Abstract
There is considerable interest in understanding how contents within the gut wall (including microbiome) can activate sensory nerve endings in the gut that project to the central nervous system. However, we have only recently begun to understand the location and characteristics of extrinsic spinal afferent nerve endings that innervate the lower gastrointestinal (GI) tract. Our aim is to identify the nerve endings in the mouse distal colon that arise from single spinal afferent neurons. C57BL/6 mice were anaesthetised and single dorsal root ganglia (DRG) between lumbosacral L6–S1 were injected with dextran biotin. Mice recovered for 7 days. Animals were then euthanized and whole colons removed, fixed and stained for calcitonin-gene-related-peptide (CGRP). Single spinal afferent nerve axons were identified entering the distal colon that ramified along many rows of myenteric ganglia, often giving rise to varicose nerve endings. These same axons bifurcated in the circular muscle giving rise to 4–5 groups of branching-type intramuscular endings, where each group of endings was separated by ~ 370 μm in the rostro-caudal axis and projected 1.2 mm around the circumference. As spinal afferent axons bifurcated, their axons often showed dramatic reductions in diameter. Here, we identified in the distal colon, the characteristics of nerve endings that arise from single colorectal-projecting axons with cell bodies in DRG. These findings suggest that a population of sensory neurons in DRG can potentially detect sensory stimuli simultaneously via different morphological types of endings that lie in both colonic smooth muscle and myenteric ganglia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is considerable interest in understanding how sensory stimuli from within the gastrointestinal (GI) tract are detected and relayed to the central nervous system (i.e., brain and spinal cord). The sensory nerve endings of the vagus nerve have been well characterized in the gut (Berthoud et al. 1997; Berthoud and Powley 1992; Harsanyiova et al. 2019; Powley et al. 2016; Powley and Phillips 2011; Powley et al. 2011; Ratcliffe et al. 2011; Serlin and Fox 2019). This is not the case for spinal afferents, which provide the major, or sole extrinsic afferent innervation to the lower GI tract and other visceral organs (Brierley et al. 2018; Brookes et al. 2013). The reason why spinal afferent endings have been so poorly understood is because it has been difficult to selectively label only the spinal afferents.
It is well accepted that most sensation from the lower GI tract is carried by spinal afferents, not vagal afferents (Brierley et al. 2018). This knowledge is based largely on lesion experiments where pain reflex responses, such as the visceromotor response (VMR) are abolished by lesioning spinal afferents to the lower visceral organs, such as the colon, when preserving the vagal afferent supply (Kyloh et al. 2011; Traub 2000). In the lower GI tract, the detection of noxious and innocuous sensory stimuli into nerve action potentials is encoded predominantly, if not exclusively, by spinal afferent neurons, whose cell bodies lie in dorsal root ganglia (DRG) (Gebhart and Bielefeldt 2016; Kyloh et al. 2011; Traub 2000; Zagorodnyuk et al. 2011). Although it is well known that the cell bodies of spinal afferents reside in DRG, understanding where the nerve endings of spinal afferent innervate has been much more challenging. This is because unlike vagal sensory ganglia, DRG are difficult to expose as a survival surgery. Only recently has a survival surgery technique been developed to selectively identify the nerve endings of spinal afferents in visceral organs (Kyloh and Spencer 2014; Spencer et al. 2014). Using this technique, an unexpectedly complex diversity of nerve endings was uncovered in the distal colon, which showed spinal afferent-innervated multiple anatomical layers, including the muscle layers, myenteric ganglia, submucosa, submucosal ganglia and mucosa (Spencer et al. 2014). Anterograde tracing from DRG in vivo has also recently been used to identify the nerve endings of spinal afferents in the stomach (Spencer et al. 2016a) and bladder (Spencer et al. 2018). Compared to the colon, there was noticeably less diversity in the different types of spinal afferent endings in these organs (Spencer et al. 2016b). The reasons for this are unknown. While different morphological types of spinal afferent endings have been identified in these visceral organs, only recently has it become clear that single neurons in DRG can give rise to different morphological types of endings (Spencer et al. 2020). A recent study showed that single non-peptidergic DRG neurons could innervate the distal colon and provide sites of innervation to the myenteric plexus, submucosa and crypts in the mucosa, while another class of neuron was identified that was peptidergic and innervated the circular muscle, myenteric plexus and submucosa (Spencer et al. 2020).
The aim of the study is to identify novel populations of nerve endings arising from single colorectal-projecting DRG neurons. To do this, we injected minute quantities of neuronal tracer unilaterally into single DRG at either L6 or S1, to minimize the number of axons labelled. Using this approach, we were able to identify in a small cohort of animals (about 7% of animals) the location and morphological types of nerve endings that emanated from a single spinal afferent axon innervating the distal colon. Here, we reveal a single population of a peptidergic colorectal-projecting afferent axon that can be described as a “myenteric-muscular” ending. This type of nerve ending is potentially capable of detecting and transmitting sensory stimuli from both the circular muscle and myenteric ganglia at the same time to the central nervous system (CNS).
Methods
Mice of the C57BL/6 strain (30–60 days old) obtained from the College of Medicine and Public Health Animal Facility (Flinders University of South Australia) were anaesthetised by isoflurane inhalation and an incision along the dorsal surface was made approximately 10 mm in the rostro-caudal axis. Lumbosacral (L6 or S1) DRG were exposed unilaterally and biotinylated dextran biotin (10–20%; Cat #D1956; Molecular Probes, Eugene, OR, USA) was injected (~ 50 nL) using glass micropipettes (inner diameter ~ 5 μm; Cat #TW150-4; World Precision Instruments, Sarasota, FL, USA). Micropipettes were advanced into L6 or S1 DRG using a micromanipulator (#M-4001002; Narishige, Setagaya-ku, Tokyo, Japan). Each DRG was injected using a custom-made nitrogen-driven spritz system (Biomedical Engineering, Flinders University of South Australia) that applied pulses of nitrogen to the pipette for 1 s duration at 0.3 Hz, 10–15 psi, for periods of 5–10 min. Following injection of DRGs, the skeletal muscle adjacent to the vertebral column was sutured, then the skin closed using 7-mm wound clips (Cat #12032-07; Fine Science Tools, North Vancouver, British Columbia, Canada). Animals were given a period of 7 days to recover, at which point they were euthanized by isoflurane overdose. A midline laparotomy was made and the full-length colon cut along the mesentery, then pinned mucosal side uppermost, as a sheet preparation, in phosphate-buffered saline (PBS). The whole colon preparation was then fixed for 4–6 h in 4% paraformaldehyde. Preparations were then washed in dimethyl sulfoxide (DMSO) for 3 × 10-min washes, immersed for 3 h in Cy3-conjugated Streptavidin (1:400 dilution from neat; Cat #016-160-084; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA), then incubated in primary antibody (rabbit anti-CGRP; 1:2000 dilution from neat; Cat #T-4032; Peninsula Laboratories International Inc., San Carlos, CA, USA) for 2 days. The whole colon was then washed (3 × 10 min in PBS) followed by incubation in secondary antibody (donkey anti-rabbit Cy5; Cat #711–175-152; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) at 1:200 dilution (from neat) for 2 h. Preparations were then washed again (3 × 10 min in PBS) and mounted, serosal side uppermost, on glass slides. Slides were viewed with an Olympus IX71 epifluorescent microscope (Shinjuku-ku, Tokyo, Japan) using appropriate laser wavelengths and images captured with a CoolSNAP™ camera (Roper Scientific, Tucson, AZ, USA) and AnalySIS Image 5.0 computer software (Olympus-SIS, Münster, Germany). The characteristics of spinal afferent endings, including their axons, were analysed using Image J software (National Institutes of Health, Bethesda, MD, USA). The use of “n” in Table 1 and the “Results” refers to the number of observations that were made and “N” refers to the number of animals that were studied. All procedures were approved by the Animal Welfare Committee of Flinders University of South Australia (approvals #784/11 and #861/13) and were performed in strict accordance with the National Health and Medical Research Council (NH&MRC) Australian code for the care and use of animals for scientific purposes (eighth edition, 2013) and recommendations from the NH&MRC Guidelines to promote the wellbeing of animals used for scientific purposes (2008).
Classification of different morphological types of spinal afferent nerve ending
The different morphological types of endings were classified according to the same scheme that was described in Spencer et al. (2014). In brief, “simple-type” endings were readily identified by having single unbranched terminating axons, or single axons that branched into single axons. “Branching-type” endings comprise multiple branching axons that lay parallel to one another in a circumferential axis around the colon. In a myenteric ganglion, intraganglionic varicose endings (IGVEs) consisted of axons that ramified around neurons within any given myenteric ganglion and had varicose terminal endings.
Results
In 81 mice, ~ 50 nL of dextran biotin was injected into single DRG (either L6 or S1 unilateral) and mice allowed to recover post-surgery for 7 days. When whole colons were visualized after fixation and staining, the vast majority of preparations showed multiple anterogradely labelled axons and endings in the distal colon that entered from different locations and hence arose from multiple different neurons in DRG. However, in only six of the total cohort of mice studied was it possible to identify single spinal afferent axons entering the distal colon and follow the full course of these axons to their nerve endings without interference from other labelled neighbouring axons in the field of view. Hence, we describe here the characteristics of at least one class of a peptidergic colorectal-projecting DRG sensory axon that innervates the terminal ~ 20 mm of distal colon and innervates both the circular muscle layer and the myenteric plexus.
Single axons with branching-type nerve endings in the circular muscle layer
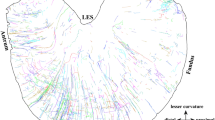
After entering the colon, single axons were found to ramify through many rows of myenteric ganglia and give off collateral bifurcations into neighbouring myenteric ganglia (Figs.1, 2). In myenteric ganglia, the nerve endings were varicose in nature and these have been described previously as IGVEs (Fig. 2a) (Spencer et al. 2014). Interestingly, the single spinal afferent axons that gave rise to the IGVEs also gave rise to another different morphological type of nerve ending in the circular muscle. These endings usually ramified parallel to the circular muscle (Figs. 1c, d and 2d) and were previously described as branching-type endings (Spencer et al. 2014); analogous to the intramuscular arrays described for vagal afferent endings in the stomach (Powley et al. 2016). However, in some animals, not all intramuscular axons ran parallel to the muscle fibres. For example, in Figs. 1(d) and 3 many of the intramuscular axons lay orthogonal to the circular muscle layer.
Characteristics of a single spinal afferent axon that innervates the myenteric plexus and circular muscle. a shows a single-parent axon on the left-hand side of the image that has two major bifurcations. The box labelled b is shown in expanded scale in panel b. Panel b shows that the axon diameter decreases substantially (see arrow) before it branches further into two distinct axon trajectories. The nerve ending shown in the upper part of b lies in a myenteric ganglion. This is demonstrated at higher magnification in Fig. 2(a). The axon indicated by the arrow in b was found to innervate a single myenteric ganglion and the circular muscle layer. The nerve ending in box c in panel a is shown in expanded scale in panel c. This axon innervates the circular muscle. The nerve ending in d of panel a is shown in expanded scale in panel d. The box labelled e in panel a is shown in expanded scale in panel e. The major decrease in diameter from the parent axon in e is shown as it bifurcates into a much finer axon, which ends in the circular muscle (shown in panel d)
CGRP immunoreactivity of nerve endings shown in Fig. 1. a shows a varicose spinal afferent ending in a myenteric ganglion. b shows the CGRP-immunoreactivity of the image in a. c superimposed image of b and c. d shows the intramuscular ending in the circular muscle layer with varicose terminal endings. This ending arises from a bifurcating axon that is shown in box b in panel a. Panel e shows the CGRP-immunoreactivity of the image in d. f shows the superimposed image of d and e. The arrows indicate this nerve ending is CGRP-immunoreactive and hence, peptidergic
Characteristics of intramuscular spinal afferent endings in the circular muscle. a shows a single axon that bifurcates into the circular muscle encompassing a spatial field of approximately 800 μm in the rostro-caudal axis and 700 μm in the circumferential axis. Numerous fine varicose endings run both parallel to the alignment of the circular muscle cells and some axons lie in the rostro-caudal axis. In panel a, the region indicated by the arrow b is shown in expanded scale in b. The region indicated in c is shown in expanded scale in c and f. Panel d shows an expanded scale of region labelled d in panel a. Panel e shows expanded scale of region e in panel a
Single spinal afferent axons classified as branching-type endings in the circular muscle consisted of a mean of 4.5 ± 2.4 (n = 17; N = 6) discrete groups of endings (Figs. 4, 5) (Table 1). Each group of branching-type intramuscular ending in the circular muscle was separated from other groups of branching-type endings that arose from the same axon, by a mean distance of 372 ± 72 μm (n = 72; N = 6) (Table 1). Each group of intramuscular branching-type ending in the circular layer consisted of a mean of 15 ± 1.4 axons (n = 24; N = 6), where the axon terminal diameter was 1.6 ± 0.03 μm (n = 72; N = 6) (Table 1). One unexpected finding of this study was the rapid changes in diameter of single spinal afferent axons between their terminal endings and their point of entry (e.g., see arrow in Fig. 1b, e). The arrow in these figures indicates how dramatically that axon diameter can change at only one bifurcation point. In Fig. 6(b), the sudden changes in axon terminal diameters is apparent. In this example, the parent axon is 1.7 μm. This then bifurcates into intramuscular axons in the circular muscle that vary from 0.9 to 1.4 μm (Fig. 6b).
Multiple branching-type intramuscular endings in the circular muscle originate from a single parent axon. a shows the groups of intramuscular endings in the circular muscle from a single axon. b shows the branching-type endings run parallel to the circular muscle (expanded from the region indicated b in panel a). c shows an expanded segment from the region indicated by arrow c in panel a
Nerve endings in the circular muscle and myenteric ganglion that arise from a single colorectal projecting peptidergic spinal afferent neuron with cell body in L6 or S1 DRG. a shows a single axon that traverses about 3.5 mm caudally after entering the colon. There are three discrete intramuscular branching-type endings in the circular muscle that arise from this parent axon, indicated by arrows b and c. The regions indicated by arrows b and c are shown on expanded scale in panels b and c, respectively. Panel d shows a higher magnification of part of the image shown in panel c, where the bifurcation occurs. Panel e shows the terminal ending in a myenteric ganglion. This ending is shown in higher magnification in Fig. 7
Changes in diameter of a single spinal afferent axon as it passes through rows of myenteric ganglia and has side branches that innervate the circular muscle. a the axon is approximately 1.6 μm until it reaches the myenteric ganglion it terminates in, where the axon diameter is reduced by about half. The box in panel a is shown in expanded scale in panel b. In b, the parent axon that runs along the myenteric ganglia is up to approximately twice the diameter as the fine varicose–branched endings in the circular muscle layer. This shows that axon terminal diameters are inconsistent and change rapidly as axons bifurcate
The total mean surface area covered by multiple discrete intramuscular endings in the circular muscle that all arose from a single-parent axon was 1240 ± 240 μm (N = 6) around the circumferential axis and 1083 ± 211 μm (N = 6) in the rostro-caudal axis (Figs. 4, 5; Table 1). When analysis was made of single groups of intramuscular branching-type endings in the circular muscle layer, it was found that they projected a mean distance of 259 ± 59 μm in the rosto-caudal axis and 572 ± 82 μm circumferentially around the colon (n = 17; N = 6). However, we did visualize single axons that projected around 2.5 mm caudally and only had a maximum of three groups of intramuscular branched endings (Fig. 5). In three of six animals tested, the single axons that gave rise to branching-type endings in the circular muscle and IGVEs in myenteric ganglia were found to be CGRP-immunoreactive (Figs. 2, 7).
CGRP immunoreactivity of the myenteric endings of colorectal-projecting spinal afferents that are shown in Fig. 5(e). a shows the myenteric varicose nerve ending that originates from a single axon in Fig. 5(a). This single axon enters the myenteric ganglion and branches in fine varicose terminals. b shows the CGRP immunoreactivity of the image in panel a. The single varicosities indicated by arrows in panel ai are shown also by the arrows in bi. This comparison reveals these varicosities are CGRP immunoreactive. c shows a superimposed image of panels a and b. ci shows a superimposed image of panels ai and bi
Discussion
In this study, we identified the nerve endings that arise from single spinal afferent neurons in DRG that innervate the distal colon of mice. In only ~ 7% of animals injected with dextran biotin into single-DRG was it possible to follow the course of a single axon in the distal colon to its endings, without visual interference from other labelled axons (from other neurons). In this small cohort of animals, we had the unique opportunity to characterize the trajectory of single peptidergic axons after their entry site in the distal colon and determine the location and morphologies of the nerve endings. A major observation of this study is the identification that single peptidergic spinal afferent neurons that enter the distal colon can give rise to axons that innervate the myenteric ganglia and circular muscle, with two different morphological types of endings (Fig. 8). This is the first time that a single sensory neuron in DRG has been shown to innervate the myenteric ganglia and the circular muscle layer, without any other detectable collateral endings. Whether multiple types of peptidergic sensory neurons in DRG (i.e., different sizes and neurochemical classes) can also give rise to the single peptidergic axons identified here that give off the same types of nerve endings (i.e., myenteric ganglia and circular muscle) remains unknown. There is evidence that spinal afferent neurons can exert an efferent function in visceral organs (Bartho et al. 2008). It is possible that in addition to serving an afferent function, some of the nerve endings of spinal afferents identified here in the myenteric ganglia or circular muscle could exert an efferent role.
Diagrammatic representation of the location of spinal afferent nerve endings innervating the mouse distal colon that arises from one major class of peptidergic colon-projecting DRG neuron. The findings of this study reveal one major class of peptidergic sensory neuron that was found to innervate the myenteric ganglia (MG) and circular muscle (CM). SC, spinal cord; DRG, dorsal root ganglia, LM: longitudinal muscle; SMG, submucosal ganglia; SM, submucosa; M, mucosa
The current study confirms earlier reports that branching-type intramuscular endings exist in the circular muscle of mouse colon (Kyloh and Spencer 2014; Spencer et al. 2014). The findings here also reveal that a single-parent axon can give rise to multiple branching-type endings in the circular muscle (typically 4–5 groups of branched-type endings) that were separated in the rostro-caudal axis by ~ 370 μm. Each branching-type ending consisted of a single-parent axon that branched into a mean of 15 axons that ran parallel to the circular muscle fibres around the circumference of the distal colon. Fine varicosities were common on their axon terminal endings. The most similar analogy to the intramuscular circular muscle endings is the intramuscular arrays that arise from vagal sensory ganglia (Fox et al. 2000; Powley et al. 2015; Powley and Phillips 2002). It is tempting to speculate that the functional role of branching-type endings in the mouse distal colon is to encode low-threshold wide-dynamic range levels of circumferential stretch (Spencer et al. 2008; Zagorodnyuk et al. 2011). This is based on the knowledge that 91% of CGRP-immunoreactive rectal afferent axons in the mouse colorectum express the capsaicin receptor, TRPV1 (Sharrad et al. 2015) and are largely activated by low thresholds while also being potently activated by capsaicin (Spencer et al. 2008). However, this notion requires further investigation.
A recent anterograde tracing study from DRG in mice identified that single peptidergic spinal afferent neurons could innervate the distal colon with nerve endings in the circular muscle, myenteric plexus and submucosa (Spencer et al. 2020). Another population of non-peptidergic neurons innervated the mucosal crypts, myenteric ganglia and submucosa (Spencer et al. 2020). How the function of these neurons differs from those identified here in the current study remains unclear. Other studies have shown from direct intracellular electrophysiological recordings from peptidergic spinal afferent neurons (in DRG) that innervate the mouse distal colon have different firing properties in response to colorectal distension (Hibberd et al. 2016). It is known that a large population of peptideric spinal afferent neurons that project to the distal colon are low threshold, wide dynamic range mechanosensory afferents (Hibberd et al. 2016).
Characteristics of single spinal afferent neurons with nerve endings in the circular muscle and myenteric ganglia
A notable feature of spinal afferent axons was that they lacked varicosities as they entered the colon. Once they passed through the serosal layer, single axons commonly weaved through rows of myenteric ganglia, at which point they usually developed varicosities. As these axons passed through many rows of myenteric ganglia, they showed little circumferential displacement. In some cases, these axons had terminal endings in myenteric ganglia that were varicose, known as IGVEs (Spencer et al. 2014). These axons then bifurcated and developed, as mentioned, an average of 4–5 branching type endings in the circular muscle. Many branching-type endings ran parallel to the circular muscle, while others did not. Why there are inconsistencies in the axon projections is unclear—perhaps these axons respond to elongation and circumferential length.
Characteristics of spinal afferent axons in the colon
A surprising observation of this study was the sudden changes in axon terminal diameters, as axons bifurcated. For example, in Figs. 1(b,e) and 6(b), there are major reductions in axon diameter as axons branch into the circular muscle layer. It is unclear why the diameters of spinal afferents changed so rapidly. If the conduction velocity in these regions is directly proportion to axon diameter, then one would expect action potential velocities to be much slower near the sites of sensory transduction, then speed up considerably as they enter the dorsal horn of the spinal cord.
Conclusion
The current study shows that a population of peptidergic spinal afferent neurons that innervates the mouse distal colon have single axons that bifurcate many times, giving off varicose endings in myenteric ganglia (IGVE) and a different morphological type of ending (intramuscular branching-type ending) in the circular muscle. In this regard, we consider this type of spinal afferent to be a “myenteric-muscular” ending. The sensory stimuli these types of endings respond to await further investigation.
References
Bartho L, Benko R, Holzer-Petsche U, Holzer P, Undi S, Wolf M (2008) Role of extrinsic afferent neurons in gastrointestinal motility. Eur Rev Med Pharmacol Sci 12(Suppl 1):21–31
Berthoud HR, Powley TL (1992) Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol 319:261–276
Berthoud HR, Patterson LM, Neumann F, Neuhuber WL (1997) Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 195:183–191
Brierley SM, Hibberd TJ, Spencer NJ (2018) Spinal afferent innervation of the colon and rectum. Front Cell Neurosci 12:467
Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP (2013) Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol 10:286–296
Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL (2000) Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J Comp Neurol 428:558–576
Gebhart GF, Bielefeldt K (2016) Physiology of visceral pain. Compr Physiol 6:1609–1633
Harsanyiova J, Ru F, Zatko T, Kollarik M, Hennel M (2019) Vagus nerves provide a robust afferent innervation of the mucosa throughout the body of the esophagus in the mouse. Dysphagia. https://doi.org/10.1007/s00455-019-10051-8
Hibberd TJ, Kestell GR, Kyloh MA, Brookes SJ, Wattchow DA, Spencer NJ (2016) Identification of different functional types of spinal afferent neurons innervating the mouse large intestine using a novel CGRPalpha transgenic reporter mouse. Am J Physiol Gastrointest Liver Physiol. https://doi.org/10.1152/ajpgi.00462.2015
Kyloh M, Spencer NJ (2014) A novel anterograde neuronal tracing technique to selectively label spinal afferent nerve endings that encode noxious and innocuous stimuli in visceral organs. Neurogastroenterol Motil 26:440–444
Kyloh M, Nicholas S, Zagorodnyuk VP, Brookes SJ, Spencer NJ (2011) Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Front Neurosci 5:16
Powley TL, Phillips RJ (2002) Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes? I. Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol Gastrointest Liver Physiol 283:G1217–G1225
Powley TL, Phillips RJ (2011) Vagal intramuscular array afferents form complexes with interstitial cells of Cajal in gastrointestinal smooth muscle: analogues of muscle spindle organs? Neuroscience 186:188–200
Powley TL, Spaulding RA, Haglof SA (2011) Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol 519:644–660
Powley TL, Hudson CN, McAdams JL, Baronowsky EA, Phillips RJ (2015) Vagal intramuscular arrays: the specialized mechanoreceptor arbors that innervate the smooth muscle layers of the stomach examined in the rat. J Comp Neurol
Powley TL, Hudson CN, McAdams JL, Baronowsky EA, Phillips RJ (2016) Vagal intramuscular arrays: the specialized mechanoreceptor arbors that innervate the smooth muscle layers of the stomach examined in the rat. J Comp Neurol 524:713–737
Ratcliffe EM, Farrar NR, Fox EA (2011) Development of the vagal innervation of the gut: steering the wandering nerve. Neurogastroenterol Motil 23:898–911
Serlin HK, Fox EA (2019) Abdominal vagotomy reveals majority of small intestinal mucosal afferents labeled in nav 1.8cre-rosa26tdTomato mice are vagal in origin. J Comp Neurol
Sharrad DF, Hibberd TJ, Kyloh MA, Brookes SJ, Spencer NJ (2015) Quantitative immunohistochemical co-localization of TRPV1 and CGRP in varicose axons of the murine oesophagus, stomach and colorectum. Neurosci Lett 599:164–171
Spencer NJ, Kerrin A, Singer CA, Hennig GW, Gerthoffer WT, McDonnell O (2008) Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience 153:518–534
Spencer NJ, Kyloh M, Duffield M (2014) Identification of different types of spinal afferent nerve endings that encode noxious and innocuous stimuli in the large intestine using a novel anterograde tracing technique. PLoS One 9:e112466
Spencer NJ, Kyloh M, Beckett EA, Brookes S, Hibberd T (2016a) Different types of spinal afferent nerve endings in stomach and esophagus identified by anterograde tracing from dorsal root ganglia. J Comp Neurol 524:3064–3083
Spencer NJ, Zagorodnyuk V, Brookes SJ, Hibberd T (2016b) Spinal afferent nerve endings in visceral organs: recent advances. Am J Physiol Gastrointest Liver Physiol 311:G1056–G1063
Spencer NJ, Greenheigh S, Kyloh M, Hibberd TJ, Sharma H, Grundy L, Brierley SM, Harrington AM, Beckett EA, Brookes SJ, Zagorodnyuk VP (2018) Identifying unique subtypes of spinal afferent nerve endings within the urinary bladder of mice. J Comp Neurol 526:707–720
Spencer NJ, Kyloh MA, Travis L, Dodds KN (2020) Identification of spinal afferent nerve endings in the colonic mucosa and submucosa that communicate directly with the spinal cord: the gut-brain axis. J Comp Neurol
Traub RJ (2000) Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. Neuroreport 11:2113–2116
Zagorodnyuk VP, Kyloh M, Nicholas S, Peiris H, Brookes SJ, Chen BN, Spencer NJ (2011) Loss of visceral pain following colorectal distension in an endothelin-3 deficient mouse model of Hirschsprung's disease. J Physiol 589:1691–1706
Funding
Experiments in this study were supported by an NHMRC Project grant #1156416 to NJS and Australian Research Council (ARC) grant #DP190103628 NJS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Spencer, N.J., Kyloh, M.A., Travis, L. et al. Sensory nerve endings arising from single spinal afferent neurons that innervate both circular muscle and myenteric ganglia in mouse colon: colon-brain axis. Cell Tissue Res 381, 25–34 (2020). https://doi.org/10.1007/s00441-020-03192-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03192-y