Abstract
In numerous mammalian species, the nose harbors several compartments populated by chemosensory cells. Among them, the Grueneberg ganglion (GG) located in the anterior nasal region comprises sensory neurons activated by given substances. In rodents, in which the GG has been best studied, these chemical cues mainly include heterocyclic compounds released by predators or by conspecifics. Since some of these substances evoke fear- or stress-associated responses, the GG is considered as a detector for alerting semiochemicals. In fact, certain behavioral and physiological reactions to alarm pheromones and predator-secreted kairomones are attenuated in the absence of a functional GG. Intriguingly, GG neurons are also stimulated by cool temperatures. Moreover, ambient temperatures modulate olfactory responsiveness in the GG, indicating that cross-talks exist between the transduction pathways mediating chemo- and thermosensory signaling in this organ. In this context, exploring the relevant molecular cascades has demonstrated that some chemosensory transduction elements are also crucial for thermosensory signaling in the GG. Finally, for further processing of sensory information, axons of GG neurons project to the olfactory bulb of the brain where they innervate distinct glomerular structures belonging to the enigmatic necklace glomeruli. In this review, the stimuli activating GG neurons as well as the underlying transduction pathways are summarized. Because these stimuli do not exclusively activate GG neurons but also other sensory cells, the biological relevance of the GG is discussed, with a special focus on the role of the GG in detecting alarm signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Grueneberg ganglion (GG) was first described by Hans Grüneberg in 1973 as a cluster of cells residing in the most rostral region of the nose in mammals (Grüneberg 1973). Although the GG has been initially thought to be closely associated with the nervus terminalis, more recent studies have revealed that its neuronal cells express the olfactory marker protein (OMP) (Fig. 1a–d), indicating that the GG is part of the olfactory system (Grüneberg 1973; Fuss et al. 2005; Koos and Fraser 2005; Fleischer et al. 2006a; Roppolo et al. 2006; Storan and Key 2006). The existence of a GG (or a related organ) has been reported for several species belonging to different orders of mammals, such as Rodentia, Insectivora, Edentata, and Carnivora (Grüneberg 1973; Tachibana et al. 1990; Brechbühl et al. 2014). However, it is still elusive if primates, including humans, are also equipped with a GG, although some findings suggest that a GG-related organ might also exist in humans, at least in human embryos (Grüneberg 1973). Hitherto, the GG has been mainly investigated in the house mouse (Mus musculus); consequently, most of the features and physiological processes characteristic of the GG that are summarized in this review pertain to the murine GG.
The GG is composed of clusters of OMP-positive cells in the most rostral nasal region. a–d Sections through the head of a neonatal mouse incubated with an antiserum against OMP (green) and counterstained in blue with 4′,6-diamidino-2-phenylindole (DAPI) or Toto-3. a On a sagittal section, the OMP-expressing GG neurons in the rostral area of the nose are stained (arrow). In a more caudal region, olfactory neurons in the MOE as well as their axonal processes projecting to the OB are labeled. b The coronal section according to the section plane denoted by the broken line in a reveals that the GG is a bilateral organ encompassing clusters of OMP-positive neurons that are situated between the nasal roof (NR), the nasal cavity (NC), and the septum. c High magnification image from a coronal section depicting a cluster of OMP-expressing GG neurons. d Higher magnification of a sagittal section through the rostral region of the murine nose. Besides the somata of OMP-positive GG neurons, the antiserum against OMP also labels their axons (arrows) that run in caudal direction (R, rostral; C, caudal) (data from Fleischer et al. 2006b; with kind permission of John Wiley and Sons). Scale bars: a = 500 μm; b = 200 µm; c = 10 µm; d = 100 μm
In the anterior nasal region, the GG encompasses cell clusters that are situated bilaterally in the nasal vestibule. In this compartment of the nose, the GG is embedded in a connective tissue encircled by the septum, the nasal roof, and a thin epithelial layer that lines the lumen of the nasal cavity (Fig. 1a–c). Thus, unlike other olfactory subsystems in the mammalian nose harboring OMP-expressing sensory neurons, such as the main olfactory epithelium (MOE), the septal organ, and the vomeronasal organ (VNO), the cells of the GG do not reside in an epithelium (Grüneberg 1973; Fuss et al. 2005; Koos and Fraser 2005; Fleischer et al. 2006a; Roppolo et al. 2006; Storan and Key 2006; Brechbühl et al. 2014; reviewed by Fleischer and Breer 2010; Fleischer 2014). With respect to its cells, the GG comprises neuronal and non-neuronal cell types; the latter (termed satellite or ensheathing cells) reveal a glia-like phenotype and largely envelope the neuronal cells in the GG (Tachibana et al. 1990; Brechbühl et al. 2008; Chehrehasa et al. 2018). The neurons of the GG (usually designated as GG neurons or GG cells), however, are characterized by the expression of OMP and neuronal markers, including βIII tubulin and protein gene product 9.5 (PGP9.5) (Fleischer et al. 2006a; Brechbühl et al. 2008, 2014). Each of the nearly 800 GG neurons in mice extends an axonal process (Fig. 1d) that projects to the olfactory bulb (OB) of the brain (Fuss et al. 2005; Koos and Fraser 2005; Fleischer et al. 2006a; Roppolo et al. 2006; Storan and Key 2006; Matsuo et al. 2012; Bumbalo et al. 2017a).
Responsiveness to distinct chemical cues in the GG and its relevance for detecting predator odors and alarm pheromones
The axonal projection to the OB as well as expression of OMP indicate an olfactory function of the GG (Fuss et al. 2005; Koos and Fraser 2005; Fleischer et al. 2006a; Roppolo et al. 2006; Storan and Key 2006). However, contrary to olfactory neurons in other nasal compartments, neurons in the GG are not endowed with an apical dendrite and their cilia do not reach the surface of the nasal epithelium, suggesting that these neurons lack a direct access to the nasal lumen (Roppolo et al. 2006; Brechbühl et al. 2008). Moreover, as the connective tissue harboring the GG in the anterior nasal region is overlaid by a (keratinized) epithelium (Brechbühl et al. 2008), it is uncertain how chemical stimuli reach GG cells. Brechbühl and co-workers (2008) have observed that the epithelial layer overlying the GG is permeable for some compounds. Thus, chemostimuli might gain access to the GG via diffusional processes. In fact, experiments utilizing tissue sections or live mice have disclosed that the GG is activated by chemicals from the environment (Brechbühl et al. 2008; Mamasuew et al. 2011a). In the latter approaches, expression of the activity-dependent gene c-Fos was utilized to monitor responsiveness of murine GG neurons to odorous compounds. Subsets of GG neurons were found to respond to certain pyrazine derivatives, notably 2,3-dimethylpyrazine (2,3-DMP), 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, and 2,3,5-trimethylpyrazine (Mamasuew et al. 2011a). However, several other substances tested in these experiments, including some related pyrazines, did not stimulate GG neurons, indicating that the GG serves as an olfactory subsystem activated by a limited set of chemicals (Mamasuew et al. 2011a). Yet, in studies based on the expression of c-Fos or calcium imaging, a couple of additional compounds have been identified that also evoke responses in subpopulations of GG neurons, including 2-ethyl-3,5-dimethylpyrazine, 2,3-lutidine (2,3-dimethylpyridine), 2,4-lutidine, 3,4-lutidine, 2-s-butyl-4,5-dihydrothiazole (SBT), 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), 2-propylthietane (2-PT), 1,3-dithiolane, and trimethylene sulfide (Mamasuew et al. 2011a; Brechbühl et al. 2013a, 2013b, 2015; Pérez-Gómez et al. 2015; Chao et al. 2018). Interestingly, most of the GG-activating substances identified so far are heteroaromatic or at least heterocyclic compounds, such as pyrazines, pyridines, thiazoles, and thiazolines. Experiments with chemicals activating the murine GG (2,3-DMP, SBT, and TMT) revealed that these substances also stimulate GG neurons of Norway rats and Golden hamsters, whereas they only poorly elicit responses in the GG of Mongolian gerbils. In consequence, it seems that GG responsiveness to certain chemicals is at least partially species-dependent (Brechbühl et al. 2014).
With respect to substances stimulating GG neurons, it is noteworthy that they also activate chemosensory cells in other olfactory organs, in particular in the MOE and in the VNO (Mamasuew et al. 2011a; Brechbühl et al. 2013a); thus, these ligands are not specific to the GG. This aspect raises the question why (some) mammals are equipped with a GG although the relevant ligands are also detected through other chemosensory organs. There is currently no clear answer to this issue; however, from a functional point of view, the peculiar axonal projection pattern of GG neurons to certain glomeruli in the bulb (as discussed in more detail below) might channel important sensory information acquired via the GG into separate cerebral centers for subsequent neuronal processing. In this regard, it is worthy of note that several of the heterocyclic nitrogen- and/or sulfur-containing compounds that activate GG neurons (e.g., 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, 3-ethyl-2,5-dimethylpyrazine, 2,4-lutidine, 2-PT, 2,3,5-trimethylpyrazine, and TMT) are released by predators (ferret, fox, mountain lion, and wolf, respectively). Because these chemical cues induce avoidance, fear-associated behaviors, and/or an elevated blood pressure in rodents, they can be considered as predator-secreted kairomones (Vernet-Maury et al. 1984; Wallace and Rosen 2000; Zhang et al. 2005; Kobayakawa et al. 2007; Osada et al. 2013; Brechbühl et al. 2015; Sievert and Laska 2016). Therefore, the GG might serve as a sensor for substances warning rodents against carnivores. With a view to the detection of alerting compounds, the GG has been reported to be critical for perceiving alarm pheromones, i.e., chemicals specifically released to signal alarm or danger to conspecifics (Brechbühl et al. 2008; Debiec and Sullivan 2014). In particular, the GG ligand SBT has been found to function as an important component of the alarm pheromone in mice that stimulates numerous GG neurons and evokes stress- and fear-related responses, including increased serum concentrations of stress hormones, elevated blood pressure, and freezing behavior (Brechbühl et al. 2013a; Chao et al. 2018). Importantly, the GG does not only contribute to the detection of given alerting substances, such as alarm pheromones and predator scents, its activation is furthermore essential for the fear and stress responses elicited by these compounds (Brechbühl et al. 2013a; Pérez-Gómez et al. 2015; Chao et al. 2018). Thus, the functional relevance of the GG might not only lie in its reception of alerting substances that simultaneously activate neurons in the MOE but beyond that in its importance for evoking the adequate physiological and behavioral reactions. With a view to behavior controlled by the GG, most recently, it has been demonstrated that also food choice is influenced in a GG-dependent manner when food is odorized with alarming substances (e.g., SBT, 2-PT, or TMT) that activate GG neurons, leading to the avoidance of such food sources (Brechbühl et al. 2020). For the acquisition of food preferences, a process termed social transmission of food preference (STFP) is of outstanding importance. During STFP, rodents utilize chemical substances present in urine, feces, and breath to communicate information related to food. Notably carbon disulfide, a compound of rodent breath, promotes STFP in rodents when paired with food odors (Bean et al. 1988; Galef et al. 1988; Galef 2012). The so-called GC-D neurons, a subpopulation of olfactory neurons in the MOE, are considered critical for establishing STFP by detecting relevant odorants, including carbon disulfide and a few other substances released by conspecifics (Leinders-Zufall et al. 2007; Munger et al. 2010; Arakawa et al. 2013; Kelliher and Munger 2015). Intriguingly, STFP can be erased in a GG-dependent manner by alerting compounds activating GG neurons. Thus, the GG is also implicated in controlling contextual food choice (Brechbühl et al. 2020).
Finally, analyzing the chemical structure of heterocyclic GG ligands in more detail, it was noted lately that they share some structural similarities with certain bitter tastants, such as 6-propyl-2-thiouracil (PTU), 2-ethylpyrazine (2-EP), 6-methyl-2-thiouracil, and methimazole. Testing these substances in calcium imaging experiments, PTU, 2-EP, and 6-methyl-2-thiouracil were found to stimulate larger subpopulations of GG neurons. The volatile compound 2-EP also induced fear-associated behaviors in mice in a GG-dependent manner, whereas PTU failed to elicit such behavioral responses, possibly due to its limited volatility (Moine et al. 2018). However, whether biologically relevant sources of 2-EP (and related bitter tastants activating the GG) exist in the natural habitat of mice is still elusive.
Chemosensory signal transduction mechanisms in the GG
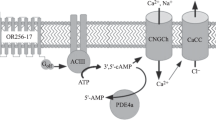
The observation that substances activating cells in the GG also stimulate subsets of neurons in the MOE (Mamasuew et al. 2011a; Brechbühl et al. 2013a) might indicate that chemosensory cells in both organs utilize similar transduction cascades. However, while detection of odorants in neurons of the MOE largely relies on odorant receptors, the adenylyl cyclase ACIII, the G protein Golf, and the cyclic nucleotide-gated (CNG) channel subunit CNGA2 (Brunet et al. 1996; Wong et al. 2000; Firestein 2001; Mombaerts 2004a; Zheng and Zagotta 2004), these signaling proteins are mostly absent from the GG (Fleischer et al. 2006b; Mamasuew et al. 2010). Instead, numerous GG neurons express a distinct CNG subunit, CNGA3 (Liu et al. 2009; Mamasuew et al. 2010). Functional experiments have disclosed that CNGA3 is essential for stimulation of GG neurons by 2,3-DMP, 2,3-lutidine, and 2-PT because responsiveness to these chemicals is decreased in the GG and in the respective bulbar glomeruli of mice lacking CNGA3 (Mamasuew et al. 2011b; Hanke et al. 2013; Pérez-Gómez et al. 2015; Bumbalo et al. 2017a). Consistent with the strong activation of CNGA3 by cyclic guanosine monophosphate (cGMP) (Dai et al. 2013; Kaupp and Seifert 2002), CNGA3-positive GG neurons co-express the cGMP-generating guanylyl cyclase subtype G (GC-G), and the cGMP-hydrolyzing phosphodiesterase (PDE) type PDE2A (Fleischer et al. 2009; Liu et al. 2009; Mamasuew et al. 2010). In GG neurons, the CNGA3 and the GC-G proteins reside in filiform and whip-like subcellular structures, probably the cilia of these cells (Liu et al. 2009; Schmid et al. 2010; Mamasuew et al. 2011b); these findings are reminiscent of the localization of olfactory signaling elements in cilia from sensory neurons in the MOE (Menco 1997; Menco et al. 1997; Meyer et al. 2000; Strotmann et al. 2004).
The belonging of GC-G to the family of the receptor/transmembrane guanylyl cyclases led to speculations that GC-G could serve as a receptor in GG cells by directly binding to (odorous) ligands via its extracellular domain (Chao et al. 2018), similar to other members of this protein group (Leinders-Zufall et al. 2007; Duda and Sharma 2008; Cockerham et al. 2009). Heterologous expression of GC-G has disclosed that the murine alarm pheromone component SBT indeed binds to the extracellular domain and subsequently enhances the enzymatic activity of GC-G. Moreover, GG neurons from GC-G-deficient mice only poorly respond to SBT, indicating that GC-G functions as a pheromone receptor in the GG (Chao et al. 2018). This concept was further corroborated by the observation that heterologous expression of GC-G and its effector, the cGMP-activated channel CNGA3, renders cells responsive to SBT, suggesting that these two signaling proteins are sufficient to elicit a SBT-evoked response (Chao et al. 2018). Importantly, in mice lacking GC-G, not only reactivity to SBT in the GG is decreased but also the behavioral and physiological responses that are usually induced in mice by this compound, such as freezing, avoidance, increased blood pressure, and elevated serum levels of the stress hormone corticosterone (Chao et al. 2018). Finally, the high percentage of SBT-reactive GG cells (> 70%) also corresponds with the expression of GC-G (and its effector CNGA3) in the vast majority of the neurons in this organ (Fleischer et al. 2009; Liu et al. 2009; Mamasuew et al. 2010; Brechbühl et al. 2013a; Chao et al. 2018).
In addition to SBT, GC-G is also critical for GG responsiveness to other odorants, including 2,3-DMP and 2,3-lutidine (Mamasuew et al. 2011b; Hanke et al. 2013). This observation is in line with the finding that in the GG, 2,3-DMP stimulates exclusively GC-G/CNGA3-positive neurons (Mamasuew et al. 2011a). Whether GC-G also serves as a receptor for 2,3-DMP, 2,3-lutidine, and other GG ligands, similar to SBT, is unclear. Alternatively, GC-G might “only” function as a downstream signaling element for the detection of these substances in the GG. In any case, the cGMP-associated signal transduction proteins GC-G and CNGA3 play an important role for the reception of given odorants in GG neurons (Mamasuew et al. 2011b; Hanke et al. 2013; Pérez-Gómez et al. 2015; Chao et al. 2018). However, some odorants might be detected via the GG independent of GC-G and CNGA3, e.g., responsiveness to the predator odorant TMT is not affected in GG neurons from CNGA3-deficient mice (Pérez-Gómez et al. 2015). Consequently, additional transduction pathways seem to exist in the GG mediating the detection of relevant odorants. Consistent with this concept, expression of different G protein-coupled receptors (GPCRs) belonging to distinct groups of chemosensory receptor proteins has been described for the GG (Fleischer et al. 2006b, 2007; Moine et al. 2018). For instance, a small subpopulation of GG neurons are endowed with certain trace amine-associated receptors (TAARs) (Fleischer et al. 2007), a group of GPCRs whose members are mostly expressed in subsets of olfactory cells in the MOE and which are therefore considered as olfactory receptors (Liberles and Buck 2006; Liberles 2015). Although expression of several TAARs has been documented for the GG, only one TAAR subtype appears to be expressed per cell (Fleischer et al. 2007). While some TAARs (TAAR2, TAAR4, and TAAR5) are expressed by very few cells in the GG, others (TAAR6 and TAAR7a/d) are expressed in clearly higher numbers of GG neurons; yet, the percentage of GG cells equipped with these TAAR types is rather moderate (Fleischer et al. 2007). Hitherto, the functional implications of TAAR-positive GG neurons are unknown, notably for two reasons. First, for the murine TAAR6 and TAAR7a/d, cognate ligands have not been identified. Second, TAAR-expressing GG cells seem to lack the transduction elements GC-G, CNGA3, and PDE2A that are required for the activation of GG neurons by several GG ligands (e.g., 2,3-DMP, 2-PT, and SBT) (Fleischer et al. 2009; Mamasuew et al. 2010, 2011b; Pérez-Gómez et al. 2015; Chao et al. 2018); these findings argue against an involvement of neurons endowed with TAARs in the detection of such compounds. In contrast to the relatively few TAAR-positive cells, numerous GG neurons express receptor V2r83 (also termed V2R2 or Vmn2r1) (Fleischer et al. 2006b), a member of the V2R family of olfactory receptors that is likewise abundant in many sensory neurons of the VNO (Martini et al. 2001). While the V2r83-positive GG neurons apparently lack expression of TAARs, they are equipped with GC-G, CNGA3, and PDE2A (Fleischer et al. 2007, 2009; Mamasuew et al. 2010; Matsuo et al. 2012). Thus, the V2r83-expressing GG neurons are most likely implicated in the reception of GG-activating substances whose detection relies on GC-G and CNGA3. Receptor V2r83 has been shown to be activated in a heterologous expression system by some hydrophobic amino acids, including isoleucine, leucine, and valine (DeMaria et al. 2013). So far, a stimulation of GG neurons by such amino acids has not been described. In addition, V2r83 is supposed to be critical for the expression of other V2R types (Akiyoshi et al. 2018). Whether such a function also applies to V2r83 in the GG is uncertain; especially since expression of other V2Rs has not been documented for GG neurons.
Regarding the expression of GPCRs in the GG, besides olfactory receptors of the TAAR and the V2R family, most recently, some bitter taste receptors belonging to the TAS2R (also called T2R) family were reported to be expressed in the GG. Notably the TAS2R subtype TAS2R143 was found to be present in numerous GG neurons (Moine et al. 2018). Heterologous expression of this receptor in human embryonic kidney (HEK) cells rendered these cells responsive to GG ligands, such as SBT, 2-PT, TMT, and 2-EP (Moine et al. 2018). These results indicate that TAS2R143 could operate as a chemosensory receptor in GG neurons contributing to the activation by certain heterocyclic chemicals. However, for none of the chemosensory GPCR types expressed in the GG (TAARs, V2r83, and TAS2Rs), it has been demonstrated that they are indeed involved in the reception of odorous compounds in GG neurons; particularly because the indication for an implication of these GPCRs in GG chemosensory signaling comes from experiments with other cells or heterologous expression systems. The notion that chemosensory GPCRs might be functional in GG neurons is supported by the expression of different α-subunits from heterotrimeric G proteins, including Gi, Go, and gustducin, in large subsets of GG neurons (Fleischer et al. 2006b; Moine et al. 2018). Yet, similar to the GPCRs, there is currently no experimental evidence that these G proteins are actually crucial for detecting odorants in the GG.
Taken together, previous studies have unraveled a number of receptors and downstream signal transduction proteins that are or seem to be implicated in the detection of environmental chemicals via the GG (Fig. 2). Interestingly, in many GG neurons, more than one receptor type appears to be expressed per cell; noteworthy, these receptors can even belong to different receptor classes, as already shown for the V2r83-positive GG neurons that co-express GC-G (Fleischer et al. 2009). Co-expression of several chemosensory receptor proteins is in marked contrast to olfactory neurons in other nasal compartments, in particular the MOE, in which only one receptor type is assumed to be expressed per cell (Mombaerts 2004b). Based on the co-expression of different receptors, distinct transduction pathways might operate in parallel in a single GG neuron to enable reception of multiple odorants. In fact, several odorants stimulate broad subpopulations of cells in the GG and individual GG neurons have been reported to respond to various of these compounds (Mamasuew et al. 2011a; Brechbühl et al. 2013a, 2015; Pérez-Gómez et al. 2015; Chao et al. 2018). Thus, with respect to the activation of GG neurons via substances released by predators or functioning as alarm pheromones, the GG might not be dedicated to the discrimination of different odorants but rather solely conveys specific alerting signals to the brain. This concept is consistent with the very limited number of GG cells and would be reminiscent of gustatory cells on the tongue for bitter tasting substances that co-express several receptors of the TAS2R/T2R family (Adler et al. 2000; Fuss et al. 2005; Fleischer et al. 2006a, 2007; Behrens et al. 2007). In this context, it is interesting to note that similar to the GG, also gustatory cells for bitter tastants detect alerting signals because many bitter tasting substances are noxious. Food containing larger quantities of bitter tastants, for which discrimination via the gustatory system does not seem to be required, elicits given innate behaviors with a protective function, such as spitting and the termination of eating. Similarly, it is conceivable that the alerting compounds detected via the GG do not have to be discriminated; as mentioned above, these substances induce protective innate reactions, including freezing, avoidance, and further fear-/stress-associated responses and behaviors (Brechbühl et al. 2013a; Chao et al. 2018).
Chemo- and thermosensory signaling elements in the GG. In the GG, appropriate odorants are considered to bind to receptor proteins residing in the cell membrane. Chemosensory receptors expressed in GG neurons comprise heptahelical GPCRs, including V2r83, TAARs (e.g., TAAR6 and TAAR7a/d), and TAS2Rs (e.g., TAS2R143), as well as the transmembrane guanylyl cyclase GC-G. Upon binding of cognate odorous ligands, the heptahelical receptor types are supposed to interact with heterotrimeric G proteins expressed in GG neurons (Gi, Go, and gustducin), which might subsequently affect unknown downstream signaling pathways. Binding of odorants (notably the alarm pheromone compound SBT) to GC-G enhances the formation of cGMP. Increased cGMP concentrations most likely evoke opening of the CNGA3 channel, inducing an influx of positively charged ions (presumably Na+ and Ca2+), thus eliciting membrane depolarization. Finally, cGMP is hydrolyzed by PDE, probably the subtype PDE2A. Alike given odorants, cool temperatures also trigger elevated enzymatic activity of GC-G, leading to activation of CNGA3. In addition to stimulation of GC-G, cool temperatures are also thought to inactivate the potassium channel TREK-1 that is co-expressed with CNGA3 and GC-G in thermosensory GG neurons. Closing of TREK-1 is considered to contribute to coolness-induced depolarization of the cell membrane. In this schematic representation, arrows with broken lines denote signaling mechanisms in GG neurons that have not been demonstrated so far. The arrow with the dotted line indicates a signaling process that has not been confirmed by experiments using transgenic knockout animals
In studies investigating the expression of chemosensory transduction elements in the GG and/or the responsiveness of GG neurons to appropriate chemical stimuli, animals (mostly mice) of distinct ages were used, ranging from pups to adults. Some of the observations made in these studies may only hold true for given developmental stages, but not for others. For example, based on in situ hybridization experiments, the expression of mRNA for receptor V2r83 and several TAARs in GG neurons was found to be higher in perinatal stages than in adults (Fleischer et al. 2007). Yet, it is unknown if the amount of the corresponding receptor proteins is also decreased in the GG of adults. Alternatively, it is conceivable that the protein level for these transduction elements has already reached a peak level in the GG of adults; thus, only a reduced amount of mRNA in GG neurons of adults is still required to replace the relevant protein molecules degraded in the course of turnover. In this regard, studies utilizing specific antibodies on GG tissue sections have confirmed expression of some transduction proteins involved in GG chemosensory signaling, including CNGA3 and GC-G, in GG neurons of both adult and neonatal mice (Liu et al. 2009; Mamasuew et al. 2010, 2011b; Schmid et al. 2010; Brechbühl et al. 2014). Accordingly, these findings suggest that GG responses to given stimuli that rely on these transduction elements are observable in both adults and pups. In fact, using calcium imaging, odorant-induced GG responses have been described for adults and neonates (Brechbühl et al. 2008, 2013a, 2013b, 2014, 2015; Pérez-Gómez et al. 2015; Chao et al. 2018; Moine et al. 2018). However, detailed analyses in which GG responses to given chemostimuli were comprehensively compared between individuals of different ages in calcium imaging experiments have not been conducted. Consequently, it could be possible that certain odorants evoke differential responses in the GG of animals from different ages. Interestingly, GG responses to 2,3-DMP monitored by the expression of c-Fos were clearly stronger in neonates than in juveniles and adults (Mamasuew et al. 2011a). Yet, it cannot be excluded that this observation is just due to a potentially lower ability of GG neurons in adults to express c-Fos upon stimulation with a given odorant (or any other appropriate stimulus).
Activation of GG neurons by cool temperatures
The presumably most intriguing trait of the GG is that besides the above-described responsiveness to given chemical cues, most of its neurons (~ 75%) are also activated by cool ambient temperatures (Mamasuew et al. 2008, 2010; Schmid et al. 2010; Brechbühl et al. 2013b; Chao et al. 2015). Such coolness-induced responses in the GG of living mice were observed at temperatures lower than about 27 °C (Stebe et al. 2014). GG neurons responsive to coolness comprise the V2r83-positive cells, while the TAAR-expressing neurons are not stimulated by cool temperatures (Mamasuew et al. 2008). Activation by coolness might be the reason for GG neurons being situated in the most anterior region of the nose that is subjected to remarkable temperature variations when the ambient temperature changes (Brechbühl et al. 2013b; Chao et al. 2015). Cool temperatures do not only activate GG cells but also the relevant glomeruli in the OB (Bumbalo et al. 2017a), strongly suggesting that coolness induces electrical signals in GG neurons that are transferred via their axonal processes to the bulb; therefore, the GG is regarded as a dual sensory organ.
In search for signal transduction elements contributing to coolness-induced responses in the GG, it was found that GG neurons lack expression of the transient receptor potential channel subtype TRPM8 (Fleischer et al. 2009), a thermosensory protein that is critical for activating neurons in trigeminal and dorsal root ganglia by cool temperatures (Bautista et al. 2007; Dhaka et al. 2007). Instead, many neurons in the GG express TREK-1, a potassium channel inactivated at cool temperatures (Maingret et al. 2000; Stebe et al. 2014). In TREK-1-deficient mice, GG stimulation by coolness is decreased, indicating that inactivation of TREK-1 at cool temperatures elicits depolarization of the cell membrane (Stebe et al. 2014) (Fig. 2). Although this observation reveals a role of TREK-1 as a thermosensor in GG neurons, it has to be noted that even in mice lacking expression of TREK-1, responses to cool temperatures in the GG are clearly detectable (Stebe et al. 2014), suggesting that an additional thermosensory protein contributes to coolness-induced activation of GG neurons. In this context, it has to be pointed out that the (TREK-1-positive) coolness-sensitive GG neurons express V2r83 as well as CNGA3 and GC-G (Mamasuew et al. 2008, 2010; Fleischer et al. 2009; Stebe et al. 2014). Interestingly, in mice deficient for GC-G, responsiveness to cool temperatures in the GG is markedly reduced (Chao et al. 2015). Likewise, elimination of CNGA3 leads to a considerable decrease in coolness-induced GG responses (Mamasuew et al. 2010); consistently, GG signals evoked by cool temperatures are also diminished by the CNG channel inhibitor L-cis diltiazem (Brechbühl et al. 2013b). Yet, heterologous expression of CNGA3 in HEK cells does not render these cells responsive to cool temperatures, arguing against the notion that CNGA3 itself serves as a thermosensory protein. However, when the cGMP-sensitive ion channel CNGA3 was co-expressed with the cGMP-producing enzyme GC-G in HEK cells, responses to coolness were detectable, indicating that a cGMP pathway mediates coolness-evoked signaling in GG neurons via activation of GC-G by cool temperatures (Chao et al. 2015) (Fig. 2). Indeed, contrary to related transmembrane guanylyl cyclases, enzymatic activity of heterologously expressed GC-G is substantially enhanced at cool temperatures, reaching a maximum at ~ 15 °C; consequently, GC-G can function as a thermosensor (Chao et al. 2015). The molecular processes underlying the unusual activation of GC-G by coolness are still uncertain. However, unlike the activation by the pheromonal substance SBT (Chao et al. 2018), only the intracellular domains of GC-G appear to be essential for stimulation by cool temperatures, whereas the extracellular domains of GC-G are dispensable for this reactivity (Chao et al. 2015). Moreover, cool temperatures promote dimerization/oligomerization of GC-G but not of other guanylyl cyclase types (Chao et al. 2015). Since dimerization/oligomerization has been proposed to be crucial for cGMP synthesis by transmembrane guanylyl cyclases (Thompson and Garbers 1995; Wilson and Chinkers 1995; reviewed in Lucas et al. 2000), coolness-induced dimerization/oligomerization might cause the elevated enzymatic activity of GC-G at cool temperatures.
In summary, two distinct thermosensors, GC-G and TREK-1, seem to operate simultaneously in coolness-sensitive GG neurons (Fig. 2). Such a co-expression of distinct thermosensors is not unique for the GG but has been also described for thermosensitive neurons in the trigeminal ganglion that express TREK-1 along with the coolness-stimulated channel TRPM8 (Yamamoto et al. 2009).
Although cool temperatures elicit strong signals in GG neurons independent of the presence of appropriate odorants (Schmid et al. 2010; Brechbühl et al. 2013b; Chao et al. 2015), the biological relevance of coolness-evoked responses in the GG is unclear; in particular because the GG is not essential for given behaviors triggered by cool temperatures in mice, including thermotaxis and huddling (Brechbühl et al. 2013b). So far, only coolness-induced ultrasound vocalization in mouse pups was found to be impaired in individuals with a GG deficient in the detection of cool temperatures (Chao et al. 2015). Such ultrasound calls, which are frequently generated by rodent pups on exposure to cool temperatures, are of particular importance for the poikilothermic mouse pups to attract the warmth-giving homeothermic dam (Allin and Banks 1971; Okon 1971; Oswalt and Meier 1975; Blumberg et al. 1992; Szentgyörgyi et al. 2008). Consistently, strong (odorant-independent) responses to cool temperatures were observed in GG neurons of neonatal mice in calcium imaging experiments and in experiments monitoring the expression of c-Fos (Mamasuew et al. 2008; Chao et al. 2015). The approaches based on expression of c-Fos have also suggested that coolness-evoked GG reactivity might be more intense in pups than in adults since stronger cooling was required to elicit responses in the GG of adults (Mamasuew et al. 2008). However, for these experiments, it cannot be excluded that the coolness-induced expression of c-Fos was weaker in the GG of adults than in neonates simply because it is easier to cool the nasal tissue in a poikilothermic pup than in a homeothermic adult mouse in which this tissue is constantly warmed up by the bloodstream. In this regard, unfortunately, coolness-evoked responses in the GG of adult animals have been barely investigated using other techniques, such as calcium imaging. Thus, it is at present uncertain whether activation of GG neurons by cool temperatures is as strong in adults as in pups. However, immunohistochemical studies have revealed that the expression of transduction proteins sufficient for thermosensory signaling, including TREK-1 as well as GC-G and CNGA3, is not confined to GG neurons of neonates but is also clearly detectable in adult mice (Liu et al. 2009; Stebe et al. 2014). Thus, these latter observations support the view that the GG of adults is responsive to cool temperatures, similar to the GG of pups.
With a view to the significance of coolness-induced GG stimulation, it has to be noted that odorant-evoked GG responses are boosted at cool temperatures (Mamasuew et al. 2011a; Brechbühl et al. 2013b). Therefore, it is tempting to speculate that the key importance of GG sensitivity for cool temperatures does not lie in functioning as a thermosensory organ in addition to other sensory systems involved in the perception of ambient temperatures, but rather in modulating chemosensory responses. Such a modulatory effect might be achieved via cross-talks between chemo- and thermosensory transduction pathways. With respect to interactions between distinct sensory cascades, some olfactory transduction elements, such as GC-G and CNGA3, are shared by chemo- and thermosensory signaling in GG neurons (Mamasuew et al. 2010, 2011b; Hanke et al. 2013; Chao et al. 2015) (Fig. 2).
Cross-talks between chemo- and thermosensory signaling in the GG and their potential functional importance
In contrast to the coolness-induced enhancement of olfactory responses in the GG (Mamasuew et al. 2011a; Brechbühl et al. 2013b), warmth reduces odorant-evoked reactivity in GG neurons (and in the corresponding glomeruli of the OB) but not in the MOE (Bumbalo et al. 2017b). Thus, ambient temperatures sensitize (coolness) or desensitize (warmth) chemosensory responsiveness in GG neurons. Currently, it is unknown why odorant-stimulated GG responses are temperature-dependent. In this context, it has to be taken into account that mice are mainly active at night when temperatures are usually lower than in the daytime. According to this nocturnal lifestyle, mice are presumably more vulnerable during the night upon leaving their warm nests. In light of the above-described relevance of the GG for detecting substances associated with danger and fear (alarm pheromones and predator scents), it is tempting to speculate that a very sensitive perception of such alerting compounds is presumably not required in the relatively safe (and warm) nest but outside of it. Hence, GG neurons could utilize temperature changes to adjust their chemosensory sensitivity, i.e., the sensitivity is increased at the cooler temperatures of the night and diminished during the warmer daytime that mice spend rather inactive in their nest. Furthermore, it can be speculated that a coolness-evoked increase in the chemosensory sensitivity of GG neurons might compensate for a probably reduced volatility of GG-activating substances during the cooler night hours that could impair the detection of these chemicals. On this matter, compared to the house mouse and the Norway rat, for the Golden hamster, only a rather low ability of GG neurons to respond to cool temperatures has been observed while GG responsiveness to given odorants (SBT, TMT, 2-PT, and 2,3-DMP) is largely similar in hamsters, mice, and rats (Brechbühl et al. 2014). Remarkably, unlike the mostly nocturnal house mouse and Norway rat, the Golden hamster is diurnal in its natural habitat (Gattermann et al. 2008). Thus, for hamsters, one might speculate that sensitizing GG detection of alerting odorants via cool temperatures could be unnecessary since they predominantly spend the cooler nighttime hours in their safe burrow (Gattermann et al. 2008).
Regarding the desensitization of chemosensory GG responses at warm temperatures (Bumbalo et al. 2017b), it has to be considered that several GG-stimulating compounds are emitted by mice themselves, notably dimethylpyrazines and the alarm pheromone substance SBT (Jemiolo et al. 1989; Brechbühl et al. 2013a). In consequence, these substances are most probably also present in the (warm) nests of mice in which strong and continuous responses to alerting semiochemicals that activate GG neurons might be misplaced. This notion is corroborated by the observation that the diminished olfactory reactivity in the GG at warm temperatures is positively correlated with exposure time, indicating that warmth could foster adaptational processes in GG cells (Mamasuew et al. 2011b). While such an adaptation does not take place at cool temperatures, at warmer conditions, it can be observed within a few minutes (Mamasuew et al. 2011b; Bumbalo et al. 2017b). Therefore, in summary, temperature-dependent modulation of chemosensory responses does not solely sensitize the GG for certain alerting odorants in a cooler and presumably more hazardous setting beyond the nest, but also rapidly desensitizes GG neurons in warmer and safer surroundings, such as the burrow, where intense and persisting reactions to such compounds are potentially not required.
The molecular processes mediating temperature-dependent regulation of olfactory sensitivity in the GG are largely uncertain. However, for the warmth-evoked desensitization of chemosensory responses in the GG, TREK-1 might be of special importance because this thermosensitive potassium channel is opened at warmer temperatures (Maingret et al. 2000), inducing membrane hyperpolarization that could hamper chemosensory signaling. Conversely, inactivation of TREK-1 at cool temperatures (Maingret et al. 2000) might elicit a membrane depolarization that could presensitize GG neurons for stimulation by appropriate odorants. In addition to TREK-1, it is conceivable that also GC-G contributes to the temperature-affected modulation of olfactory signaling in the GG. For instance, the enhanced dimerization/oligomerization of GC-G at cool temperatures (Chao et al. 2015) might facilitate odorant-induced stimulation of cGMP synthesis for those GG-activating compounds that utilize GC-G as a receptor or downstream signaling element. Hence, detailed future studies are required to unravel the molecular background of cross-talks between chemo- and thermosensory signaling in GG neurons. Such thorough analyses could also provide more detailed insights why odorant-adapted GG neurons are still fully responsive to cool temperatures (Mamasuew et al. 2011b), suggesting that the olfactory transduction proteins affected by adaptational mechanisms might be irrelevant for thermosensation in the GG.
Axonal processes of GG neurons innervate necklace glomeruli in the bulb
In general, olfactory neurons in the nose of mammals are equipped with an axon that terminates in round- or oval-shaped neuropil structures of the OB termed glomeruli where synaptic contacts with dendrites of downstream projection neurons are established (reviewed by Zou et al. 2009). The axonal processes of GG neurons (henceforth called GG axons) (Fig. 1d) coalesce, forming a single or a few bundles that run ipsilaterally in a posterior direction. After penetrating the cribriform plate, these bundles project along the medial side of the OB to approach a more posterior bulbar region in which they furcate into two branches. One branch runs from medial to more ventral regions of the bulb, while the other projects across the dorsal aspect of the OB to reach lateral and finally ventral bulbar areas. Thus, GG axons collectively form an almost annular trajectory around the posterior part of the bulb (Fig. 3a) (Fuss et al. 2005; Koos and Fraser 2005; Fleischer et al. 2006a; Roppolo et al. 2006; Storan and Key 2006; Matsuo et al. 2012; Bumbalo et al. 2017a). The trajectory of GG axons in the OB reveals several globular swellings (Fig. 3) (Fuss et al. 2005; Koos and Fraser 2005; Roppolo et al. 2006; Matsuo et al. 2012; Bumbalo et al. 2017a). Hitherto, these structures have been sparsely investigated, yet the presence of the presynaptic marker synaptotagmin strongly indicates that they indeed represent glomeruli in which synapses are formed between the terminals of GG axons and downstream interneurons (Matsuo et al. 2012). This concept is supported by the observation that exposure of the GG to adequate stimuli in live animals leads to activation of cells in the OB that are directly adjacent to these structures, most likely periglomerular cells. Therefore, the globular structures in the OB that are innervated by GG axons have been termed Grueneberg glomeruli (Matsuo et al. 2012; Bumbalo et al. 2017a). Detailed studies have disclosed that GG axons innervate in a quite stereotypical pattern nine Grueneberg glomeruli residing in medial, dorsal, lateral, and ventral areas of the OB (Fig. 3b, c) (Bumbalo et al. 2017a). Concerning the total number of Grueneberg glomeruli, however, it has to be taken into account that these experiments were conducted with transgenic animals in which the axons of only the GC-G-expressing GG neurons were labeled (Bumbalo et al. 2017a). As a consequence, there might be additional glomeruli in the OB that are innervated by GC-G-negative GG cells.
Axonal projection pattern of GG neurons. a Simplistic drawing of a sagittal section through the head of a mouse. The location of the MOE is marked in blue. The GG, the trajectory of its axons as well as the glomeruli these axons innervate in the OB (Grueneberg glomeruli) are given in green color. In the caudal region of the OB, the axonal fibers originating from the GG divide into two branches. Some of the fibers take a medial to ventral route, whereas others run across the dorsal bulbar area before they approach lateral and ventral regions of the OB. The inset shows a schematic representation of the coronal section plane through the OB that is denoted by the rectangle circumscribed by the dashed line (d, dorsal; m, medial). Fibers originating from the GG as well as the corresponding Grueneberg glomeruli (dots) are indicated in green. The axonal processes of GG neurons penetrate the OB at medial sites and almost entirely encircle the OB in its posterior region in such a manner that these glomeruli seem to be interconnected. b, c Visualization of GG axonal projections on coronal sections through the OB of a pup from a transgenic mouse line (GC-G/GFP) in which the green fluorescent protein (GFP) is expressed in GC-G-positive cells; accordingly, axons of GC-G-expressing GG neurons and Grueneberg glomeruli (highlighted by arrows) are endowed with GFP. Because GC-G expression in the head of mice is restricted to GG neurons (Fleischer et al. 2009; Chao et al. 2015), only these cells and their axons are GFP-positive in the head of transgenic GC-G/GFP mice. Localization of GFP was uncovered by staining with a GFP-specific antibody (green). The images depicted represent a superposition of 8 (b) or 5 (c) individual micrographs (d: dorsal; l: lateral; v: ventral; m: medial). Sites at which GG axons invade the OB are indicated by the arrowheads in b. d Higher magnification of one of the ventral Grueneberg glomeruli shown in b. GFP-positive axons pass the glomerulus heading for a subsequent one. e, f High magnification images of a (dorsal) glomerulus formed by axonal terminals from GC-G/GFP-positive GG neurons. The glomerulus is surrounded by DAPI-labeled cells (blue) and its outline is highlighted by the dashed line in f (data from Bumbalo et al. 2017a; by courtesy of Springer Nature). Scale bars: b, c = 500 µm; d, f = 50 µm
The probably most striking feature of the Grueneberg glomeruli is that they seem to be interconnected by fibers (Fig. 3b–d) that are presumably composed of GG axons passing through one Grueneberg glomerulus to approach subsequent ones (Fuss et al. 2005; Koos and Fraser 2005; Roppolo et al. 2006; Storan and Key 2006; Matsuo et al. 2012; Bumbalo et al. 2017a). For olfactory neurons, such a projection pattern is rather unusual; yet, it is reminiscent of the abovementioned GC-D neurons in the MOE whose axons converge on a special group of up to 40 interconnected glomeruli in the OB. The peculiar arrangement of these interconnected glomeruli encircling the OB resembles beads on a string; therefore, such glomeruli have been designated as necklace glomeruli (Juilfs et al. 1997; Leinders-Zufall et al. 2007; Walz et al. 2007). According to their similar arrangement, also the Grueneberg glomeruli are regarded as necklace glomeruli although necklace glomeruli innervated by GC-D neurons are obviously segregated from Grueneberg glomeruli (Fuss et al. 2005; Koos and Fraser 2005; Roppolo et al. 2006; Storan and Key 2006; Matsuo et al. 2012).
The transfer of sensory input from the GG to the OB and the subsequent coding of this information in the OB has been barely examined so far. It has been observed that an appropriate chemical cue evokes electrical signals in the GG that could be conveyed via GG axons to the OB of the brain (Hanke et al. 2013). In line with this concept, chemo- or thermosensory stimulation of the GG, as described above, elicits activation of Grueneberg glomeruli in the OB (Bumbalo et al. 2017a). In these experiments, when mice were exposed to the GG-activating odorant 2,3-DMP or cool temperatures, most if not all of the Grueneberg glomeruli were stimulated (Bumbalo et al. 2017a). This finding is remarkable because in the MOE, a given odorant evokes responses in only a subpopulation of the sensory neurons; accordingly, only a subset of the glomeruli in the bulb is stimulated, leading to a so-called chemotopic or topographic map of activated glomeruli in the OB that encodes the chemosensory information (Buck 1996, 2000; Mori et al. 1999; Bozza and Mombaerts 2001). This discrepancy between coding the information from the GG (activation of most or even all Grueneberg glomeruli) and coding sensory input from the MOE (stimulation of only a subset of the glomeruli connected to the MOE) is compliant with the concept that the GG could be rather dedicated to the mere detection of alarm signals than to the discrimination of different substances. How a single odorant can activate most or even all Grueneberg glomeruli is currently unclear. One explanation could be that this odorant induces responses in the majority of the GG neurons. In fact, it has been described that a single odorant can stimulate very large subpopulations of these cells (Brechbühl et al. 2013a). Alternatively or in addition, a single GG neuron might innervated different Grueneberg glomeruli simultaneously via branching of its axonal process; a notion consistent with the observation that Grueneberg glomeruli seem to be interconnected.
Conclusions and future directions
While the GG was widely ignored after its initial discovery, in recent years, it has been investigated at least in mice in more detail. These studies have disclosed that the GG is implicated in the reception of given alerting semiochemicals (alarm pheromones and predator scents). Importantly, detection of these compounds via the GG is critical for eliciting the corresponding behavioral and physiological reactions that are frequently associated with stress and/or fear. Intriguingly, with respect to its sensory capacity, it has been found that the GG is not only activated by certain chemical cues but also by cool temperatures, indicating that it serves as a dual sensory organ. Despite these major findings, a series of aspects related to the GG remain elusive. For instance, it is currently unknown whether a GG exists in only given mammalian species or in most/all mammals. Notably, with regard to its relevance for evoking stress and fear responses, it would be interesting to examine if a GG or a functional equivalent in another olfactory subsystem is also present in the nose of humans; however, the observation that the gene encoding GC-G, a protein essential for chemo- and thermosensory signaling in GG neurons of mice, is considered a pseudogene in humans might contradict this notion (Kuhn 2016). On the molecular level, it is unclear which transduction elements contribute to the temperature-dependent modulation of chemosensory signaling in the GG. Moreover, concerning the significance of ambient temperatures for GG stimulation, the biological importance of the odorant-independent activation of GG neurons by cool temperatures is still enigmatic. Finally, the neuronal wiring of the GG and especially its connectivity with cerebral centers controlling fear- and stress-associated responses await further investigation. Thus, future studies targeting the GG and its downstream neuronal circuitry might provide a better understanding of how the olfactory system processes alerting signals in order to enable mammals to avoid predation or other hazards.
Abbreviations
- 2,3-DMP:
-
2,3-Dimethylpyrazine
- 2-EP:
-
2-Ethylpyrazine
- 2-PT:
-
2-Propylthietane
- cGMP:
-
Cyclic guanosine monophosphate
- CNG:
-
Cyclic nucleotide-gated
- CNGA3:
-
Cyclic nucleotide-gated channel A3
- GC-G:
-
Guanylyl cyclase subtype G
- GFP:
-
Green fluorescent protein
- GG:
-
Grueneberg ganglion
- GPCR(s):
-
G protein-coupled receptor(s)
- HEK:
-
Human embryonic kidney
- MOE:
-
Main olfactory epithelium
- OB:
-
Olfactory bulb
- OMP:
-
Olfactory marker protein
- PDE:
-
Phosphodiesterase
- PDE2A:
-
Phosphodiesterase 2A
- PTU:
-
6-Propyl-2-thiouracil
- SBT:
-
2-S-butyl-4,5-dihydrothiazole
- STFP:
-
Social transmission of food preference
- TAAR(s):
-
Trace amine-associated receptor(s)
- TAS2R/T2R:
-
Bitter taste receptor
- TMT:
-
2,5-Dihydro-2,4,5-trimethylthiazoline
- VNO:
-
Vomeronasal organ
References
Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS (2000) A novel family of mammalian taste receptors. Cell 100:693–702
Akiyoshi S, Ishii T, Bai Z, Mombaerts P (2018) Subpopulations of vomeronasal sensory neurons with coordinated coexpression of type 2 vomeronasal receptor genes are differentially dependent on Vmn2r1. Eur J Neurosci 47:887–900
Allin JT, Banks EM (1971) Effects of temperature on ultrasound production by infant albino rats. Dev Psychobiol 4:149–156
Arakawa H, Kelliher KR, Zufall F, Munger SD (2013) The receptor guanylyl cyclase type D (GC-D) ligand uroguanylin promotes the acquisition of food preferences in mice. Chem Senses 38:391–397
Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448:204–208
Bean NJ, Galef BG, Mason JR (1988) The effect of carbon disulphide on food consumption by house mice. J Wildl Manage 52:502–507
Behrens M, Foerster S, Staehler F, Raguse JD, Meyerhof W (2007) Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J Neurosci 27:12630–12640
Blumberg MS, Efimova IV, Alberts JR (1992) Ultrasonic vocalizations by rat pups: the primary importance of ambient temperature and the thermal significance of contact comfort. Dev Psychobiol 25:229–250
Bozza TC, Mombaerts P (2001) Olfactory coding: revealing intrinsic representations of odors. Curr Biol 11:R687-690
Brechbühl J, de Vallière A, Wood D, Nenniger Tosato M, Broillet MC (2020) The Grueneberg ganglion controls odor-driven food choices in mice under threat. Commun Biol 3:533
Brechbühl J, Klaey M, Broillet MC (2008) Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science 321:1092–1095
Brechbühl J, Klaey M, Moine F, Bovay E, Hurni N, Nenniger-Tosato M, Broillet MC (2014) Morphological and physiological species-dependent characteristics of the rodent Grueneberg ganglion. Front Neuroanat 8:87
Brechbühl J, Moine F, Broillet MC (2013b) Mouse Grueneberg ganglion neurons share molecular and functional features with C. elegans amphid neurons. Front Behav Neurosci 7:193
Brechbühl J, Moine F, Klaey M, Nenniger-Tosato M, Hurni N, Sporkert F, Giroud C, Broillet MC (2013a) Mouse alarm pheromone shares structural similarity with predator scents. Proc Natl Acad Sci U S A 110:4762–4767
Brechbühl J, Moine F, Tosato MN, Sporkert F, Broillet MC (2015) Identification of pyridine analogs as new predator-derived kairomones. Front Neurosci 9:253
Brunet LJ, Gold GH, Ngai J (1996) General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron 17:681–693
Buck LB (1996) Information coding in the vertebrate olfactory system. Annu Rev Neurosci 19:517–544
Buck LB (2000) The molecular architecture of odor and pheromone sensing in mammals. Cell 100:611–618
Bumbalo R, Lieber M, Lehmann E, Wolf I, Breer H, Fleischer J (2017b) Attenuated chemosensory responsiveness of the Grueneberg ganglion in mouse pups at warm temperatures. Neuroscience 366:149–161
Bumbalo R, Lieber M, Schroeder L, Polat Y, Breer H, Fleischer J (2017a) Grueneberg glomeruli in the olfactory bulb are activated by odorants and cool temperature. Cell Mol Neurobiol 37:729–742
Chao YC, Chen CC, Lin YC, Breer H, Fleischer J, Yang RB (2015) Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures. EMBO J 34:294–306
Chao YC, Fleischer J, Yang RB (2018) Guanylyl cyclase-G is an alarm pheromone receptor in mice. EMBO J 37:39–49
Chehrehasa F, Jacques A, St John JA, Ekberg JAK (2018) The Grueneberg olfactory organ neuroepithelium recovers after injury. Brain Res 1688:65–72
Cockerham RE, Leinders-Zufall T, Munger SD, Zufall F (2009) Functional analysis of the guanylyl cyclase type D signaling system in the olfactory epithelium. Ann N Y Acad Sci 1170:173–176
Dai G, Peng C, Liu C, Varnum MD (2013) Two structural components in CNGA3 support regulation of cone CNG channels by phosphoinositides. J Gen Physiol 141:413–430
Debiec J, Sullivan RM (2014) Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc Natl Acad Sci U S A 111:12222–12227
DeMaria S, Berke AP, Van Name E, Heravian A, Ferreira T, Ngai J (2013) Role of a ubiquitously expressed receptor in the vertebrate olfactory system. J Neurosci 33:15235–15247
Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A (2007) TRPM8 is required for cold sensation in mice. Neuron 54:371–378
Duda T, Sharma RK (2008) ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun 367:440–445
Firestein S (2001) How the olfactory system makes sense of scents. Nature 413:211–218
Fleischer J (2014) The Grueneberg ganglion: a cool chemodetector. Chemosense 15:3–19
Fleischer J, Breer H (2010) The Grueneberg ganglion: a novel sensory system in the nose. Histol Histopathol 25:909–915
Fleischer J, Hass N, Schwarzenbacher K, Besser S, Breer H (2006a) A novel population of neuronal cells expressing the olfactory marker protein (OMP) in the anterior/dorsal region of the nasal cavity. Histochem Cell Biol 125:337–349
Fleischer J, Mamasuew K, Breer H (2009) Expression of cGMP signaling elements in the Grueneberg ganglion. Histochem Cell Biol 131:75–88
Fleischer J, Schwarzenbacher K, Besser S, Hass N, Breer H (2006b) Olfactory receptors and signalling elements in the Grueneberg ganglion. J Neurochem 98:543–554
Fleischer J, Schwarzenbacher K, Breer H (2007) Expression of trace amine-associated receptors in the Grueneberg ganglion. Chem Senses 32:623–631
Fuss SH, Omura M, Mombaerts P (2005) The Grueneberg ganglion of the mouse projects axons to glomeruli in the olfactory bulb. Eur J Neurosci 22:2649–2654
Galef BG (2012) A case study in behavioral analysis, synthesis and attention to detail: social learning of food preferences. Behav Brain Res 231:266–271
Galef BG, Mason JR, Preti G, Bean NJ (1988) Carbon disulfide: a semiochemical mediating socially-induced diet choice in rats. Physiol Behav 42:119–124
Gattermann R, Johnston RE, Yigit N, Fritzsche P, Larimer S, Ozkurt S, Neumann K, Song Z, Colak E, Johnston J, McPhee ME (2008) Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Lett 4:253–255
Grüneberg H (1973) A ganglion probably belonging to the N. terminalis system in the nasal mucosa of the mouse. Z Anat Entwicklungsgesch 140:39–52
Hanke W, Mamasuew K, Biel M, Yang RB, Fleischer J (2013) Odorant-evoked electrical responses in Grueneberg ganglion neurons rely on cGMP-associated signaling proteins. Neurosci Lett 539:38–42
Jemiolo B, Andreolini F, Xie TM, Wiesler D, Novotny M (1989) Puberty-affecting synthetic analogs of urinary chemosignals in the house mouse, Mus domesticus. Physiol Behav 46:293–298
Juilfs DM, Fülle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA (1997) A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci U S A 94:3388–3395
Kaupp UB, Seifert R (2002) Cyclic nucleotide-gated ion channels. Physiol Rev 82:769–824
Kelliher KR, Munger SD (2015) Chemostimuli for guanylyl cyclase-D-expressing olfactory sensory neurons promote the acquisition of preferences for foods adulterated with the rodenticide warfarin. Front Neurosci 9:262
Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H (2007) Innate versus learned odour processing in the mouse olfactory bulb. Nature 450:503–508
Koos DS, Fraser SE (2005) The Grueneberg ganglion projects to the olfactory bulb. NeuroReport 16:1929–1932
Kuhn M (2016) Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev 96:751–804
Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD (2007) Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci U S A 104:14507–14512
Liberles SD (2015) Trace amine-associated receptors: ligands, neural circuits, and behaviors. Curr Opin Neurobiol 34:1–7
Liberles SD, Buck LB (2006) A second class of chemosensory receptors in the olfactory epithelium. Nature 442:645–650
Liu CY, Fraser SE, Koos DS (2009) Grueneberg ganglion olfactory subsystem employs a cGMP signaling pathway. J Comp Neurol 516:36–48
Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA (2000) Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52:375–414
Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honoré E (2000) TREK-1 is a heat-activated background K(+) channel. EMBO J 19:2483–2491
Mamasuew K, Breer H, Fleischer J (2008) Grueneberg ganglion neurons respond to cool ambient temperatures. Eur J Neurosci 28:1775–1785
Mamasuew K, Hofmann N, Breer H, Fleischer J (2011a) Grueneberg ganglion neurons are activated by a defined set of odorants. Chem Senses 36:271–282
Mamasuew K, Hofmann N, Kretzschmann V, Biel M, Yang RB, Breer H, Fleischer J (2011b) Chemo- and thermosensory responsiveness of Grueneberg ganglion neurons relies on cyclic guanosine monophosphate signaling elements. Neurosignals 19:198–209
Mamasuew K, Michalakis S, Breer H, Biel M, Fleischer J (2010) The cyclic nucleotide-gated ion channel CNGA3 contributes to coolness-induced responses of Grueneberg ganglion neurons. Cell Mol Life Sci 67:1859–1869
Martini S, Silvotti L, Shirazi A, Ryba NJ, Tirindelli R (2001) Co-expression of putative pheromone receptors in the sensory neurons of the vomeronasal organ. J Neurosci 21:843–848
Matsuo T, Rossier DA, Kan C, Rodriguez I (2012) The wiring of Grueneberg ganglion axons is dependent on neuropilin 1. Development 139:2783–2791
Menco BP (1997) Ultrastructural aspects of olfactory signaling. Chem Senses 22:295–311
Menco BP, Cunningham AM, Qasba P, Levy N, Reed RR (1997) Putative odour receptors localize in cilia of olfactory receptor cells in rat and mouse: a freeze-substitution ultrastructural study. J Neurocytol 26:691–706
Meyer MR, Angele A, Kremmer E, Kaupp UB, Muller F (2000) A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc Natl Acad Sci U S A 97:10595–10600
Moine F, Brechbühl J, Nenniger Tosato M, Beaumann M, Broillet MC (2018) Alarm pheromone and kairomone detection via bitter taste receptors in the mouse Grueneberg ganglion. BMC Biol 16:12
Mombaerts P (2004a) Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci 5:263–278
Mombaerts P (2004b) Odorant receptor gene choice in olfactory sensory neurons: the one receptor-one neuron hypothesis revisited. Curr Opin Neurobiol 14:31–36
Mori K, Nagao H, Yoshihara Y (1999) The olfactory bulb: coding and processing of odor molecule information. Science 286:711–715
Munger SD, Leinders-Zufall T, McDougall LM, Cockerham RE, Schmid A, Wandernoth P, Wennemuth G, Biel M, Zufall F, Kelliher KR (2010) An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr Biol 20:1438–1444
Okon EE (1971) The temperature relations of vocalization in infant Golden hamsters and Wistar rats. J Zool 164:227–237
Osada K, Kurihara K, Izumi H, Kashiwayanagi M (2013) Pyrazine analogues are active components of wolf urine that induce avoidance and freezing behaviours in mice. PLoS ONE 8:e61753
Oswalt GL, Meier GW (1975) Olfactory, thermal, and tactual influences on infantile ultrasonic vocalization in rats. Dev Psychobiol 8:129–135
Pérez-Gómez A, Bleymehl K, Stein B, Pyrski M, Birnbaumer L, Munger SD, Leinders-Zufall T, Zufall F, Chamero P (2015) Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Curr Biol 25:1340–1346
Roppolo D, Ribaud V, Jungo VP, Lüscher C, Rodriguez I (2006) Projection of the Grüneberg ganglion to the mouse olfactory bulb. Eur J Neurosci 23:2887–2894
Schmid A, Pyrski M, Biel M, Leinders-Zufall T, Zufall F (2010) Grueneberg ganglion neurons are finely tuned cold sensors. J Neurosci 30:7563–7568
Sievert T, Laska M (2016) Behavioral responses of CD-1 mice to six predator odor components. Chem Senses 41:399–406
Stebe S, Schellig K, Lesage F, Breer H, Fleischer J (2014) The thermosensitive potassium channel TREK-1 contributes to coolness-evoked responses of Grueneberg ganglion neurons. Cell Mol Neurobiol 34:113–122
Storan MJ, Key B (2006) Septal organ of Grüneberg is part of the olfactory system. J Comp Neurol 494:834–844
Strotmann J, Levai O, Fleischer J, Schwarzenbacher K, Breer H (2004) Olfactory receptor proteins in axonal processes of chemosensory neurons. J Neurosci 24:7754–7761
Szentgyörgyi H, Kapusta J, Marchlewska-Koj A (2008) Ultrasonic calls of bank vole pups isolated and exposed to cold or to nest odor. Physiol Behav 93:296–303
Tachibana T, Fujiwara N, Nawa T (1990) The ultrastructure of the ganglionated nerve plexus in the nasal vestibular mucosa of the musk shrew (Suncus murinus, insectivora). Arch Histol Cytol 53:147–156
Thompson DK, Garbers DL (1995) Dominant negative mutations of the guanylyl cyclase-A receptor. Extracellular domain deletion and catalytic domain point mutations. J Biol Chem 270:425–430
Vernet-Maury E, Polak EH, Demael A (1984) Structure-activity relationship of stress-inducing odorants in the rat. J Chem Ecol 10:1007–1018
Wallace KJ, Rosen JB (2000) Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav Neurosci 114:912–922
Walz A, Feinstein P, Khan M, Mombaerts P (2007) Axonal wiring of guanylate cyclase-D-expressing olfactory neurons is dependent on neuropilin 2 and semaphorin 3F. Development 134:4063–4072
Wilson EM, Chinkers M (1995) Identification of sequences mediating guanylyl cyclase dimerization. Biochemistry 34:4696–4701
Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR (2000) Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27:487–497
Yamamoto Y, Hatakeyama T, Taniguchi K (2009) Immunohistochemical colocalization of TREK-1, TREK-2 and TRAAK with TRP channels in the trigeminal ganglion cells. Neurosci Lett 454:129–133
Zhang JX, Soini HA, Bruce KE, Wiesler D, Woodley SK, Baum MJ, Novotny MV (2005) Putative chemosignals of the ferret (Mustela furo) associated with individual and gender recognition. Chem Senses 30:727–737
Zheng J, Zagotta WN (2004) Stoichiometry and assembly of olfactory cyclic nucleotide-gated channels. Neuron 42:411–421
Zou DJ, Chesler A, Firestein S (2009) How the olfactory bulb got its glomeruli: a just so story? Nat Rev Neurosci 10:611–618
Acknowledgments
The author is indebted to Heinz Breer and Jürgen Krieger for generous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Ethical approval
This review does not contain any previously unpublished studies with human participants or animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fleischer, J. The Grueneberg ganglion: signal transduction and coding in an olfactory and thermosensory organ involved in the detection of alarm pheromones and predator-secreted kairomones. Cell Tissue Res 383, 535–548 (2021). https://doi.org/10.1007/s00441-020-03380-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03380-w