Abstract
Abnormal activation of Wnt signaling has been demonstrated in the wound healing process and the pathogenesis of fibrotic disorders, with Wnt4 specifically identified as having a key role in the pathogenesis of renal, pulmonary and liver fibrosis. Wnt4 also was found to be upregulated by transforming growth factor-β1 (TGF-β1) in fetal and postnatal murine fibroblasts and bone marrow mesenchymal cells, suggesting an underlying cooperation between Wnt4 and TGF-β1 in fibrosis. However, the specific roles of Wnt4 in TGF-β1-induced skin myofibroblast transition and hypertrophic scar formation remain unclear. In the present study, we first observed reduced Wnt4 expression in hypertrophic scar tissue compared with that in normal skin tissue. Following upregulation by TGF-β1, Wnt4 inhibited the TGF-β1-induced transdifferentiation of fibroblasts into myofibroblasts. Using fibroblast-populated collagen lattice contraction assays, we showed that the increased contractility induced by TGF-β1 was significantly blocked by exogenous Wnt4 and the α-smooth muscle actin (α-SMA) expression was decreased in fibroblasts in the collagen lattices. In addition, knockdown of Wnt4 resulted in further increases in α-SMA and collagen I expressions. Further investigation showed that Wnt4 could inhibit the autocrine effect of TGF-β1 as well as block the phosphorylation of Smad3 and ERK but not of AKT or JNK. Lastly, using hypertrophic scar–derived fibroblasts, we showed that the elevated α-SMA and collagen I levels were markedly reduced after treatment with Wnt4. Taken together, our results suggest that Wnt4 negatively regulates TGF-β1-induced fibroblast activation, which may represent a novel therapeutic strategy for the treatment and prevention of hypertrophic scars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertrophic scar tissue, which typically develops after severe burn and/or traumatic injury of the skin, is a fibrotic disorder of cutaneous wound healing that manifests as abnormal deposition of extracellular matrix (ECM) proteins and contraction of the granulation tissue, which leads to impaired appearance of the skin as well as function problems (Finnerty et al. 2016). Despite the extensive research that has been carried out, the exact mechanisms underlying hypertrophic scar formation remain incompletely understood and accordingly, the therapeutic methods also are not satisfactory (Friedstat and Hultman 2014; Gold et al. 2014; Hutchenreuther and Leask 2016; Zhang et al. 2017).
Myofibroblasts, which originate largely from transdifferentiation of fibroblasts, have been reported to play a central role during hypertrophic scar formation (Diegelmann and Evans 2004; Tomasek et al. 2002). Although fibroblasts are present in the relatively quiescent state of normal skin tissue, these cells undergo a phenotype change into myofibroblasts in response to skin injury. The most widely accepted molecule marker of myofibroblasts is upregulated α-smooth muscle actin (α-SMA). With their increased ECM protein synthesis and increased contractile activity, myofibroblasts can facilitate wound healing and tissue repair (Diegelmann and Evans 2004; Hutchenreuther and Leask 2016; Tomasek et al. 2002). The myofibroblasts are removed via apoptosis once the normal wound healing process is completed and their persistence leads to the excessive synthesis and deposition of collagen proteins, which contribute to fibrotic disorders, such as hypertrophic scar, liver fibrosis, renal fibrosis and lung fibrosis (Lemoinne et al. 2016; Song et al. 2016; Sun et al. 2016; Thannickal 2012; Yazdani et al. 2017; Zhou et al. 2013). Thus, blockade of the fibroblast-to-myofibroblast transition has become an important anti-fibrosis strategy (Reddy et al. 2014; Rezvani et al. 2016; Yazdani et al. 2017).
Although the exact mechanisms remain unknown, current evidence indicates that transforming growth factor-β1 (TGF-β1) plays a crucial role in the transition of fibroblasts into myofibroblasts (Mia and Bank 2016; Scharenberg et al. 2014; Tomasek et al. 2002; Wang et al. 2016a). The signaling downstream of TGF-β1 is mainly mediated by the Smad family of proteins (Cutroneo 2007). Much research has shown upregulated expression levels of TGF-β1, TGF-β receptors and Smad proteins in hypertrophic scars and organ fibrosis, further indicating the important role of TGF-β/Smad signaling in the pathogenesis of fibrotic disorders and making this pathway a potential therapeutic target (Cutroneo 2007; Hsu et al. 2010; Wu et al. 2012). Moreover, recent studies have found that TGF-β1 cooperates with Wnt signaling in regulating various biological and pathological processes (Castellone and Laukkanen 2017; George 2009; Liu et al. 2012).
Multiple studies have demonstrated the involvement of abnormal activation of Wnt signaling and excessive expression of Wnt proteins in wound healing and fibrotic disorders (Guo et al. 2012; Wei et al. 2011), with Wnt4 specifically implicated in the pathogenesis of organ fibrosis, including that of the kidneys, lungs and liver (Chen et al. 2016; Li et al. 2017; Rajasekaran et al. 2017). In the skin of mice, the Wnt4 expression is higher in fetal skin than in postnatal skin and Wnt4-null skin exhibits dermal fibroplasias, suggesting a possible anti-fibrotic role of Wnt4 (Saitoh et al. 1998). In contrast, increased Wnt4 expression is observed in the early stages of wound healing in postnatal mice and in murine fibroblasts after induction of injury by fibrin degradation products, as well as in bone marrow mesenchymal cells stimulated with TGF-β1, suggesting a possible pro-fibrotic role for Wnt4 (Labus et al. 1998). More recently, Wnt4 was shown to be upregulated by TGF-β1 in fetal and postnatal murine fibroblasts (Colwell et al. 2006), indicating the underlying cooperation between TGF-β1 and Wnt4 in the regulation of fibroblast activation. However, the exact effects of Wnt4 on the TGF-β1-induced transition of fibroblasts into myofibroblasts as well as in scar formation remain poorly understood.
In the present study, we investigate the effects of Wnt4 on the TGF-β1-induced phenotype change of fibroblasts into myofibroblasts, as well as the potential underlying mechanism. Our results show that Wnt4 negatively regulates the TGF-β1-induced fibroblast activation, which may have been, in part, mediated by inhibition of the autocrine function of TGF-β1 and blocking of Smad3 and ERK phosphorylation. Therefore, our findings indicate that Wnt4 may play a crucial role in fibroblast activation and the pathogenesis of hypertrophic scar formation, providing insight into a potential therapeutic target for preventing such scarring.
Materials and methods
Materials
Recombinant human TGF-β1 was obtained from PeproTech (London, UK), and human recombinant Wnt4 was obtained from R&D (Minneapolis, MN, USA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco (Grand Island, NY, USA) and TRIzol Reagent was obtained from Invitrogen (Carlsbad, CA, USA). Rabbit polyclonal antibody against collagen (Col) type I was purchased from Abcam (Cambridge, MA, USA). Mouse monoclonal antibody against α-SMA, rabbit polyclonal antibody against β-actin and DAPI (4′,6-diamidino-2-phenylindole) were obtained from Boster Biological Technology Co. (Boster, Wuhan, China). Rabbit polyclonal antibodies against β-catenin, JNK, ERK1/2, p38 MAPK, phospho-JNK, phospho-ERK1/2 and phospho-p38MAPK were obtained from Cell Signaling Technology Inc. (Beverly, MA, USA). Anti-mouse IgG-Cy3 antibody was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Polyvinylidene fluoride (PVDF) membranes and Immobilon Western Chemiluminescent HRP Substrate were purchased from the Millipore Corp (Bedford, MA, USA). The TGF-β1 enzyme-linked immunosorbent assay (ELISA) kit was purchased from Neobioscience (Shenzhen, China).
Cell culture
Hypertrophic scar and normal skin tissues were obtained from patients undergoing plastic surgery in our department with their written informed consent. Each sample was divided into two portions for cell culture and RNA extraction. Fibroblasts were isolated and cultured as previously reported (Liu et al. 2012). Briefly, dermal parts were minced and incubated in a solution of collagenase type I (0.1 mg/mL) at 37 °C for 3 h to separate the fibroblasts. The fibroblasts were culture-pelleted and grown in DMEM supplemented with 10% fetal calf serum (Gibco), 100 U/mL penicillin and 100 U/mL streptomycin at 37 °C in a 5% (v/v) CO2 humidified atmosphere. Cells from passages 3–5 were used for subsequent experiments. For stimulation of the transition to myofibroblasts, upon reaching 80–90% confluence in 60-mm dishes, fibroblasts were starved for 12 h in serum-depleted medium and then stimulated with TGF-β1 (10 ng/mL), Wnt4 (10 ng/mL), or both at indicated concentrations.
RNA isolation and real-time PCR
Total RNA from scar and normal skin tissues, cultured cells and collagen lattices was extracted using TRIzol Reagent according to the manufacturer’s instruction. Then, 500 ng of total RNA was used to synthesize cDNA. Real-time polymerase chain reaction (PCR) was performed using the Bio-Rad IQ5 Real-Time System (Bio-Rad, Hercules, CA, USA) with the SYBR® Premix Ex Taq™ II kit (TaKaRa, Dalian, China). The PCR cycling conditions were as follows: initial denaturation at 95 °C for 30 s, followed by 40 cycles with denaturation at 95 °C for 10 s, annealing at 60 °C for 10 s and elongation at 72 °C for 15 s. The expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was measured and used to normalize the expression levels of other mRNAs. All PCR experiments were performed in triplicate. The primer pairs used are listed in Supplementary Table 1.
Western blotting
For analysis of protein expression by western blotting, 40 μg of total protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes. After blocking with 5% nonfat milk, the membranes were incubated with antibodies for rabbit anti-human Col I (dilution, 1:400), β-catenin (1:800), JNK (1:1000), ERK1/2 (1:1000), p38 (1:1000), phosphorylated (p)-JNK (1:1000), pERK1/2 (1:2000), p-p38 (1:1000), β-actin (1:400), or mouse anti-human α-SMA (1:400) overnight at 4 °C. Horseradish peroxidase–conjugated goat anti-rabbit IgG (1:3000) or goat anti-mouse IgG (1:3000) was used as the secondary antibody. Proteins were visualized with an enhanced chemiluminescence system using FluorChem FC (Alpha Innotech) and the densities of the bands were analyzed with the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunocytochemistry
Fibroblast samples were fixed with 90% ethanol and 1 μL/mL Triton X-100 was used to permeabilize the membranes. After blocking with 3% bovine serum albumin at 37 °C for 30 min, the samples were incubated with monoclonal mouse anti-human α-SMA antibody (1:100) at 4 °C overnight. The anti-mouse IgG-Cy3 antibody (1:1000) was used as the secondary antibody and DAPI was used for nuclear staining. Fluorescence within the cells was visualized under a fluorescence microscope (Olympus IX71, Tokyo, Japan) and analyzed by ImageJ software.
Fibroblast-populated collagen lattice contraction assays
Rat tail tendons were obtained and type I collagen was extracted as previously described (Bell et al. 1979; Liu et al. 2012; Shi et al. 2013). A 500-μL suspension containing 1 × 105 cells, 1 mg/mL collagen, TGF-β1 (10 ng/mL) and Wnt4 (20 ng/mL) was added in triplicate into 24-well plates and then incubated at 37 °C for 1 h to allow the mixture to gel. One milliliter of serum-free DMEM supplemented with TGF-β1 (10 ng/mL), Wnt4 (20 ng/mL), or both was added gently. After incubation at 37 °C for 24 h, the gels were detached mechanically from the side of the wells. Images were captured at 0, 4, 8, 12, 24 and 48 h after release. The surface area of each gel at each time point was measured using the ImageJ software and normalized to the original surface area.
Construction and infection of Wnt4 siRNA Lentivirus
The siRNA Lentivirus for Wnt4 was constructed by Shanghai GenePharma Co., Ltd. (GenePharma, Shanghai, China). Upon reaching 60–70% confluence, the fibroblasts were infected with Lentivirus at a multiplicity of infection (MOI) of 20 according to the manufacturer’s instructions. TGF-β1 (10 ng/mL) was added 48 h after infection and total RNA or protein was extracted 48 h later.
Enzyme-linked immunosorbent assay
The fibroblasts were seeded into 96-well plates and stimulated with TGF-β1 (10 ng/mL) for 24 h. When they reached 80–90% confluence, they were serum-starved for 12 h. The culture medium was then exchanged with DMEM containing Wnt4 (20 ng/mL) or DMEM only for 24 h. The amount of TGF-β1 in the supernatant from the fibroblast samples was analyzed using a TGF-β1 ELISA kit, according to the manufacturer’s instructions. TGF-β1 concentrations were calculated from respective standard curves.
Statistical analysis
The experimental data are presented as mean ± standard error values. Student’s T test was used for the comparisons between two groups and analysis of variance (ANOVA) was used for multi-group comparisons. All statistical tests were performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) and differences for which P < 0.05 were considered statistically significant.
Results
Wnt4 expression is decreased in hypertrophic scars and fibroblasts derived from scar tissues
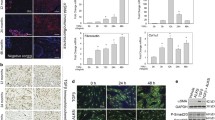
Six hypertrophic scar samples were obtained from patients for PCR analysis of the expressions of Col I, TGF-β1, and Wnt4 relative to levels in healthy skin. We observed that Col I and TGF-β1 expressions in hypertrophic scar tissues increased significantly compared with those in normal skin tissues (Fig. 1a, a′). The expression level of Wnt4 mRNA was examined in scar tissues as well as in scar tissue–derived fibroblasts. The Wnt4 mRNA expression in the hypertrophic scar tissue samples was lower than that in the normal skin tissue (Fig. 1a″) and consistently, the Wnt4 mRNA expression in hypertrophic scar–derived fibroblasts was lower than that in normal fibroblasts (Fig. 1a‴).
Wnt4 expression in hypertrophic scar tissue and during TGF-β1-induced fibroblast activation. The mRNA expression levels of Col I (a), TGF-β1 (a′) and Wnt4 (a″) and in six hypertrophic scar samples and normal skin tissue samples, as well as Wnt4 (a‴) in isolated fibroblasts, were measured by real-time PCR. (b) Protein expression levels of α-SMA and Col I in normal skin–derived fibroblasts stimulated with TGF-1, as determined by western blotting. (c–d″). Immunocytochemical analysis of the α-SMA expression in normal skin–derived fibroblasts after TGF-1 stimulation. Images show double-stained fibroblasts. Red: α-SMA; blue: nuclear staining with DAPI. Scale bar = 50 μm. (e) The mRNA expression of Wnt4 in normal skin–derived fibroblasts stimulated with TGF-1, as determined by real-time PCR. Results represent mean ± SEM (n = 3). *P < 0.05, **P < 0.01
Wnt4 is upregulated by TGF-β1 during fibroblast activation
To investigate the expression pattern of Wnt4 during the TGF-β1-induced transition of fibroblasts into myofibroblasts, fibroblasts isolated from normal skin were stimulated with TGF-β1 for 48 h. As demonstrated by the results of real-time PCR and western blotting in Fig. 1(b) and Supplementary Fig. 1, TGF-β1 stimulation led to significantly increased expression of α-SMA and Col I, as well as an increased number of α-SMA-positive fibroblasts on immunohistochemical staining and an increase in the corresponding optical density, indicating successful induction of fibroblast activation (Fig. 1c–d″). Moreover, the Wnt4 expression during the TGF-β1-induced fibroblast activation showed a rapid and significant increase with a peak value at 4 h (25.3-fold increased expression, P < 0.01) after TGF-β1 stimulation, followed by a decrease from 8 h (19.6-fold, P < 0.01) to 48 h, with the expression level remaining 5-fold greater than the basal level at 48 h (Fig. 1e).
Wnt4 negatively regulates the TGF-β1-induced fibroblast activation
To evaluate the effects of Wnt4 on the TGF-β1-induced fibroblast activation, the mRNA and protein expressions of α-SMA and Col I by fibroblasts after stimulation by Wnt4 at concentrations of 1, 10, 20, or 100 ng/mL in combination with TGF-β1 at 10 ng/mL were assessed. We observed that the increased mRNA and protein levels of α-SMA and Col I stimulated by TGF-β1 were downregulated significantly by Wnt4 in a dose-dependent manner (Fig. 2a, a′, b and Supplementary Fig. 2). In addition, immunocytochemical staining showed that the TGF-β1-induced increases in the number of α-SMA-positive fibroblasts and corresponding optical density was markedly blocked by Wnt4 application, indicating that Wnt4 has inhibitory effects on fibroblast activation (Fig. 2c–g′).
Regulation of TGF-β1-induced fibroblast activation by Wnt4. Effect of Wnt4 on TGF-β1-induced upregulation of α-SMA (a) and Col I (a′) mRNA, as determined by real-time PCR. (b) Effect of Wnt4 on TGF-β1-induced α-SMA and Col I proteins, as examined by western blotting. (c–f″) Immunocytochemical analysis of TGF-β1-induced α-SMA expression with Wnt4 treatment. Images show double-stained fibroblasts. Red: α-SMA; blue: nuclear staining with DAPI. Scale bar = 50 μm. (g–g″) Quantitative analysis of the optical density of α-SMA staining and the percentage of α-SMA-positive fibroblasts. Results represent mean ± SEM (n = 3). *P < 0.05 vs. control, **P < 0.01 vs. control, #P < 0.05 vs. TGF-β1-stimulated fibroblasts, ##P < 0.01 vs. TGF-β1-stimulated fibroblasts
Wnt4 inhibits TGF-β1-induced contractile activity of fibroblasts
Increased contractile ability is an important feature of myofibroblasts that is related to the α-SMA expression. Collagen lattice contraction assays were performed to evaluate the change in the contractility of fibroblasts when stimulated with TGF-β1 and Wnt4. As shown in Fig. 3(a, b), TGF-β1 stimulation led to a marked decrease in the size of the collagen lattices compared with the control group, indicating the increased contractility of fibroblasts embedded in collagen lattices after stimulation by TGF-β1. Conversely, Wnt4 treatment alone did not have an obvious effect on the size of the collagen lattices. However, with Wnt application, the decrease in the collagen lattice size induced by TGF-β1 was rescued. To further evaluate whether the change in fibroblast contractility was associated with the α-SMA expression, the α-SMA mRNA expression within the lattice samples was measured. As expected, the α-SMA expression by fibroblasts within the collagen lattices was significantly increased after TGF-β1 stimulation but downregulated by Wnt4 (Fig. 3c).
Inhibition of the TGF-β1-induced contractile activity of skin fibroblasts by Wnt4. (a) Effect of 20 mM Wnt4 on the contractility of fibroblasts in the basal state and after stimulation by TGF-β1. Representative images from triplicate experiments are shown. (b) Quantification of the contraction area of the collagen lattices. (c) The α-SMA mRNA expression in fibroblasts cultured in collagen lattices, as determined by real-time PCR. Results represent mean ± SEM (n = 3). **P < 0.01 vs. control, ##P < 0.01 vs. TGF-β1-stimulated fibroblasts
Knockdown of Wnt4 results in further increases in α-SMA and Col I expressions
To further elucidate the role of Wnt4 in TGF-β1-induced fibroblast activation, the effects of Wnt4 knockdown in fibroblasts on the mRNA and protein expressions of α-SMA and Col I were examined. As shown in Fig. 4 (a, a', b) and Supplementary Fig. 3, α-SMA and Col I expressions at both the mRNA and protein levels were increased to even higher levels after TGF-β1 stimulation of fibroblasts with Wnt4 knockdown compared with levels in normal fibroblasts after TGF-β1 stimulation. These results provide additional evidence that Wnt4 negatively regulates the TGF-β1-induced fibroblast activation.
Effects of Wnt4 knockdown on α-SMA and Col I expressions and effects of Wnt4 treatment on the autocrine loop of TGF-β1 and myofibroblast transition. mRNA expression levels of α-SMA (a) and Col I (a′) in normal skin–derived fibroblasts after knockdown of Wnt4, as determined by real-time PCR. (b) α-SMA and Col I protein expressions after knockdown of Wnt4, as determined by western blotting. α-SMA mRNA (c) and protein (c′) expressions after Wnt4 and TGF-β stimulation, as determined by real-time PCR and western blotting, respectively. TGF-β1 mRNA and protein expressions after Wnt4 and TGF-β stimulation, as determined by real-time PCR (d) and ELISA (d′), respectively. *P < 0.05 vs. control, **P < 0.01 vs. control, #P < 0.05 vs. TGF-β1-stimulated fibroblasts, ##P < 0.01 vs. TGF-β1-stimulated fibroblasts
Wnt4 inhibits the autocrine expression of TGF-β1 and reverses the phenotype change of fibroblasts
Previous research has shown that TGF-β1 can positively regulate its own expression via an autocrine or positive feedback loop, thereby enhancing fibroblast activation and aggravating fibrosis. To evaluate the effects of Wnt4 on the autocrine loop of TGF-β1, after TGF-β1 stimulation, fibroblasts were treated with Wnt4 or medium without Wnt4, and the mRNA and protein expression levels of α-SMA were analyzed. The results showed that the α-SMA expression, which was induced by TGF-β1 stimulation for 24 h, increased further with the removal of TGF-β1 and no addition of Wnt4. Interestingly, this increase in the α-SMA expression was blocked by Wnt4 treatment for 24 h. Moreover, application of Wnt4 for 48 h after 48 h of TGF-β1 stimulation reversed the increased expression of α-SMA induced by TGF-β1 (Fig. 4(c, c′) and Supplementary Fig. 3). The effects of Wnt4 on the TGF-β1 expression were also examined after the same treatment conditions. Both the TGF-β1 mRNA expression and the secreted TGF-β1 protein level showed obvious increases after TGF-β1 stimulation, which were significantly inhibited by Wnt4 (Fig. 4d, d′).
Wnt4 influences the phosphorylation of Smad3 and ERK but not of JNK or AKT
Because the pro-fibrotic effects induced by TGF-β1 have been reported to be mediated by Smads, ERK, JNK and AKT, we evaluated the effects of Wnt4 on the phosphorylation of Smad3, MAPKs and AKT. First, we observed that TGF-β1 stimulation led to a significant increase in p-Smad3 from 15 min with a peak level at 60 min (Supplementary Fig. 4) and similar results were observed for p-ERK and p-AKT. p-JNK was the only tested protein to show only a slight increase after TGF-β1 stimulation. We then evaluated the effects of Wnt4 on the phosphorylation of Smad3, ERK, JNK and AKT at 60 min after stimulation with TGF-β1 and Wnt4. The TGF-β1-induced phosphorylation of both Smad3 (Fig. 5a, a′) and ERK (Fig. 5b, b′) was blocked by Wnt4, whereas Wnt4 had no obvious effects on the phosphorylation of JNK or AKT (Supplementary Fig. 5b).
Effects of Wnt4 on TGF-β1-induced phosphorylation of Smad3 and ERK and on the expressions of α-SMA and Col I in fibroblasts harvested from hypertrophic scar samples. p-Smad3/Smad3 (a, a′) and p-ERK/ERK (b, b′) protein expressions after TGF-β1 and Wnt4 stimulation. (a, b) Representative images of western blots. (a′, b′) Ratios of phosphorylated protein/total protein, plotted as mean values from triplicate experiments. Col I (c) and α-SMA (c′) mRNA expressions in fibroblasts derived from hypertrophic scar samples after Wnt4 stimulation, as determined by real-time PCR. (d–d″) α-SMA and Col I protein expressions in hypertrophic scar–derived fibroblast after Wnt4 treatment, as examined by western blotting. (d) Representative images of western blots. (d′, d″) Relative expression levels of Col I and α-SMA proteins, plotted as mean values from triplicate experiments. P < 0.05 vs. control, **P < 0.01 vs. control, #P < 0.05 vs. TGF-β1-stimulated fibroblasts; ##P < 0.01 vs. TGF-β1-stimulated fibroblasts
Wnt4 downregulates the excessive expressions of α-SMA and Col I in hypertrophic scar–derived fibroblasts
We next investigated the effects of Wnt4 on protein expression in fibroblasts derived from hypertrophic scar samples. The scar-derived fibroblasts were stimulated with Wnt4 at varying concentrations (1, 10, 20, or 100 ng/mL) for 48 h after serum starvation for 12 h and then, the mRNA and protein expression levels of α-SMA and Col I were examined. The results showed that both α-SMA and Col I were downregulated at the mRNA (Fig. 5c, c′) and protein (Fig. 5d–d″) levels by Wnt4 in a dose-dependent manner.
Discussion
Both the TGF-β and Wnt signaling pathways play significant roles in the regulation of fibroblast function, wound healing and hypertrophic scar formation (Guo et al. 2012; Scharenberg et al. 2014; Tomasek et al. 2002; Wang et al. 2016a; Wei et al. 2011). In the current study, we investigated the effects of Wnt4 on the TGF-β1-induced activation of fibroblasts and explored the possible mechanism. We first observed that the TGF-β1 expression was greater in hypertrophic scar tissue than in normal skin, whereas the Wnt4 expression was lower in hypertrophic scar tissue than in normal skin. Furthermore, treatment with TGF-β1 induced fibroblast activation and in this process, the Wnt4 expression also showed a remarkable increase, peaking at 4 h, which is consistent with a previous report (Colwell et al. 2006). These results suggest that Wnt4 might be involved in the TGF-β1-induced transition of fibroblasts to myofibroblasts.
Myofibroblasts, which are primarily derived by transdifferentiation of fibroblasts, facilitate wound healing through elevated ECM secretion and contraction. However, persistence of myofibroblasts in the wound area leads to fibrotic diseases, including renal fibrosis, lung fibrosis and hypertrophic scar formation (Diegelmann and Evans 2004; Tomasek et al. 2002). TGF-β1 signaling is believed to play a crucial role in the pathogenesis of fibrotic disease and recent studies have found that cross -talk between TGF-β1 and Wnt signaling is involved in wound healing and tissue fibrosis (Castellone and Laukkanen 2017; George 2009; Liu et al. 2012).
The Wnt protein family consists of 19 highly conserved, secreted morphogenic glycoproteins that are highly expressed primarily during embryonic development, after which they disappear or are expressed at low levels in adult skin and organs (Bastakoty and Young 2016; Reddy et al. 2001). The expression of Wnt proteins is known to be upregulated after injury (Aisagbonhi et al. 2011; Labus et al. 1998; Lyu and Joo 2006). Among the Wnt proteins, Wnt1 and Wnt4 have been reported to play a significant role in the pathogenesis of organ fibrosis (Maarouf et al. 2016; Surendran et al. 2002; Zhong et al. 2017). Wnt1 mainly contributes to the development of lung fibrosis and is not expressed in skin tissue (Labus et al. 1998; Liu et al. 2012). Wnt4 was shown to be more highly expressed in fetal skin than in postnatal skin of mice and knockout of Wnt4 can lead to dermal fibroplasia (Saitoh et al. 1998). In the kidney, Wnt4 can inhibit renal fibrosis induced by high glucose (Ho et al. 2012). These results suggest a possible anti-fibrotic role of Wnt4 in skin and organs. On the contrary, the Wnt4 expression was found to be upregulated by fibrin degradation products in murine fibroblasts and by TGF-β1 stimulation in bone marrow mesenchymal cells (Labus et al. 1998). Previous research using different renal injury models found that Wnt4 is activated in interstitial cells expressing α-SMA-positive and ECM proteins during kidney fibrosis and exogenous Wnt4 induced myofibroblast differentiation in vitro, as evidenced by the increased expression of α-SMA (DiRocco et al. 2013). These results indicate a pro-fibrotic role of Wnt4. Thus, no consensus has been reached regarding the role of Wnt4 in the fibroblast-to-myofibroblast transition and the tissue fibrosis.
In the present study, we investigated the role of Wnt4 in TGF-β1-induced skin fibroblast activation. First, we found that the Wnt4 expression was increased in these cells after TGF-β1 stimulation, with a peak expression increase of 25.3-fold at 4 h followed by a slow decline to 5-fold greater than the baseline expression by 48 h, suggesting that Wnt4 might be involved in TGF-β1-induced fibroblast activation. To further investigate the role of Wnt4 in this process, Wnt4 was applied to the fibroblasts in combination with TGF-β1. Interestingly, the increase in α-SMA and Col I expressions in TGF-β1-treated fibroblasts, representing the fibroblast-to-myofibroblast transition, was inhibited by Wnt4 in a dose-dependent manner. Moreover, Wnt4 knockdown led to further increases in α-SMA and Col I expressions in TGF-β1-treated fibroblasts, suggesting that Wnt4 upregulation in response to TGF-β1 treatment might negatively regulate the TGF-β1-induced fibroblast activation. Increased contractility, which is associated with the α-SMA expression, is an important feature of myofibroblasts. In 3D collagen lattice contraction assays, when added with TGF-β1, Wnt4 blocked the increased contractile ability of fibroblasts and a further experiment showed that this blockade was due to downregulation of α-SMA in the fibroblasts. These observations also indicate that Wnt4 negatively regulated the TGF-β1-induced fibroblast transition.
It has been reported that TGF-β1 can promote its own expression, as well as that of Smad proteins, which represents an important positive feedback loop during the pathogenesis of fibrotic diseases (Dabiri et al. 2008; Popova et al. 2010). In the current study, we observed that when the fibroblasts were cultured for 24 h without TGF-β1 after TGF-β1 stimulation for 24 h, both TGF-β1 and α-SMA expressions showed further increases, suggesting the existence of the autocrine loop of TGF-β1. Notably, this increased expression of TGF-β1 was blocked by Wnt4. Moreover, when the fibroblasts were incubated with Wnt4 for 48 h after TGF-β1 stimulation for 48 h, the α-SMA expression also was significantly downregulated, indicating that Wnt4 could inhibit the fibroblast-to-myofibroblast transition.
Prior studies have indicated that the regulation of α-SMA and Col I expressions by TGF-β1 is mediated via Smad, JNK, ERK and AKT (Fan et al. 2015; He et al. 2015; Jiang et al. 2017; Saito et al. 2017; Wang et al. 2016b). Thus, we further investigated the effects of Wnt4 on the expression and activation of these signaling proteins. After TGF-β1 stimulation, the phosphorylation of Smad3, ERK and AKT was significantly increased, whereas that of JNK was only slightly upregulated by TGF-β1. Furthermore, the increases in Smad3 and ERK phosphorylation were remarkably blocked by Wnt4. However, the changes in JNK and AKT phosphorylation were not significantly influenced by Wnt4. These results indicate that although Smad3, ERK, AKT and JNK could be activated by TGF-β1 during the fibroblast-to-myofibroblast transition, the negative regulatory effects of Wnt4 on the TGF-β1-induced fibrotic response may be mediated primarily through inhibition of Smad3 and ERK phosphorylation.
Increased ECM synthesis is a key feature of myofibroblasts but when it persists beyond the levels needed for wound healing, excessive scarring can occur. In the current study, we observed that the Wnt4 expression was decreased in both hypertrophic scar tissues and scar-derived fibroblasts. Thus, we further investigated the effects of exogenous Wnt4 on scar-derived fibroblasts. Interestingly, the increased expressions of α-SMA and Col I in these tissues and cells were downregulated by Wnt4 in a dose-dependent manner. These results suggest that the low expression level of Wnt4 may contribute to the formation of hypertrophic scars and delivery of Wnt4 may also reverse the abnormal phenotype of scar-derived fibroblasts, which represents a potential therapeutic strategy for the prevention and treatment of hypertrophic scarring.
Taken together, our results suggest that in human skin fibroblasts, Wnt4 plays a negative regulatory role in TGF-β1-induced fibroblast activation by inhibiting the expressions of α-SMA and Col I as well as blocking the autocrine effect of TGF-β1, possibly by mediating the phosphorylation of Smad3 and ERK. Further research is warranted to investigate this mechanism as a possible therapeutic target along with the potential of Wnt delivery as a treatment strategy for fibrotic conditions, specifically hypertrophic scarring.
References
Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK (2011) Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech 4:469–483
Bastakoty D, Young PP (2016) Wnt/beta-catenin pathway in tissue injury: roles in pathology and therapeutic opportunities for regeneration. FASEB J 30:3271–3284
Bell E, Ivarsson B, Merrill C (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A 76:1274–1278
Castellone MD, Laukkanen MO (2017) TGF-beta1, WNT, and SHH signaling in tumor progression and in fibrotic diseases. Front Biosci (Schol Ed) 9:31–45
Chen X, Shi C, Meng X, Zhang K, Li X, Wang C, Xiang Z, Hu K, Han X (2016) Inhibition of Wnt/beta-catenin signaling suppresses bleomycin-induced pulmonary fibrosis by attenuating the expression of TGF-beta1 and FGF-2. Exp Mol Pathol 101:22–30
Colwell AS, Krummel TM, Longaker MT, Lorenz HP (2006) Wnt-4 expression is increased in fibroblasts after TGF-beta1 stimulation and during fetal and postnatal wound repair. Plast Reconstr Surg 117:2297–2301
Cutroneo KR (2007) TGF-beta-induced fibrosis and SMAD signaling: oligo decoys as natural therapeutics for inhibition of tissue fibrosis and scarring. Wound Repair Regen 15(Suppl 1):S54–S60
Dabiri G, Tumbarello DA, Turner CE, Van de Water L (2008) Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-beta1 autocrine loop. J Invest Dermatol 128:2518–2525
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9:283–289
DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD (2013) Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol 24:1399–1412
Fan C, Dong Y, Xie Y, Su Y, Zhang X, Leavesley D, Upton Z (2015) Shikonin reduces TGF-beta1-induced collagen production and contraction in hypertrophic scar-derived human skin fibroblasts. Int J Mol Med 36:985–991
Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN (2016) Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 388:1427–1436
Friedstat JS, Hultman CS (2014) Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann Plast Surg 72:S198–S201
George SJ (2009) Regulation of myofibroblast differentiation by convergence of the Wnt and TGF-beta1/Smad signaling pathways. J Mol Cell Cardiol 46:610–611
Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibel J, Murcia C, International Advisory Panel on Scar M (2014) Updated international clinical recommendations on scar management: part 2--algorithms for scar prevention and treatment. Dermatol Surg 40:825–831
Guo Y, Xiao L, Sun L, Liu F (2012) Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res 61:337–346
He T, Bai X, Yang L, Fan L, Li Y, Su L, Gao J, Han S, Hu D (2015) Loureirin B inhibits hypertrophic scar formation via inhibition of the TGF-beta1-ERK/JNK pathway. Cell Physiol Biochem 37:666–676
Ho C, Lee PH, Hsu YC, Wang FS, Huang YT, Lin CL (2012) Sustained Wnt/beta-catenin signaling rescues high glucose induction of transforming growth factor-beta1-mediated renal fibrosis. Am J Med Sci 344:374–382
Hsu YC, Chen MJ, Yu YM, Ko SY, Chang CC (2010) Suppression of TGF-beta1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res 302:717–724
Hutchenreuther J, Leask A (2016) A tale of two orgins: do myofibroblasts originate from different sources in wound healing and fibrosis? Cell Tissue Res 365:507–509
Jiang X, Huang B, Yang H, Li G, Zhang C, Yang G, Lin F, Lin G (2017) TGF-beta1 is involved in vitamin D-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells by regulating the ERK/JNK pathway. Cell Physiol Biochem 42:2230–2241
Labus MB, Stirk CM, Thompson WD, Melvin WT (1998) Expression of Wnt genes in early wound healing. Wound Repair Regen 6:58–64
Lemoinne S, Thabut D, Housset C (2016) Portal myofibroblasts connect angiogenesis and fibrosis in liver. Cell Tissue Res 365:583–589
Li Z, Zhou L, Wang Y, Miao J, Hong X, Hou FF, Liu Y (2017) (Pro)renin receptor is an amplifier of Wnt/beta-catenin signaling in kidney injury and fibrosis. J Am Soc Nephrol 28:2393–2408
Liu J, Wang Y, Pan Q, Su Y, Zhang Z, Han J, Zhu X, Tang C, Hu D (2012) Wnt/beta-catenin pathway forms a negative feedback loop during TGF-beta1 induced human normal skin fibroblast-to-myofibroblast transition. J Dermatol Sci 65:38–49
Lyu J, Joo CK (2006) Expression of Wnt and MMP in epithelial cells during corneal wound healing. Cornea 25:S24–S28
Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, Gauvin D, Hou X, Kramann R, Humphreys BD (2016) Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol 27:781–790
Mia MM, Bank RA (2016) The pro-fibrotic properties of transforming growth factor on human fibroblasts are counteracted by caffeic acid by inhibiting myofibroblast formation and collagen synthesis. Cell Tissue Res 363:775–789
Popova AP, Bozyk PD, Goldsmith AM, Linn MJ, Lei J, Bentley JK, Hershenson MB (2010) Autocrine production of TGF-beta1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Phys Lung Cell Mol Phys 298:L735–L743
Rajasekaran MR, Kanoo S, Fu J, Bhargava V, Mittal RK (2017) Wnt-beta catenin signaling pathway: a major player in the injury induced fibrosis and dysfunction of the external anal sphincter. Sci Rep 7:963
Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE (2001) Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev 107:69–82
Reddy AT, Lakshmi SP, Zhang Y, Reddy RC (2014) Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages. FASEB J 28:5299–5310
Rezvani M, Espanol-Suner R, Malato Y, Dumont L, Grimm AA, Kienle E, Bindman JG, Wiedtke E, Hsu BY, Naqvi SJ, Schwabe RF, Corvera CU, Grimm D, Willenbring H (2016) In vivo hepatic reprogramming of myofibroblasts with AAV vectors as a therapeutic strategy for liver fibrosis. Cell Stem Cell 18:809–816
Saito S, Zhuang Y, Shan B, Danchuk S, Luo F, Korfei M, Guenther A, Lasky JA (2017) Tubastatin ameliorates pulmonary fibrosis by targeting the TGFbeta-PI3K-Akt pathway. PLoS One 12:e0186615
Saitoh A, Hansen LA, Vogel JC, Udey MC (1998) Characterization of Wnt gene expression in murine skin: possible involvement of epidermis-derived Wnt-4 in cutaneous epithelial-mesenchymal interactions. Exp Cell Res 243:150–160
Scharenberg MA, Pippenger BE, Sack R, Zingg D, Ferralli J, Schenk S, Martin I, Chiquet-Ehrismann R (2014) TGF-beta-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J Cell Sci 127:1079–1091
Shi JH, Guan H, Shi S, Cai WX, Bai XZ, Hu XL, Fang XB, Liu JQ, Tao K, Zhu XX, Tang CW, Hu DH (2013) Protection against TGF-beta1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Arch Dermatol Res 305:341–352
Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, Reetz J, Brandes S, Dai Z, Putzer BM, Arauzo-Bravo MJ, Steinemann D, Luedde T, Schwabe RF, Manns MP, Scholer HR, Schambach A, Cantz T, Ott M, Sharma AD (2016) Direct reprogramming of hepatic myofibroblasts into hepatocytes in vivo attenuates liver fibrosis. Cell Stem Cell 18:797–808
Sun YB, Qu X, Caruana G, Li J (2016) The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 92:102–107
Surendran K, McCaul SP, Simon TC (2002) A role for Wnt-4 in renal fibrosis. Am J Physiol Ren Physiol 282:F431–F441
Thannickal VJ (2012) Mechanisms of pulmonary fibrosis: role of activated myofibroblasts and NADPH oxidase. Fibrogenesis Tissue Repair 5:S23
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3:349–363
Wang S, Meng XM, Ng YY, Ma FY, Zhou S, Zhang Y, Yang C, Huang XR, Xiao J, Wang YY, Ka SM, Tang YJ, Chung AC, To KF, Nikolic-Paterson DJ, Lan HY (2016a) TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget 7:8809–8822
Wang Z, Han Z, Tao J, Wang J, Liu X, Zhou W, Xu Z, Zhao C, Ju X, Wang Z, Tan R, Gu M (2016b) Transforming growth factor-beta1 induces endothelial-to-mesenchymal transition via Akt signaling pathway in renal transplant recipients with chronic allograft dysfunction. Ann Transplant 21:775–783
Wei J, Melichian D, Komura K, Hinchcliff M, Lam AP, Lafyatis R, Gottardi CJ, MacDougald OA, Varga J (2011) Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum 63:1707–1717
Wu CS, Wu PH, Fang AH, Lan CC (2012) FK506 inhibits the enhancing effects of transforming growth factor (TGF)-beta1 on collagen expression and TGF-beta/Smad signalling in keloid fibroblasts: implication for new therapeutic approach. Br J Dermatol 167:532–541
Yazdani S, Bansal R, Prakash J (2017) Drug targeting to myofibroblasts: implications for fibrosis and cancer. Adv Drug Deliv Rev
Zhang J, Li Y, Bai X, Li Y, Shi J, Hu D (2017) Recent advances in hypertrophic scar. Histol Histopathol 33:27–39
Zhong X, Tu YJ, Li Y, Zhang P, Wang W, Chen SS, Li L, Chung AC, Lan HY, Chen HY, Li GS, Wang L (2017) Serum levels of WNT1-inducible signaling pathway protein-1 (WISP-1): a noninvasive biomarker of renal fibrosis in subjects with chronic kidney disease. Am J Transl Res 9:2920–2932
Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ (2013) Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123:1096–1108
Funding
This work was supported by the National Natural Science Foundation of China (No. 81201470, No. 81772071, No. 81501684), the Natural Science Foundation of Shaanxi Province (No. 2017JQ8031) and the China Postdoctoral Science Foundation (No. 2014M562600).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Written informed consent was obtained from each patient.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All the experiments, including the collection of skin and scar tissue samples from patients, were approved by the ethics committee of Xijing Hospital, The Fourth Military Medical University (Approval No. KY20183245).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
(PNG 186 kb)
Supplementary Fig. 2
(PNG 94 kb)
Supplementary Fig. 3
(PNG 296 kb)
Supplementary Fig. 4
(PNG 178 kb)
Supplementary Fig. 5
(PNG 152 kb)
Supplementary Fig. 6
(PNG 186 kb)
Supplementary Table 1
(DOC 703 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Zhao, B., Zhu, H. et al. Wnt4 negatively regulates the TGF-β1-induced human dermal fibroblast-to-myofibroblast transition via targeting Smad3 and ERK. Cell Tissue Res 379, 537–548 (2020). https://doi.org/10.1007/s00441-019-03110-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03110-x