Abstract
The morphology of the deep pineal gland of the Sprague Dawley rat was investigated by serial block face scanning electron microscopy. Cells were three-dimensionally (3-D) reconstructed using the software Fiji TrackEM. The deep pineal gland consisted of 2–5 layers of electron-lucent pinealocytes, with a euchromatic nucleus, endowed with one or two processes. Laterally, the deep pineal merged with the habenula and the stria medullaris thalami, via an intermediate area containing cells with more electron-dense cytoplasm and an indented nucleus with heterochromatin. Neither nerve terminals nor capillaries were observed in the deep pineal itself but present in the intermediate parts of the gland. The deep pineal was in contact with the third ventricle via the pineal and suprahabenular recesses. The ependymal lining in these recesses was an epithelium connected by tight junctions between their lateral cell membranes. Several intraventricular nerve terminals were in contact with the ependyma. 3-D reconstructions showed the ependymal cells endowed with long slender process penetrating the underlying pineal parenchyma. Few “tanocyte-like” ependymal cells, endowed with a process, reaching the subarachnoid space on the inferior surface of the deep pineal were observed. In addition, pinealocyte and astrocyte processes, often connected by gap junctions, bordered the inferior surface. In summary, the rat deep pineal gland is a neuroendocrine structure connected to the habenula. We here report specialized ependymal cells that might transmit signals from the cerebrospinal fluid to the deep pineal parenchyma and a “trans-pineal tanocyte-like cell” that connects the ventricular system with the subarachnoid space.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pineal complex of the rodents is part of the epithalamus and consists of a large superficial pineal gland and a smaller deep pineal gland connected by a slender stalk (Vollrath 1981). The superficial pineal gland is located on the surface of the brain in the most caudal part of the longitudinal cerebral fissure between the two hemispheres. The deep pineal gland is situated in the caudal roof of the third ventricle between the posterior and habenular commissures (Vollrath 1981; Møller and Baeres 2002). An evagination of the third ventricle, the pineal recess, extends into the parenchyma of the deep pineal (Møller and Baeres 2002). Furthermore, a second evagination of the third ventricle, the suprapineal recess, evaginates from the third ventricle, anterior to the habenular commissure, and extends in dorsal and caudal directions superior to the deep pineal gland (Ribas 1977). Laterally, the deep pineal is merging with the stria medullaris thalami, an epithalamic fiber bundle from the septal area, and hypothalamus terminating in the habenula.
Earlier investigations have questioned whether the deep pineal gland in rodents is a functional part of the pineal complex and is synthesizing and releasing the hormone melatonin as the superficial pineal gland (Moore 1975). However, studies using immunohistochemistry and in situ hybridization have shown that the pinealocytes in the deep pineal gland contain the biochemical machinery necessary for melatonin synthesis (Ribelayga et al. 1998; Karlsen et al. 2013; Rath et al. 2006, 2013, 2016).
Melatonin produced by the deep pineal might be secreted to the blood stream. However, the close relationship to the cerebrospinal fluid (CSF) in the third ventricle raises the question, whether melatonin and additional factors, such as peptides, in the deep pineal are also released directly to the CSF, as has been shown in ungulates (Tricoire et al. 2003a, b).
The deep pineal gland is also of interest in relation to the innervation of the pineal complex of the rat. Although the sympathetic innervation originating in the superior cervical ganglion is a functionally important innervation (Klein et al. 1997), a parasympathetic input to the gland from the sphenopalatine and otic ganglia is also present (Shiotani et al. 1986; Møller 1999). Furthermore, an innervation from small perikarya in the sensory trigeminal ganglion has been demonstrated in several studies (Møller 1999; Møller and Baeres 2002). Finally, an innervation from the brain (central innervation) has been demonstrated in several rodents by use of in vivo neuronal tracings (Korf and Wagner 1980; Møller and Korf 1983a), lesion studies (Rønnekleiv and Møller 1979; Møller and Korf 1983b), and electrophysiology (Rønnekleiv et al. 1980; Semm et al. 1981; Dafny 1983). However, the cell types and nerve fibers of the deep pineal gland, as well as their relation to the ventricular system and the subarachnoid surface of the brain in fissura transversa cerebri, are still poorly understood.
The advent of serial block face scanning electron microscopy has made it possible to produce a detailed ultrastructural analysis of the deep pineal gland. We have in this investigation analyzed series of about 500 serial block faces, 30 nm apart, of the Sprague Dawley rat deep pineal gland obtained by a serial block face scanning electron microscope (FEI Teneo VS). We provide a detailed description of the cell types in the central part of the deep pineal gland and in the lateral areas connected to the habenula and stria medullaris thalami. The 3-D reconstructions reveal that some ependymal cells were endowed with processes penetrating the pineal parenchyma and other ependymal cells possessed a cellular process extending to the subarachnoid space on the inferior part of the deep pineal.
Methods
Light microscopy
Three Sprague Dawley male rats weighing 200 g were anesthetized with tribromethanol (40 mg/100 g body weight) and vascularly perfused via the left ventricle of the heart with 100 ml phosphate-buffered saline (PBS) with heparin (1500 units (6.72 mg)/100 ml; Sigma # H3393-50 Ku) for 1 min. The right atrium was opened for the venous efflux before the start of the perfusion. This was followed by perfusion with 150 ml 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 5 min. The brains were removed and postfixed in the same fixative overnight in the fridge, cryoprotected in 25% sucrose in water for 48 h, and frozen in crushed carbon dioxide. Sixteen-micrometer-thick coronal sections of the brains were cut in a Leica cryostat at − 18 °C and mounted on glass slides. The sections were stained with 0.1% cresyl violet, dried for 1 h at 40 °C, and cover slipped with Pertex®.
Electron microscopy
Transmission electron microscopy
Five Sprague Dawley male rats were anesthetized with tribromethanol and perfused as described above with PBS followed by 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 5 min. The epithalamus with the superficial pineal was isolated and stored overnight in the fridge in the same fixative. The tissue was subsequently processed for serial block face scanning according to Deerinck et al. 2010 (https://ncmir.ucsd.edu/sbem-protocol).
The epithalamic tissue blocks were trimmed and postfixed in 2% OsO4 and 1.5% ferrocyanide in 0.15 M cacodylate buffer (pH 7.2), on ice for 2 h. Following 2 × 10-min rinse in double-distilled water (dd water) at room temperature, the tissue blocks were impregnated with a 1% TCH-solution (0.1 g thiocarbohydrazide in 10 ml dd water) for 40 min at room temperature, followed by impregnation in 2% OsO4 in dd water for 1 h. After 3 × 10-min rinse in dd water, the tissue blocks were impregnated in 0.02 M lead nitrate in 0.03 M sodium aspartate in dd water (pH adjusted to 5.5) at 60 °C for 60 min. Finally, the tissue was impregnated with 1% uranyl acetate in dd water in the fridge overnight. Following 3 × 10-min rinse in dd water, the blocks were dehydrated in an increasing ethanol series (70, 90, 96, and 100% ethanol) and via a mixture of Epon Hard® and propylene oxide (EH/pro: 25, 50, and 75%) transferred to 100% Epon Hard and polymerized for 48 h at 60 °C.
Overview sections, 2 µm in thickness, were cut in the coronal and sagittal planes and stained with toluidine blue. From the overview section, optimal fields were selected, and the blocks were trimmed for thin sectioning. Sections 60 nm in thickness were cut by use of an ultra-microtome (Leica UC7) and imaged in a CM 100 TEM (Philips) operated at 80 kV and equipped with a Veleta camera (Olympus) with a resolution of 2048 × 2048 pixels and the iTEM software package.
Serial block face scanning electron microscopy
Tissue blocks with the deep pineal gland were trimmed to approximately a 300 µm × 300 µm block face with less than 2-mm depth using a razor blade and polished using a conventional ultra-microtome and a diamond knife. The blocks were mounted using Epo-Tek EE129-4 Adhesive (EMS #12,670-EE, Ted Pella) to a 3-mm head diameter aluminum-mounting pin. The pinned sample was subsequently sputter coated with gold (20 nm) and transferred to the stage of a Teneo VS (FEI), aligned to the knife, and sequentially sectioned and block face imaged. This technique, based on the work of Denk and Horstman (2004), allows a backscattered electron image of the block face to be collected after each diamond knife cut. In total, 790 images were recorded giving an overall volume of 84 µm × 84 µm × 28 µm. This relates to a 6.7 nm × 6.7 nm × 30 nm voxel size.
Analysis
Image analysis of the serial sections obtained in the serial block face scanning electron microscope was performed using the software package, Image J (Schneider et al. 2012), with the plugin Track EM (https://imagej.net/software/fiji/).
Results
Gross anatomy and light microscopy appearance
In a dorsal view of the rat diencephalic-mesencephalic area, the pineal complex is seen as a long slender superficial pineal gland, located between the superior colliculi, connected via a pineal stalk to the deep pineal gland (Fig. 1). The deep pineal gland itself is attached to the posterior and habenular commissures in the caudal part of the third ventricle (Fig. 1).
Superior view of the rostral midbrain and diencephalon showing the epithalamic area with the pineal complex of the rat. “Rostral” indicates the rostral end of the tissue block and “Caudal” the caudal end. The superficial pineal gland (SP) is located between the superior colliculi (Sup.coll.) and is connected to the deep pineal gland (Deep pineal) via a pineal stalk, which is hidden in the pial tissue between the two structures. The stria medullaris thalami is seen on both sides of the third ventricle (3.ventr.) merging caudally in the posterior commissure (Post.com.). Scale bar 1 mm
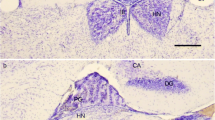
In Nissl-stained coronal cryostat sections through the posterior and habenular commissures of the rat, the pineal and suprahabenular recesses are seen below and above the habenular commissure, respectively (Fig. 2a, b). In the most rostral sections (Fig. 2a), the deep pineal gland constitutes 3–5 layers of pinealocytes located lateral to cells of the subcommissural organ. In more caudal sections (Fig. 2b), the cells of the subcommissural organ have disappeared, and all the cells dorsal to the posterior commissure are part of the deep pineal gland (Fig. 2b).
Coronal cryostat sections of the deep pineal gland of the rat. a Rostral section showing the subcommissural organ (SCO) located both ventral and dorsal to the posterior commissure (pc). Some pinealocytes (Deep pineal) are located lateral to the dorsal SCO bordering the ventricular system in the pineal recess (pr). Hc habenular commissure, shr suprahabenular recess. Cresyl violet staining. Scale bar 200 µm. b Caudal section through the deep pineal gland attached to the posterior commissure (pc). The subcommissural organ has disappeared in the pineal recess (pr) and only pinealocytes (Deep pineal) are bordering the ventricular system. hc habenular commissure, shr suprahabenular recess, 3.ventr. third ventricle. Cresyl violet staining. Scale bars 100 µm
In a coronal 2-µm-thick Epon-embedded section (Fig. 3a) just rostral to the posterior commissure, the medial and lateral habenular nuclei can be observed lateral to the third ventricle. A strongly myelinated fiber tract, the stria medullaris thalami, which is a projection from the septal nuclei, rostral hypothalamus, and rostral thalamic nuclei, is located on the dorsal part of the habenular nuclei (Fig. 3a). In a more caudal section (Fig. 3b), the stria medullaris is located lateral to the supracommissural part of the subcommissural organ and deep pineal gland (Fig. 4a) still connected to the superior part of the posterior commissure. This connection to the posterior commissure disappears in sections about 60 µm more caudal (Fig. 4c), where the most ventrally located pinealocytes in the deep pineal border the subarachnoid space in fissura transversa cerebri (Fig. 4c).
Montage of the coronal section of the habenular and subcommissural areas of the rat. a Dorso-caudal part of the third ventricle (3.ventr.) of the rat with the medial (mh) and lateral (lh) habenular nuclei. The stria medullaris thalami (Str. med.) is located on the dorsal part of the habenular nuclei. Two-micrometer-thick Epon embedded section. Toluidine blue staining. Scale bar 200 µm. b Slightly more caudal section through the subcommissural organ (SCO) located on both ventral and dorsal to the posterior commissure (pc). Two-micrometer-thick Epon embedded section. Toluidine blue staining. Shr suprahabenular recess, 3.ventr. third ventricle. Scale bar 200 µm
Montage of light and electron micrographs of coronal sections of the deep pineal gland of the rat. a Section showing the projection from the stria medullaris thalami (smp) lateral to the deep pineal gland. pc posterior commissure, shr suprahabenular recess. Two-micron-thick Epon embedded section. Toluidine blue staining. Scale bar 100 µm. b Electron micrograph of part of the area seen in a. A pinealocyte (P) with electron-lucent cytoplasm is located beneath the ependymal cells (E) bordering the suprahabenular recess (shr). smp projection of stria medullaris thalami. Scale bars 5 µm. c Coronal Sect. 20 µm caudal to the section in a. The deep pineal gland (Dp) is no longer attached to the posterior commissure (pc) but is abutting fissura transversa cerebri (ftc). SCO subcommissural organ, smp projection of stria medullaris thalami. Two-micrometer-thick Epon embedded section. Toluidine blue staining. Scale bar 400 µm. d Electron micrograph of the deep pineal gland seen in c. The electron-lucent pinealocytes with euchromatin (P) are located beneath the mitochondria-rich, more electron-dense ependymal cells (E). A transitional cell (T) is seen beneath the pinealocytes. On the inferior surface of the deep pineal gland, towards the fissura transversa cerebri (ftc), a pial cell (pi) is located in the subarachnoid space. shr suprahabenular recess. Scale bar, 10 µm
Electron micrographs of cells and nerve fibers in the rat deep pineal gland. a Overview electron micrograph of a coronal section of the rat deep pineal gland. Electron-lucent pinealocytes (P) are located below myelinated fibers of the posterior commissure (pc). A pinealocyte (yellow cell) has been 3-D reconstructed and is shown in the insert in the left side of the picture. A pial cell (pi) is located on the subarachnoid surface of the deep pineal in fissura transversa cerebri (ftc). T transitional cell. Scale bar 5 µm. The cell marked in yellow was 3-dimenstionally reconstructed using Fiji TrackEM (300 slices in the Z direction, with 30-nm distance between contiguous slices), and a 3-dimensional view projected on the first orthoslice is seen in the inset in the left corner of the micrograph. The nucleus is labelled red. Scale bar 5 µm. b Electron micrograph of an area of the deep pineal close to the posterior commissure. Several pinealocytes (P) are observed. A transitional cell (T) and an oligodendrocyte (oli) are also present. An annulate lamella (Al) is located below the oligodendrocyte. Scale bar 5 µm. c Electron micrograph from the lateral part of the deep pineal gland. A pinealocyte (P) is located beneath ependymal cells (E). Transitional cells (T) with more electron-dense cytoplasm and nuclei with heterochromatin are seen. shr suprahabenular recess. Scale bar 5 µm. d Electron micrograph from the lateral part of the pineal gland. A myelinated nerve fiber (m) and several unmyelinated fibers area present together with transitional cells (T). Scale bar 5 µm. e Electron micrograph from the lateral part of the deep pineal gland. A terminal process (arrow) containing small dense core vesicles and mitochondria is opposing a transitional cell (T). Scale bar 2.5 µm
Electron micrographs showing nerve terminals in the lateral part of the rat deep pineal gland bordering the projections of the stria terminalis thalami. a Sympathetic nerve terminals with small dense core transmitter vesicles. One sympathetic terminal (A) makes synaptic contact with a cellular process. Due to the density of the cytoplasm, the process probably originates from a transitional cell. A thin cellular process (B) separates a sympathetic terminal from the transitional cell above the terminal. Scale bar 2 µm. b Unmyelinated nerve fiber with a bouton en passant (A). A terminal with small and large dense core granules (B) makes synaptoid contacts with a transitional cell (T). Scale bar 0.5 µm. c Nerve terminal with small clear vesicles (arrow) making a synaptic contact with a transitional cell (T). Scale bar, 2 µm. d Myelinated nerve fiber endowed with a bouton en passant (arrow) after losing its myelin sheet. Scale bar 5 µm
Overview electron micrographs of the surface of the deep pineal gland towards the suprahabenular recess (shr). a Ependymal cells (E) with many cytoplasmic extensions projecting into the cerebrospinal fluid of the suprahabenular recess (shr) are seen. Many pinealocytes (P) are located beneath the ependyma. Scale bar 5 µm. b Montage showing the 3-D reconstruction of the ependymal cell (E) is also seen in a. The ependymal cell seen in the insert in the right lower corner was reconstructed using Fiji TrackEM (225 slices in the Z direction, with 30-nm distance between continuous slices). The insert in the left lower corner shows the same cell projected on the first orthoslice. shr suprahabenular recess. Scale bar 2.5 µm. c 3-D reconstruction of an ependymal cell (shown in magenta color) lining the surface towards the suprahabenular recess (shr). Note the long slender cellular process (arrow) extending towards the stria medullaris thalami. The cell was reconstructed from 428 serial, 30-nm-thick sections, and displayed projected on the first orthoslice. Scale bar 5 µm
Electron micrographs of the ependymal and pial surfaces of the rat deep pineal gland. a Luminal part of ependymal cells with many cytoplasmic projections. The luminal parts of the lateral ependymal membranes are connected by tight junctions (arrows). csf cerebrospinal fluid. Scale bar 500 nm. b Luminal part of ependymal cells with cytoplasmic projections and a kinocilium (arrow). csf cerebrospinal fluid. Scale bar 250 nm. c Intraventricular nerve terminal (arrow) in close apposition to an ependymal cell (E). csf cerebrospinal fluid. Scale bar 1 µm. d Secretion of an electron-dense product (arrow) by the ependymal cells into the cerebrospinal fluid (csf). Scale bar 1 µm. e Pial surface of the rat deep pineal gland towards the subarachnoid space in fissura transversa cerebri (ftc). A cellular process with many filaments (Fi) is bordering the surface, which is covered by a basal lamina (arrows). Scale bar 1 µm. f Surface of the deep pineal towards the subarachnoid surface in fissura cerebri transversa (fct). Thin lamellar cellular processes (arrows) are located beneath the surface. A cellular process with many filaments (Fi) is also present. Scale bar 2 µm
Light and electron micrographs of sagittal sections of the deep pineal gland illustrating the “trans-pineal tanocyte-like ependymal cells.” a Two-micrometer-thick Epon-embedded section of the epithalamic area of the Sprague Dawley rat. The deep pineal (Dp) is located between the habenular (hc) and posterior (pc) commissures. SCO subcommissural organ, 3.ventr. third ventricle. Toluidine blue staining. Scale bar, 100 µm. b Higher magnification of the rat deep pineal gland shown in the parallelogram in a. The deep pineal is bordering the pineal recess (pr) to the left in the micrograph, and fissura transversa cerebri (ftc) to the right in the photomicrograph. The arrow marked tE points toward the subarachnoid process of the trans-pineal tanocyte-like ependymal cell seen in the electron micrograph in c. hc habenular commissure, pc posterior commissure. Toluidine blue staining. Scale bar 20 µm. c A trans-pineal tanocyte-like ependymal cell (tE) with the luminal part (arrow) in contact with the cerebrospinal fluid in the pineal recess (pr) and a process (arrowhead) reaching the subarachnoid space in fissura transversa cerebri (ftc). p pinealocyte. Scale bar 5 µm. d Three-dimensional view of the reconstructed trans-pineal tanocyte-like ependymal cell (tE) using Fiji TrackEM (300 sections in the Z direction, with 30-nm distance between contiguous sections) projected on the last orthogonal slice in the series. ftc fissura transversa cerebri. P pinealocyte, pr pineal recess. Scale bar 5 µm. e Higher magnification of part of the trans-pineal tanocyte-like ependymal cell (tE) seen in c. Several terminals (arrows) are abutting the process of the cell. Scale bar 2 µm. f The 3-D reconstructed trans-pineal tanocyte-like ependymal cell seen in d viewed from the last section of the series. Scale bar 5 µm
Ultrastructural appearance
Cell types in the rat deep pineal gland
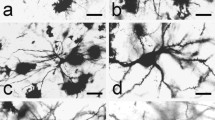
The pinealocytes in the deep pineal gland were by transmission electron microscopy (TEM) characterized by an electron-lucent cytoplasm, an ovoid nucleus with only minor indentations and homogenous euchromatin in the nucleus (Figs. 4b, d and 5a-c). The cytoplasm of pinealocytes contained the classical organelles such as mitochondria, Golgi apparatus, smooth and rough endoplasmic reticulum, and many clear vesicles (Fig. 5a-c). In addition, several annulate lamellae were observed (Fig. 5b). However, the presence of dense core secretory vesicles was rare, and acervuli, lipid droplets, and synaptic ribbons were not detected. In addition to the electron-lucent pinealocytes with euchromatin, cells with a denser cytoplasm and heterochromatin in the nucleus were present (Figs. 5d, e and 6a-d). We named such cells transitional cells. The cells were more numerous in the intermediate area located in the lateral parts of the deep pineal, where few pinealocytes were present. In addition, electron-dense oligodendrocytes (Fig. 5b) could be observed in the lateral parts, often located between the myelinated nerve fibers. Astroglial cells, recognized due to the presence of many neurofilaments in the cytoplasm, were present, and processes from these cells were seen bordering the subarachnoid space (Fig. 8e, f).
Nerve fibers and terminals in the rat deep pineal gland
In the central part of the deep pineal gland inferior to the posterior commissure (Fig. 5a, b), some myelinated and unmyelinated nerve fibers and nerve terminals were present (Fig. 5a, b) without making synaptic contacts or terminal boutons in this area. In the lateral areas of the deep pineal gland, nerve terminals were present. Some of the nerve terminals were sympathetic (Fig. 6a), containing small ovoid transmitter vesicles with an eccentric-located dense core. Other terminals were non-sympathetic terminals containing clear vesicles without a dense core (Fig. 6c). Some of the unmyelinated fibers were endowed with boutons en passant (Fig. 6b). Few myelinated fibers terminated as unmyelinated fibers with boutons en passant (Fig. 6d). It was in our series of thin sections not possible to determine the origin of any of the nerve terminals encountered.
The surfaces of the rat deep pineal gland
The cellular lining of the deep pineal toward the cerebrospinal fluid (CSF) in the suprahabenular and pineal recesses consisted of electron-dense ependymal cells with many mitochondria. The cells did not constitute a simple columnar epithelium, but consisted of cells with an irregular cell body from which few processes extended into the deep pineal parenchyma (Fig. 7a-c). Some ependymal cells were endowed with very long thin processes extending into the deep pineal parenchyma (Fig. 7c) between the pinealocytes and interstitial cells. We were not able to detect any synapses or specialized membrane connections between these processes and the cells in apposition to the process. From the luminal surface or the ependymal cells, kinocilia and small cytoplasmic projections emerged (Fig. 8a, b). Tight junctions located between the luminal parts of the lateral membranes of the ependymal cells (Fig. 8a) were present. An electron-dense material, secreted from the luminal and apical parts of the ependymal membranes, was located as a surface coat on the ependymal cells (Fig. 8d). Terminals of intraventricular nerve fibers were located on the surface of some ependymal cells (Fig. 8c).
The surface of the deep pineal, abutting the subarachnoid space in fissura transversa cerebri, was lined with an electron-dense basal lamina (Fig. 8e, f). On the pineal side of the basal lamina, astrocytic foot processes were present (Fig. 8e) interdigitating with processes from the pinealocytes themselves (Fig. 8e). Single pial cells were located on the subarachnoid side of the basal lamina (Fig. 5a). Gap junctions were observed between the cells bordering this surface.
In the sagittal sections of the deep pineal gland (Fig. 9a-f), some ependymal cells were extending all the way from the ependymal surface of the deep pineal gland to the subarachnoid space in fissura transversa cerebri (Fig. 9c and d). This “trans-pineal” ependymal cell consisted of a large ependymal part, containing the nucleus, extending a thinner process towards the subarachnoid surface (Fig. 9c-f). Close to the subarachnoid space, bouton-like structures with clear vesicles were observed to make contact to the process (Fig. 9e). We have named the ependymal cell a “trans-pineal tanocyte-like cell.” However, no vascular system was present in fissura transversa cerebri.
Discussion
The deep pineal gland is a transition area between the habenula and pineal gland
The pineal gland of rodents is termed a pineal complex because it consists of three parts: a large superficial pineal gland, a deep pineal gland, and a stalk connecting the two structures (Vollrath 1981). This morphology differs from that of most other mammals, where the pineal gland is situated on the dorsal part of the brain stem with only a short stalk attaching the gland to the meso-diencephalic area of the brain (Vollrath 1981; Møller and Baeres 2002). The volume of the deep pineal gland is small compared to that of the superficial pineal gland, and this volume varies considerably among rodents (Boeckmann 1980). In the rat, the deep pineal, also termed lamina intercalaris (Kappers 1960; Björklund et al. 1972), has a volume between 1.5 and 3.0% of the total pineal complex (Vollrath 1981).
In this study by TEM imaging, the pinealocytes of the deep pineal gland of the Sprague Dawley rat exhibited an electron-lucent cytoplasm and a nucleus-containing euchromatin. As seen in the light microscopic coronal sections of this study, pinealocytes of the deep pineal gland were mostly located in the midline area, dorsal to the posterior commissure, and merged laterally with the projections of the stria medullaris thalami and the medial habenular habenula via an intermediate area. The majority of cells in the intermediate area exhibited a denser cytoplasm and an indented nucleus with heterochromatin. We have named these cells “transitional cells.” The morphology of the cells in the intermediate area supports the concept that some cells in the rat deep pineal represent a transitional cell type between an endocrine pinealocyte and a neuron. The presence of such a transitional cell type in the rat deep pineal-habenular area has been indicated in several previous studies. Thus, investigations of gene expression in the rat deep pineal gland by use of in situ hybridization and immunohistochemical visualization of proteins have shown that the transcripts and proteins present in the pinealocytes are not expressed in all the cells (Rath et al. 2006, 2013 and 2016). On the other hand, some cells in the stria medullaris thalami and the medial habenular nucleus express pinealocyte markers. Thus, after treating rats with a monoamine oxidase inhibitor and using the classical Falck-Hillarp histofluorescence technique (Falck et al. 1962), a strong serotonin fluorescence, a marker for pinealocytes, is observed in cells located in the stria medullaris thalami. Furthermore, cells immunoreactive for the S-antigen, a marker for pinealocytes, are present in the medial habenular nucleus of the Djungarian hamster (Korf et al. 1986) and mouse (Korf et al. 1990; Brednow and Korf 1998). Therefore, some of the cells in the rodent deep pineal-habenular area may represent a transitional cell type between a neuroendocrine cell and a neuron.
The pinealocytes in the deep pineal of the Sprague Dawley rat contained the classical organelles, e.g., mitochondria, Golgi apparatus, smooth and rough endoplasmic reticulum, and clear vesicles described in the pinealocytes of the superficial pineal gland (Karasek 1992). However, the number of the larger 50–100-nm dense core vesicles considered to contain an up to now unidentified peptide (Haldar-Misra and Pévet 1983) was low. In the superficial pineal gland of the rat, these dense core vesicles are present, but the number varies considerably among rodent species with a high number in the mouse and hamster (Juillard 1979) and a moderate number in the rat (Vollrath 1981). Synaptic ribbons, a highly distinctive rod-like organelle, present in photoreceptive cells of lower vertebrates and in secretory pinealocytes (Vollrath 1973; King and Dougherty 1982; Jastrow et al. 1997), were in this study not observed in the pinealocytes of the deep pineal gland. The frequency of synaptic ribbons present in the pinealocytes of the superficial gland also varies between species; e.g., the organelle is more common in the pinealocytes of guinea pigs than that in rats (Mcnulty and Fox 1992). Based on the ultrastructural appearance of the pinealocytes in the rat deep pineal gland, our data suggest that the amount of peptide, synthesized in this part of the pineal complex, is low. An interesting organelle observed in some pinealocytes of the deep pineal is the annulate lamella. Annulate lamellae are common organelles in the hamster pinealocytes (Bucana et al. 1971), but are also present in pinealocytes of other mammals including the albino rat (Wolfe 1965). Annulate lamellae are porous stacked membranes located in the cytoplasm often connected with one part to the endoplasmic reticulum and the opposite part to the nuclear envelope. Immunohistochemical analysis of the proteins in the annulate lamellae indicates an important role in nucleocytoplasmic trafficking (Rawe et al. 2003). In our stack of sections, we were able to follow the annulate lamellae in Fig. 5b from its origin in the cytoplasm before disappearing again (Sect. 186–287, 30-nm thick, total 3.030 µm). However, in the investigated cell, the annulate lamellae were connected to neither the endoplasmic reticulum nor the nuclear membrane.
The 3-dimensional reconstructions of the pinealocytes in the rat deep pineal gland, made in this study by serial block face scanning of the tissue block in the Teneo VS electron microscope, showed the pinealocytes to be cells with a polygonal shape endowed with one or two cellular processes. Pinealocytes are often described as astrocytic-like, star-shaped cells with several processes emerging from the cell body. This description is based on silver staining of the human pineal gland (Del-Río Hortega 1922; Scharenberg and Liss 1965). However, staining of pinealocytes in the superficial pineal of the rat has not confirmed such a morphology. Therefore, a 3-dimensional reconstruction of the cells in the superficial pineal is necessary for obtaining a detailed information about the complex cellular morphology and connections of the rat superficial pineal gland.
The deep pineal gland is now recognized as a functional part of the pineal complex. Thus, in the hamster, melatonin has been measured in dissected deep pineal tissue by use of radioimmunoassay, and the concentration of melatonin was shown to exhibit a circadian rhythm with a peak during the nighttime (Sheridan and Rollag 1983). In the Wistar rat, mRNA encoding the enzyme hydroxyindole-O-methyltransferase (HIOMT), now called acetylserotonin O-methyltransferase (ASMT), an enzyme transforming N-acetylserotonin to melatonin, has by in situ hybridization been shown to be present in both the superficial pineal gland, the pineal stalk, and the deep pineal gland (Ribelayga et al. 1998). Furthermore, several studies using immunohistochemistry and in situ hybridization have shown that both the superficial pineal gland and the cells in the deep pineal gland of the Sprague Dawley rat express proteins and transcription factors important for synthesis and regulation of melatonin synthesis (Karlsen et al. 2013; Rath et al. 2006, 2013, 2016). This raises the question how the melatonin synthesis in the deep pineal gland is regulated and how melatonin reaches its target cells in the brain.
Nerve fibers of multiple origins innervate the rat deep pineal gland
The synthesis of melatonin in the rat superficial pineal gland is stimulated by postganglionic sympathetic nerve fibers with perikaryal origin in the superior cervical ganglion of the sympathetic trunk (Kappers 1960; Møller 1992). The released noradrenaline is targeting β-adrenergic receptors on the cell membrane of the pinealocyte (Klein et al. 1983). Such sympathetic nerve endings were present in the intermediate area of rat deep pineal gland and easily recognized due to the ovoid-shaped 40–60-nm transmitter vesicles containing a small dense core and few larger dense core granules. The large dense core vesicles in the sympathetic nerve terminals in the superficial pineal of rodents such as the rat (Zhang et al. 1991; Mikkelsen and Møller 1999) and gerbil (Shiotani et al. 1986) contain neuropeptide Y (NPY). Neuropeptide Y is inhibiting the noradrenalin-stimulated secretion of melatonin in the pineal gland (Olcese 1991).
Sympathetic nerve terminals were, in this study of the Sprague Dawley rat, not observed in the central part of the rat deep pineal between the pinealocytes. This is in contrast to ultrastructural studies of the deep pineal gland of the mouse, where sympathetic nerve terminals were located between the pinealocytes in the deep pineal gland (Møller 1981). However, sympathetic nerve terminals were clearly present in lateral parts of the Sprague Dawley rat deep pineal. Thus, noradrenaline might still target the pinealocytes due to the short diffusion distance in the deep pineal gland of this species.
Parasympathetic nerve terminals with clear transmitter vesicles, containing large dense core granules, were also observed in the intermediate parts of the rat deep pineal. Such terminals are also present in the superficial rat pineal gland (Rønnekleiv and Møller 1979), and acetylcholine is inhibiting secretion of melatonin from the gland (Phansuwan-Pujito et al. 1999). The dense core granules in the cholinergic nerve fibers contain vasoactive intestinal peptide (VIP) (Møller et al. 1987) and have been in rodents traced back to the parasympathetic sphenopalatine and otic ganglia (Shiotani et al. 1986). VIP via binding to receptors on the pinealocytes stimulates melatonin secretion (Yuwiler 1983). In other nerve terminals, the large dense core granules contain pituitary adenylate cyclase-activating peptide (PACAP) (Liu and Møller 2000), calcitonin gene-related peptide, and substance P (Shiotani et al. 1986; Matsushima et al. 1999). These intrapineal nerve fibers have by retrograde in vivo tracings been shown to originate from small perikarya in the trigeminal ganglion (Shiotani et al. 1986; Reuss 1999; Møller and Liu 1999). The presence of an innervation from the trigeminal ganglion has also been shown by use of anterograde in vivo tracings (Larsen et al. 1998). PACAP is stimulating secretion of melatonin via PACAP receptors in the pinealocyte cell membrane (Simonneaux et al. 1993). The influence of CGRP and substance P on pineal physiology has not been elucidated. It might be possible that CGRP and SP include VIP and PACAP target receptors on the vasculature of the gland. An innervation of a neuroendocrine structure with nerve fibers originating in the sensory trigeminal ganglion is a new concept in neuroanatomy.
In this study, we observed myelinated nerve fibers in the lateral part of the deep pineal that were losing the myelin sheet and subsequently displaying boutons en passant. Such nerve fibers are non-sympathetic fibers, but we were not able to determine the location of the cell bodies from which these fibers originate. However, most likely, these fibers originate from nerve cell bodies outside the pineal complex because, in spite of the presence of intrapineal neuronal perikarya in the pineal of several mammals, e.g., human, monkey, ferret (David and Herbert 1973), rabbit (Romijn 1975), and cotton rat (Sakai et al. 1994), such neurons have never been observed in the pineal gland of the Sprague Dawley rat.
In the stria terminalis thalami itself, nerve cell bodies were present. These neurons are probably extensions of the neurons in the medial habenular nucleus. Classical axo-dendritic synapses with clear transmitter vesicles in the presynaptic boutons were observed on the stria medullaris neurons. However, whether the neurons in the stria medullaris thalami innervate the pineal gland was not solved in this study.
The surface of the deep pineal gland towards the CSF is bordered by a tight ependyma with long processes into the deep pineal parenchyma
The rat deep pineal gland is bordering the ventricular system in the pineal and suprahabenular recesses. In several species, pinealocytes emerging between the ependymal cells contact the CSF, e.g., in the rhesus monkey (Hewing 1980), golden hamster (Hewing 1978), guinea pig (Hewing 1980), sheep (Tricoire et al. 2003a), and Mongolian gerbil (Hewing 1982; Welsh 1983). Furthermore, physiological date in the sheep supports a release of melatonin directly to the CSF. Thus, in the sheep, the concentration of melatonin is higher in the pineal recess than that in the ventral part of the third ventricle (Tricoire et al. 2003b).
However, in this study of the deep pineal of the rat, such a CSF-contacting pinealocyte surface towards the ventricular system was not observed. In this species, the border area towards the pineal and suprapineal recesses consisted of electron-dense ependymal cell, endowed with many kinocilia and cytoplasmic projections, and tight junctions connecting the luminal part of the lateral membranes of the ependymal cells.
Our study showed a much-diversified morphology of the cells in the ependymal lining towards the ventricular lumen. Thus, our 3-D reconstruction of pinealocytes shows that some of the cells are endowed with the long slender processes penetrating the subependymal layer. This indicates that signals from the CSF could be transmitted to pinealocytes and intermediate cells in the deep pineal. We were in this study not able to detect any membrane specialization of these processes. This might be due to the resolution limits of the serial block face scanning electron microscope. However, we observed processes in the pineal parenchyma terminating on capillaries in the deep pineal gland itself. However, limited by the number of sections in our analyzed stack, we could not with certainty show that these processes originate from ependymal cells.
In the sagittal section of the deep pineal, we observed ependymal cells, endowed with cilia and cytoplasmic processes into the CSF, projecting a process through the pineal parenchyma terminating on the inferior pial surface in fissura transversa cerebri. We have named this cell a “trans-pineal tanocyte-like ependymal cell.” However, no vascular system, comparable to the venous portal system in the median eminence (Korf and Møller 2021), was present. Such a “trans-pineal tanocyte-like ependymal cell” might still transmit signals from the CSF to the surface of the brain.
In addition to the processes of the “trans-pineal ependymal cell,” the subarachnoid surface of the deep pineal consisted of processes from astrocytes and pinealocytes connected by gap junctions and covered by a basal lamina and some pial cells.
In summary, this paper shows that the deep pineal gland of the Sprague Dawley rat is a neuroendocrine area with a transitional zone towards the habenula and the stria medullaris thalami. The innervation is mostly located in the transitional zone receiving nerve fibers of multiple origins. The presence of specialized ependymal cells indicates that signals from the CSF might participate in the regulation of the cells in the deep pineal gland. Furthermore, a “trans-pineal tanocyte-like ependymal cell” might be a deep pineal analogue to the tanocytes in the infundibular recess of the hypothalamus.
References
Björklund A, Owman Ch, West KA (1972) Peripheral sympathetic innervation and serotonin cells in the habenular region of the rat brain. Z Zellforsch 127:570–579
Boeckmann D (1980) Morphological investigation of the deep pineal of the rat. Cell Tissue Res 210:283–294. https://doi.org/10.1007/BF00237616 (PMID: 7407872)
Brednow K, Korf HW (1998) Morphological and immunocytochemical features of the pineal organ of C3H and C57BL mice at different stages of postnatal development. Cell Tissue Res 292:521–530. https://doi.org/10.1007/s004410051081 (PMID: 9582409)
Bucana CD, Nadakavukaren MJ, Frehn JL (1971) Annulate lamellae in hamster pineal gland. Tissue Cell 3:405–411. https://doi.org/10.1016/s0040-8166(71)80042-3 (PMID: 18631562)
Dafny N (1983) Evidence that the rat pineal has neuronal connections via the pineal stalk. Exp Neurol 79:858-861. https://doi.org/10.1016/0014-4886(83)90048-1
David GFX, Herbert J (1973) Experimental evidence for a synaptic connection between habenula and pineal ganglion in the ferret. Brain Res 64:327–343
Deerinck TJ, Bushong EA, Thor A, Ellisman MH (2010) NCMIR methods for 3D EM: a new protocol for preparation of biological specimens for serial blockface scanning electron microscopy. Center for Research in Biological Systems and the National Center for Microscopy and Imaging Research, University of California, San Diego, La Jolla, CA, USA https://ncmir.ucsd.edu/sbem-protocol
Denk W, Horstmann H (2004) Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol 2:e329. https://doi.org/10.1371/journal.pbio.0020329
Del Río-Hortega P (1922) Constitución histologica de la glandula pineal. Arch Neurol 3:359–389
Falck B, Hillarp N-Å, Thieme G, Torp A (1962) Fluoresence of catecholamines and related compounds with formaldehyde. J Histochem Cytochem 10:348–354. https://doi.org/10.1177/10.3.348
Haldar-Misra C, Pévet P (1983) Influence of melatonin on the process of protein and/or peptide secretion in the pineal gland of the rat and hamster. An in vitro study. Cell Tissue Res 231:73–82. https://doi.org/10.1007/BF00215775
Hewing M (1978) A liquor contacting area in the pineal recess of the golden hamster (Mesocricetus auratus). Anat Embryol (berl) 153:295–304. https://doi.org/10.1007/BF00315932
Hewing M (1980) Cerebrospinal fluid-contacting area in the pineal recess of the vole (Microtus agrestis), guinea pig (Cauia cobaya), and rhesus monkey (Macaca mulatta). Cell Tissue Res 209:473–484. https://doi.org/10.1007/BF00234759
Hewing M (1982) Pinealocytes contacting the cerebrospinal fluid of the suprapineal recess in the Mongolian gerbil (Meriones unguiculatus). Cell Tissue Res 222:177–185. https://doi.org/10.1007/BF00218298
Jastrow H, Von Mach M-A, Vollrath L (1997) The shape of synaptic ribbons in the rat pineal gland. Cell Tissue Res 287:255–261. https://doi.org/10.1007/s004410050750
Juillard M-T (1979) The proteinaceous content and possible physiological significance of dense-cored vesicles in hamster and mouse pinealocytes. Ann Biol Anim Biochim Biophys 19:413–428
Kappers JA (1960) The development, topographical relations and innervation of the epiphysis cerebri in the albino rat. Zeitschrift ZellforschMikrosk Anat 52:163–221. https://doi.org/10.1007/BF00338980
Karasek M (1992) Ultrastructure of the mammalian pinealocyte under natural and experimental conditions: quantitative aspects. Micr Res Tech 21:116–123. https://doi.org/10.1002/jemt.1070210204
Karlsen AS, Rath MF, Rohde K, Toft T, Møller M (2013) Developmental and diurnal expression of the synaptosomal-associated protein 25 (Snap25) in the rat pineal gland. Neurochem Res 38:1219–1228. https://doi.org/10.1007/s11064-012-0918-7
King TS, Dougherty WJ (1982) Effect of denervation on “synaptic” ribbon populations in the rat pineal gland. J Neurocytol 1:19–22. https://doi.org/10.1007/bf01258002
Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Begay V, Falcon J, Cahill GM, Cassone VM, Baler R (1997) The melatonin rhythm generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res 52:307–357 (PMID: 9238858)
Klein D, Sugden D, Weller JL (1983) Postsynaptic a-adrenergic receptors potentiate the padrenergic stimulation of pineal serotonin N-acetyltransferase Proc Natl Acad Sci USA 80599–80603 https://doi.org/10.1073/pnas.80.2.599
Korf HW, Wagner U (1980) Evidence for a nervous connection between the brain and the pineal organ in the guinea pig. Cell Tissue Res 209:505–510. https://doi.org/10.1007/BF00234762
Korf HW, Møller M (2021) The median eminence and infundibular nucleus; window for endocrine and metabolic communication. Handbook of clinical neurology, Vol.180 (3rd series). The human hypothalamus: middle and posterior region (DF Swaab, PJ Lucassen, F Krei, R Buijs, and A Saleh, Editors), pp. 227–251 Elsevier, BV
Korf HW, Oksche A, Ekström P, Gery I, Zigler JS Jr (1986) Klein DC (1986) Pinealocyte projections into the mammalian brain revealed with S-antigen antiserum. Science 231:735–737. https://doi.org/10.1126/science.3454660
Korf HW, Sato T, Oksche A (1990) Complex relationships between the pineal organ and the medial habenular nucleus-pretectal region of the mouse as revealed by S-antigen immunocytochemistry. Cell Tissue Res 261:493–500. https://doi.org/10.1007/BF00313528
Larsen PJ, Enquist LW, Card JP (1998) Characterization of the multisynaptic neuronal control of the rat pineal gland using viral transneuronal tracing. Eur J Neurosci 10:128–145. https://doi.org/10.1046/j.1460-9568.1998.00003.x
Liu W, Møller M (2000) Innervation of the rat pineal gland by PACAP-immunoreactive nerve fibers originating in the trigeminal ganglion: a degeneration study. Cell Tissue Res 301:369–373. https://doi.org/10.1007/s004410000251
Matsushima S, Sakai Y, Hira Y (1999) Peptidergic peripheral nervous systems in the mammalian pineal gland. Microsc Res Tech 46:265–280. https://doi.org/10.1002/(SICI)1097-0029(19990815/01)46:4/5%3c265::AID-JEMT4%3e3.0.CO;2-S
Mcnulty JA, Fox LM (1992) Pinealocyte synaptic ribbons and neuroendocrine function. Microsc Res Techn 21:175–187. https://doi.org/10.1002/jemt.1070210302
Mikkelsen JD, Møller M (1999) Neuropeptide Y in the mammalian Pineal gland. Microsc Res Techniq 46:239–256. https://doi.org/10.1002/(SICI)1097-0029(19990815/01)46:4/5%3c239::AID-JEMT2%3e3.0.CO;2-2
Moore RY (1975) Indolamine metabolism in the intact and denervated pineal, pineal stalk and habenula. Neuroendocrinol 19:323–330. https://doi.org/10.1159/000122453 (PMID: 1241605)
Møller M (1981) The ultrastructure of the deep pineal gland of the Mongolian gerbil and mouse: granular vesicle localization and innervation. In: Matthews CD, RF seemark, (eds) Pineal Function. Elsevier, Amsterdam, pp 257–266
Møller M (1992) Fine structure of the pinealopetal innervation of the mammalian pineal gland. Microsc Res Techn 21:188–204. https://doi.org/10.1002/jemt.1070210303
Møller M (1999) Introduction to mammalian pineal innervation. Microsc Res Techniq 46:235–238. https://doi.org/10.1002/(SICI)1097-0029(19990815/01)46:4/5%3c235::AID-JEMT1%3e3.0.CO;2-9
Møller M, Korf HW (1983a) The origin of central pinealopetal nerve fibers in the Mongolian gerbil as demonstrated by the retrograde transport of horseradish peroxidase. Cell Tissue Res 230:273–287. https://doi.org/10.1007/bf00213805
Møller M, Korf HW (1983b) Central innervation of the pineal organ of the Mongolian gerbil. A histochemical and lesion study. Cell Tissue Res 230:259–272. https://doi.org/10.1007/BF00213804
Møller M, Liu W (1999) Innervation of the rat pineal gland by nerve fibres originating in the sphenopalatine, otic and trigeminal ganglia. A retrograde in vivo neuronal tracing study. Reprod Nutr Dev 39:345–353. https://doi.org/10.1051/rnd:19990307
Møller M, Baeres FMM (2002) The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res 309:139–150. https://doi.org/10.1007/s00441-002-0580-5
Møller M, Cozzi B, Schröder H, Mikkelsen JD (1987) The peptidergic innervation of the mammalian pineal gland. In: Fundamentals and clinics in pineal research (GP Trentini, C de Gaetani, P Pévet, eds.). Serono Symposia Publication nr. 44, pp. 71 77. New York: Raven Press 1987
Olcese J (1991) Neuropeptide Y: an endogenous inhibitor of norepinephrine-stimulated melatonin secretion in the rat pineal gland. J Neurochem 57:943–947. https://doi.org/10.1111/j.1471-4159.1991.tb08241.x
Phansuwan-Pujito P, Møller M, Govitrapong P (1999) Cholinergic innervation and function in the mammalian pineal gland. Microsc Res Tech 46:281–195. https://doi.org/10.1002/(SICI)1097-0029(19990815/01)46:4/5%3c281::AID-JEMT5%3e3.0.CO;2-N
Rath MF, Coon SL, Amaral FG, Weller JL, Møller M, Klein DC (2016) Melatonin synthesis: acetylserotonin o-methyltransferase (ASMT) is strongly expressed in a subpopulation of pinealocytes in the male rat pineal gland. Endocrinol 157:2028–2040. https://doi.org/10.1210/en.2015-1888
Rath MF, Muñoz E, Ganguly S, Morin F, Shi Q, Klein DC, Møller M (2006) Expression of the Otx2 homeobox gene in the developing mammalian brain: embryonic and adult expression in the pineal gland. J Neurochem 97:556–566. https://doi.org/10.1111/j.1471-4159.2006.03773.x
Rath MF, Rohde K, Klein DC, Møller M (2013) Homeobox genes in the rodent pineal gland: roles in development and phenotype maintenance. Neurochem Res 3:1100–1111. https://doi.org/10.1007/s11064-012-0906-y
Rawe VY, Olmedo SB, Nodar FN, Ponzio R, Sutovsky P (2003) Abnormal assembly of annulate lamellae and nuclear pore complexes coincides with fertilization arrest at the pronuclear stage of human zygotic development. Hum Reprod 28:576–582. https://doi.org/10.1093/humrep/deg114 (PMID: 12615828)
Reuss S (1999) Trigeminal innervation of the mammalian pineal gland. Microsc Res Tech 46:305–309. https://doi.org/10.1002/(SICI)1097-0029(19990815/01)46:4/5%3c305::AID-JEMT7%3e3.0.CO;2-#
Ribas, JL (1977) The Rat Epithalamus I. Correlative Scanning-Transmission Electron Microscopy of Supraependymal Nerves. Cell Tiss. Res. 182, 1-16. https://doi.org/10.1016/0006-8993(77)90631-x
Ribelayga C, Gauer F, Pévet P, Simonneaux V (1998) Distribution of hydroxyindole-O-methyltransferase mRNA in the rat brain: an in situ hybridisation study. Cell Tissue Res 291:415–421.
Romijn HJ (1975) Structure and innervation of the pineal gland of the rabbit, Oryctolagus cuniculus (L.). Cell Tissue Res 157:25–51. https://doi.org/10.1007/bf00223229
Rønnekleiv OK, Møller M (1979) Brain-pineal nervous connections in the rat: an ultrastructure study following habenular lesion. Exp Brain Res 37:551–562. https://doi.org/10.1007/BF00236823
Rønnekleiv OK, Kelly MJ, Wuttke W (1980) Single unit recordings in the rat pineal gland: evidence for habenulo-pineal neural connections. Exp Brain Res 39:187–192. https://doi.org/10.1007/BF00237549
Sakai Y, Hira Y, Oomori Y, Daikoku S, Matsushima S (1994) Immunohistochemical studies on sympathetic and non-sympathetic nerve fibers and neuronal cell bodies in the pineal gland of cotton rats, Sigmodon hispidus. Arch Histol Cytol 57:47–58. https://doi.org/10.1679/aohc.57.47
Scharenberg K, Liss L (1965) The histologic structure of the human pineal body. Prog Brain Res 10:193–217. https://doi.org/10.1016/s0079-6123(08)63452-4 (PMID: 14281603)
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Semm P, Schneider T, Vollrath L (1981) Morphological and electrophysiological evidence for habenular influence on the guinea-pig pineal gland. J Neural Transm 150:247–266. https://doi.org/10.1007/BF01249146
Sheridan MN, Rollag MD (1983) Development and melatonin content of the deep pineal gland in the Syrian hamster. Am J Anat 168:145–156. https://doi.org/10.1002/aja.1001680204
Shiotani Y, Yamano M, Shiosaka S, Emson PC, Hillyard CJ, Girgis S, MacIntyre I (1986) Distribution and origins of substance P (SP)-, calcitonin gene-related peptide (CGRP)-, vasoactive intestinal polypeptide (VIP)-, and neuropeptide Y (NPY)-containing nerve fibers in the pineal gland of gerbils. Neurosci Lett 70:87–192. https://doi.org/10.1016/0304-3940(86)90461-1
Simonneaux V, Ouichou A, Pévet P (1993) Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates melatonin synthesis from rat pineal gland. Brain Res 603:148–152. https://doi.org/10.1016/0006-8993(93)91313-h
Tricoire H, Malpaux B, Møller M (2003a) Morphological indications for a direct secretion of melatonin from the pineal gland to the third ventricle of the sheep. A light and electron microscopical study of the pineal recess. J Comp Neurol 456:39–47. https://doi.org/10.1002/cne.10477
Tricoire H, Møller M, Chemineau P, Malpaux B (2003b) Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reproduction, Suppl 61:311–321 (PMID: 14635944)
Vollrath L (1973) Synaptic ribbons of a mammalian pineal gland circadian changes. Cell Tissue Res 145:171–183. https://doi.org/10.1007/bf00307386
Vollrath L (1981) The pineal organ. Hdb mikr Anat Mensch, VI/7 (A Oksche, L Vollrath, eds) Springer: Berlin Heidelberg New York.
Welsh MG (1983) CSF-contacting pinealocytes in the pineal recess of the Mongolian gerbil: a correlative scanning and transmission electron microscope study. Am J Anat 166:483–493. https://doi.org/10.1002/aja.1001660408 (PMID: 6858943)
Wolfe DE (1965) The epiphyseal cell: an electron-microscopic study of its intercellular relationship and intracellular morphology in the pineal body of the Albino rat. Prog Brain Res 10:332–386. https://doi.org/10.1016/s0079-6123(08)63460-3 (PMID: 14281612)
Yuwiler A (1983) Vasoactive intestinal peptide stimulation of pineal serotonin-N-acetyltransferase activity: general characteristics. J Neurochem 41:146–153. https://doi.org/10.1111/j.1471-4159.1983.tb11826.x.PMID
Zhang E, Mikkelsen JD, Møller M (1991) Tyrosine hydroxylase-and neuropeptide Y-immunoreactive nerve fibers in the pineal complex of untreated rats and rats following removal of the superior cervical ganglia. Cell Tissue Res 265:63–71. https://doi.org/10.1007/BF00318140
Funding
This work was supported by the Velux foundation, grant no. 37840 to Morten Møller, the Independent Research Fund Denmark, grant no. 8020-00037B (to MFR), and the Lundbeck Foundation, grant no. R344-2020–261 (to MFR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were performed in accordance with the guidelines of EU directive 2010/63/EU. The study was approved by the Danish Animal Experiments Inspectorate (authorization number 2017–15-0201–01190) and by the Faculty of Health and Medical Sciences, University of Copenhagen (authorization number P21-146).
Informed consent
The informed consent of the participants in this study was obtained verbally and confirmed by e-mail.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Møller, M., Midtgaard, J., Qvortrup, K. et al. An ultrastructural study of the deep pineal gland of the Sprague Dawley rat using transmission and serial block face scanning electron microscopy: cell types, barriers, and innervation. Cell Tissue Res 389, 531–546 (2022). https://doi.org/10.1007/s00441-022-03654-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03654-5