate change effects on temperate forests. PhD dissertation, Department of Plant Biology, North Carolina State University
McLaughlin RE (1962) Effect of temperature upon larval mortality of armyworm, Pseudaletia unipuncta (Haworth). J Insect Pathol 4:279
Menzel A, Sparks TH, Estrella N, Roy DB (2006) Altered geographic and temporal variability in phenology in response to climate change. Glob Ecol Biogeogr 15:498–504
Molnar AV, Tokolyi J, Vegvari Z, Sramko G, Sulyok J, Barta Z (2012) Pollination mode predicts phenological response to climate change in terrestrial orchids: a case study from central Europe. J Ecol 100:1141–1152
Monteith JL (1995) A reinterpretation of stomatal responses to humidity. Plant Cell Environ 18:357–364
Newton LA, Runkle ES (2009) High-temperature inhibition of flowering of Phalaenopsis and Doritaenopsis orchids. Hortic Sci 44:1271–1276
NOAA (2012) State of the climate: national overview for February 2012, vol. 2013. NOAA National Climatic Data Center
Oishi AC, Oren R, Stoy PC (2008) Estimating components of forest evapotranspiration: a footprint approach for scaling sap flux measurements. Agric For Meteorol 148:1719–1732
Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schafer KVR (1999) Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ 22:1515–1526
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Pelini SL, Bowles FP, Ellison AM, Gotelli NJ, Sanders NJ, Dunn RR (2011) Heating up the forest: open-top chamber warming manipulation of arthropod communities at Harvard and Duke Forests. Methods Ecol Evol 2:534–540
Pfeifer M, Wiegand K, Heinrich W, Jetschke G (2006) Long-term demographic fluctuations in an orchid species driven by weather: implications for conservation planning. J Appl Ecol 43:313–324
Posé D, Verhage L, Ott F, Yant L, Mathieu J, Angenent GC, Immink RGH, Schmid M (2013) Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature 503:414–417
Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Annu Rev Ecol Syst 16:179–214
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30:1035–1040
Sherry RA, Zhou XH, Gu SL, Arnone JA, Schimel DS, Verburg PS, Wallace LL, Luo YQ (2007) Divergence of reproductive phenology under climate warming. Proc Natl Acad Sci USA 104:198–202
Skillman JB (1994) Environmental effects on photosynthesis and photoinhibition in evergreen perennials native to a seasonal habitat. PhD dissertation, Duke University, Durham
Smith NG, Dukes JS (2013) Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Glob Change Biol 19:45–63
Smith JL, Hunter KL, Hunter RB (2002) Genetic variation in the terrestrial orchid Tipularia discolor. Southeast Nat 1:17–26
Snow AA, Whigham DF (1989) Costs of flower and fruit production in Tipularia discolor (Orchidaceae). Ecology 70:1286–1293
Sparks TH, Jeffree EP, Jeffree CE (2000) An examination of the relationship between flowering times and temperature at the national scale using long-term phenological records from the UK. Int J Biometeorol 44:82–87
Swarts ND, Dixon KW (2009) Terrestrial orchid conservation in the age of extinction. Ann Bot 104:543–556
Tissue DT, Skillman JB, McDonald EP, Strain BR (1995) Photosynthesis and carbon allocation in Tipularia discolor (Orchidaceae), a wintergreen understory herb. Am J Bot 82:1249–1256
Totland Ø, Alatalo JM (2002) Effects of temperature and date of snowmelt on growth, reproduction, and flowering phenology in the arctic/alpine herb, Ranunculus glacialis. Oecologia 133:168–175
Trenberth KE, Fasullo J, Smith L (2005) Trends and variability in column-integrated atmospheric water vapor. Clim Dyn 24:741–758
Way DA, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–688
Way DA, Sage RF (2008) Elevated growth temperatures reduce the carbon gain of black spruce [Picea mariana (Mill.) B.S.P.]. Glob Change Biol 14:624–636
Went FW (1953) The effect of temperature on plant growth. Annu Rev Plant Physiol Plant Mol Biol 4:347–362
Whigham DF (1984) Biomass and nutrient allocation of Tipularia discolor (Orchidaceae). Oikos 42:303–313
Whigham DF, McWethy M (1980) Studies on the pollination ecology of Tipularia discolor (Orchidaceae). Am J Bot 67:550–555
Will RE, Wilson SM, Zou CB, Hennessey TC (2013) Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest-grassland ecotone. New Phytol 200:366–374
Wu ZT, Dijkstra P, Koch GW, Penuelas J, Hungate BA (2011) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob Change Biol 17:927–942
Yamasaki T, Yamakawa T, Yamane Y, Koike H, Satoh K, Katoh S (2002) Temperature acclimation of photosynthesis and related changes in photosystem II electron transport in winter wheat. Plant Physiol 128:1087–1097
Yamori W, Hikosaka K, Way DA (2014) Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth Res 119:101–117
Yu HY, Luedeling E, Xu JC (2010) Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proc Natl Acad Sci USA 107:22151–22156
Zimmerman JK (1990) Role of pseudobulbs in growth and flowering of Catasetum viridiflavum (Orchidaceae). Am J Bot 77:533–542
Zimmerman JK, Whigham DF (1992) Ecological functions of carbohydrates stored in corms of Tipularia discolor (Orchidaceae). Funct Ecol 6:575–581
Acknowledgments
We thank Mark Boudreau and Lauren Nichols for maintaining the experimental site and Jesse Nippert for processing the C isotope data. The Hoffmann lab group, three anonymous reviewers, and Joy Ward provided helpful comments on the manuscript. The experimental warming site is funded by a US DOE PER award (DE-FG02-08ER64510) to R. R. Dunn, A. M. Ellison, N. J. Gotelli, and N. J. Sanders. This study was funded by the National Institute of Climate Change Research (NICCR-DE-FC02-06ER64156), and this publication was developed under STAR Fellowship Assistance Agreement no. F09A10379 awarded by the US Environmental Protection Agency (EPA). It has not been formally reviewed by EPA. The views expressed in this publication are solely those of R. M. Marchin, and EPA does not endorse any products or commercial services mentioned in this publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Joy K. Ward.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marchin, R.M., Dunn, R.R. & Hoffmann, W.A. Are winter-active species vulnerable to climate warming? A case study with the wintergreen terrestrial orchid, Tipularia discolor . Oecologia 176, 1161–1172 (2014). https://doi.org/10.1007/s00442-014-3074-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3074-8