Abstract
Brain-derived neurotrophic factor (BDNF) is known to control a wide variety of brain functions, ranging from memory formation to food intake. However, since the BDNF levels are extremely low in the nervous system, the dynamics in neurons from intracellular trafficking to secretion is absolutely complicated; the understanding is not fully promoted. We here review the findings of those critical mechanisms from intracellular trafficking to the secretion of BDNF. Furthermore, to solve this issue, technological advances for the detection, measurement, and imaging of this growth factor are essential. We believe that this review helps the study of these complex but critical mechanisms of BDNF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The neurotrophin family
Neurotrophins (NTs) historically emerged as a family of polypeptides for promoting neuronal survival and differentiation and have recently been studied as a class of modulators for synaptic transmission and plasticity (Bibel and Barde 2000; Park et al. 2008). In the 1950s, the group of Levi-Montalcini and Hamburger found that when a mouse sarcoma tumor was implanted near the spinal cord of the developing chicken the neurons exhibited neurite outgrowth (Levi-Montalcini and Hamburger 1953). The extensive efforts after the finding led to the successful identification of a growth factor namely nerve growth factor (NGF) (Bocchini and Angeletti 1969; Cohen 1960; Cohen and Levi-Montalcini 1956). Their discovery of NGF played a pioneering role in the studies of developmental neurobiology, which raised a new hypothesis that target-derived secreted proteins control neurite growth and neuronal survival.
Over 20 years after the discovery of NGF, Barde et al. (1982) isolated a neuronal survival-eliciting factor from the pig brain, named brain-derived neurotrophic factor (BDNF) (Barde et al. 1982). In 1989, the primary structure of BDNF and its expression in the brain was identified (Leibrock et al. 1989). Interestingly, it was demonstrated that BDNF is highly homologous to NGF at the level of the amino acid sequence, and using a technology of polymerase chain reaction (PCR), neurotrophin-3 (NT-3) (Maisonpierre et al. 1990), and neurotrophin-4/5 (NT-4/5) (Ip et al. 1992), whose amino acid sequence is very homologous to NGF and BDNF, was identified one after another. These four NTs and their genes showed marked homology to each other in terms of sequence and structure (Lessmann et al. 2003). These discoveries of NTs family have provided novel insights into the formation of neuronal communication during the development of the nervous system and into synaptic plasticity, memory, and learning in the adult brain. Interestingly, it was demonstrated that, for the formation of specific neuronal communications spatially and temporally, one or more NTs are required. Furthermore, there are long- and short-term actions of NTs. The long-term NT action depends on gene regulation, whereas for short-term effects, including chemotrophic effects on developing neurons and synaptic transmissions, the activation of cytoplasmic effectors by NTs was shown to be required.
NT receptors and their signaling
There have been prolonged efforts to identify the receptors of NTs. Two distinct classes of NT receptors have been discovered. The first receptor, the p75 NT receptor (p75NTR), is a member of the tumor necrosis factor (TNF) receptor family (Chao 2003). p75NTR was initially discovered as a low-affinity NGF receptor but was found to bind BDNF, NT-3, and NT-4/5 with a similar affinity (for review, (Chao 2003)). The extracellular domain of p75NTR has cysteine-rich motifs. The cytoplasmic domain of p75NTR includes a “death” domain, similar to that of the TNF receptor family (He and Garcia 2004; Liepinsh et al. 1997). Interestingly, while p75NTR does not contain a catalytic kinase motif, it interacts with many proteins that transmit signals for controlling neuronal survival and differentiation (Chao 2003; Hempstead 2014). The p75NTR receptor activates three major signaling pathways (for review, (Hempstead 2002)) (also see articles by Korte et al., Brigadski et al., and Mobley et al., in this special issue). First, NF-kappa B activation leads to the transcription of multiple genes, some of which promote neuronal survival. Second, activation of the Jun kinase pathway similarly controls the activation of several genes, some of which promote neuronal apoptosis. Third, ligand engagement of p75NTR, through the regulation of Rho activity, controls the motility of growth cone.
The second class of NT receptors is the tropomyosin-related kinase (Trk) family of receptor tyrosine kinases (TrkA, TrkB, and TrkC) (for review, (Reichardt 2006)). Each NT binds to Trk receptor in a specific manner: NGF binds to TrkA, BDNF and NT-4/5 to TrkB, and NT-3 to TrkC. The Trk receptors have a trans-membrane region that spans the plasma membrane and a cytoplasmic domain that has tyrosine kinase activity. Binding of a NT to a specific Trk receptor activates its tyrosine kinase, leading to the activation of phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and phospholipase C-γ (PLC-γ) pathways. Notably, it was demonstrated that mutation of TrkB receptors at the PLC-γ docking site, but not the Shc site, impaired hippocampal long-term potentiation (LTP) (Minichiello et al. 2002). Many additional adaptors for p75NTR and Trk receptors have been reported. Information about the two types of NT receptors and their signaling mechanisms has been extensively reviewed by others (for review, (Chao 2003, Hempstead 2002, Ibanez and Simi 2012, Reichardt 2006)) and will not be further discussed in this review.
A critical mechanism for NT action: secretion and intracellular transportation of NT
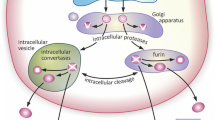
NTs are initially synthesized as precursors composed of pre-pro-neurotrophins. The precursor proteins are structurally composed of a signal sequence, a pro-domain, and the mature domain. To produce the mature and bioactive form, the signal sequence and the next N-terminal fragment or the “pro-domain” are proteolytically processed by intracellular and/or extracellular proteolytic enzymes (e.g., furin, pro-hormone convertase, and plasmin) (Lee et al. 2001; Lu et al. 2005; Pang and Lu 2004; Seidah et al. 1996). Several reports explored the intracellular mechanism of NT processing. The N-terminal pro-domain is proteolytically processed in either trans-Golgi by furin or secretory granules by intracellular proteinases to generate a mature form of neurotrophins while in non-neuronal cells, neurotrophins are constitutively secreted (Seidah et al. 1996).
It was previously reported that the BDNF secretion is controlled by both constitutive and activity-regulated secretion pathways in cultured neurons (Mowla et al. 1999). The activity-dependent secretion of BDNF is controlled in a neuronal activity- and Ca2+-dependent manner (for review, (Lessmann and Brigadski 2009)) (also see Brigadski and Lessmann in this special issue). There is a large study indicating that the activity-dependent secretion of BDNF is a critical mechanistic step in controlling synaptic transmission and long-term synaptic plasticity (Park and Poo 2013). Furthermore, the exonic Val66Met polymorphism, in which an amino acid residue substitution alters valine to methionine at codon 66 within the BDNF pro-domain, has been reported to be a functional single-nucleotide polymorphism (SNP) that results in deficient BDNF translocation and secretion as well as impaired episodic memory in human (Egan et al. 2003). This article first showed that the BDNF itself and its mechanism of secretion and translocation is important for the brain function in human. We next review the articles that study the secretion and translocation of BDNF.
Biogenesis and transport of BDNF-containing vesicles
Like other growth factors, BDNF protein is produced as a precursor polypeptide, proBDNF (32 kDa), in the rough endoplasmic reticulum (rER), and is processed to mature BDNF (〜 14 kDa) during the sorting pathway from the rER-Golgi (processed by furin) to dense core vesicle (DCV) like secretory vesicles (by proconvertases), and extracellularly by plasmin and metalloproteinases MMP3 and MMP7 (for reviews, see Lessmann and Brigadski (2009), Song et al. (2017). BDNF synthesized in the rER is transported into the trans-Golgi network (TGN) and is packed and stored in DCVs.
Granin (chromogranin and secretogranin), which plays a potential regulatory molecule for the packing of BDNF in DCVs, is a highly acidic, Ca2+-binding, and prone-to-aggregate protein. Several members of this family are abundantly localized in DCVs and are possibly involved in the sorting and packing of peptide precursors into DCVs (Bartolomucci et al. 2011). Secretogranin II (SCG2/chromogranin C) of the granin family is localized in BDNF vesicles in hippocampal neurons.
There are two sorting receptors for BDNF to the regulatory secretion pathway: a VPS10 domain family protein sortilin (SORT1), which interacts with the pro-domain of BDNF, and carboxypeptidase E (CPE), which interacts with BDNF in the TGN. Sortilin (SORT1), a member of the Vps10p sorting receptor family specifically interacts with the pro-domain to sort BDNF to the regulated secretory pathway (Chen et al. 2005). The lipid-raft-associated sorting receptor CPE likely sorts proBDNF into regulated pathway vesicles for activity-dependent secretion in cortical and hippocampal neurons (Lou et al. 2005). CPE also binds dynactin that recruits kinesins kinesin family member 1A (KIF1A and kinesin family member 1A (KIF3A) (plus-end microtubule-based motors) or dynein (a minus-end-directed motor) for driving bidirectional (anterograde and retrograde) transport of BDNF vesicle to maintain vesicle homeostasis and secretion in hippocampal neurons (Park et al. 2008).
Quantitative measurement and immunocytochemistry of endogenous BDNF
While many aspects of the BDNF role in the brain have been understood, the subcellular localization and concentration of this releasable protein should be determined carefully. One important reason is that endogenous levels of BDNF are very low. Therefore, to understand the mechanism underlying spatial and temporal actions of BDNF, the methodology of the quantitative measurement, immunocytochemistry, and imaging of BDNF should be convincing.
For quantification of endogenous BDNF, a two-site enzyme-linked immunosorbent assay (ELISA) is used, although there are some methodological issues to apply this method to human samples. One problem is that the BDNF levels in serum, plasma, and whole blood are divergent (see Karege et al. 2005). A method to obtain reliable measurements of human serum BDNF has been proposed by comparing six commercially available kits (Polacchini et al. 2015). Several factors including rodent strain, extraction buffer used, and dilution of the sample are pointed out (Angelucci et al. 2003; Angelucci et al. 2000).
To investigate the intracellular localization of endogenous BDNF, not only highly sensitive antibody but control experiment, for instance, a comparison of the immunoreactive signal between wild-type mice and its littermate lacking BDNF gene is required. Recently, two reports clearly showed the intracellular localization of endogenous BDNF clearly. Dieni et al. (2012) demonstrated a beautiful immunocytochemical localization of endogenous BDNF in the adult mouse hippocampus (Dieni et al. 2012). They carefully investigated the distribution of BDNF at both light microscopic and ultrastructural levels using three lines of transgenic animals. As a negative control, they performed their study using a line conditionally lacking BDNF gene in adult neurons (Rauskolb et al. 2010). In the report of Dieni et al. (2012), they suggested an anterograde mode of action of BDNF, which contrasts with the long-established retrograde model of NGF in the peripheral nervous system.
For the detection of endogenous BDNF, molecular tagging is also useful. In the study using Bdnf-hemagglutinin (HA) knock-in mouse line, Yang et al. (2009) showed that proBDNF was releasable at the prenatal stage and still detectable in adulthood (Yang et al. 2009). Interestingly, they also observed highest levels of proBDNF and neurotrophin receptor p75 at the perinatal stage and their expression levels declined in adulthood (Yang, Siao, Nagappan, Marinic, Jing, McGrath, Chen, Mark, Tessarollo, Lee, Lu and Hempstead, 2009), suggesting that the biological action of proBDNF, through the activation of p75, is distinct form that of BDNF (Barker 2009). Recently, Harward et al. (2016) performed electron microscopy to examine BDNF localization in a mouse line in which a C-terminal HA epitope tag was added to the Bdnf-coding sequence (Bdnf-HA) (Yang, Siao, Nagappan, Marinic, Jing, McGrath, Chen, Mark, Tessarollo, Lee, Lu and Hempstead, 2009). An immune-electron microscopy study using a highly sensitive antibody against the HA-tag showed the presence of endogenous BDNF not only in axons but also in dendrites and spines of CA1 pyramidal cells of these mice (Harward et al. 2016).
Using specificity controls, Dieni et al. (2012) showed that antibodies reacting either with BDNF or its pro-peptide (BDNF pro-peptide, ~ 17 kDa) both stained large dense core vesicles in excitatory presynaptic terminals of the adult mouse hippocampus, indicating that the BDNF and its pro-peptide are co-stored in and is able to be co-secreted from the same BDNF vesicles (Dieni, Matsumoto, Dekkers, Rauskolb, Ionescu, Deogracias, Gundelfinger, Kojima, Nestel, Frotscher and Barde, 2012). In line with this finding, Uegaki et al. (2017) showed that the BDNF pro-peptide binds to mature BDNF with high affinity on a BIAcore sensor chip (Uegaki et al. 2017). Furthermore, they showed that this interaction was more enhanced at acidic pH than at neutral pH (Uegaki, Kumanogoh, Mizui, Hirokawa, Ishikawa and Kojima, 2017), suggesting that BDNF and its pro-peptide are present together in intracellular compartments such as trafficking vesicles.
Biochemical detection and imaging of BDNF release
How is BDNF secreted from neurons? To address this question, a variety of BDNF release assay and imaging techniques has been developed. To measure the released BDNF levels, the biochemical method is technically solid. In particular, specific antibody and sensitive immunoassay methods were recently useful. The release from cells into culture media can conventionally be measured for BDNF (Anastasia et al. 2013, Katoh-Semba et al. 1999, Kolbeck et al. 1999, Matsumoto et al. 2008, Rauskolb, Zagrebelsky, Dreznjak, Deogracias, Matsumoto, Wiese, Erne, Sendtner, Schaeren-Wiemers, Korte and Barde, 2010), its pro-peptide (BDNF pro-peptide) (Dieni, Matsumoto, Dekkers, Rauskolb, Ionescu, Deogracias, Gundelfinger, Kojima, Nestel, Frotscher and Barde, 2012, Mizui et al. 2019, Mizui et al. 2015), and proBDNF (Yang, Siao, Nagappan, Marinic, Jing, McGrath, Chen, Mark, Tessarollo, Lee, Lu and Hempstead, 2009). To detect the endogenous BDNF pro-peptide in Western blotting, membrane fixation with glutaraldehyde was technically effective (Dieni, Matsumoto, Dekkers, Rauskolb, Ionescu, Deogracias, Gundelfinger, Kojima, Nestel, Frotscher and Barde, 2012, Matsumoto et al. 2008, Mizui et al. 2019, Mizui et al. 2015).
The cell imaging methods utilizing the expression of recombinant BDNF fused to fluorescent proteins such as green fluorescent protein (GFP) and its derivatives are a most used approach (Table 1). The luminal side of vesicles is acidic, so that fluorescence of pH-sensitive GFP derivatives such as pHluorin extinct inside of the vesicles, and is emitted upon exocytosis due to being exposed to the neutral solution via its release out of cells. If fusion pore is already open (i.e., the intra-vesicular pH becomes neutral), pHluorin fluorescence is unquenched even in the absence of release. For the purpose to measure small amounts of intrinsic BDNF, imaging animal models carrying modified BDNF have also been developed (Table 1).
By monitoring fluorescent changes of BDNF-GFP before and after exocytosis, it was shown that BDNF is activity dependently released at synaptic sites of cultured rat hippocampal neurons in response to depolarization induced by application of high KCl (Kojima et al. 2001), of cultured mouse cortical neurons in response to picrotoxin (Kohara et al. 2001), and of cultured rat hippocampal neurons in response to either high K+/glutamate or electrical stimulation (depolarization and tetanus) (Hartmann et al. 2001). Interestingly, BDNF-GFP expressed in axons of cultured mouse cortical neurons was dominantly transported in an anterograde direction, whereas that in dendrites mostly stayed or fluctuated back and forth within a short distance (Adachi et al. 2005). Notably, the Val66Met polymorphism affects the activity-dependent release of BDNF. Met variants showed lower activity-dependent release from cultured rat hippocampal neurons, while its constitutive release is unchanged (Egan et al. 2003).
Imaging of dendritic BDNF release
Hippocampal neurons in vivo are normally exposed to tonic activities ranging from 1 to 20 Hz and brief high-frequency (50 Hz) bursting activities (Bland 1986). Under these conditions, BDNF-GFP heterologously overexpressed in cultured rat hippocampal neurons is secreted predominantly from the dendrite and acts on presynaptic terminals as a retrograde signal or on the postsynaptic cell as an autocrine factor (see Brigadski and Lessmann in this special issue). The dendritic release at postsynaptic and extra-synaptic sites is elicited by Ca2+ influx via ionotropic glutamate receptor (iGluR) or differential voltage current conveyor (DVCC) in rat hippocampal neurons (Hartmann et al. 2001) (also see Brigadski and Lessmann 2020 in this special issue). The kinetics of depolarization (50 mM KCl)-induced postsynaptic BDNF-EGFP release was analyzed using a similar culture system (time constant τ = 307 ± 78 s; half decay time t1/2 = 288 ± 4 s) (Brigadski et al. 2005). Interestingly, BDNF is a sticky protein and the release time became faster (τ = 81 ± 22 s; t1/2 = 52 ± 14 s) after treatment with monensin (to neutralize intra-vesicular pH) prior to depolarization, suggesting a possible effect of pH-dependent peptide condensation in the acidic luminal condition on vesicular exocytosis. These authors also showed that BDNF-EGFP showed slower release time course than three other EGFP-fused NTs (NT-3, NGF, and NT-4) and that BDNF and NT-3 are more efficiently sorted to the regulated dendritic release, whereas NGF and NT-4 are more efficiently sorted to constitutive release (Brigadski et al. 2005). The activity-dependent dendritic release of BDNF-GFP was also shown to be triggered by spontaneous back-propagating action potentials (bAPs) that induce Ca2+ influx through voltage-dependent calcium channels (VDCCs) (Kuczewski et al. 2008). In cultured rat hippocampal neurons transfected with BDNF-pHluorin, 10-Hz stimulation (1 min, 300 pulses) triggers full-collapse vesicle fusion and substantial BDNF release at the dendrite presumably by triggering bAPs and Ca2+ influx via voltage-gated Ca2+ channels (VGCCs) (Matsuda et al. 2009). In CA1 pyramidal neurons of acute rat hippocampal slices, a theta burst of postsynaptic action potentials (APs) elicited BDNF-dependent postsynaptic timing-dependent long-term potentiation (t-LTP, a physiologically relevant type of synaptic plasticity that results from the repeated sequential firing of APs in pre- and postsynaptic neurons) that relied on postsynaptic BDNF release and its autocrine action at the postsynaptic CA1 neurons (Edelmann et al. 2015). The authors noted that although Dieni et al. (2012) does not significantly detect BDNF in dendritic compartments of CA1 neurons by immunochemistry (Dieni et al. 2012) (see the beginning of Chapter 8), endogenous levels of BDNF in CA1 dendrites—while sufficient to affect t-LTP—might be undetectable levels by antibody detection. In cultured rat hippocampal slices, biolistically transfected with BDNF-pHluorin, the rapid release is evoked N-methyl-d-aspartate receptor (NMDAR)-calcium/calmodulin-dependent protein kinase II (CaMKII) dependently from dendritic spines stimulated with uncaged glutamate, which activates an autocrine signaling system (Harward et al. 2016). In acute hippocampal slices of BDNF-GFP knock-in (KiBE) mice, 70% of BDNF-GFP vesicles are localized in dendrites and their release shows about 30-s delay after depolarization and continues for more than 100 s thereafter (Leschik et al. 2019).
Imaging of axonal BDNF release
Dieni et al. (2012) provided the evidence showing by using specific antibodies (see Chapter 5) that both BDNF and its pro-peptide are released from axons in an anterograde fashion rather than from postsynaptic dendrites/spines in the adult hippocampus (Dieni et al. 2012). They noted that the use of in vitro cultured neurons transfected with BDNF constructs probably grow in very different conditions compared with in vivo ones and express significantly higher levels of BDNF detectable in the adult hippocampus, which may cause ectopic localization of exogenous BDNF. Recently, Leschick et al. (2019) developed a knock-in mouse line which Bdnf exon 9 sequence was fused with constitutively fluorescent EGFP protein (KiBE) and visualized that the BDNF-EGFP-containing vesicles in pyramidal cell dendrites of hippocampal slices prepared from the imaging mouse model KiBE (Leschik et al. 2019). This study is a kind of challenging because, as described earlier, BDNF levels contained in neurons and tissues are not plenty, and the content depends on cell type, states and development, and circadian rhythms analyzed (Hamatake et al. 2011). Given such difficulty, the development of technology for precisely visualizing the localization and release of BDNF should be important to address the remaining yet important questions, including the relationship between axonal-presynaptic release and dendritic-postsynaptic release, in the field of BDNF biology.
Immunohistochemical studies demonstrated that BDNF protein derived from anterograde axonal transport was detected in axonal processes and preferentially stored in terminals within the innervation targets of rat brain (Conner et al. 1997). BDNF was also shown to be anterogradely transported in catecholaminergic axon terminals projected to rat lateral septum (Fawcett et al. 2000). Moreover, heterogeneously expressed BDNF-GFP moved in axons of cultured mouse cortical neurons in the anterograde direction, though some moved retrogradely, and transferred to postsynaptic neurons in an activity-dependent manner (Kohara et al. 2001). Immuno-electron microscopic study demonstrated that BDNF- and pro-domain-containing DCV-like vesicles are abundantly localized to excitatory presynaptic terminals, such as mossy fiber terminals, of the adult mouse hippocampus, indicating an anterograde mode of BDNF action (Dieni et al. 2012).
By using in situ ELISA technique, it was shown that 100-Hz tetanus and theta burst stimulation rather than low-frequency stimulation triggered the more efficient release of native BDNF from newborn rat hippocampal neurons by either Ca2+ influx via the N-type VDCC or Ca2+ release from the internal store (Balkowiec and Katz 2002). In CA3-CA1 synapses of hippocampal slices, presynaptic BDNF release from the Shaffer collaterals of CA3 neurons is physiologically required to recruit the presynaptic (200 Hz- or theta burst-induced long-term potentiation, LTP), but not postsynaptic (50 Hz-induced LTP) module of plasticity (Zakharenko et al. 2003). Presynaptically released BDNF was suggested to act on TrkB at the presynaptic terminals in an autocrine mode or at postsynaptic spines in an anterograde mode. In cultured rat hippocampal neurons transfected with BDNF-pHluorin, the activity-dependent axonal release was also reported (Matsuda et al. 2009). A brief spiking activity (10 Hz, 300 pules) triggers a transient fusion pore opening followed by immediate retrieval of vesicles, resulting in very little partial BDNF release at the axon. However, full-vesicular fusion with BDNF secretion could occur at the axon when the neuron was stimulated by prolonged high-frequency activity (required sustained high-frequency tonic or bursting activities for a few minutes) (Matsuda et al. 2009).
Regulatory molecules underlying the exocytosis of BDNF vesicles (Fig. 1)
BDNF is activity dependently released via exocytosis of DCV-like secretory vesicles. In the same way as exocytosis of synaptic vesicles (SVs), BDNF is released to the outside of the cells through fusion pore that is formed by membrane fusion between the secretory vesicles and the plasma membrane. The basic machineries for SV exocytosis, including the soluble NSF attachment protein receptor (SNARE) proteins and associated regulatory molecules, are well studied and are thought to be similarly utilized in exocytosis of BDNF vesicles. Although in this way the same regulatory molecules and some different isoforms seem to be involved in exocytosis of BDNF vesicles, the detailed molecular machineries that precisely tune the release of BDNF which is so effective even in very small amounts are yet unclear.
Molecular components underlying the exocytosis of DCV containing BDNF and pro-peptide. The exocytosis of DCV is implemented by SNARE complex (syntaxin 1A, SNAP25, and synaptobrevin-2 [Syb2]/VAMP2) and consists of three steps: docking, priming, and membrane fusion. ① Docking: DCV is recruited to the release site on the plasma membrane by several proteins—e.g., Rab3a, RIM1/2, and Munc13. proBDNF is included in and transported by the vesicle prior to the docking step—i.e., through the rER-TGN trafficking steps during which a fraction of proBDNF is luminally processed into pro-peptide and mBDNF. ② Priming: Munc13, in turn, plays a key role in stabilizing SNARE component(s), which renders DCV ready to be released. CAPS2 (and/or CAPS1, presumably) is also involved in the priming step in a different manner from Munc13. Specific phospholipids of the plasma membrane, such as PIP2, are likely to support the molecular functions of these priming proteins. ③ Fusion: following the Ca2+ influx form outside the cell via VDCC and/or NMDAR, as well as the Ca2+ release from internal stores via IP3 receptor (IP3R) or ryanodine receptor (RyR), a Ca2+ sensor synaptotagmin (Syt) on the DCV membrane controls the timing of vesicle release. Consequently, SNARE complex executes DCV exocytosis by bringing together the plasma membrane and DCV membrane mechanically, resulting in spilling out BDNF, pro-peptide, or proBDNF enclosed in DCV. Released proBDNF is extracellularly processed by specific proteases. Release of BDNF is controlled positively by CAPS2/1 (by interacting with syntaxin) and SNAP47 (by binding to Syb2/VAMP2 and SNAP25) and negatively by Syt-IV and ARMS/Kidins220
Three SNARE proteins localized to vesicle membrane (synaptobrevin/VAMP) and target membrane (syntaxin and SNAP25) are the main machinery for vesicle fusion at the active zone or the release site membrane and consist of multiple isoforms of each member. Their isoforms synaptobrevin/VAMP2 (Syb2) and synaptosomal-associated protein (SNAP25) mediate the vesicular release of BDNF-pHluorin in axons and dendrites of cortical neurons (Shimojo, Courchet, Pieraut, Torabi-Rander, Sando, Polleux and Maximov, 2015). Remarkably, axonal secretion of BDNF is also regulated by SNAP47, an atypical member of the SNAP family (Shimojo, Courchet, Pieraut, Torabi-Rander, Sando, Polleux and Maximov, 2015). The interaction of mammalian uncoordinated-13 (Munc13) and Rab3-interacting molecule 1 (RIM1) is thought to target Rab3-containing SVs to the active zone. The N-terminal domain of RIM1/2 interacts with both Rab3A anchoring to DCV membrane and Munc13 binding to PIP2 distributed within the release site membrane. RIM1/2 is an essential Ras-related protein 3A (Rab3A) effectors and is essential for DCV fusion for the regulated release of neuropeptides in hippocampal neurons and organize DCV fusion by positioning/activating acronym for Munc13 and recruiting DCVs through Rab3 interactions (Persoon et al. 2019). Although the release of NPY-pHluorin was analyzed in this study, the release of BDNF seems to be similarly conducted by utilizing this kind of machinery.
In addition to the vesicle targeting and fusion machineries, some positive and negative regulators for the exocytosis pathway of BDNF vesicles have been identified, by which BDNF release is thought to be finely tuned. Synaptotagmin (Syt) is an integral membrane protein on SVs and acts as a Ca2+ sensor to trigger the SNARE-mediated vesicle fusion. Its isoform syaptotagmin-IV (Syt-IV) localizes to BDNF vesicles and can interact with SNARE proteins, while it fails to bind to phosphatidylinositol bisphosphate2 (PIP2)-containing plasma membrane in response to Ca2+, resulting in the inhibition of dendritic and axonal BDNF release BDNF release (Dean et al. 2009). Therefore, Syt-IV acts as a negative regulator for exocytosis of BDNF vesicles and modulates synaptic function and long-term potentiation (Dean et al. 2009; Dean et al. 2012). Ankyrin repeat-rich membrane spanning protein ARMS/Kindins220 is a scaffold protein that interacts with multiple proteins including Trk and p75 receptors and negatively regulates BDNF release by interacting with Syt-IV (López-Benito et al. 2018).
The Ca2+-dependent activator protein for secretion (CAPS) family proteins (CAPS1 and CAPS2) are thought to act at the priming step of vesicle exocytosis by interacting with the SNARE proteins (syntaxin1/SANP25/VAMP2) and PIP2 (James and Martin 2013). CAPS2 is the first molecule that has been shown to act as a positive regulator for BDNF release from cortical, hippocampal, and cerebellar neurons (Sadakata et al. 2007a; Sadakata et al. 2004). Notably, the deficiency of CAPS2 in mice showed impaired late phase of LTP and reduced number of cortical and hippocampal neurons, which was ameliorated by application of exogenous BDNF (Sadakata et al. 2007a; Sadakata et al. 2007b). CAPS2 promotes the depolarization-induced release of BDNF-pHluorin from main axons and their extra-synaptic sites of cultured mouse hippocampal neurons, in terms of kinetics, frequency, and amplitude (Sadakata et al. 2012; Shinoda et al. 2011) and axons of cerebellar granule cells (Sadakata et al. 2014; Sadakata et al. 2012). Another paralog CAPS1 is also involved in regulating the BDNF release. The knockdown of CAPS1 by shRNA eliminated the VAMP-2-dependent docking and evoked exocytosis of fusion-competent BDNF-EGFP vesicles in PC12 cells (Kabachinski et al. 2016). Acute single-cell knockdown of CAPS1 in cultured rat hippocampal neurons leads to a strong reduction in the number of fusion-competent secretory granules and to a significant decrease of released BDNF-GFP (Eckenstaler et al. 2016).
Conclusions
Even a trace amount of BDNF exerts a variety of crucial roles in the development and function of the nervous system. Therefore, the high-sensitive detection of in vivo local BDNF release used to be difficult in many cell types and neural circuits. Recent studies have overcome this difficulty by developing various sophisticated detection methods and have documented kinetics of activity-dependent, Ca2+-dependent release of BDNF from dendrites and axons in neurons and glial cells. Molecular mechanisms underlying dendritic-postsynaptic release and axonal-presynaptic release, however, remain to be elucidated. Several molecules such as CAPS2 and Syt-IV, which facilitates and inhibits BDNF release respectively, have been identified so far. Unraveling the release ratios of BDNF-associated peptides (BDNF, its precursor proBDNF and its pro-domain called BDNF pro-peptide) will also be informative to the understanding of their functional significances in brain function and dysfunction.
References
Adachi N, Kohara K, Tsumoto T (2005) Difference in trafficking of brain-derived neurotrophic factor between axons and dendrites of cortical neurons, revealed by live-cell imaging. BMC Neurosci 6:42
Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS, Hempstead BL, Bracken C (2013) Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun 4:2490
Angelucci F, Aloe L, Vasquez PJ, Mathe AA (2000) Mapping the differences in the brain concentration of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in an animal model of depression. Neuroreport 11:1369–1373
Angelucci F, Aloe L, Jimenez-Vasquez P, Mathe AA (2003) Lithium treatment alters brain concentrations of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor in a rat model of depression. Int J Neuropsychopharmacol 6:225–231
Balkowiec A, Katz DM (2002) Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci 22:10399–10407
Barde YA, Edgar D, Thoenen H (1982) Purification of a new neurotrophic factor from mammalian brain. EMBO J 1:549–553
Barker PA (2009) Whither proBDNF? Nat Neurosci 12:105–106
Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SR (2011) The extended granin family: structure, function, and biomedical implications. Endocr Rev 32:755–797
Bibel M, Barde YA (2000) Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14:2919–2937
Bland BH (1986) The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol 26:1–54
Bocchini V, Angeletti PU (1969) The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A 64:787–794
Brigadski T, Hartmann M, Lessmann V (2005) Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. J Neurosci 25:7601–7614
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299–309
Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS (2005) Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci 25:6156–6166
Cohen S (1960) Purification of a nerve-growth promoting protein from the mouse salivary gland and its neuro-cytotoxic antiserum. Proc Natl Acad Sci U S A 46:302–311
Cohen S, Levi-Montalcini R (1956) A nerve growth-stimulating factor isolated from snake venom. Proc Natl Acad Sci U S A 42:571–574
Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S (1997) Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 17:2295–2313
Dean C, Liu H, Dunning FM, Chang PY, Jackson MB, Chapman ER (2009) Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat Neurosci 12:767–776
Dean C, Liu H, Staudt T, Stahlberg MA, Vingill S, Bückers J, Kamin D, Engelhardt J, Jackson MB, Hell SW, Chapman ER (2012) Distinct subsets of Syt-IV/BDNF vesicles are sorted to axons versus dendrites and recruited to synapses by activity. J Neurosci 32:5398–5413
Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundelfinger ED, Kojima M, Nestel S, Frotscher M, Barde YA (2012) BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol 196:775–788
Eckenstaler R, Lessmann V, Brigadski T (2016) CAPS1 effects on intragranular pH and regulation of BDNF release from secretory granules in hippocampal neurons. J Cell Sci 129:1378–1390
Edelmann E, Cepeda-Prado E, Franck M, Lichtenecker P, Brigadski T, Lessmann V (2015) Theta burst firing recruits BDNF release and signaling in postsynaptic CA1 neurons in spike-timing-dependent LTP. Neuron 86:1041–1054
Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269
Fawcett JP, Alonso-Vanegas MA, Morris SJ, Miller FD, Sadikot AF, Murphy RA (2000) Evidence that brain-derived neurotrophic factor from presynaptic nerve terminals regulates the phenotype of calbindin-containing neurons in the lateral septum. J Neurosci 20:274–282
Hamatake M, Miyazaki N, Sudo K, Matsuda M, Sadakata T, Furuya A, Ichisaka S, Hata Y, Nakagawa C, Nagata K, Furuichi T, Katoh-Semba R (2011) Phase advance of the light-dark cycle perturbs diurnal rhythms of brain-derived neurotrophic factor and neurotrophin-3 protein levels, which reduces synaptophysin-positive presynaptic terminals in the cortex of juvenile rats. J Biol Chem 286:21478–21487
Hartmann M, Heumann R, Lessmann V (2001) Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J 20:5887–5897
Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan E, Laviv T, Hempstead BL, Yasuda R, McNamara JO (2016) Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature 538:99–103
He XL, Garcia KC (2004) Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science 304:870–875
Hempstead BL (2002) The many faces of p75NTR. Curr Opin Neurobiol 12:260–267
Hempstead BL (2014) Deciphering proneurotrophin actions. Handb Exp Pharmacol 220:17–32
Ibanez CF, Simi A (2012) p75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci 35:431–440
Ip NY, Ibanez CF, Nye SH, McClain J, Jones PF, Gies DR, Belluscio L, Le Beau MM, Espinosa R 3rd, Squinto SP et al (1992) Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci U S A 89:3060–3064
James DJ, Martin TF (2013) CAPS and Munc13: CATCHRs that SNARE vesicles. Front Endocrinol (Lausanne) 4:187
Kabachinski G, Kielar-Grevstad DM, Zhang X, James DJ, Martin TF (2016) Resident CAPS on dense-core vesicles docks and primes vesicles for fusion. Mol Biol Cell 27:654–668
Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G (2005) Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry 57(9):1068–72
Katoh-Semba R, Takeuchi IK, Inaguma Y, Ito H, Kato K (1999) Brain-derived neurotrophic factor, nerve growth and neurotrophin-3 selected regions of the rat brain following kainic acid-induced seizure activity. Neurosci Res 35:19–29
Kohara K, Kitamura A, Morishima M, Tsumoto T (2001) Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science 291:2419–2423
Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, Matsumoto T, Katoh-Semba R, Nawa H, Hatanaka H (2001) Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J Neurosci Res 64:1–10
Kolbeck R, Bartke I, Eberle W, Barde YA (1999) Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J Neurochem 72:1930–1938
Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M (2009) Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain 2:27
Kuczewski N, Porcher C, Ferrand N, Fiorentino H, Pellegrino C, Kolarow R, Lessmann V, Medina I, Gaiarsa JL (2008) Backpropagating action potentials trigger dendritic release of BDNF during spontaneous network activity. J Neurosci 28:7013–7023
Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294:1945–1948
Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde YA (1989) Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341:149–152
Leschik J, Eckenstaler R, Endres T, Munsch T, Edelmann E, Richter K, Kobler O, Fischer KD, Zuschratter W, Brigadski T, Lutz B, Lessmann V (2019) Prominent postsynaptic and dendritic exocytosis of endogenous BDNF vesicles in BDNF-GFP knock-in mice. Mol Neurobiol 56:6833–6855
Lessmann V, Brigadski T (2009) Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res 65:11–22
Lessmann V, Gottmann K, Malcangio M (2003) Neurotrophin secretion: current facts and future prospects. Prog Neurobiol 69:341–374
Levi-Montalcini R, Hamburger V (1953) A diffusible agent of mouse sarcoma, producing hyperplasia of sympathetic-ganglia and hyperneurotization of viscera in the chick embryo. J Exp Zool 123:233–287
Liepinsh E, Ilag LL, Otting G, Ibanez CF (1997) NMR structure of the death domain of the p75 neurotrophin receptor. EMBO J 16:4999–5005
López-Benito S, Sánchez-Sánchez J, Brito V, Calvo L, Lisa S, Torres-Valle M, Palko ME, Vicente-García C, Fernández-Fernández S, Bolaños JP, Ginés S, Tessarollo L, Arévalo JC (2018) Regulation of BDNF release by ARMS/Kidins220 through modulation of synaptotagmin-IV levels. J Neurosci 38:5415–5428
Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP (2005) Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron 45:245–255
Lu B, Pang PT, Woo NH (2005) The yin and yang of neurotrophin action. Nat Rev Neurosci 6:603–614
Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD (1990) NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron 5:501–509
Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM (2009) Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci 29:14185–14198
Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA (2008) Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci 11:131–133
Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M (2002) Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 36:121–137
Mizui T, Ishikawa Y, Kumanogoh H, Lume M, Matsumoto T, Hara T, Yamawaki S, Takahashi M, Shiosaka S, Itami C, Uegaki K, Saarma M, Kojima M (2015) BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proc Natl Acad Sci U S A 112:E3067–E3074
Mizui T, Hattori K, Ishiwata S, Hidese S, Yoshida S, Kunugi H, Kojima M (2019) Cerebrospinal fluid BDNF pro-peptide levels in major depressive disorder and schizophrenia. J Psychiatr Res 113:190–198
Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS, Murphy RA (1999) Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci 19:2069–2080
Nakajima T, Sato M, Akaza N, Umezawa Y (2008) Cell-based fluorescent indicator to visualize brain-derived neurotrophic factor secreted from living neurons. ACS Chem Biol 3:352–358
Pang PT, Lu B (2004) Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev 3:407–430
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14(1):7–23
Park JJ, Cawley NX, Loh YP (2008) A bi-directional carboxypeptidase E-driven transport mechanism controls BDNF vesicle homeostasis in hippocampal neurons. Mol Cell Neurosci 39:63–73
Persoon CM, Hoogstraaten RI, Nassal JP, van Weering JRT, Kaeser PS, Toonen RF, Verhage M (2019) The RAB3-RIM pathway is essential for the release of neuromodulators. Neuron 104(1065–1080):e1012
Polacchini A, Metelli G, Francavilla R, Baj G, Florean M, Mascaretti LG, Tongiorgi E (2015) A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Sci Rep 5:17989
Rauskolb S, Zagrebelsky M, Dreznjak A, Deogracias R, Matsumoto T, Wiese S, Erne B, Sendtner M, Schaeren-Wiemers N, Korte M, Barde YA (2010) Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci 30:1739–1749
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond Ser B Biol Sci 361:1545–1564
Sadakata T, Mizoguchi A, Sato Y, Katoh-Semba R, Fukuda M, Mikoshiba K, Furuichi T (2004) The secretory granule-associated protein CAPS2 regulates neurotrophin release and cell survival. J Neurosci 24:43–52
Sadakata T, Kakegawa W, Mizoguchi A, Washida M, Katoh-Semba R, Shutoh F, Okamoto T, Nakashima H, Kimura K, Tanaka M, Sekine Y, Itohara S, Yuzaki M, Nagao S, Furuichi T (2007a) Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci 27:2472–2482
Sadakata T, Washida M, Iwayama Y, Shoji S, Sato Y, Ohkura T, Katoh-Semba R, Nakajima M, Sekine Y, Tanaka M, Nakamura K, Iwata Y, Tsuchiya KJ, Mori N, Detera-Wadleigh SD, Ichikawa H, Itohara S, Yoshikawa T, Furuichi T (2007b) Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J Clin Invest 117:931–943
Sadakata T, Shinoda Y, Oka M, Sekine Y, Sato Y, Saruta C, Miwa H, Tanaka M, Itohara S, Furuichi T (2012) Reduced axonal localization of a Caps2 splice variant impairs axonal release of BDNF and causes autistic-like behavior in mice. Proc Natl Acad Sci U S A 109:21104–21109
Sadakata T, Kakegawa W, Shinoda Y, Hosono M, Katoh-Semba R, Sekine Y, Sato Y, Saruta C, Ishizaki Y, Yuzaki M, Kojima M, Furuichi T (2014) Axonal localization of Ca2+-dependent activator protein for secretion 2 is critical for subcellular locality of brain-derived neurotrophic factor and neurotrophin-3 release affecting proper development of postnatal mouse cerebellum. PLoS One 9:e99524
Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA (1996) Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett 379:247–250
Shimojo M, Courchet J, Pieraut S, Torabi-Rander N, Sando R 3rd, Polleux F, Maximov A (2015) SNAREs controlling vesicular release of BDNF and development of callosal axons. Cell Rep 11:1054–1066
Shinoda Y, Sadakata T, Nakao K, Katoh-Semba R, Kinameri E, Furuya A, Yanagawa Y, Hirase H, Furuichi T (2011) Calcium-dependent activator protein for secretion 2 (CAPS2) promotes BDNF secretion and is critical for the development of GABAergic interneuron network. Proc Natl Acad Sci U S A 108:373–378
Song M, Martinowich K, Lee FS (2017) BDNF at the synapse: why location matters. Mol Psychiatry 22:1370–1375
Uegaki K, Kumanogoh H, Mizui T, Hirokawa T, Ishikawa Y, Kojima M (2017) BDNF binds its pro-peptide with high affinity and the common Val66Met polymorphism attenuates the interaction. Int J Mol Sci 18
Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL (2009) Neuronal release of proBDNF. Nat Neurosci 12:113–115
Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A (2003) Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron 39:975–990
Funding
This work was supported by the Japan Science and Technology Agency Core Research for Evolutional Science and Technology (CREST) (to M.K. and T.F.); AMED under Grant Number JP20lm0203012j0002 (to M.K.); a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (17K07073) (to M.K.) and (17H03563) (to T.F.); and The NOVARTIS Foundation (Japan) for the Promotion of Science (T.F.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics statement
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kojima, M., Ishii, C., Sano, Y. et al. Journey of brain-derived neurotrophic factor: from intracellular trafficking to secretion. Cell Tissue Res 382, 125–134 (2020). https://doi.org/10.1007/s00441-020-03274-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03274-x