Abstract
The central nervous system (CNS) of Drosophila is comprised of the brain and the ventral nerve cord (VNC), which are the homologous structures of the vertebrate brain and the spinal cord, respectively. Neurons of the CNS arise from neural stem cells called neuroblasts (NBs). Each neuroblast gives rise to a specific repertory of cell types whose fate is unknown in most lineages. A combination of spatial and temporal genetic cues defines the fate of each neuron. We studied the origin and specification of a group of peptidergic neurons present in several abdominal segments of the larval VNC that are characterized by the expression of the neuropeptide GPB5, the GPB5-expressing neurons (GPB5-ENs). Our data reveal that the progenitor NB that generates the GPB5-ENs also generates the abdominal leucokinergic neurons (ABLKs) in two different temporal windows. We also show that these two set of neurons share the same axonal projections in larvae and in adults and, as previously suggested, may both function in hydrosaline regulation. Our genetic analysis of potential specification determinants reveals that Klumpfuss (klu) and huckebein (hkb) are involved in the specification of the GPB5 cell fate. Additionally, we show that GPB5-ENs have a role in starvation resistance and longevity; however, their role in desiccation and ionic stress resistance is not as clear. We hypothesize that the neurons arising from the same neuroblast lineage are both architecturally similar and functionally related.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurons are a highly diverse group of cells that display a large variety of shapes, connectivity patterns and molecular profiles. In the central nervous system (CNS) of Drosophila, there are three large groups of neurons: the interneurons, which transform sensory input into behavioural responses; the motor neurons, which respond to the behavioural responses via precise synaptic connections to specific muscles; and the neuroendocrine, peptidergic neurons, which have diffuse terminals that release hormonal or paracrine signals to regulate and integrate diverse body functions such as growth, metabolism, reproduction, ion homeostasis, circadian rhythms and behaviour (Nassel 2002). Similar to vertebrates, neuronal clusters follow a developmental logic in which specific clusters arise from the same neuronal stem cell, the neuroblast (NB).
Nearly all of the NBs in the Drosophila brain and ventral nerve cord (VNC) have already been individually identified (Doe 1992; Urbach and Technau 2003; Birkholz et al. 2013). The VNC is composed of about 30 NBs per hemineuromere (Doe 1992), although fewer NBs are found in the anterior gnathal and terminal abdominal segments (Bossing et al. 1996; Schmidt et al. 1997; Birkholz et al. 2013). Each NB undergoes multiple rounds of asymmetric cell division, and with each division, it self-renews and generates a secondary precursor cell called the ganglion mother cell (GMC) that terminally divides to generate two neurons or glia. Through successive cell divisions, each NB produces unique and highly diverse progeny (Bossing et al. 1996). In the embryonic VNC, all NBs undergo temporal changes by expressing temporal transcription factors (TTFs) (Kambadur et al. 1998; Doe 2017). Each TTF defines a temporal identity window for the NB and its descendant GMC. Notch signalling, via a Notch-ON or Notch-OFF system, then mediates GMC division to produce two sibling cells with distinct identities (Jan and L. Y. 1994). As a result, cell diversity is generated both among and within NB lineages.
After embryonic neurogenesis, most NBs die, while the others enter a mitotically quiescent state until second larval instar when they reactivate to generate postembryonic neurons that will form the adult nervous system. During this phase, each NB and its immature progeny form cell clusters whose axonal projections group to specific larval regions. Each axon group identifies the neurons of a hemilineage (Truman et al. 2010). In Drosophila, approximately 23 NBs per hemineuromere are reactivated in the thoracic segments, but only three or four are reactivated in the abdominal segments (Birkholz et al. 2015).
Recently, Birkholz et al. (2015) used Flybow to track primary lineages in the VNC and reported a correspondence between embryonic and postembryonic lineages in the thoracic segments. Lacin and Truman (2016) subsequently established that the neurons born during late embryonic phases are genetic and morphologically similar to those born postembryonically. To assess the functional role of hemilineage neurons, Harris et al. (2015), using TRP1 channel activation, showed that each hemilineage appears to have a modular function as each lineage’s cells can be associated with particular behavioural responses, such as postural changes, rhythmic movements related to walking or flight and the take-off response.
Although detailed studies of thoracic secondary NB lineages have been conducted, little has been reported on the abdominal lineages. Only three postembryonic NBs have been identified in the abdominal hemiganglia: ventrolateral (vl), ventromedial (vm) and dorsolateral (dl) (Truman and Bate 1988). More recently, these NBs have been correlated to NB5-3, NB5-2 and NB3-5, respectively (Birkholz et al. 2015; Lacin and Truman 2016). Although different marker genes are known to characterize distinct NB lineages, thus allowing their identification, little is known about the fate of the neuronal progeny. Only the vl NB (NB5-3) is known as a progenitor of abdominal leucokinergic neurons (ABLKs), which are generated in two phases, an embryonic and a postembryonic, separated by a quiescent period (Estacio-Gomez et al. 2013). ABLKs are characterized by their production of the neurohormone leucokinin (LK), which is involved in the induction of fluid secretion by Malpighian tubules, feeding regulation and ionic stress tolerance (Nassel 2002; Al-Anzi et al. 2010; Lopez-Arias et al. 2011; Zandawala et al. 2018). ABLKs also produce the diuretic hormone DH44 (Zandawala et al. 2018).
Here, we analyse the origin and specification of a group of peptidergic neurons that produce the glycoprotein GPB5, the GPB5-expressing neurons (hereafter, GPB5-ENs). GPB5 forms a heterodimeric hormone with the glycoprotein GPA2. Both GPB5 and GPA2, which are also present in vertebrates, belong to the large family of glycoproteins that also includes LH, FSH and TSH hormones (for review, Rocco and Paluzzi (2016)). Studies in Aedes aegypti suggest that GPA2/GPB5 functions in feeding and hydromineral balance (Rocco et al. 2017), while, in Drosophila, GPA2/GPB5 is hypothesized to act as an anti-diuretic in the hindgut (Sellami et al. 2011). We present data supporting the hypothesis that neurons from the same VNC abdominal NB lineage have not only similar neuroanatomical characteristics but also similar physiological roles.
Material and methods
D. melanogaster stocks
All lines were maintained on a standard D. melanogaster diet at 22 °C, 55% humidity and a 12:12 h light/dark photoperiod. A complete list of the fly strains used, and their sources, is provided in Table 1. To assess the persistence of GPB5-ENs in adults, a flip-out method was used by crossing a GPB5-GAL4 line with UAS-GFP;tubGAL80ts;UASflp,Act>stop>βgal/TM6B. To facilitate the random action of the GAL4 driver at the beginning of development, the cross was maintained at 29 °C. GPB5-ENs in which UAS-flp is active mediate the recombination of the Act>>βgal cassette and will permanently express actin associated to βgal for the duration of the neuron’s life, regardless of GPB5 production.
Immunohistochemistry
Immunohistochemistry of larval and adult CNS tissue was performed as described by Herrero et al. (Herrero et al. 2014). Primary and secondary antibodies and dilutions (in BBT) used in this study are shown in Table 2. Briefly, larvae and adults were dissected in PBS, fixed for 30 min and 1 h, respectively, in ice-cold 4% formaldehyde followed by 10 min in ice-cold methanol. Samples were then washed with BBT (PBS with 0.1% BSA and 0.3% Tween-20) for 1 h and incubated with diluted primary antibody overnight at 4 °C. After several rinses with BBT, samples were incubated with secondary antibodies conjugated to Alexa Fluor (488, 555 or 647) at room temperature for 1.5 h. After several rinses with BBT, samples were mounted in Vectashield (Vector Labs).
Confocal imaging and analysis
Confocal images were taken on a Zeiss LSM Meta 510 confocal microscope. Stacks of 0.5 to 2-μm spacing were collected and processed with the Zeiss LSM software and then edited in Adobe Photoshop.
Antibody production
To generate the anti-GPB5 antibody, two rats were immunized with the peptide GSRAIMVGADTKNLDY, which includes the sequence of the mature peptide. A terminal Cys residue was added to couple the peptide to the keyhole limpet hemocyanin carrier protein. After two rounds of immunizations, the rats were bled and the resulting sera tested for GPB5-specific staining of the larval CNS.
The LK antibody was generated by immunizing four mice with the peptide NSVVLGKKQRFHSWGC, which corresponds to the mature peptide sequence described by Terhzaz et al. (1999). As in the GPB5 immunization, a terminal Cys residue was added to couple the peptide to the keyhole limpet hemocyanin carrier protein. After five rounds of immunizations, the mice were bled and the sera tested for LK-specific staining of the larval CNS.
Stress resistance assays
Five- to 6-day-old male flies were separated into groups of 25 individuals with three replicates for each GPB5RNAi and parental genotype. They were kept at 25 °C and 55% humidity under a 12:12 light/dark photoperiod. The vials were scored daily for survival, and every 3 h for desiccation or 6 h for starvation and ionic stress, until all flies were dead. For the desiccation assays, flies were kept in empty vials and, for the starvation assays, in vials containing 5 ml of medium consisting of only 0.5% agar. For the ionic stress assays, flies were kept in vials containing 20% sucrose, 5% dry yeast and 2% agar supplemented with 4% NaCl.
Longevity
Males were collected within 24 h of eclosion and separated into groups of 25. They were raised at 25 °C and 60% humidity under a 12:12 light/dark cycle. Groups were scored and transferred to fresh vials with food every 2 days.
Statistical analyses
One-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test, was used to compare the three genotypes used in the stress resistance assays. An unpaired Student’s t test was used for pair-wise comparisons. All resistance stress curves were compared using the Mantel–Cox log rank test. All statistical analyses were performed using GraphPad Prism.
Results
Identification of the GPB5-ENs
To determine the precise expression pattern of the GPB5 peptide in the VNC, we generated a polyclonal antibody that recognizes the mature peptide and used it to label the CNS. In general, the expression pattern of the antibody mirrored that of the GPB5-GAL4 transgenic reporter line (GPB5-GAL4 UAS-GFP) at all stages of larval development (Fig. 1).
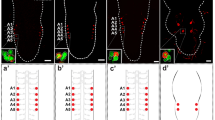
Localization of GPB5 in the embryonic, larval and adult CNS of Drosophila and its schematic representation. The expression pattern of GPB5 was characterized using a GPB5-Gal4 enhancer line driving the GFP reporter (green) or an anti-GPB5 specific antibody (red). a, a′ GPB5-positive neurons beginning to be present at embryonic stage 17. Visualization of emergent neurons is only possible with the GFP reporter, and not all pairs simultaneously express the reporter. b, b′ GPB5 expression in four pairs of neurons per hemiganglion in a first-instar larva. The expression pattern of anti-GPB5 matches that of the reporter line (inset). c, c′ GPB5 expression in the CNS of an early pupa. GPB5 expression in neurons in hemisegments A3 and A4 is no longer observed (see anti-GPB5 signal decay in the inset and the small black dots in the schematic). d, d′ GPB5 expression in the abdominal ganglia of the adult CNS. Only four neurons are observed in each hemiganglion. Inset: GPB5 antibody expression (red). e, f Neurons expressing GPB5 (green) in a larval (e) and adult (f) CNS. Arrows indicate neurons expressing βgal (red) but not GPB5 in GPB5-GAL4>UGFPtub-GAL80(ts); UflpAct>>βgal (see text). Scale bars 50 μμm
GPB5-Gal4 reporter expression is first detected at late embryonic stage 17 in several ventrolateral cells of abdominal segments A1–4 (Fig. 1a, a′). However, immunostaining with the peptide antibody does not detect GPB5 at this stage. Characterization of the extent of reporter expression across embryos demonstrates that GPB5 is restricted to 16 ventrolateral cells, two per hemisegment, in these abdominal segments. This pattern is more evident at larval stages, in which it is maintained (Fig. 1b, b′), and is consistent with the pattern described by Sellami et al. (2011). Immunostaining of larvae with the peptide antibody, which is now detectable, confirms GPB5 expression in a pattern coincident with the one observed with the GPB5-Gal4 line (Fig. 1b). At an early pupal stage [0–12 h after puparium formation (APF)], we observed that the GPB5-expressing cells in segments A3 and A4 are smaller in size than those in the other segments (Fig. 1c, c′). At later stages of pupal development, GPB5-expressing cells are only detected in segments A1 and A2. This expression is maintained in the adult (Fig. 1d, d′).
To determine whether the cells in segments A3 and A4 that lose GPB5 expression die or only stop expressing the neuropeptide, we traced GPB5 expression in flip-out clones that permanently express β-galactosidase, even when the peptide is no longer detected (GPB5-GAL4 UAS-GFP tubG80ts; UAS-Flp Act>stop>βGal lines at 29 °C). We observed that these cells are still alive at larval and adult stages; therefore, they have only lost GPB5 expression (Fig. 1e, f), suggesting that they have adopted a different fate.
Neuronal anatomy of GPB5-ENs
To study the detailed neuroanatomy of the GPB5-ENs, and to identify possible neuronal circuit connections, we labelled the presynaptic and postsynaptic compartments using the somatodendritic specific marker DenMark and the terminal presynaptic marker Synaptobrevin::GFP (Syt::GFP) driven by GPB5-GAL4 (GPB5-GAL4>DenMark UASsyt:eGFP). In third-instar larvae, we detected strong DenMark expression in neuronal somas and extensive neurite arborizations projecting both anterior and posterior to the cell bodies (Fig. 2a, b). All of the dendritic neurites form bilateral dendritic arborizations, which join in the dorsal medial tracts from the A1 segment. The posterior-most GPB5-ENs (in A4) extend their posterior dendritic axons to the segmental nerve. Presynaptic Syt::GFP is observed in axonal projections from somas to the medial dorsal axon tract that ascends to the suboesophageal ganglion (located in the ventral region of the brain). By contrast, in adults, extensive Syt::GFP is found in thoracic and abdominal neuropils (Fig. 2c, d), and the DenMark signal is weaker (Fig. 2d). Only short and limited arborization is observed in the anterior-most abdominal segments, due to the presence of fewer GPB5-ENs in the posterior-most segments. Taken together, these results suggest that GPB5-ENs integrate information from other abdominal neurons, and regulate or modulate both information processed in the suboesophageal ganglion of the larva and motor and autonomic responses in the adult through thoracic and abdominal centres, respectively.
Organization of GPB5-EN cell bodies, dendrites and axons in the larval and adult CNS. GPB5-GAL4>DenMark,UASsyt:eGFP was used to double label dendrites (DenMark; in red) and axons (synaptobrevin-GFP; in green). a, b Larval CNS dendrites extend to adjacent GPB5s and synaptobrevin-GFP directs GFP to the synaptic terminals in the dorsal medial neuropil. b′, b″ Higher power views of the box in b showing the neuronal processes labelled with DenMark (b′) and GFP (b″). c, d In the adult CNS ventral ganglia, dendritic extensions are concentrated in the first segments of the abdominal ganglia (d) and terminal axon projections extend to the thoracic ganglia (c). d′, d″ Higher power views of the box in d showing the neuronal processes labelled with DenMark (d′) and GFP (d″). Scale bars 100 μm
NB5-3 is the progenitor of the GPB5-ENs
We next wanted to identify the progenitor NB of the GPB5-ENs. Due the close proximity of the GPB5-ENs and the ABLKs (Fig. 3), we postulated that they could be generated from the same progenitor NB. The progenitor NB of the ABLKs was originally identified as NB5-5 based on the combined RNA expression of gooseberry (gsb)-lacZ, huckebein (hkb)-lacz and unplugged (unpg)-lacZ (Doe 1992; Benito-Sipos et al. 2010), markers which were considered distinctive to NB5-5 (Doe 1992). However, later studies showed that the NB that generates embryonic ABLKs also generates ABLKs during larval stages (Estacio-Gomez et al. 2013) and that NB5-5 dies at the end of embryogenesis (Lacin and Truman 2016), indicating that this NB cannot be the progenitor of ABLKs. More recently, it was demonstrated that the abdominal NBs that undergo quiescence at late embryonic stages and then resume proliferation at larval stages originate from embryonic NB3-5, NB5-2 and NB5-3 (Lacin and Truman 2016), suggesting that the ABLKs originate from one of these three NBs. Given these findings, several authors have proposed NB5-3 as the ABLK progenitor NB (Estacio-Gomez et al. 2013; Alvarez-Rivero et al. 2017). The only caveat is that ABLKs express hkb-lacZ, which was not originally assigned as a marker of NB5-3 (Chu-LaGraff et al. 1995). We hypothesize that this is due to a late expression of the reporter line in ABLK neurons but not in its progenitor NB.
Spatial distribution of GPB5-ENs and ABLKs in the larval and adult CNS. General distribution of leucokinin (anti-Lk; red) and GPB5 (GPB5-GAL4>GFP; green)-expressing neurons in a third instar larva (a) and an adult CNS (b). Colocalization of Lk and GFP was not observed, clearly demonstrating that the two markers label distinct types of neurons that are in very close proximity (see inset details). Scale bars 100 μm
To test our hypothesis, we looked at the expression of the following row-5 NB markers in the GPB5-ENs at embryonic stage 17: gsb-lacZ, Runt, eyeless (ey)-Gal4, unpg-lacZ, which all mark NB5-3, hkb-lacZ and ladybird (lbe)-Gal4 (Doe 1992) (Lacin and Truman 2016)) (Fig. 4). We observed expression of the known NB5-3 markers in the GPB5-ENs (Fig. 4a–d), consistent with the hypothesis that NB5-3 is the progenitor of the GPB5-ENs. When we examined the expression of the NB5-4 and 5-5 marker hkb-lacZ and the NB5-6 marker lbe-Gal4, we found that GBP5-ENs (and ABLKs) are positive for hkb-lacZ (Fig. 4e) but negative for lbe-Gal4 (Fig. 4g). Even though NB5-3 does not express hkb-lacZ, the colocalization pattern of NB5-3 markers in the ABLKs and GPB5-ENs suggests that these neurons originate from the same progenitor NB, namely NB5-3. To further support a common origin, we tested whether klumpfuss (klu-lacZ), which is required for specification of the ABLKs (Benito-Sipos et al. 2010), is also expressed in GPB5-ENs (Fig. 4f). We observed colocalization of klu-lacz and GPB5, suggesting klu may also be involved in the specification of the GPB5-ENs. The expression pattern of row 5 markers is summarized in Fig. 4h.
Genetic factor characteristics of NB5-3 are also expressed in GPB5 and ABLK neurons. a–f Expression of GPB5 (green), Lk (blue) and the row 5 NB markers (red): a eyGAL4>cherry, b Runt, c gsblacz, d unpg-lacz, e hkb-lacz, f klu-lacz and g lbeGAL4>GFP in the ventral ganglia of stage 17 embryos. Individual channels of insets are shown at the bottom of each panel; a′, b, c′, d′, e′, f′ and g′ show GPB5-ENs; a″, b″, c″, d″, e″, f″ and g″ show 5 NB marker expression; and a‴, b‴, c‴, d‴, e‴, f‴ and g‴ show ABLKs. Blue and green circles in the NB marker channel indicate the ABLKs and GBP5-ENs, respectively. h Summary of the observed colocalization of row 5 NB markers in the different NBs and in the GPB5 and ABLK neurons. Note hkb is a known marker of NB5-4 and NB5-5 but not of NB5-3 (Benito-Sipos et al. 2010; Doe 1992), despite being expressed in both GPB5-ENs and ABLKs. Scale bars 25 μm
GPB5-ENs are generated in the Pdm temporal window
In the embryonic VNC, NBs sequentially express the following set of transcription factors that define temporal windows: Hunchback (Hb), Krüppel (Kr), Nubbin/Pdm2 (Pdm), Castor (Cas) and Grainy head (Grh) (Kambadur et al. 1998; Isshiki et al. 2001; Brody and Odenwald 2000). The expression of these factors is a key component of the combinatorial genetic code that gives identity to the progeny generated in each temporal window, which, in turn, usually inherits the expression of the same factor. We examined the expression of these temporal genes in the GPB5-ENs and found that only Pdm is expressed in these neurons (Fig. 5a–d), demonstrating that the GPB5-ENs are generated in the Pdm temporal window. The ABLKs are subsequently generated in the Cas/Grh window (Benito-Sipos et al. 2010).
Expression of temporal transcription factors during NB development reveals the lineage origin of the GPB5-ENs. Protein expression of GPB5 and/or LK and the TTF krulacz (a), pdm-GAL4>cherry (b), caslacz (c) and grhlacz (d) at embryonic stage 17. Individual channels of insets are shown to the right of each panel; a′, b′, c′ and d′ show temporal factor expression; a″, b″, c″ and d″ show GPB5-ENs; and b‴ and c‴ show ABLKs. Blue and green circles in the temporal factors channel indicate the ABLKs and GPB5.ENs, respectively. GPB5 only colocalizes with the pdm marker, and, as previous described (Benito-Sipos et al. 2010), LK colocalizes with cas. GPB5 immunostaining is shown in b, while GPB5-GAL4>GFP is shown in a, c and d. Scale bars 10 μm
We next examined the expression of GPB5 in various mutants (Fig. 6). In agreement with our observation and compared with wild type (Fig. 6a), GPB5-ENs are lost in Pdm mutants (Df(2R)ED773) (Fig. 6b). We also checked whether overexpression of Pdm in neurons (elav-Gal4), neuroblasts (insc-Gal4) or neuroectoderm (sca-Gal4) could generate additional GPB5-ENs, but we did not observe an increase in the number of GPB5-ENs (Fig. 6c). The number of GPB5-ENs is also unaffected in cas, grh and nab mutants (results not shown).
Altered pattern of GPB5 expression in different mutant backgrounds. a–c The wild-type expression of GPB5 (a), the loss of expression in a pdm deficiency mutant (b) and normal expression in a pdm gain-of-function line (c) at first larval instar. Effects on GPB5 fate due to the loss of function of hkb (d) and klu (e) and the overexpression of klu (f) in first instar larval CNS. g unpg loss-of-function larva showing no changes in GPB5 expression. Inset in e shows a higher power view of the increased number of GPB5s in the absence of klu. h Quantification of the observed number of GPB5-positive cells per hemiganglion (n ≥ 10 CNSs for all genotypes). Asterisks denote a significant difference compared with the control (Student’s t test, p ≤ 0.0001). Scale bars 10 μm
The genes hkb and klu are involved in the specification of GPB5-ENs
To explore which other genes might be involved in the specification of GPB5-ENs, we checked the requirements of klu, hkb and unpg. In the hkb mutant, GBP5 expression is completely absent (Fig. 6d), whereas, in the klu mutant, more GPB5-positive cells are observed, with some hemineuromeres having three or four positive cells (Fig. 6e). Overexpression of klu (elav-GAL4 UAS-klu) leads to a loss of GPB5 expression (Fig. 6f). Finally, GPB5 expression is not altered in unpg mutants (Fig. 6g). Quantification of the number of GPB5-ENs in the different mutant/overexpression lines is summarized in Fig. 6h. Taken together, these results suggest that hkb and klu are involved in different aspects of GPB5-EN specification. While hkb expression is necessary for their specification, klu seems to be required to represses the GPB5 fate. Interestingly, klu is also required to specify the ABLK fate (Benito-Sipos et al. 2010).
Notch signalling does not affect the GPB5-ENs
Since the two GPB5-ENs present in each hemisegment are equal in appearance and in embryonic origin, we next wanted to determine whether they are sibling cells generated from the same GMC or, alternatively, cells generated from different GMCs within the Pdm temporal window. During a GMC division, Notch signalling triggers a binary cell fate decision that generates two neuronal progenies: a Notch-ON and a Notch-OFF. This unequal activation of Notch signalling is mediated by the asymmetric segregation of Numb to one of the two daughter cells, blocking Notch signalling and inducing the B-fate (Notch-OFF). In the sibling cell, which does not receive Numb, Notch signalling is activated and the cell adopts the A-fate (Notch-ON) (Spana and Doe 1996; Kumar et al. 2009).
To test whether Notch is involved in the specification of GPB5-ENs, we studied the effect of different mutant conditions of the Notch signalling pathway on their specification (Fig. 7). First, we overexpressed a constitutively active version of the Notch receptor (UAS-Nintra) in all NBs using a pan-neuronal driver (elav-GAL4) or in the progenitor NB using pdm-Gal4. We expected to observe either lost or duplication of GPB5-ENs in these lines compared with wild type (Fig. 7a); however, we did not observe any significant change in the number of GPB5-ENs in any of the experiments (Fig. 7b). Next, we analysed numb and mastermind (Mam) mutants. Mam encodes a transcriptional cofactor that interacts with the intracellular part of the Notch receptor to activate transcription. Likewise, we did not observe any significant differences in the number of GPB5-ENs in these two mutant lines (Fig. 7c, d). We also assessed possible apoptosis of the GMC progeny by overexpressing the apoptotic inhibitor p35 under the control of GPB5-GAL4 or elav-GAL4; however, no changes in the number of GBP5-ENs were observed in any of the experiments (results not shown). Quantification of the number of GPB5-ENs in the different mutant/overexpression lines is summarized in Fig. 7e. We conclude that the two GPB5-ENs present in each hemisegment are sibling cells, generated from the same GMC, that do not adopt different fates in response to Notch signalling, at least regarding GPB5 expression.
Notch signalling is not involved in the determination of GPB5 sibling neurons. Compared to the wild-type control (a), none of the analysed Notch mutants, including elavGAL4>Nintra (b), numb (c) and mam (d), show any changes in GPB5 expression. a, b The CNS of first instar larvae and c, d, of stage 17 embryos. e Quantification of the number of GPB5-positive cells observed in the different genotypes (n ≥ 10 CNSs analysed for each genotype). Scale bars 10 μm
Possible functions of GPB5-ENs
To investigate the possible function of the GPB5 peptide in the physiology and behaviour of the adult fly, we knocked down its expression by overexpressing GPB5-RNAi. The overexpression of this construct with GPB5-GAL4 has been reported to completely eliminate peptide expression in larvae (Sellami et al. 2011). However, in our hands, we still observed GPB5 expression in one or two neurons per ganglion with this construct (results not shown). Therefore, we used the pan-neuronal driver elav-GAL4 to overexpress GPB5-RNAi, which knocks out GPB5 expression in all adult ganglia but does not affect Lk expression in the ABLKs, confirming it as a suitable construct (Fig. 8a, b).
Survival in response to stress and the longevity of GPB5 knockout flies generated using elav-GAL4 > GPB5-RNAi. a, b Expression pattern of GPB5-ENs (red) and ABLKs (green) in the adult ventral ganglion of wild type (a) and knockout elavGAL4>GPBRNAi,UAS-dicer (b) flies. Note the complete lack of GPB5 expression in the knockout flies. c– f Graphs showing the survival of GPB5 knockout flies (blue line) compared with control flies, i.e., the parental lines UAS-GPB5RNAi and elavGAL4>dicer (red and green lines, respectively) under different stress conditions. Under desiccation (c) and ionic stress (d) conditions, clear differences are not observed between the knockout line and the control lines. However, under starvation conditions (e) and in terms of longevity (f), a significant increase in survival is observed in the knockout line. The significant differences observed in the desiccation and ionic stress assays correspond to only one of the parental lines. Data are presented as survival curves, and the error bars represent standard error (****p < 0.0001, as assessed by a Mantel–Cox log-rank test)
The GPB5/GPA2 peptide is hypothesized to play an anti-diuretic role based on the expression of its receptor in tissues involved in water retention, such as the salivary gland, Malpighian tubules and hindgut (Sellami et al. 2011). To check for a possible role of the peptide in the regulation of water content, we analysed the mortality of GPB5 knockout individuals under conditions of desiccation or ionic stress. The parental GAL4 and UAS lines served as controls in all stress resistance experiments. In the desiccation assay, we observed a significant increase in survival in the GPB5 knockout individuals compared with the UAS control line and with the first 30 h of observation of the GAL4 line (Fig. 8c). Interestingly, the UAS parental line showed a marked decrease in survival compared with the other two lines, representing the most significant difference observed in this assay. No significant differences in survival were observed with the GPB5 knockout flies in the ionic stress assay compared with the control lines, although a significant difference was observed between the two parental lines (Fig. 8d). In fact, in all of these assays, higher mortality was observed in the UAS parental line compared with the GAL4 parental line. Given this, we cannot consider the statistical differences of the GPB5 knockout line compared with the control lines as relevant in these assays.
We also examined the mortality of GPB5 knockout flies under starvation conditions and their overall longevity. The results of the respective assays showed a significant increase in the survival and the longevity of GPB5 knockout flies (Fig. 8e, f). In summary, the response to desiccation and ionic stress do not seem to involve GPB5. However, GPB5 does appear to play a role in the response to nutritional restriction and in the regulation of life span, as indicated by the starvation and longevity assays.
Discussion
In this study, we confirmed the pattern of GPB5-ENs described by Sellami et al. (2011). These neurons are present as four pairs of neurons per hemiganglion in segments A1–4 of the larva CNS and in two pairs of neurons per hemiganglion in segments A1 and A2 of the adult CNS. We also showed that larval GPB5-ENs from segments A3 and A4 that stop expressing GPB5 peptide do not die, but rather persist in adults as dedifferentiated or reprogrammed cells. The neuroanatomy of GPB5-ENs presents bilaterally distributed dendritic arborizations that connect each segment, and axonal projections that ascend through the medial dorsal tract in the VNC of the larva. In the adult, GPB5-EN dendritic processes are reduced, and axonal presynaptic terminals extend to thoracic ganglia.
From our analysis of the GPB5-EN progenitor NB, we found that these neurons express all the markers of NB5-3 and also hkb-lacz, a marker that is only expressed in NB5-4 and NB5-5 (Doe 1992). Similar results led us to conclude in a previous report that ABLK neurons originated from the NB5-5 lineage (Benito-Sipos et al. 2010). However, subsequent studies showed that additional ABLK neurons originate during larval neurogenesis from the same embryonic progenitor NB (Estacio-Gomez et al. 2013; Alvarez-Rivero et al. 2017) and that, while NB5-3 is present in the larva, NB5-5 undergoes apoptosis in the embryo (Lacin and Truman 2016). Given these findings, we proposed that NB5-3 is the progenitor of ABLK neurons and that hkb expression is not inherited from the progenitor NB but rather represents de novo expression in the neurons. This also appears to be the case in the GPB5-ENs, as evidenced by our expression analysis; therefore, we conclude that NB5-3 is the GPB5-EN progenitor NB. We further conclude from our analysis of expression and requirement of temporal genes that GPB5-ENs are generated in the Pdm temporal window. We also found that the genes klu and hkb are involved in GPB5-EN specification.
GMCs divide to generate two postmitotic cells that usually acquire two different fates, which are driven by the asymmetric activation of Notch in one of the cells. Loss- or gain-of-function mutations of the Notch pathway transform the fate of a cell into that of the sibling cell. In our case, the fate of GPB5-ENs was not affected in mutants of the Notch signalling pathway. Thus, we consider the two GPB5-ENs of each hemisegment as sibling cells that emerge from the same GMC, and the expression of the peptide does not allow us to distinguish between them.
Overall, we confirmed the lineage relationship between two groups of peptidergic neurons, ABLKs and GPB5-ENs, which are specified in two different temporal windows of the same progenitor NB (Fig. 9). We also observed that they have a similar cellular morphology and spatial distribution and share expression of several genes involved in their specification. Also, they both appear to play functional roles in the response to starvation resistance.
Metamorphic changes in GPB5-EN neurite projections
During metamorphosis, many of the larval neurons persist into adulthood; however, they often change their morphology and functions or undergo programmed cell death (PCD). Interestingly, GBP5-ENs (and ABLKs) have a different arborization pattern in the larva versus the adult. In the larva, more dendritic arbours are present, projecting both anteriorly and posteriorly from the cell bodies, but not in the two pairs of adult neurons. Also, the axonal projections of these neurons extend and concentrate through the medial dorsal tract, projecting to the suboesophageal ganglion in the larva but through the thoracic-abdominal ganglion in the adult.
In the adult VNC, postembryonic neurogenesis produces many neurons in each thoracic neuromere that, during metamorphosis, extend primary neurites into specific, tightly fasciculated tracts that remain largely intact throughout metamorphosis. The detailed anatomical description of the adult VNC is currently viewed as containing 16 distinct neuropils (Namiki et al. 2018), which include motor neurons that control legs, wings and neck movements; sensory neurons from the legs, wings and halteres (Tuthill and Wilson 2016); and descending neurons that transmit information from the brain. GPB5-ENs project extensively to adult VNC neuropils where they likely help regulate or modulate motor output and sensory input. However, larval GPB5-EN function appears to be more related to physiological functions integrated in the suboesophageal ganglion, such as feeding or drinking.
GPB5-ENs dedifferentiate or are reprogrammed during postlarval stages
One major cellular event of CNS metamorphosis is the PCD of larval neurons that will no longer be necessary in ensuing life stages (Veverytsa and Allan 2013). More than 300 larval cells, primarily localized in the VNC, undergo PCD shortly after puparium formation in Drosophila (Lee et al. 2018). However, little is known about the alternative to PCD: larval neurons changing their identity in the adult. It is now clear that differentiated cells can be reprogrammed to a pluripotent state (Froldi and Cheng 2016) or to an alternative differentiated fate (Vierbuchen et al. 2010). Although many studies have determined the factors involved in maintaining differentiated neurons in the Drosophila CNS (for a review, see Froldi and Cheng (2016)), nothing is known about how neurons are reprogrammed. Our findings suggest that the larval GPB5-ENs in the A3 and A4 neuromeres, after switching off neuropeptide expression, dedifferentiate or are reprogrammed in the adult. Despite the difficulty of obtaining clones of reprogrammed larval GPB5-ENs in the adults, a very interesting next step would be to determine the new identities of these cells, which will help us to understand the aim of this complex process.
Early and late temporal factors control different neuropeptide fates from the same NB
We identified the GPB5-ENs as a group of early neurons born from abdominal NB lineage 5-3 within the Pdm temporal window. The same NB later gives rise to the ABLK neurons within the Cas/Grh temporal windows, as previously described (Estacio-Gomez et al. 2013; Alvarez-Rivero et al. 2017). Interestingly, the ABLKs are produced in two distinct waves of neurogenesis separated by a NB quiescent stage, while GPB5-ENs are generated in a single wave. Despite differences in temporal origin (from the same NB progenitor) and neuropeptide nature, the anatomical structure of the two types of neurons is similar in both the larva and the adult. Postembryonic hemilineage neurons from a single thoracic NB are known to share an anatomical ground plan and to have a role in motor functions related to changes in posture, walking or wing waving, among others (Harris et al. 2015). However, nearly nothing is known of the relationships among lineages derived from the same abdominal NB. We showed that primary (GPB5s) and secondary (ABLKs) neurons are present from first larval instar to adulthood, forming a small cluster of cells in larval neuromeres A1–4 and adult segments A1 and A2. The neurite bundles derived from both lineages can be easily recognized in larvae but not in adults. Perhaps the three-dimensional arrangements of the bundles cannot be sufficiently resolved in the adult due to differences in neuropeptide localization during the development of the abdominal ganglion. Precisely how the common structural plan is regulated in both ABLKs and GPB5-ENs remains to be determined, as does the relationship between timing of birth and neuropeptide production.
Role of klu, hkb and Notch signalling in the specification of GPB5-ENs
To better characterize the cell fate specification of GPB5-ENs and given their close relationship with ABLKs, we examined several of the ABLK determinants previously described by Benito-Sipos et al. (Benito-Sipos et al. 2010). These authors showed that klu mutants lack ABLKs and, thus, its requirement for ABLK fate specification. In contrast, we showed that klu mutants have a greater number (three or four) of GPB5-ENs. Considering that GPB5-ENs differentiate prior to ABLKs (Fig. 9), loss of klu might lead to the extension of the GPB5 fate to the following round of NB division. These findings are similar to those described for NB4-2 in which klu acts to differentiate between the first two GMCs derived from this lineage, leading to the differential specification of cell fates in the neurons from the first versus second GMC, and the generation of neuronal diversity (Yang et al. 1997). Although we did not determine the fate of the neurons born from the GMC that follows the one that generates the GPB5-ENs, our results suggest that it is the ABLKs. Chu-LaGraff et al. (Chu-LaGraff et al. 1995) also implicated hkb in the proper differentiation of motoneurons arising from the NB4-2 lineage and its GMCs. Bossing et al. (Bossing et al. 1996) showed that hkb is required for correct axon pathfinding in neurons derived from NB1-1 and NB2-2. Interestingly, they also observed defects in NB lineages that do not express hkb such as the processes of NB2-1-derived neurons and a decrease in the number of cells originating from NB6-1. Although hkb expression does not characterize NB5-3 identity itself, its expression in both GPB5-ENs and ABLKs clearly indicates a role for this gene in this NB lineage, thus making it a marker that can be used to distinguish NB5-3-derived cells. Although we have observed that hkb loss of function results in the loss of GPB5 expression, its precise role in the differentiation of these neurons needs to be further explored.
Notch signalling is involved in the development of many cell types, regulating cellular behaviours ranging from differentiation and proliferation to migration and death (for a recent review, see Henrique and Schweisguth (2019)). However, dissecting the specific roles that Notch signalling plays in a developmental process can be challenging. In the case of the ABLKs, Notch signalling is not required to specify the neuronal fate (Benito-Sipos et al. 2010); however, once the ABLK fate has been specified, Notch signalling supresses PCD and the cells are in a Notch-ON state. Overexpression of constitutively active forms of Notch or numb mutants rescues the Notch-OFF siblings of the ABLKs that normally undergo PCD. Interestingly, despite its role in the ABLKs, Notch signalling does not appear to play any role in the GPB5-ENs.
ABLKs and GPB5-ENs share physiological roles
The ABLKs are hypothesized to regulate resistance to desiccation, starvation and ionic stress, possibly indirectly in response to some diuretic activity (Zandawala et al. 2018). Similar to the ABLKs, we found that when the GPB5 neuropeptide is absent, starvation resistance (and potentially desiccation resistance) increases significantly. As previously mentioned, an antidiuretic function for GPB5-ENs in the Drosophila hindgut has been previously suggested (Sellami et al. 2011). Moreover, the localization of the GPA2/GPB5 glycoprotein hormone receptor LGR-1 in different regions of the Aedes aegypti digestive tract suggests a role for these neuropeptides in feeding, digestion and hydromineral balance in insects (Rocco et al. 2017). The two hormones differentially contribute to hydromineral and nutritional balance: Lk regulates feeding and water content through the CNS and the Malpighian tubules, while GPA2/GPB5 regulates the absorption of solutes and water through the digestive tract.
In this study, we also found that longevity increases significantly in GPB5 loss-of-function flies compared with their parents. This result contrasts with the high mortality of eclosing flies derived from crosses between GPB5Gal4 and UAS-reaper that was observed by Sellami et al. (Sellami et al. 2011). We also observed that high mortality in GPB5 loss-of-function flies in the first days after eclosion; however, the differences were not significant. Sellami et al. (Sellami et al. 2011) suggested that GPA2/GPB5 is most important during early larval development and that larvae that are able to withstand the loss of GPA2/GPB5 are more fit to survive in conditions in which lethality is reduced. Consistent with this idea, our results suggest that the increase in starvation resistance promoted by the loss of GPB5 may lead to a decrease in nutritive and hydric requirements and, hence, a longer lifespan.
In summary and resembling the functional and anatomical ground plan of the thoracic neuronal lineages of the Drosophila CNS, we have shown that two sets of abdominal neuroendocrine cells, the ABLKs and the GPB5-ENs, not only originate from the same NB progenitor but also may both function, albeit antagonistically, in hydrosaline regulation and survival.
References
Al-Anzi B, Armand E, Nagamei P, Olszewski M, Sapin V, Waters C, Zinn K, Wyman RJ, Benzer S (2010) The leucokinin pathway and its neurons regulate meal size in Drosophila. Curr Biol 20:969–978
Alvarez-Rivero J, Moris-Sanz M, Estacio-Gomez A, Montoliu-Nerin M, Diaz-Benjumea FJ, Herrero P (2017) Variability in the number of abdominal leucokinergic neurons in adult Drosophila melanogaster. J Comp Neurol 525:639–660
Baonza A, Garcia-Bellido A (2000) Notch signaling directly controls cell proliferation in the Drosophila wing disc. Proc Natl Acad Sci U S A 97:2609–2614
Benito-Sipos J, Estacio-Gomez A, Moris-Sanz M, Baumgardt M, Thor S, Diaz-Benjumea FJ (2010) A genetic cascade involving klumpfuss, nab and castor specifies the abdominal leucokinergic neurons in the Drosophila CNS. Development 137:3327–3336
Birkholz O, Rickert C, Berger C, Urbach R, Technau GM (2013) Neuroblast pattern and identity in the Drosophila tail region and role of doublesex in the survival of sex-specific precursors. Development 140:1830–1842
Birkholz O, Rickert C, Nowak J, Coban IC, Technau GM (2015) Bridging the gap between postembryonic cell lineages and identified embryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Biol Open 4:420–434
Bossing T, Udolph G, Doe CQ, Technau GM (1996) The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev Biol 179:41–64
Brody T, Odenwald WF (2000) Programmed transformations in neuroblast gene expression during Drosophila CNS lineage development. Dev Biol 226:34–44
Calleja M, Moreno E, Pelaz S, Morata G (1996) Visualization of gene expression in living adult Drosophila. Science 274:252–255
Cheah PY, Chia W, Yang X (2000) Jumeaux, a novel Drosophila winged-helix family protein, is required for generating asymmetric sibling neuronal cell fates. Development 127:3325–3335
Chu-LaGraff Q, Schmid A, Leidel J, Bronner G, Jackle H, Doe CQ (1995) Huckebein specifies aspects of CNS precursor identity required for motoneuron axon pathfinding. Neuron 15:1041–1051
Doe CQ (1992) Molecular markers for identified neuroblasts and ganglion mother cells in the Drosophila central nervous system. Development 116:855–863
Doe CQ (2017) Temporal patterning in the Drosophila CNS. Annu Rev Cell Dev Biol 33:219–240
Doe CQ, Technau GM (1993) Identification and cell lineage of individual neural precursors in the Drosophila CNS. Trends Neurosci 16:510–514
Estacio-Gomez A, Moris-Sanz M, Schafer AK, Perea D, Herrero P, Diaz-Benjumea FJ (2013) Bithorax-complex genes sculpt the pattern of leucokinergic neurons in the Drosophila central nervous system. Development 140:2139–2148
Froldi F, Cheng LY (2016) Understanding how differentiation is maintained: lessons from the Drosophila brain. Cell Mol Life Sci 73:1641–1644
Grosskortenhaus R, Robinson KJ, Doe CQ (2006) Pdm and Castor specify late-born motor neuron identity in the NB7-1 lineage. Genes Dev 20:2618–2627
Harris, R. M., B. D. Pfeiffer, G. M. Rubin, and J. W. Truman. 2015. Neuron hemilineages provide the functional ground plan for the Drosophila ventral nervous system, Elife, 4
Henrique, D., and F. Schweisguth. 2019. Mechanisms of notch signaling: a simple logic deployed in time and space, Development, 146
Herrera SC, Martin R, Morata G (2013) Tissue homeostasis in the wing disc of Drosophila melanogaster: immediate response to massive damage during development. PLoS Genet 9:e1003446
Herrero P, Estacio-Gomez A, Moris-Sanz M, Alvarez-Rivero J, Diaz-Benjumea FJ (2014) Origin and specification of the brain leucokinergic neurons of Drosophila: similarities to and differences from abdominal leucokinergic neurons. Dev Dyn 243:402–414
Isshiki T, Pearson B, Holbrook S, Doe CQ (2001) Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell 106:511–521
Jan YN, L. Y. (Jan. 1994) Neuronal cell fate specification in Drosophila. Curr Opin Neurobiol 4:8–13
Kambadur R, Koizumi K, Stivers C, Nagle J, Poole SJ, Odenwald WF (1998) Regulation of POU genes by castor and hunchback establishes layered compartments in the Drosophila CNS. Genes Dev 12:246–260
Kumar A, Bello B, Reichert H (2009) Lineage-specific cell death in postembryonic brain development of Drosophila. Development 136:3433–3442
Lacin H, Truman JW (2016) Lineage mapping identifies molecular and architectural similarities between the larval and adult Drosophila central nervous system. Elife 5:e13399
Lee G, Kim J, Kim Y, Yoo S, Park JH (2018) Identifying and monitoring neurons that undergo metamorphosis-regulated cell death (metamorphoptosis) by a neuron-specific caspase sensor (Casor) in Drosophila melanogaster. Apoptosis 23:41–53
Lopez-Arias B, Dorado B, Herrero P (2011) Blockade of the release of the neuropeptide leucokinin to determine its possible functions in fly behavior: chemoreception assays. Peptides 32:545–552
Mellerick DM, Kassis JA, Zhang SD, Odenwald WF (1992) Castor encodes a novel zinc finger protein required for the development of a subset of CNS neurons in Drosophila. Neuron 9:789–803
Namiki S, Dickinson MH, Wong AM, Korff W, Card GM (2018) The functional organization of descending sensory-motor pathways in Drosophila. Elife 7:e34272
Nassel DR (2002) Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol 68:1–84
Nassel DR, Cantera R, Karlsson A (1992) Neurons in the cockroach nervous system reacting with antisera to the neuropeptide leucokinin I. J Comp Neurol 322:45–67
Rocco, DA, Paluzzi JV (2016) Functional role of the heterodimeric glycoprotein hormone, GPA2/GPB5, and its receptor, LGR1: an invertebrate perspective. Gen Comp Endocrinol 234:20–27
Rocco DA, Kim DH, Paluzzi JV (2017) Immunohistochemical mapping and transcript expression of the GPA2/GPB5 receptor in tissues of the adult mosquito, Aedes aegypti. Cell Tissue Res 369:313–330
Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM (1997) The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol 189:186–204
Sellami A, Agricola HJ, Veenstra JA (2011) Neuroendocrine cells in Drosophila melanogaster producing GPA2/GPB5, a hormone with homology to LH, FSH and TSH. Gen Comp Endocrinol 170:582–588
Spana EP, Doe CQ (1996) Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 17:21–26
Terhzaz S, O'Connell FC, Pollock VP, Kean L, Davies SA, Veenstra JA, Dow JA (1999) Isolation and characterization of a leucokinin-like peptide of drosophila melanogaster. J Exp Biol 202:3667–3676
Truman JW, Bate M (1988) Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol 125:145–157
Truman JW, Moats W, Altman J, Marin EC, Williams DW (2010) Role of Notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster. Development 137:53–61
Tuthill JC, Wilson RI (2016) Mechanosensation and adaptive motor control in insects. Curr Biol 26:R1022–R1R38
Udolph G, Rath P, Chia W (2001) A requirement for Notch in the genesis of a subset of glial cells in the Drosophila embryonic central nervous system which arise through asymmetric divisions. Development 128:1457–1466
Ulvklo C, MacDonald R, Bivik C, Baumgardt M, Karlsson D, Thor S (2012) Control of neuronal cell fate and number by integration of distinct daughter cell proliferation modes with temporal progression. Development 139:678–689
Urbach R, Technau GM (2003) Molecular markers for identified neuroblasts in the developing brain of Drosophila. Development 130:3621–3637
Veverytsa L, Allan DW (2013) Subtype-specific neuronal remodeling during Drosophila metamorphosis. Fly (Austin) 7:78–86
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463:1035–1041
Yang X, Bahri S, Klein T, Chia W (1997) Klumpfuss, a putative Drosophila zinc finger transcription factor, acts to differentiate between the identities of two secondary precursor cells within one neuroblast lineage. Genes Dev 11:1396–1408
Zandawala M, Marley R, Davies SA, Nassel DR (2018) Characterization of a set of abdominal neuroendocrine cells that regulate stress physiology using colocalized diuretic peptides in Drosophila. Cell Mol Life Sci 75:1099–1115
Acknowledgements
We are grateful to JA Veenstra for providing the GPB5-Gal4 and UAS-GPB5RNAi flies and Beatriz Fraile for her technical help in the laboratory. We thank the Bloomington Stock Center for providing fly stocks and the Confocal Microscopy Service of the CBM-SO for technical imaging assistance. We appreciate Melinda Modrell’s assistance with the English language.
Funding
This work was supported by a Spanish Ministerio de Ciencia e Innovación (grant number BFU2014-53761 (to F.J.D-B.)) and by institutional grants from the Fundación Ramón Areces and Banco de Santander to the CBM-SO.
Author information
Authors and Affiliations
Contributions
All authors had full access to all of the data in the study and take responsibility for the accuracy of the data analysis. Study concept and design: FJDB and PH. Acquisition of data: LDP, LMP and PH. Analysis and interpretation of data: LDP, LMP, FJDB and PH. Drafting of manuscript: PH. Obtained funding: FJDB. Study supervision: FJDB and PH.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
All procedures performed in this study involving animals were in accordance with the ethical standards of the institution at which the studies were conducted (Consejo Superior de Investigaciones Cientificas/Spanish National Research Council). The only animal used by the authors is insects (Drosophila melanogaster). The use of insects as an experimental system does not require ethical approval.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Díaz-de-la-Peña, L., Maestro-Paramio, L., Díaz-Benjumea, F.J. et al. Temporal groups of lineage-related neurons have different neuropeptidergic fates and related functions in the Drosophila melanogaster CNS. Cell Tissue Res 381, 381–396 (2020). https://doi.org/10.1007/s00441-020-03231-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03231-8