Abstract
Experimental autoimmune orchitis (EAO) is a chronic inflammatory disorder that causes progressive spermatogenic impairment. EAO is characterized by high intratesticular levels of nitric oxide (NO) and tumor necrosis factor alpha (TNFα) causing germ cell apoptosis and Sertoli cell dysfunction. However, the impact of this inflammatory milieu on the spermatogenic wave is unknown. Therefore, we studied the effect of inflammation on spermatogonia and preleptotene spermatocyte cell cycle progression in an EAO context and through the intratesticular DETA-NO and TNFα injection in the normal rat testes. In EAO, premeiotic germ cell proliferation is limited as a consequence of the undifferentiated spermatogonia (CD9+) cell cycle arrest in G2/M and the reduced number of differentiated spermatogonia (c-kit+) and preleptotene spermatocytes that enter in the meiotic S-phase. Although inflammation disrupts spermatogenesis in EAO, it is maintained in some seminiferous tubules at XIV and VII–VIII stages of the epithelial cell cycle, thereby guaranteeing sperm production. We found that DETA-NO (2 mM) injected in normal testes arrests spermatogonia and preleptotene spermatocyte cell cycle; this effect reduces the number of proliferative spermatogonia and the number of preleptotene spermatocytes in meiosis S-phase (36 h after). The temporal inhibition of spermatogonia clonal amplification delayed progression of the spermatogenic wave (5 days after) finally altering spermatogenesis. TNFα (0.5 and 1 µg) exposure did not affect premeiotic germ cell cycle or spermatogenic wave. Our results show that in EAO the inflammatory microenvironment altered spermatogenesis kinetics through premeiotic germ cell cycle arrest and that NO is a sufficient factor contributing to this phenomenon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orchitis involves spermatogenesis impairment and is a cause of infertility in men, systemic and ascending canalicular infections, trauma or toxicants are considered etiological factors (Fijak et al. 2018). Although inflammation and infection of the urogenital tract (mainly epididymitis) are usually associated with orchitis (Fijak et al. 2018), a recent study showed that the presence of pathogens and inflammatory signs within the urogenital tract was unrelated to testicular leukocyte infiltration in infertile patients (Pilatz et al. 2019). Preliminary results in testicular biopsies of patients with idiopathic infertility showed a negative correlation between the number of CD45+ immune cells and the number of undifferentiated spermatogonia (Gónzalez et al. 2018).
Inflammatory lesions in testes of infertile men occur together with signs of tubular damage, such as partial or complete loss of germinal epithelium, germ cell sloughing, thickening of the seminiferous tubules (STs) wall (Duan et al. 2011; Frungieri et al. 2002; Meinke et al. 2000), and reduced mitotic activity of spermatogonia (Steger et al. 1998).
Experimental autoimmune orchitis (EAO) is a model of autoimmune-based chronic testicular inflammation leading to severe damage of seminiferous epithelium and infertility. EAO is helpful for understanding disease development and progression mechanisms leading to spermatogenesis impairment and finally azoospermia (Lustig et al. 2020). This disease is characterized by the infiltration of the interstitium by immune cells (mainly macrophages, dendritic cells, and T cells), the formation of autoantibodies against testicular antigens, production of proinflammatory mediators, and dysregulation of steroidogenesis (Lustig et al. 2020). Proinflammatory cytokines (TNFα, NO, and IL6) highly produced by testicular macrophages impair spermatogenesis via germ cell apoptosis and Sertoli cell dysfunction (Suescun et al. 2003; Theas et al. 2008; Jarazo-Dietrich et al. 2012, 2015; Rival et al. 2006; Pérez et al. 2012). Testicular oxidative stress in orchitis results from increased activity and expression of nitric oxide synthase (Jarazo-Dietrich et al. 2012), in fact inhibition of the enzyme activity prevents EAO development (Jarazo-Dietrich et al. 2015).
However, their involvement in premeiotic germ cells [spermatogonia and preleptotene spermatocytes (PLs)] behavior has been little explored. Hakovirta et al. (1995) demonstrated in vitro that IL6 inhibited DNA synthesis of differentiated type A and B spermatogonia and PLs. Our group verified in vitro that NO and TNFα arrest the cell cycle of differentiated spermatogonia B GC-1 cell line reducing the number of cells in the mitotic metaphase (Ferreiro et al. 2019).
In the present work, we studied the impact of the inflammatory microenvironment on spermatogenesis disruption, focusing on premeiotic germ cells, undifferentiated (CD9+) differentiated (c-Kit+) spermatogonia, and PLs behavior in EAO. We also evaluated the involvement of NO and TNFα in spermatogonia and PLs proliferation and apoptosis and spermatogenesis progression.
Material and methods
Animals
Adult male inbred Wistar rats were purchased from Bioterio Central, Facultad de Farmacia y Bioquímica (Buenos Aires, Argentina). Animals were housed at 22 °C with a 12 h light, 12 h dark cycle and fed standard food pellets and water ad libitum.
Induction of EAO
Rats of the experimental (EAO) group were immunized with testicular homogenate (TH) prepared as previously described (Doncel et al. 1989). Briefly, rats were injected three times with 200 mg of TH/dose/rat at 14-day intervals. TH (0.4 ml) emulsified with complete Freund’s adjuvant (CFA, 0.4 ml) (Sigma-Aldrich, St. Louis, MO, USA) was injected intradermally in footpads and at multiple sites near ganglion regions. The first two immunizations were followed by an i.v. injection of 0.5 ml Bordetella pertussis (strain 10,536; Instituto Malbrán, Buenos Aires, Argentina) containing 10 × 1010 microorganisms and the third by i.p. injection of 5 × 109 microorganisms. EAO rats were killed 50–60 and 70–80 days after the first immunization. Normal untreated rats were killed simultaneously with EAO rats. Testes were removed and weighed and processed for the studies described below.
Histopathology
Testes Sections (4–5 µm thick) fixed in Bouin were rehydrated and stained with hematoxylin–eosin. Seminiferous tubules at stage VII–VIII of the epithelial cycle were identified by the presence of mature elongated spermatids (stage 19) and residual bodies in the lumen and stage XIV by the presence of meiotic metaphases (Leblond and Clermont 1952).
Animals immunized to induce orchitis develop focal lesion 50–60 days from the first immunization and a more severe lesion at 70–80 days (Doncel et al. 1989). We developed an EAO score (0–10) (Jarazo-Dietrich et al. 2015) in order to quantify the extent and severity of testicular damage that occurs in EAO based on the percentage of seminiferous tubules with germ cell damage, the degree of germ cell sloughing (partial or total) and the testicular weight–body weight ratio (TW/BW). Testicular weigh/BW is reduced in rats with germ cell sloughing. Although there are exceptions, i.e., testis with edema, both testis generally present similar degree of histopathological damage.
The seminiferous tubule (ST) area was calculated as the area of a circle (π x radio2) from testis section microphotographs; the radio was calculated from the average of the largest diameter and the smallest diameter of the tubule divided by two. ImageJ software was used to process and analyze the images. Seminiferous tubules were classified into four categories depending on the total area as previously described (Caneguim et al. 2009): (a) reduced (measuring 19 × 103 μm2–51 × 103 μm2), (b) small (measuring 51 × 103 μm2–83 × 103 μm2), (c) medium (measuring 83 × 103 μm2–115 × 103 μm2), and (d) large (measuring 115 × 103 μm2–147 × 103 μm2). The frequency of tubules according in each category was calculated for the groups studied. We classified and recorded 100 STs/section in 3 to 4 non-consecutive sections.
Isolation of germ cells for flow cytometry
Germ cells were isolated from the testis as previously reported (Theas et al. 2006) with some modifications, following published methods for quantification of testicular Brdu+ germ cells by flow cytometry (Krishnamurthy et al. 2000) (van Kroonenburgh et al. 1985). Albuginea was removed together with large blood vessels and testis was subjected to enzymatic digestion with collagenase (0.23 mg/ml, Worthington Biochemical Corporation, Freehold, NJ, USA) in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) (Sigma–Aldrich) for 30 min at 34 °C in a Dubnoff shaking water bath. After adding cold PBS to inactivate collagenase, seminiferous tubules were allowed to settle 3–5 min at 4 °C. The supernatant containing interstitial cells was discarded and seminiferous tubules were resuspended in 7 ml PBS containing 1% inactivated fetal bovine serum (FBS) and mechanically dissociated to a single-cell suspension using a plastic Pasteur pipette. Debris was removed by two steps of filtration through a nylon filter pore size 41 μm (Millipore, Bedford, MA). The cell suspension was passed through a 25-gauge, 1-in needle and resuspended in PBS 1% inactivated FBS, counted and centrifuged at 300 × g for 10 min at 4 °C.
Study of premeiotic germ cells in phase S by flow cytometry
Normal and EAO rats were injected i.p. with 100 mg/kg of 5-Bromo-2 Deoxyuridine (BrdU, Sigma-Aldrich) 2 h before killing. This BrdU pulse enables the study of proliferating testicular germ cells (Takubo et al. 2008). 2 × 106 cells in 50 μl of 0.15 M cold NaCl were fixed and permeabilized with 950 μl of absolute ethanol (Merck) for 30 min at 4 °C. Cells were centrifuged, washed, and treated with 1% paraformaldehyde (PFA), 0.01% Tween-20 in PBS for 1 h at room temperature (RT). Then, the cells were centrifuged and incubated with 100 U/µl DNAsa (Sigma-Aldrich) in 0.15 M NaCl, 4.2 mM MgCl2 buffer pH 5.0 for 10 min at RT. To detect the BrdU incorporated, cells were incubated with an anti-BrdU-PE antibody (PE Mouse Anti-BrdU Set 556029) or with isotype control (Mouse IgG1, κ, PE Mouse Anti-BrdU Set 556028) for 40 min at RT. Lastly, the cells were fixed in 1% PFA for 10 min and stored at 4 °C in the dark until analyzed in a Becton and Dickinson FACSCalibur (Becton and Dickinson Company, BD, Franklin Lakes, NJ, USA) flow cytometer; 100,000 to 200,000 cells/tube were acquired.
Study of premeiotic germ cells in phase S in normal and EAO testis by immunofluorescence
Frozen testis Sections (4–5 µm thick) were fixed in 4% PFA for 10 min at RT. Antigen were retrieved by heating in a microwave (3 min at 750 W, three times) in 10 mM sodium citrate buffer, pH 6.0. Sections were permeabilized with 0.1% Triton X-100 in PBS for 4 min at 4 °C; non-specific labeling was prevented by preincubating the sections with blocking solution (0.1% Triton X-100 and 3% BSA) for 30 min at RT. Then, sections were incubated with an anti-BrdU antibody (1:100, GE Healthcare Cell Proliferation Kit) or buffer provided in the kit (negative control) for 18 h at 4 °C followed by an anti-mouse FITC secondary antibody (10 µg/ml, Vector Labs Burlingame, CA, USA) for 2 h at RT in the dark. Sections were mounted with Vectashield antifade mounting medium with DAPI (Vector Labs) and observed in an Axiophot epifluorescence microscope.
Thirty STs in three non-consecutive sections of the poles and the middle regions of each testis were analyzed to quantify BrdU+ STs. BrdU+ premeiotic germ cells (spermatogonia and PLs) were counted in 50 BrdU+ STs/section. Seminiferous tubules at VII–VIII stages were identified through DAPI staining by the presence of spermatozoa and residual bodies (Leblond and Clermont 1952) and BrdU+ preleptone spermatocytes were counted.
Analysis of apoptotic germ cells by TUNEL assay
TUNEL assay was performed as previously reported (Jarazo-Dietrich et al. 2015). Briefly, testis Sections. (4–5 μm thick) were deparaffinized and hydrated by successive series of ethanol and then irradiated in a microwave oven (370 W for 5 min) in 10 mM sodium citrate buffer, pH 6.0, and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in 0.1% sodium citrate for 5 min at 4 °C. Non-specific labeling was prevented by incubating sections with blocking solution (2% blocking reagent in 150 mM NaCl and 100 mM maleic acid, pH 7.5). After 10-min incubation with terminal deoxynucleotidyl transferase (TdT) buffer (buffer TdT, 1 × ; CoCl2, 1 × , Roche), DNA was 3′-end labeled with digoxigenin-11-dideoxy-uridine triphosphate (4 μM Dig-ddUTP, Roche) by incubation with TdT (0.4 U/μl, Roche) in TdT buffer for 1 h at 37 °C. As assay control, the TdT enzyme was replaced by the same volume of TdT buffer. Sections were then incubated with blocking solution for 30 min at RT and after that with an alkaline phosphatase-conjugated anti-digoxigenin antibody (0.375 mU/μl, Roche) for 2 h at RT. Endogen phosphatase activity was prevented by incubation with 1 mM levamisole (Sigma-Aldrich). Alkaline phosphatase substrates, nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP, Roche) were added for 1 h. This reaction was stopped by washing with Tris–EDTA buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0). Sections were rinsed in 95% ethanol for 24 h at RT, light counterstained with eosin, dehydrated by successive series of ethanol and mounted.
Study of undifferentiated CD9 spermatogonia cell cycle by flow cytometry
3 × 106 cells were immunostained with a mouse anti-CD9 antibody (50 µg/ml, BD Pharmingen) or isotype for 30 min at 4 °C. The primary antibody was detected by incubation with an anti-mouse FITC antibody (10 µg/ml, Vector Labs) for 30 min at 4 °C. Then, cells were washed in PBS, fixed with 70% ethanol (Merck) by gentle vortexing and stored overnight at –20 °C; the following day the cells were washed in PBS and the DNA was stained with propidium iodide (PI) (50 μg/ml, Sigma-Aldrich) in the presence of RNAse (50 μg/ml, Sigma-Aldrich). At least 8 × 105 cells were acquired in a FACSCalibur (BD Biosciences) flow cytometer. No changes in FITC distributions occurred after ethanol and RNAse treatment and PI staining. The presence of FITC labeling did not affect DNA distributions of cells identified by a membrane antigen (Braylan et al. 1982).
CD9+ spermatogonia in G1, S, and G2 were quantified in regions corresponding to 2 C, S, and 4 C DNA content respectively (Krishnamurthy et al. 2000) (Online Resource 1).
Indirect immunoperoxidase for detection of undifferentiated CD9 spermatogonia
Sagittal testis sections (4–5 µm thick) from normal and EAO rats fixed in 10% formol were deparaffinized and rehydrated. Antigens were retrieved by heating sections in a microwave (370 W 5 min) in 10 mM sodium citrate buffer, pH 6.0. Endogenous peroxidase was blocked with 0.3% H2O2 in methanol for 30 min. Testis sections were washed in PBS and incubated with 5% normal goat serum (Vector Labs) for 45 min at RT, followed by incubation with 20% biotin blocking solution (SP-2001, Vector Labs) for 25 min at RT. Sections were incubated with a rabbit monoclonal antibody anti-CD9 (2 μg/µl) (ab92726 Abcam, Cambridge, Madison) in PBS for 48 h at 4 °C in a humidified chamber and reacted with a biotinylated goat anti-rabbit (7.5 μg/ml) (Vector Labs) diluted in PBS for 1 h at RT in the dark. The reaction was amplified with a Vectastain Elite ABC Kit (PK-6200, Vector Labs), and the reaction product was visualized by adding diaminobenzidine substrate (SK-4100, Vector Labs). As negative controls sections were incubated with isotype IgG control. Sections were counterstained with hematoxylin and mounted using DPX mounting medium (Sigma-Aldrich).
Indirect immunoperoxidase for detection of differentiated c-Kit spermatogonia
Sagittal testis sections (4–5 µm thick) from normal and EAO rats fixed in 4% PFA were deparaffinized and rehydrated. Endogenous peroxidase was blocked with 0.3% H2O2 in methanol for 30 min. Testis sections were washed in PBS and incubated with Blotto (0.1% TBS-Tween, 5% nonfat dry milk) and Avidin blocking solution (SP-2001, Vector Labs) for 30 min at RT. Sections were incubated with goat polyclonal antibody anti-c-Kit (8 μg/ml) (sc-1494 Santa Cruz Biotechnology) in TBS 0.01% Triton X-100 (Sigma-Aldrich) with 10% normal horse serum, 0.05% BSA, and 20% biotin blocking solution (SP-2001, Vector Labs) overnight at 4 °C in a humidified chamber. A biotinylated horse anti-goat rat adsorbed (7.5 μg/ml) (Vector Labs) was diluted in PBS with 5% Blotto for 1 h at RT. The reaction was amplified with a Vectastain Elite ABC Kit (PK-6200, Vector Labs), and the reaction product was visualized by adding diaminobenzidine substrate (SK-4100, Vector Labs). As negative controls sections were incubated with isotype IgG control. Sections were counterstained with hematoxylin and mounted using DPX mounting medium (Sigma-Aldrich).
Intratesticular DETA-NO injection to study premeiotic germ cells in the S-phase and spermatogenesis
NO donor DETA-NOate releases NO at a slow rate over a prolonged period (t1/2 ~ 20 h) (DETA-NO, Cayman Chemical Company). DETA-NO was dissolved 2 h before use in sterile saline solution to achieve a steady concentration of NO in solution. Normal rats were anesthetized with ketamine (100 mg/kg, i.p)-xylazine (10 mg/kg, i.p) and DETA-NO (2 mM in sterile saline solution) was injected intratesticularly in the testis parenchyma with a 30-gauge needle in a final volume of 100 μl. Testes (contralateral) injected with the same volume of saline solution were used as controls. Testes were injected once (into the upper third of the testis) or twice (upper and bottom third of the testis) at 18-h intervals and rats were killed 36 h and 120 h (5 days, d) after the first injection. Two hours before euthanasia, BrdU (100 mg/kg) was injected i.p (Muñoz-Velasco et al. 2013). Testes were removed and frozen for double immunofluorescence (BrdU-TUNEL).
Another group of normal rats was injected in the same conditions as described above and rats were sacrificed 60 days after the first injection. Testes were removed, fixed in Bouin by perfusion embedded in paraffin and sections stained with hematoxylin–eosin for histopathological analysis. The ST area was defined as previously reported (Caneguim et al. 2009); the number of STs at stage VII-VIII and XIV of the epithelial cycle was determined according to the major well-defined cell associations (Leblond and Clermont 1952).
Intratesticular TNFα injection to study premeiotic germ cells in the S-phase and spermatogenesis
To evaluate the effect of TNFα, we used the same experimental design as for DETA-NO; normal rat testis was intratesticularly injected twice with TNFα and the contralateral testis with saline. Testes were injected once (into the upper third of the testis) or twice (upper and bottom third of the testis) with 24 h interval, rats were killed 48 h or 120 h (5 days) after the first injection. The dose of TNFα was selected based on the previous reports of intratesticular administration of this cytokine (Li et al. 2006). Two hours before killing, BrdU (100 mg/kg) was i.p. injected. Testes were removed and frozen for double immunofluorescence (BrdU-TUNEL).
Another group of normal rats was injected in the same conditions as described above and rats were killed 60 days after the first injection. Testes were removed and fixed in Bouin by perfusion and stained with hematoxylin–eosin for histopathological analysis as was described above for the DETA-NO study.
Double immunofluorescence BrdU-TUNEL
Longitudinal testis sections from rats injected with DETA-NO or TNFα (4–5 μm) were deparaffinized and hydrated and then irradiated in a microwave oven (370 W for 5 min) in 10 mM sodium citrate buffer, pH 6.0, and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in 0.1% sodium citrate for 5 min at 4 °C. Non-specific labeling was prevented by incubating sections with blocking solution [2% blocking reagent, Roche (Molecular Biochemicals GmbH) in 150 mM NaCl and 100 mM maleic acid, pH 7.5] for 30 min at RT. After 10 min incubation with TdT buffer (buffer TdT, 1 × ; CoCl2, 1 × , Roche), apoptotic DNA was 3′-end labeled with Dig-ddUTP (4 μM, Roche) by incubation with TdT (0.4 U/μl, Roche) in TdT buffer for 1 h at 37 °C. As assay control, the TdT enzyme was replaced by the same volume of TdT buffer. Sections were then incubated with blocking solution for 30 min at RT, followed by detection of Dig-ddUTP with a rhodamine conjugated anti-digoxigenin antibody (20 µg/μl, Roche) for 1 h at RT.
Sections were permeabilized with 0.1% Triton X-100 in PBS for 30 min at RT; non-specific labeling was prevented by pre-incubating the sections with blocking solution (0.1% Triton X-100 and 3% BSA) for 30 min at RT. Then, sections were incubated with an anti-BrdU antibody (1:100, GE Healthcare Cell Proliferation Kit) or buffer provided in the kit (negative control) for 18 h at 4 °C followed by an anti-mouse FITC secondary antibody (10 µg/ml, Vector Labs) 2 h at RT in the dark. Sections were mounted with VECTASHIELD antifade mounting medium with DAPI (Vector Labs) and observed in an Axiophot epifluorescence microscope.
Thirty STs in 3 non-consecutive longitudinal sections from each half of the testis were analyzed to quantify BrdU+ STs and TUNEL+ germ cells. Seminiferous tubules at VII–VIII stages were identified by DAPI staining by the presence of spermatozoa and residual bodies and STs at XIV stage were identified in hematoxylin–eosin stained sections by the presence of meiotic metaphases (Leblond and Clermont 1952). Seminiferous tubules containing BrdU+ spermatogonia were calculated by the following difference: Total BrdU+ STs – BrdU+ VII–VII STs. Spermatogonia and PLs were counted in 50 BrdU+ STs/section. The number of BrdU+ PLs was determined in STs at VII–VIII stage. The number of BrdU+ spermatogonia was determined in the remained BrdU+ STs.
Statistical analysis
Data were compared for statistical significance using GraphPad Prism version 6.0 (GraphPad Software Inc.). Data are presented as the mean ± SEM of at least two independent experiments. Depending on the experimental design, statistical analyses were done with Student’s t test, Mann–Whitney, or one-way ANOVA, followed by Bonferroni’s multiple comparisons test. Differences were considered significant when p < 0.05.
Results
In EAO spermatogenesis was preserved in some seminiferous tubules at VII-VIII and XIV stages
In focal EAO, damaged seminiferous tubules (STs) were arranged in foci intermingled with STs in which complete spermatogenesis occurred (Fig. 1c and d); in the severe phase, spermatogenesis was disrupted in most STs (Fig. 1e and f). However, in some STs with severe impairment of spermatogenesis, spermatogonia and spermatocytes were still present at the basal compartment (Fig. 1f and g). In the focal and severe EAO STs at VII–VIII stage, in which preleptotene spermatocytes (PLs) enter the meiotic S-phase and spermatozoa are released to the lumen (Huckins 1971a), are preserved, although their number was significantly reduced vs. normal testis (Fig. 1h and h’).
Histopathology and spermatogenesis in EAO. In the testis of normal rat (a and b) tubular and interstitial compartment show preserved histology. In focal EAO (55 days from the first immunization) (c and d), seminiferous tubules (STs) lose their normal arrangement. In severe EAO (75 days from the first immunization) (e–g), greater and more extensive STs damage with increased interstitial cell density is observed. Most STs presented sloughed germ cells in the lumen (asterisk); spermatogonia (black large arrowhead), spermatocytes (white arrowhead) and Sertoli cells (small black arrow head) are the main cells found in the basal compartment of severely altered STs (g). Bar indicates 100 µm in left microphotographs, 50 µm in right microphotographs and 10 µm in g. Seminiferous tubules at VII–VIII (h and h’) and XIV stages (h’’ and h’’’) were recorded in 100 STs/slide in 3 non-consecutive sections/rat (n = 3–4 rat per group). Mann–Whitney test, *p< 0.05 and ***p < 0.001 vs. respective normal
Stage XIV is the last stage of the spermatogenic wave; therefore, it is useful to understand the global impact of inflammatory microenvironment on wave progression (Hess 1990). Seminiferous tubules at stage XIV behave in a similar way to STs at VII-VIII; many were preserved but their number was significantly and progressively reduced (vs. normal rats) during EAO development (Fig. 1h’’ and h’’’).
Overall, the number of TUNEL+ basal germ cells/ST in focal and severe EAO was similar to normal rats (Fig. 2e and e’) while the number of adluminal TUNEL+ germ cells (late spermatocytes and spermatids)/ST was significantly increased in both phases of EAO (Fig. 2f and f’).
Apoptosis of basal and adluminal germ cells in EAO. TUNEL technique: representative microphotographs of testis of normal (a), experimental rats with focal (b) and severe (c) EAO; nuclei of apoptotic (TUNEL+) germ cells are blue, black arrow head indicates TUNEL+ basal germ cells. Note the increased number of adluminal TUNEL+ germ cells in EAO (blue arrow head, b and c). In the negative control TdT enzyme was replaced by incubation buffer (d). Bar indicates: 20 µm. TUNEL+ germ cells (a–d) were counted in 100 STs in 3 non-consecutive slides/rat (3–4 rats per group). Student’s t test, *p < 0.05
In EAO spermatogonia and preleptotene spermatocyte cell cycle progression is arrested
Spermatogenesis is sustained by spermatogonia which undergo nine to ten mitotic amplification divisions until they differentiates into PLs (Fayomi et al. 2018). To evaluate the overall impact of inflammation on spermatogenesis, we assessed spermatogonia and PLs in mitotic and meiotic S-phase respectively by bromodeoxyuridine (BrdU) incorporation (Krishnamurthy et al. 2000; Muñoz-Velasco et al. 2013).
The percentage of BrdU+ premeiotic germ cells, analyzed by flow cytometry, decreased in EAO rats with focal and severe orchitis compared with normal rats (Fig. 3a’’’ and b’’’).
The percentage of premeiotic germ cells in phase S is reduced in EAO. In normal and EAO rats, testicular weight (TW)–body weight (BW) index was calculated by dividing the TW of the testis used for BrdU cytometric analysis by the BW (see “Material and method” for a detailed explanation). TW/BW [mean ± SEM (n = 5–7 rats per group)]: focal EAO: 2.14 ± 0.07 × 10-3***, normal: 3.35 ± 0.163; severe EAO: 1.55 ± 0.15*** normal: 3.24 ± 0.06, p < 0.001 Student’s t test vs. respective normal. The degree of orchitis (EAO score) was confirmed by histopathological analysis of the contralateral testis in sections fixed in Bouin and stained with hematoxylin–eosin. EAO score [mean ± SEM (n = 5–7 rats per group)]: focal EAO: 4.02 ± 0.32, severe EAO: 9.00 ± 0.37. Representative dot plot (a–a’’ and b–b’’): 1 × 106 germ cells (GC) were immunostained with an anti-BrdU-PE antibody or isotype control antibody, R1 was set based on isotype control (a and b). Percentage of BrdU+premeiotic GC was obtained (a’’’ and b’’’). Testis of 5–7 rats per group was analyzed. Student´s t-test, **p < 0.01 vs. respective normal
We analyzed the number of premeiotic germ cells in S-phase present in the STs by immunofluorescence. In focal EAO, the number of STs containing BrdU+ premeiotic germ cells was similar to normal rats (Fig. 4i), although the number of BrdU+ premeiotic germ cells/ST was reduced (Fig. 4j). In severe EAO, the number of STs with BrdU+ premeiotic germ cells and the number of premeiotic germ cells/ST was reduced compared to normal rats (Fig. 4i’ and j’).
The number of premeiotic germ cells and preleptotene spermatocytes in phase S is reduced in EAO. BrdU+ seminiferous tubules (STs) containing premeiotic germ cells (GC) [spermatogonia plus preleptotene spermatocytes (PLs)] (i and i’), BrdU+ premeiotic GC/ST (j and j’), BrdU+ STs in stage VII-VIII (k and k’), and BrdU+ PLs present in VII–VIII STs (l and l’) were counted (for details see “Material and methods” section). Testis of 5–7 rats per group was analyzed. Student’s t test, *p < 0.05 and ***p < 0.001 vs. respective normal. Upper panel: representative microphotographs of testis sections processed for BrdU detection by immunofluorescence, nuclei were stained with DAPI; in the negative control (g and h) first antibody was replaced by incubation buffer. In focal EAO (b, e), damaged STs with BrdU+ premeiotic germ cells (asterisk) are observed. In severe EAO (c, f), most STs are altered, although they still home BrdU+ premeiotic germ cells. Note the reduction in the number of BrdU+ STs in EAO (b and c) vs. normal (a). Dotted lines indicate STs (stage VII–VIII) with no proliferative germ cells. Stages VII–VIII were identified by the presence of late spermatids in the lumen. Bar indicates 50 µm
In focal EAO testis both the number of BrdU+ VII–VIII STs and BrdU+ PLs/ST decreased significantly when compared to normal rats (Fig. 4k and l). In severe EAO, the few VII–VIII STs did not present BrdU+ PLs (Fig. 4k’ and l’).
We quantified the number of CD9+ spermatogonia by immunohistochemistry. CD9+ spermatogonia population was significantly reduced in focal and severe EAO vs. normal testis (Fig. 5e and e’). In severe EAO, the number of CD9+ spermatogonia was lower than in focal orchitis.
Number of undifferentiated CD9+ spermatogonia is reduced and the cell cycle is arrested in G2/M-phase in EAO.
The number of undifferentiated CD9+ spermatogonia is reduced in EAO. The number of CD9+ spermatogonia (SPG) was evaluated by indirect immunoperoxidase in formol 10% fixed testis sections. Upper panel: representative microphotographs. Isolated (large arrow head) and aligned (small arrow head) CD9+ SPG are present in the basal compartment of seminiferous tubules (STs), in normal (a and a'), focal (b and b'), and severe (c and c') EAO testis. In the negative control, the first antibody was replaced by isotype IgG control (d). Bar indicates 20 µm; insert: bar indicates 5 µm. One hundred STs in two non-consecutive sections of normal and EAO testis were analyzed. Spermatogonia showing histological characteristics of type A and localized in the basal compartment of the STs were counted (e and e’). n = 3 rats per group; data were analyzed by Student´s t-test, ***p < 0.001 vs. respective normal
Cell cycle analysis of CD9+ spermatogonia of rats with focal EAO by flow cytometry showed that the percentage of CD9+ spermatogonia at G2/M phase of the cell cycle was higher than in normal rats whereas the percentage of CD9+ spermatogonia at G1 and S-phase was similar to normal rats (Fig. 6a’’’). In rats with severe EAO, the rate of CD9+ spermatogonia at G2/M-phase was increased and the percentage of CD9+ spermatogonia at G1-phase was greatly reduced compared to normal rats (Fig. 6b’’’). These results indicate that CD9+ spermatogonia arrested the cell cycle at G2/M.
The cell cycle of undifferentiated CD9 + spermatogonia is arrested in EAO. Germ cells (GC) (3 × 106) were immunostained with anti-CD9 antibody or isotype, permeabilized, thereafter stained with propidium iodide (PI) and analyzed by flow cytometry (a–a’’ and b–b’’). Percentage of CD9+ spermatogonia (SPG) in G1, S, and G2 phase of the cell cycle was calculated in the CD9 gate (R1) determined by isotype and SPG DNA content (a’’’ and b’’’). Testis of 5 rats per group were analyzed. Student´s t-test, *p < 0.05 and ***p < 0.001 vs. respective normal
Differentiated c-Kit + spermatogonia population decreases in EAO
Differentiated A spermatogonia from A1 to A4 express the receptor for the stem cell factor (SCFR) or c-Kit (Zhang et al. 2011). The number of c-Kit+ spermatogonia significantly decreased in both phases of EAO compared to normal testis (Fig. 7e and e’); in severe EAO, the number of c-Kit+ was lower than in the focal phase of orchitis.
The number of differentiated c-Kit+ spermatogonia is reduced in EAO. The number of c-Kit+ spermatogonia (SPG) was evaluated by indirect immunoperoxidase in PFA 4% fixed testis sections. Upper panel: representative microphotographs of seminiferous tubules (STs) homing c-Kit+ SPG in normal (a), focal (b) and severe (c) EAO testis (black arrow head). In the negative control, the first antibody was replaced by isotype IgG control (d). Bar indicates 20 µm. One hundred STs in two non-consecutive sections of normal and EAO testis were analyzed. Spermatogonia displaying type A histological characteristic, localized in the basal compartment of the STs were counted (e and e’). n = 3 rats per group. Student’s t test, ***p < 0.001 vs. respective normal
NO reduced spermatogonia and preleptotene spermatocytes at S-phase and impaired spermatogenesis progression without inducing germ cell apoptosis
To evaluate the effect of NO on spermatogonia and PLs at S-phase, we used a nitric oxide donor, DETA-Nonoate (DETA-NO). DETA-NO continually releases NO for 18 h. The dose of DETA-NO was selected based in its effect on GC-1 spermatogonia B cell cycle and also because it concords with the NO produced by testicular EAO macrophages (Ferreiro et al. 2019; Jarazo-Dietrich et al. 2015).
The S-phase of undifferentiated spermatogonia A single and A aligned (syncytial chains containing 4, 8, and 16 cells) lasts 25 h, the S-phase of differentiated spermatogonia (A1-A4, Intermediate and B) 18–22 h, and the PLs S-phase lasts 81.4 h (3.4 days) (Huckins 1971a, b).
We decided to study the effect of NO release for 18 h, a time-lapse mainly affecting undifferentiated spermatogonia, and for 36 h covering undifferentiated and differentiated spermatogonia proliferation. With these time points, the effect of NO on PLs S-phase would be left unevaluated. Sustained release of NO for 36 h was achieved by injecting DETA-NO intratesticularly twice with an 18 h interval; the contralateral testis was injected with saline.
After 18 h, DETA-NO (2 mM) significantly reduced the number of STs with spermatogonia in S-phase (20%) compared with saline injected controls (Fig. 8k). After 36 h, DETA-NO (2 mM) significantly reduced the number of STs with BrdU+ spermatogonia (48%) and also the number of BrdU+ spermatogonia/ST vs. saline (Fig. 9f and f’). The greater reduction of STs with BrdU + spermatogonia in DETA-NO 2 mM after 36 h compared with DETA-NO 2 mM after 18 h might be due to the expanding spermatogonia population at S-phase covered over the prolonged NO exposure time frame. In addition, a larger area is reached by DETA-NO solution in the experimental design described. DETA-NO (2 mM) did not induce germ cell apoptosis (Fig. 8l and Fig. 9f’’).
DETA-NO reduced the number of seminiferous tubules with spermatogonia in S-phase. One testis of normal rats was injected once with DETA-NO (2 mM) and the contralateral with saline, animals were killed 18 h after the first injection (a). Testis was processed for double immunofluorescence BrdU-TUNEL, nuclei were counterstained with DAPI; in negative controls anti BrdU antibody and TdT enzyme were replaced by their respective incubation buffers (f−g and j−j''). Seminiferous tubules (STs) with BrdU+ spermatogonia (SPG) (k), BrdU+ SPG/ST (k’) and TUNEL+ germ cells (GC)/ST (l) were quantified (for details see “Material and methods” section). Student’s t test, *p < 0.05 vs. respective saline. Microphotographs: Note the reduction in the number of STs with BrdU+ premeiotic GC in testes injected with DETA-NO vs. saline (b and c). Seminiferous tubules at VII-VIII stage with no proliferative germ cells are indicated by dotted lines (b). Apoptotic germ cells (red) are indicated (h and i, arrow head). Bar indicates 50 µm
DETA-NO reduced the number of spermatogonia in S-phase. One testis of normal rats was injected with DETA-NO (2 mM) and the contralateral with saline, animals were killed 36 h after the first injection (a). Testis was processed for double immunofluorescence BrdU-TUNEL, nuclei were counterstained with DAPI; in negative controls anti BrdU antibody and TdT enzyme were replaced by their respective incubation buffers. Seminiferous tubules (ST) with BrdU+ spermatogonia (SPG) (f), BrdU+ SPG/ST (f’) and TUNEL+ germ cells (GC)/ST (f’’) were quantified (for details see “Material and methods” section). Testis of 3 rats per group was analyzed Student’s t test, *p < 0.05 and **p < 0.01 vs. respective saline. Microphotographs: Note the reduction in the number of STs with BrdU+ premeiotic germ cells, and in the number of BrdU+ cells/ST in testes injected with DETA-NO (d) vs. saline (b). Seminiferous tubules at VII-VIII stage with no proliferative germ cells are indicated by dotted lines (d). Bar indicates 50 µm
As expected, we observed no effect of DETA-NO either on PLs at S-phase or on the number of ST at stage VII–VIII after 18 and 36 h exposure (Online Resource 2).
By exposing germ cells to DETA-NO for 36 h and killing the animals 3.5 days after, we gave the PLs time to conclude the S-phase and therefore were able to study the effect of NO on these cells (Fig. 10a). DETA-NO (2 mM) significantly reduced the number of STs with BrdU+ PLs and the number of BrdU+ PLs/ST (Fig. 10i and i’). This sequence also allowed us to evaluate whether spermatogonia could overpass the inhibitory effect of DETA-NO observed after 36 h (Fig. 9f and f’). The inhibitory effect of DETA-NO (2 mM) on the number of STs with BrdU+ spermatogonia observed after 36 h was lost; the number of BrdU+ spermatogonia/ST was significantly reduced vs. saline (10% reduction) (Fig. 10h), even though it was lowered to a lesser extent compared to 36 h (44% reduction vs. saline) (Fig. 9f’). A dose-dependent effect of NO on spermatogonia recovery was observed when a higher DETA-NO (10 mM) concentration was evaluated since the inhibitory effect on the number of STs with BrdU+ spermatogonia observed after 36 h still lasted for 3.5 days [mean ± SEM (n = 3), 36 h: DETA-NO: 9.17 ± 1.89*, saline: 17.00 ± 2.28; 3.5 days: DETA-NO: 17.61 ± 1.03**, saline: 22.00 ± 1.21; Student’s t test, *p < 0.05, **p < 0.01].
DETA-NO reduced the number of spermatogonia and preleptotene spermatocyte in S-phase and impaired spermatogenesis progression. One testis of normal rats was injected twice with DETA-NO (2 mM) and the contralateral with saline, animals were killed 5 days after the first injection (a). Testis was processed for double immunofluorescence BrdU-TUNEL and nuclei were counterstained with DAPI. Seminiferous tubules (ST) with BrdU+ spermatogonia (SPG) (h), BrdU+ SPG/ST (h’), ST with BrdU+ preleptotene spermatocytes (PLs) (i), Brdu+ PLs/ST (i’) and TUNEL+ germ cells (GC)/ST (j) were quantified (for details see “Material and methods” section). Total STs at VII-VIII (j’) and XIV (j’’) stages were also recorded. The testis of 3 rats per group was analyzed and 4–7 non-consecutive slides/rat were evaluated Student’s t test, *p < 0.05, **p < 0.01, and ***p < 0.001 vs. respective saline. Microphotographs: Note the reduction in the number of BrdU+ SPG/ST and in the number of ST VII-VIII with BrdU+ PLs (indicated by dotted lines) in DETA-NO treated testis (d and f) vs. saline (b). Bar indicates 50 µm
DETA-NO did not affect the number of TUNEL+ germ cells (Fig. 10j).
To evaluate the impact of spermatogonia cell cycle arrest induced by DETA-NO, we determined the number of STs at VII-VIII (DAPI staining) and XIV stage (hematoxylin–eosin staining). DETA-NO (2 mM) significantly reduced the number of STs at VII–VIII stage (Fig. 10j’) while the number of STs at XIV stage was similar to that in saline injected testis (Fig. 10j’’).
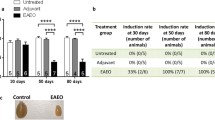
The time required for spermatogonia to convert into sperm is 53.2 days in Wistar rats (Clermont 1972). To study the effect of DETA-NO on spermatogenesis, rats were killed 60 days after the first of two intratesticular DETA-NO injections (Fig. 11a). Variations in the normal frequency of ST area might reflect spermatogenesis alteration as previously demonstrated (Linakis and Cunningham 1979). DETA-NO (2 mM) increased the percentage of STs of reduced area and decreased STs of medium area (Fig. 11b), whereas it affected neither the percentage of STs at VII-VIII and XIV stages (Fig. 11c and c’), nor the number of spermatocytes in metaphase/ST XIV (mean ± SEM, DETA-NO: 6.97 ± 0.55, saline: 6.84 ± 0.76; n = 3, Student’s t test).
DETA-NO impaired spermatogenesis progression. One testis of normal rats was injected twice with DETA-NO (2 mM) and the contralateral with saline, animals were killed 60 days after the first injection (a). Testes were fixed in Bouin and stained with H-E. Seminiferous tubules grouped according to their area (b) and STs in VII-VIII (c) and XIV (c’) stages were quantified in 100 STs in 3–4 non-consecutive slides per testis (n = 3 rats per group). Student’s t test, ***p < 0.001 vs. respective saline
TNFα did not affect spermatogonia and preleptotene spermatocytes at S-phase or spermatogenesis progression
To study the effect of TNFα on spermatogonia proliferation, one testis was injected intratesticularly with TNFα (0.5 or 1 µg) and the contralateral with saline (Fig. 12a). After 24 h, TNFα affected neither the number of STs with BrdU+ spermatogonia (Fig. 12b and b’’), the number of BrdU+ spermatogonia/ST (Fig. 12b’ and b’’’). Moreover, TNFα did not induce germ cell death by apoptosis (Fig. 12c and c’). As expected we observed no effect of TNFα (0.5 and 1 µg) on PLs at S-phase, nor on the number of STs at stage VII–VIII after 24 h action (Online Resource 3).
TNFα did not affect the number of spermatogonia in S-phase. One testis of normal rats was injected once with TNFα (0.5 or 1 µg) and the contralateral with saline, animals were killed 24 h after the injection (a). Testis was processed for double immunofluorescence BrdU-TUNEL and nuclei were counterstained with DAPI. Seminiferous tubules (ST) with BrdU+ spermatogonia (SPG) (b and b’’), BrdU+ SPG/ST (b’ and b’’’) and TUNEL+ germ cells (GC)/ST (c and c’) were quantified (for details see “Material and methods” section). The testis of 3 rats per group was analyzed. Student’s t test
To evaluate the effect of TNFα on PLs proliferation and on spermatogenesis, we used the same experimental design as for DETA-NO; normal rat testes were intratesticularly injected twice with TNFα (1 µg) and the contralateral testis with saline. Rats were killed 5 days (Fig. 13a) or 60 days after the second TNFα injection (Fig. 13d). TNFα did not affect the number of STs with BrdU+ spermatogonia (Fig. 13b), the number of BrdU+ spermatogonia/ST (Fig. 13b’), PLs proliferation (Fig. 13b’’ and b’’’). Also, TNFα did not induce germ cell death by apoptosis (Fig. 13c’’). The number of STs at VII–VIII and XIV stage (Fig. 13c and c’) was similar to that in saline injected testis. After 60 days, TNFα did not alter the normal frequency of STs (Fig. 13e) neither the percentage of STs at VII–VIII and XIV stages (Fig. 13f and f’), nor the number of spermatocytes in metaphase/ST (mean ± SEM, TNFα: 4.41 ± 1.02, saline: 5.56 ± 1.24; n = 3, Student’s t test).
TNFα affected neither the number of spermatogonia and preleptotene spermatocyte in S-phase nor spermatogenic wave. One testis of normal rats was injected twice with TNFα (1 µg) and the contralateral with saline, animals were killed 5 days (a) or 60 days (d) after the first injection. a–c’’: testis was processed for double immunofluorescence BrdU-TUNEL, nuclei were counterstained with DAPI. Seminiferous tubules (ST) with BrdU+ spermatogonia (SPG) (b), BrdU+ SPG/ST (b’), ST with BrdU+ preleptotene spermatocytes (PLs) (b’’), Brdu+ PLs/ST (b’’’) and TUNEL.+ germ cells (GC)/ST (c) were quantified (for details see “Material and methods” section). Total STs at VII-VIII (c’) and XIV (c’’) stages were also recorded. Three rats per group were analyzed and 4–7 non-consecutive slides/rat were evaluated. Student’s t test. d–f’’: testes were fixed in Bouin and stained with H-E. Seminiferous tubules were grouped according to their area and quantified (e); STs in VII-VIII (f) and XIV (f’) stages were also quantified in 100 STs in 3–4 non-consecutive sections per testis (n = 3 rats per group)
Discussion
The primary purpose of our work was to evaluate the impact of inflammatory testicular microenvironment generated in the testes of rats with EAO on spermatogonia and PLs function.
During the normal progression of spermatogenesis, spermatogonia undergo clonal expansion until differentiation into PLs that enter meiosis after passing the S-phase (Clermont 1972). In EAO, the overall proliferative potential of premeiotic germ cells is limited, leading to a reduced number of PLs entering the meiotic S-phase. Consequently, spermatogenesis progression is impaired but not completely suppressed since some STs at VII-VIII stages, still generating spermatozoa, arise.
The interrupted clonal expansion of undifferentiated CD9+ spermatogonia due to the cell cycle arrest in the G2/M phase might reduce the undifferentiated and differentiated (c-Kit+ spermatogonia) population and PLs that enter in meiosis. A direct effect of inflammation on PLs cell cycle is also possible since our results show a reduced number of PLs in the S-phase per ST. Basal germ cell apoptosis might contribute to the higher restraint of mitotic amplification of premeiotic germ cells observed in EAO. Although the number of basal apoptotic germ cells/STs is similar to normal rats, the percentage of these STs increased in orchitis (data not shown).
To explore whether NO and TNFα, interfere with the normal cell cycle of spermatogonia and PLs, DETA-NO or TNFα were injected intratesticularly, and spermatogonia and PLs at S-phase were studied at different periods after a 2-h pulse of BrdU (Muñoz-Velasco et al. 2013).
Nitric oxide arrests spermatogonia cell cycle progression, since a reduced number of spermatogonia at S-phase was detected. Nitric oxide’s inhibitory effect on spermatogonia cell cycle observed after 36 h of continuous NO release was no longer observed after 3.5 days from NO exhaustion. We propose that at this time point, spermatogonia overcome the NO induced cell cycle arrest. We demonstrated that GC-1 spermatogonia B cells recover after 72 h of NO deprivation, enabling cell cycle progression from S to G2/M and the G1 phases (Ferreiro et al. 2019).
Concerning NO effect on PL cell cycle progression, we demonstrated that NO arrests their cell cycle reducing the number of PLs in S-phase present in the STs at VII–VIII stages. Most germ cells in S-phase at stages VII–VIII are PLs, since only 1% of the A single undifferentiated spermatogonia, the smallest spermatogonia population in the testis, are in mitosis at this stage (Huckins 1971b). Differentiated spermatogonia A4, Intermediate and spermatogonia B, are mitotically active at stages I, IV, and VI (Huckins 1971a). The limited clonal expansion of spermatogonia induced by NO (observed at 36 h) might impair the spermatogenic wave reducing the frequency of STs at VII–VII stages of the epithelial cell cycle as we observed at day 5. In fact, stages IV–VII last 5.6 days (Hess et al. 1990) (Clermont 1972), almost the entire time-lapse covered by the experimental design. Spermatogonia B differentiate into PLs at VI–VII stages. This population is probably targeted by NO action since we demonstrated that NO arrests the cell cycle of spermatogonia B GC-1 cell line at S-phase, reducing the number of spermatogonia in G2/M (Ferreiro et al. 2019). In GC-1 cells, we observed that oxidative stress generated by DETA-NO (2 mM) causes DNA fragmentation and activation of p-ATMser−1981quinase (Online Resource 4). Activated p-ATMser−1981quinase (by oxidative stress and/or DNA damage) (Shiloh 2014) through a phosphorylation cascade prevents the activation of cycline-Cdk complexes leading to cell cycle arrest at G2-M and G1-S phases (Shiloh 2003). The frequency of STs at XIV stages of the epithelial cell cycle remained unchanged after day 5 of DETA-NO injection. We cannot discard an effect of NO on differentiated A2 spermatogonia proliferation in stages XII-XIII (Huckins 1971a). STs at XIV stage appear approximately 2.27 days after XII–XIII stages (Hess et al. 1990; Clermont 1972), a time frame surpassed by our experimental design.
The long-term effect of NO on spermatogenesis was evaluated after 60 days. Seminiferous tubules on transversal testis sections have different areas due to the specific germ cell associations that define the stages of the epithelial cycle (Clermont 1972; Wing and Christensen 1982). Nitric oxide increased the frequency of STs with small and reduced areas, and decreased STs with medium sizes. Temporary gaps, in the epithelial cycle progression, might explain the changes observed in ST area in NO exposed testis, as a consequence of reversible spermatogonia cell cycle inhibition. In fact, changes in the normal frequency of STs stages result from spermatogenesis kinetic impairment (Hess et al. 1990). Savitt et al. (2012) observed that the inhibition of GDNF signaling, a trophic factor maintaining SSC self-renewal, through c-ret (GDNF co-receptor) inactivation (Sharma and Braun 2018) for 2 days, reduced the number of SSC and the area of STs 44 days afterwards (Savitt et al. 2012). The authors explain that as SSC is depleted, some regions of the basal compartment of STs will be empty until refilled by an influx of new cells. This refilling depletes undifferentiated spermatogonia in another part of the tubule. Consequently, temporary pauses in the production of differentiated spermatogenic cells in specific regions of a tubule cause diverse morphologies of ST and ST area reduction (Savitt et al. 2012). Similarly, NO reversible induced spermatogonia cell cycle arrest at S-phase might generate “time-lapse gaps” in the epithelial cycle progression in the testis of normal rats.
This study observed that NO did not induce germ cell apoptosis when DETA-NO was administered intratesticularly. We previously reported that DETA-NO 2 mM induces basal germ cell apoptosis in cultures of STs segments (Jarazo-Dietrich et al. 2015). We speculate that in vitro culture of STs segments makes germ cells more vulnerable compared to an in vivo context.
Concerning TNFα, we expected an indirect in vivo effect of this cytokine on the premeiotic germ cell cycle since in the normal rat testis spermatogonia do not express TNFR1 (Suescun et al. 2003; De et al. 2015) unlike Sertoli cells and Leydig cells (Xiong and Hales 1993). Sertoli cells respond to TNFα by releasing IL6 (De et al. 2015; Riccioli et al. 1995) and the combined action of TNFα, INFγ, and IL1 induce the release of NO (Bauché et al. 1998). Nitric oxide and IL6 display inhibitory effects on spermatogonia proliferation (Ferreiro et al. 2019; Hakovirta et al. 1995). However, present results do not show an in vivo effect of TNFα on the premeiotic germ cell cycle not expressing TNFR1, contrasting with the apoptotic effect of this cytokine on spermatocytes and spermatids expressing TNFR1 (previously reported) (Theas et al. 2008; Suescun et al. 2003). Nitric oxide released by testicular macrophages increases from focal to severe orchitis (Jarazo-Dietrich et al. 2012). As we demonstrated by results of the present in vivo experiments and published results (Ferreiro et al. 2019), the behavior of premeiotic germ cells observed in EAO might result from NO’s inhibitory action on spermatogonia and or on PLs cell cycle progression. Consequently, time gaps generated by the reversible arrest of spermatogonia cell cycle would affect the progression of spermatogenic wave by greatly reducing the number of STs at VII-VIII stage.
Low spermatogenic efficiency in infertile men is due not only to impairment of postmeiotic events, but also to a decrease in the mitotic activity and number of spermatogonia (Steger et al. 1998; Hentrich et al. 2017). In fact, as also occurs in EAO, proinflammatory agents such as NO and TNFα produced by the immune cells that infiltrate the testes of infertile patients (Mayerhofer et al. 2018; Sezer et al. 2005; Frungieri et al. 2002) might limit the clonal expansion of spermatogonia. NO affecting this clonal expansion would result in a greater reduction of sperm production.
Conclusion
In conclusion, we demonstrated that inflammation arrested spermatogonia cell cycle in orchitis, reducing the number of undifferentiated CD9+ and differentiated c-Kit+ spermatogonia. This behavior limited premeiotic germ cells’ proliferative capability. Spermatogenesis progression was also compromised by the limited number of preleptotene spermatocytes able to enter the S-phase. However, the spermatogenic wave continued, as demonstrated by the presence of seminiferous tubules at the VII-VIII and XIV stages.
Spermatogonia and preleptotene spermatocytes are possible targets of NO released by inflammatory macrophages. In previous in vitro experiments, we demonstrated that NO arrests the spermatogonia cell cycle at S-phase (Ferreiro et al. 2019). Similarly, we now showed that NO also inhibited spermatogonia and the preleptotene spermatocytes cell cycle in the normal rat testes. Although our paradigm of NO spermatogonia cell cycle inhibition was temporary, the decreased function of these cells induced changes in spermatogenesis that could be observed over 60 days after the original insult.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Bauché F, Stéphan JP, Touzalin AM, Jégou B (1998) In vitro regulation of an inducible-type NO synthase in the rat seminiferous tubule cells. Biol Reprod 58:431–438. https://doi.org/10.1095/biolreprod58.2.431
Braylan RC, Benson NA, Nourse V, Kruth HS (1982) Correlated analysis of cellular DNA, membrane antigens and light scatter of human lymphoid cells. Cytometry 2:337–343. https://doi.org/10.1002/cyto.990020511
Caneguim BH, Cerri PS, Spolidório LC, Miraglia SM, Sasso-Cerri E (2009) Structural alterations in the seminiferous tubules of rats treated with immunosuppressor tacrolimus. Reprod Biol Endocrinol. https://doi.org/10.1186/1477-7827-7-19
Clermont Y (1972) Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 52:198–236. https://doi.org/10.1152/physrev.1972.52.1.198
De SK, Chen HL, Pace JL, Hunt JS, Terranova PF, Enders GC (1993) Expression of tumor necrosis factor-alpha in mouse spermatogenic cells. Endocrinology 133:389–396. https://doi.org/10.1210/endo.133.1.8319585
Doncel GF, Di Paola JA, Lustig L (1989) Sequential study of the histopathology and cellular and humoral immune response during the development of an autoimmune orchitis in Wistar rats. Am J Reprod Immunol 20:44–51. https://doi.org/10.1111/j.1600-0897.1989.tb00638.x
Duan YG, Yu CF, Novak N, Bieber T, Zhu CH, Schuppe HC, Haidl G, Allam JP (2011) Immunodeviation towards a Th17 immune response associated with testicular damage in azoospermic men. Int J Androl. https://doi.org/10.1111/j.1365-2605.2010.01137.x
Fayomi A, David S, Doungkamchan C, Orwig KE (2018) Spermatogonial stem cells and sspermatogenesis in mice, monkeys and men. Stem Cell Res 29:107–214. https://doi.org/10.1016/j.scr.2018.04.009
Ferreiro ME, Amarilla MS, Glienke L, Méndez CS, González C, Jacobo PV, Sobarzo CM, De Laurentiis A, Ferraris MJ, Theas MS (2019) The inflammatory mediators TNFα and nitric oxide arrest spermatogonia GC-1 cell cycle. Reprod Biol 19:329–339. https://doi.org/10.1016/j.repbio.2019.11.001
Fijak M, Pilatz A, Hedger MP, Nicolas N, Bhushan S, Michel V, Tung KSK, Schuppe HC, Meinhardt A (2018) Infectious, inflammatory and “autoimmune” male factor infertility: how do rodent models inform clinical practice? Hum Reprod Update 24:416–441. https://doi.org/10.1093/humupd/dmy009
Frungieri MB, Calandra RS, Lustig L, Meineke V, Köhn FM, Vogt HJ, Mayerhofer A (2002) Number, distribution pattern, and identification of macrophages in the testes of infertile men. Fertil Steril 78:298–306. https://doi.org/10.1016/S0015-0282(02)03206-5
Gónzalez L, Amarilla MS, Méndez S, Glienke L, Oxilia H, Fulco MF, Jacobo P, Sobarzo CM, TM (2018) Study of spermatogonia population in infertile patients with immune cells infiltrates. Rev Med 78:245–246
Hakovirta H, Syed V, Jégou B, Parvinen M (1995) Function of interleukin-6 as an inhibitor of meiotic DNA synthesis in the rat seminiferous epithelium. Mol Cell Endocrinol 108:193–198. https://doi.org/10.1016/0303-7207(95)03475-M
Hentrich A, Wolter M, Szardening-Kirchner C, Lüers GH, Bergmann M, Kliesch S, Konrad L (2017) Reduced numbers of Sertoli, germ, and spermatogonial stem cells in impaired spermatogenesis. Mod Pathol 30:311. https://doi.org/10.1038/modpathol.2016.194
Hess RA (1990) Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: light microscopic observations of perfusion-fixed and plastic-embedded testes. Biol Reprod 43:525–542. https://doi.org/10.1095/biolreprod43.3.525
Huckins C (1971a) Cell cycle properties of differentiating spermatogonia in adult Sprague- Dawley rats. Cell Tissue Kinet 4:139–154. https://doi.org/10.1111/j.1365-2184.1971.tb01524.x
Huckins C (1971b) The spermatogonial stem cell population. Cell Tissue Kinet 4:313–334. https://doi.org/10.1111/j.1365-2184.1971.tb01543.x
Jarazo-Dietrich S, Jacobo P, Pérez CV, Guazzone VA, Lustig L, Theas MS (2012) Up regulation of nitric oxide synthase-nitric oxide system in the testis of rats undergoing autoimmune orchitis. Immunobiology 217:778–787. https://doi.org/10.1016/j.imbio.2012.04.007
Jarazo-Dietrich S, Fass MI, Jacobo PV, Sobarzo CMA, Lustig L, Theas MS (2015) Inhibition of NOS-NO system prevents autoimmune orchitis development in rats: Relevance of NO released by testicular macrophages in germ cell apoptosis and testosterone secretion. PLoS ONE. https://doi.org/10.1371/journal.pone.0128709
Krishnamurthy H, Danilovich N, Morales CR, Sairam MR (2000) Qualitative and quantitative decline in spermatogenesis of the follicle- stimulating hormone receptor knockout (FORKO) mouse. Biol Reprod 62:1146–1159. https://doi.org/10.1095/biolreprod62.5.1146
Leblond CP, Clermont Y (1952) Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci 55:548–573. https://doi.org/10.1111/j.1749-6632.1952.tb26576.x
Li MWM, Xia W, Mruk DD, Wang CQF, Yan HHN, Siu MKY, Lui WY, Lee WM, Cheng CY (2006) Tumor necrosis factor α reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol 190:313–329. https://doi.org/10.1677/joe.1.06781
Linakis JG, Cunningham CL (1979) Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology 64:61–65. https://doi.org/10.1007/BF00427346
Lustig L, Guazzone VA, Theas MS, Pleuger C, Jacobo P, Pérez CV, Meinhardt A, Fijak M (2020) Pathomechanisms of autoimmune based testicular inflammation. Front Immunol 11:1–8. https://doi.org/10.3389/fimmu.2020.583135
Mayerhofer A, Walenta L, Mayer C, Eubler K, Welter H (2018) Human testicular peritubular cells, mast cells and testicular inflammation. Andrologia 50:e13055. https://doi.org/10.1111/and.13055
Meineke V, Frungieri MB, Jessberger B, Vogt HJ, Mayerhofer A (2000) Human testicular mast cells contain tryptase: increased mast cell number and altered distribution in the testes of infertile men. Fertil Steril. https://doi.org/10.1016/S0015-0282(00)00626-9
Muñoz-Velasco I, Ortíz R, Echeverría OM, Escobar ML, Vázquez-Nin GH (2013) Characterization of the pre-meiotic S phase through Incorporation of BrdU during spermatogenesis in the Rat. J Histochem Cytochem 61:680–689. https://doi.org/10.1369/0022155413496639
Pérez CV, Sobarzo CM, Jacobo PV, Pellizzari EH, Cigorraga SB, Denduchis B, Lustig L (2012) Loss of occludin expression and impairment of blood-testis barrier permeability in rats with autoimmune orchitis: effect of interleukin 6 on Sertoli cell tight junctions. Biol Reprod 87:1–12. https://doi.org/10.1095/biolreprod.112.101709
Pilatz A, Kilb J, Kaplan H, Fietz D, Hossain H, Schüttler CG, Diemer T, Bergmann M, Domann E, Weidner W, Wagenlehner F, Schuppe HC (2019) High prevalence of urogenital infection/inflammation in patients with azoospermia does not impede surgical sperm retrieval. Andrologia 51:1–10. https://doi.org/10.1111/and.13401
Riccioli A, Filippini A, De Cesaris P, Barbacci E, Stefanini M, Starace G, Ziparo E (1995) Inflammatory mediators increase surface expression of integrin ligands, adhesion to lymphocytes, and secretion of interleukin 6 in mouse Sertoli cells. Proc Natl Acad Sci U S A 92:5808–5812. https://doi.org/10.1073/pnas.92.13.5808
Rival C, Theas MS, Guazzone VA, Lustig L (2006) Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J Reprod Immunol. https://doi.org/10.1016/j.jri.2005.10.006
Savitt J, Singh D, Zhang C, Chen LC, Folmer J, Shokat KM, Wright WW (2012) The in vivo response of stem and other undifferentiated spermatogonia to the reversible inhibition of glial cell line-derived neurotrophic factor signaling in the adult. Stem Cells 30:732–740. https://doi.org/10.1002/stem.1028
Sezer C, Koksal IT, Usta MF, Gulkesen KH, Erdogru T, Ciftcioglu A, Baykara M (2005) Relationship between mast cell and iNOS expression in testicular tissue associated with infertility. Arch Androl 51:149–158. https://doi.org/10.1080/014850190518161
Sharma M, Braun RE (2018) Cyclical expression of GDNF is required for spermatogonial stem cell homeostasis. Dev. https://doi.org/10.1242/dev.151555
Shiloh Y (2014) ATM: Expanding roles as a chief guardian of genome stability. Exp Cell Res 329:154–161. https://doi.org/10.1016/j.yexcr.2014.09.002
Shiloh Y (2003) ATM and related protein kinases: Safeguarding genome integrity. Nat Rev Cancer 3:155–168. https://doi.org/10.1038/nrc1011
Steger K, Aleithe I, Behre H, Bergmann M (1998) The proliferation of spermatogonia in normal and pathological human seminiferous epithelium: an immunohistochemical study using monoclonal antibodies against Ki-67 protein and proliferating cell nuclear antigen. Mol Hum Reprod 4:227-233. https://doi.org/10.1093/molehr/4.3.227
Suescun MO, Rival C, Theas MS, Calandra RS, Lustig L (2003) Involvement of tumor necrosis factor-α in the pathogenesis of autoimmune orchitis in rats. Biol Reprod 68:2114-2121. https://doi.org/10.1095/biolreprod.102.011189
Takubo K, Ohmura M, Azuma M, Nagamatsu G, Yamada W, Arai F, Hirao A, Suda T (2008) Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell 2:170–182. https://doi.org/10.1016/j.stem.2007.10.023
Theas MS, Rival C, Dietrich SJ, Guazzone VA, Lustig L (2006) Death receptor and mitochondrial pathways are involved in germ cell apoptosis in an experimental model of autoimmune orchitis. Hum Reprod 21:1734–1742. https://doi.org/10.1093/humrep/del066
Theas MS, Rival C, Jarazo-Dietrich S, Jacobo P, Guazzone VA, Lustig L (2008) Tumour necrosis factor-α released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Hum Reprod 23:1865–1872. https://doi.org/10.1093/humrep/den240
van Kroonenburgh MJ, Beck JL, Scholtz JW, Hacker-Klom U, Herman CJ (1985) DNA analysis and sorting of rat testis cells using two-parameter flow cytometry. Cytometry 6:321–326. https://doi.org/10.1002/cyto.990060408
Wing T-Y, Christensen AK (1982) Morphometric studies on rat seminiferous tubules. Am J Anat 165:13–25. https://doi.org/10.1002/aja.1001650103
Xiong Y, Hales DB (1993) The role of tumor necrosis factor-a in the regulation of mouse leydig cell steroidogenesis. Endocrinology 132:2438–2444. https://doi.org/10.1210/endo.132.6.8504748
Zhang L, Tang J, Haines CJ, Feng H, Lai L, Teng X, Han Y (2011) C-Kit and its related genes in spermatogonial differentiation. Spermatogenesis 1, 186–194. https://doi.org/10.4161/spmg.1.3.17760
Acknowledgements
We thank the Instituto Nacional de Microbiología “A. Malbrán”, División Vacunas Bacterianas for their generous gift of Bordetella pertussis, and M. Imsem, and C. García for their technical assistance.
Funding
Universidad de Buenos Aires (UBA 20020150100104BA) and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT PICT 0497).
Author information
Authors and Affiliations
Contributions
María Eugenia Ferreiro, Cinthia Soledad Méndez, and Leilane Glienke: investigation, methodology, formal analysis; Maria Jimena Ferraris and Patricia Verónica Jacobo: conceptualization, review and editing; Cristian Marcelo Sobarzo: visualization; Daniel Pisera and Livia Lustig: supervision, review and editing; Maria Susana Theas: conceptualization supervision, validation, resources, original draft.
Corresponding author
Ethics declarations
Ethics approval
The use of rats followed the National Institutes of Health (NIH) guidelines for care and use of experimental animals, and ethical approval was granted by local the committee: CICUAL-Facultad de Medicina, Universidad de Buenos Aires [RES (CD): 2029/2019].
Informed consent
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maria Eugenia Ferreiro and Cinthia Soledad Méndez are co-author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferreiro, M.E., Méndez, C.S., Glienke, L. et al. Unraveling the effect of the inflammatory microenvironment in spermatogenesis progression. Cell Tissue Res 392, 581–604 (2023). https://doi.org/10.1007/s00441-022-03703-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03703-z