Abstract
The emergence of a specialized system for food digestion and nutrient absorption was a crucial innovation for multicellular organisms. Digestive systems with different levels of complexity evolved in different animals, with the endoderm-derived one-way gut of most bilaterians to be the prevailing and more specialized form. While the molecular events regulating the early phases of embryonic tissue specification have been deeply investigated in animals occupying different phylogenetic positions, the mechanisms underlying gut patterning and gut-associated structures differentiation are still mostly obscure. In this review, we describe the main discoveries in gut and gut-associated structures development in echinoderm larvae (mainly for sea urchin and, when available, for sea star) and compare them with existing information in vertebrates. An impressive degree of conservation emerges when comparing the transcription factor toolkits recruited for gut cells and tissue differentiation in animals as diverse as echinoderms and vertebrates, thus suggesting that their function emerged in the deuterostome ancestor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Before the emergence of the gastrointestinal (GI) system, food sources for unicellular and multicellular organisms were limited to small molecules that could be directly up-taken across the cell membrane. In an aquatic environment like the sea, where life evolved, the invention of a closed apparatus to digest big food particles has represented a beneficial innovation for multicellular organisms, likely fostering the evolution of complex body structures.
Three main forms of GI system with different levels of complexity have evolved in eumetazoans (Schmidt-Rhaesa 2007): i) the digestive syncytium of some Acoela and ii) the sac-shaped intestine of Cnidaria, Gnathostomulida, and Platyhelminthes, provided with one opening to the external environment for both food uptake and residue expulsion; iii) the more complex and efficient one-way gut found in most bilaterians and equipped with two openings, a mouth specialized for food uptake and an anus for expulsion of undigested matter.
The GI system forms during embryonic development through gastrulation, a complex morphogenetic process that, in triploblast bilaterians, gives rise to three embryonic germ layers (the ectoderm, the mesoderm, and the endoderm). The word “gastrulation” literally means “formation of the gut” (from the Greek, “gastrula” that means “belly”) as one of its most evident morphological results is a primitive gut tube (the “archenteron”), provided, in the case of one-way guts, with a blastoporal opening and a mouth. Gastrulation has both common and specific features in the different animals (for a detailed description refer to (Stern 2004)). For instance, differences in the fate of the blastoporal opening during gastrulation have long been used as a criterion to classify animals in protostomes (in which the blastopore gives rise to the mouth and the anus forms from a secondary opening) and deuterostomes (in which the blastopore gives rise to the anus and the mouth forms from a secondary opening). A third condition, amphistomy, described only in two species (Malakhov 1998; Treadwell 1901), consists in the formation of both mouth and anus from the blastopore, through the fusion of the central margins of the blastoporal opening. This classification has been confirmed through genome-based phylogenies but the finding of deuterostomy in a number of protostomes has challenged it and still fuels debates about the ancestral condition of the blastopore fate (Martin-Duran et al. 2016; Nielsen 2017, 2019; Nielsen et al. 2018).
At the end of gastrulation, the archenteron is a straight tube, in which the undifferentiated foregut, midgut, and hindgut territories will give rise to the highly specialized tissues and organs of the adult gut. In particular, the foregut will give rise to the most anterior structure of the gut, the pharynx/esophagus, that is specialized in food uptake and it usually presents a strong musculature allowing to hold and swallow food particles. Posterior to the pharynx is the stomach that develops from the midgut cells and it is specialized in food digestion through the secretion of digestive enzymes. Finally, the most posterior structure, the intestine, forms from the hindgut and it is responsible of completing digestion, up-taking nutrients, and expelling undigested food through the anus.
In most bilaterians, the gut tube forms entirely from the inner endoderm layer, while in some others, such as insects, the foregut and the hindgut derive from ectodermal cells. The early stages of gut development have been deeply described in animals occupying different phylogenetic positions, revealing conservation and divergence in these early events of the embryonic development (Stainier 2002). On the contrary, less is known about the molecular regulation of the gut A-P patterning and the formation of gut-associated structures such as muscles and glands like pancreas and liver. Molecular studies on gut patterning have been conducted in some protostomes (i.e., the nematode Caenorabditis elegans, the arthropod Drosophila melanogaster, the annelid Capitella teleta, and the priapulid Priapulus caudatus (Boyle et al. 2014, Lengyel and Iwaki 2002, Martin-Duran and Hejnol 2015, McGhee 2013)). However, differences in gut development modes and paucity of functional data make comparisons between protostomes and deuterostomes difficult and not always informative. Therefore, to ease comparisons in the developmental modes of different GI structures in representative animals, in this review, we focus on the development of cell and tissue types in deuterostome one-way guts, referring primarily to echinoderms (sea urchins and sea stars) and vertebrates, with the most molecular data available.
Among non-chordate deuterostomes, echinoderms represent valuable model systems in developmental biology, cell biology, and genetics, mostly for their external fertilization and development and for the transparency of eggs and embryos that facilitate direct observations of developmental processes (Arnone et al. 2015). Moreover, the echinoderm lineage diverged from chordates prior to the large-scale gene duplication events that occurred early during vertebrate evolution. Finally, the recent advances of genomic and transcriptomic analyses in several species (Cary et al. 2019; Kudtarkar and Cameron 2017) and the successful incorporation of high-throughput methods to analyze gene regulatory networks (GRNs) including biochemical assays (e.g., ChIP-Seq, DNase-Seq, and ATAC-Seq) (Lowe et al. 2017; Shashikant et al. 2018) offer a unique opportunity to study structure and evolution of the gene regulatory apparatus controlling embryonic development. Indeed, the most exhaustive characterization of a GRN for any developmental system has been developed for the sea urchin embryo and it describes the molecular events driving the specification of endomesodermal territories in time and space (Croce et al. 2011; Croce and McClay 2010; Davidson et al. 2002; Peter and Davidson 2010).
As indirect developers, echinoderms live a larval phase (the pluteus in echinoids and the bipinnaria larva in asteroids) during which they are equipped with a fully functional regionalized gut. Detailed molecular studies have partially reconstructed the main gene regulatory events driving the sea urchin larval gut development, mostly in Strongylocentrotus purpuratus, providing mechanistic explanations for the antero-posterior patterning and for the specification and development of associated gut structures such as gastrointestinal muscles and pancreatic-like cells (Andrikou et al. 2013, 2015; Annunziata and Arnone 2014; Annunziata et al. 2014; Cole et al. 2009; Perillo and Arnone 2014; Perillo et al. 2016, 2018). In addition, the discovery of pharyngeal neurons of endodermal origin (Wei et al. 2011) and the recent finding of endoderm-derived neuronal nitric oxide synthase (nNOS)-positive cells controlling pyloric sphincter relaxation through nitric oxide signaling, a mechanism known to regulate pyloric sphincter opening in vertebrates (Yaguchi and Yaguchi 2019), both contribute to highlight the potential of molecular investigations in echinoderm model system.
In this review, we describe the main discoveries in gut and gut-associated structures development in echinoderms and compare them with available information in vertebrates. Understanding the properties of gut patterning and formation of gut-associated structures in early branching deuterostomes, like echinoderms, holds the key in revealing the mechanisms driving cell type differentiation in more complex organisms such as humans, and in understanding the evolution of GI cell types.
Larval gut structure and patterning in echinoderms
Most echinoderms are indirect developers and live part of their biphasic life cycle as swimming larvae. Larvae feeding on phytoplankton (planktotrophic larvae) are present in holothurioids (auricularia), asteroids (bipinnaria) ophiuroids (ophiopluteus), and echinoids (echinopluteus), while crinoids larvae do not have a functional gut (McEdward and Miner 2001). The echinoderm larval guts are formed of a muscular esophagus, a large spherical stomach, and a tubular intestine (see the sea urchin and sea star larvae in Fig. 1(a, b)). The larval feeding behavior has been observed and described for many echinoderms (Strathmann 1975). In synthesis, the cilia of the ciliated band create a current that brings food particles close to the mouth; food particles enter the mouth of the larvae and are transported down the esophagus by ciliary beating and peristaltic contractions of muscular fibers giving rise to the bolus. The lower esophagus muscle fibers contract toward the upper esophagus causing the opening of the cardiac sphincter and allowing the passage of the bolus into the stomach. Once in the stomach, food particles are agitated and dispersed by ciliary beating causing their breakup and allowing nutrient uptake. The undigested material is then passed to the intestine through the opening of the pyloric sphincter, when present. Eventually, food residues are defecated through the anus.
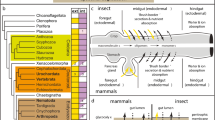
Gut patterning in echinoderms and vertebrates. Differential interference contrast (DIC) microscopy images of the sea urchin S. purpuratus (a) and the sea star P. miniata (b) larval guts in lateral view. (c) Cartoons showing the expression patterns of TFs involved in gut patterning in the sea urchin, sea star, and mouse embryonic guts. a, anus; cs, cardiac sphincter; e, esophagus; i, intestine; m, mouth; ps, pyloric sphincter; s, stomach; asterisks indicate absence of pyloric sphincter in the sea star gut. References for the expression domains for the sea urchin are: FoxA (Oliveri et al. 2006); GataE (Lee and Davidson 2004); Xlox and Cdx (Arnone et al. 2006; Cole et al. 2009); and Hox11/13b (Arenas-Mena et al. 2006); for the sea star, FoxA (Hinman et al. 2003); GataE (Hinman and Davidson 2007); Xlox and Cdx (Annunziata et al. 2013); and Hox11/13b (this work); for the mouse: FoxA (Besnard et al. 2004); Gata4/5/6 (Ayanbule et al. 2011); Xlox (Pdx) and Cdx (Zorn and Wells 2009); and Hox11/13b (Hoxa/d13) (Zorn and Wells 2009). (d-e’”) Projections of merged confocal stacks of double fluorescent in situ hybridization of P. miniata early gastrula embryos in ventral view (d, d’, d”, d’”) and lateral view (e, e’, e”, e’”) showing co-expression of Cdx (magenta) and Hox11/13b (cyan) genes. Nuclei are stained blue with 4′,6-diamidino-2-phenylindole (DAPI)
The functional specialization of the different larval gut regions has been analyzed by ultrastructural studies in two sea urchin species (Dendraster excentricus and Echinocardium cordatum) and two brittle stars (Amphipholis kochii and Ophiura sarsi) (Burke 1981; Gliznutsa and Dautov 2011; Kungurtzeva and Dautov 2001; Nezlin and Yushin 1994). In both analyzed sea urchin larvae, the esophagus is divided in two regions, one upper part that is composed of columnar densely ciliated cells and a lower part that is bulbous and it is composed of cuboidal cells with few cilia. According to (Burke 1981), the upper esophagus serves in collecting food particles and transporting them to the lower esophagus, a region devoted to the temporary storage of algal cells during the formation of the bolus, likely through the secreting mucus. The stomach is composed of two to three ultrastructurally distinct types of columnar and cuboidal epithelial cells. Two cell types are described in D. excentricus: type I cells that are distributed throughout the stomach, present numerous microvilli, and contain two types of vesicles (type A provided with fine granular substance of medium electron density; type B provided with coarse granules, myelin bodies, and multivesicular bodies); type II cells that are restricted to the anterior portion of the stomach, do not present villi, and contain within their cytoplasm remnants of whole algal cells in various stages of digestions, indicating that these cells are devoted to phagocytosis and digestion. On the other hand, Nezlin and Yushin 1994 describe three cell types in the stomach of the E. cordatum pluteus: type I and II that are the most abundant and both contain vesicles; type III that are scattered in the stomach epithelium and contain both vesicles and residues of algal cells, indicating a phagocytosis function. Finally, the intestine in D. excentricus resulted made of a squamous epithelium containing numerous vesicles and a single cilium per cell and presenting very little specialization, suggesting that it functions primarily as a conductive tube for the elimination of undigested material. Conversely, the E. cordatum intestine cells presented microvilli on the apical surface, together with vesicles of different sizes and remnants of algal cells in the cytoplasm, indicating that these cells can phagocytize food and absorb and store nutrients.
Based on these two available descriptions, secreting cells and cells devoted to phagocytosis and digestion have been found either in restricted regions of the stomach or in the entire stomach epithelium and in the intestine, making the identification of specific domains of cellular specialization in the sea urchin larval gut difficult. More ultrastructural data combined with molecular data are needed to define specific cell types and compare the gut structure in different echinoderm species.
The gut of ophiuroid larvae presents a similar morphology to the sea urchin larvae one. The esophagus is composed of a single type of cells that are columnar and present one cilium each and it is made of two parts, one bulbus and one tubular, resembling the echinoid larval esophagus. The stomach is composed of three cell types: type I and type II that are columnar cells provided with a single cilium, compose most of the stomach epithelium, and differ only in the electron density of the cytoplasm, with type I being more electron-dark; type III cells are very rare, they are found in the “dome” and “bottom” of the stomach and present a well-developed endoplasmatic reticulum and large vacuoles. The intestine cuboidal epithelium is composed of two cell types, similar to cell type I and II of the stomach, but presenting shorter and less abundant microvilli. The intestine structure of the ophiopluteus provides evidence that the process of nutrient absorption continues in the intestine like in E. cordatum (Nezlin and Yushin 1994).
Despite the differences highlighted by ultrastructural analysis, the echinoderm larval gut is composed of cells with highly specialized morphology and function, and studies in the sea urchin and sea star embryos have revealed that gut cell identity is achieved through complex molecular events during embryogenesis. Moreover, some of the transcription factors (TFs) expressed in the different gut compartments of the sea urchin larval gut show similar expression domains in the vertebrate gut domains (for a detailed description see Annunziata et al. 2014). These TFs have fundamental roles in the formation and patterning of the sea urchin and vertebrate gut and gut-associated structures. Data about TFs expression and function in the sea star larval gut are limited but highlight conservation in the recruitment of the sea urchin and vertebrate gut TFs suggesting that these genes have similar functions in the sea star gut patterning. In Fig. 1(c), we show the expression domains of TFs critical for gut differentiation such as FoxA, GataE, Xlox, Cdx, and Hox11/13b in the sea urchin, sea star, and mouse guts. Interestingly, these TFs present very similar expression domains: FoxA is expressed in the entire gut in all three animals, although in sea stars and sea urchins, its expression in the presumptive stomach of the larva is significantly lower than that of the most posterior and anterior regions of the archenteron; GataE is stomach specific in the two echinoderms and expressed in all gut compartments in the mouse; Xlox is confined to the stomach-intestine boundary in all three animals and it is expressed in the pancreas of the mouse; Cdx is expressed in the intestine and anal cells of all three animals; Hox11/13b is expressed in the very posterior cells of the gut and in the anal cells of sea urchin, sea star, and mouse guts. Interestingly, the expression of these genes is also conserved in the embryonic gut of some protostomes (Boyle and Seaver 2010; de Rosa et al. 2005; Frobius and Seaver 2006; Hui et al. 2009; Kulakova et al. 2008; Lengyel and Iwaki 2002; Martin-Duran and Hejnol 2015; McGhee 2013).
Besides the expression domains, functional data available in sea urchin and in mouse highlighted similar functions for some of these TFs, an example being the two ParaHox, Xlox and Cdx. In the sea urchin embryo, Xlox is required for the activation of Cdx in the most posterior cells of the developing gut, for pyloric sphincter formation and stomach cells differentiation, while Cdx acts through a Wnt10 signaling event to clear Xlox and midgut markers from the intestinal cells, eventually causing the separation between stomach and intestine fates (Annunziata et al. 2014; Cole et al. 2009). Interestingly, Xlox and Cdx murine homologs (Pdx1 and Cdx2, respectively) also show similar expression and function in the mouse embryonic gut. Indeed, Pdx1 plays a crucial role in pancreas development and in the maintenance of β-cells function (Monaghan et al. 1993; Stoffers et al. 1997) and in pyloric sphincter morphogenesis (Offield et al. 1996). On the other hand, Cdx2 knockout in mouse endoderm results in the loss of intestinal identity with a posterior to anterior gut transformation, leading to ectopic expression of anterior gut markers in the intestine (Gao et al. 2009).
The conservation of Xlox and Cdx expression domains in the sea star Patiria miniata larva with Xlox confined to the stomach-intestine boundary and Cdx expressed in the hindgut and anal cells, despite the absence of the pyloric sphincter in the sea star, suggests that the two ParaHox have a conserved function in the bipinnaria gut regionalization. However, functional data are needed to prove this hypothesis.
One intriguing difference in gut gene expression patterns in sea urchin and sea star embryos is the biphasic expression of the sea star Cdx. In S. purpuratus, Cdx expression starts at late gastrula and requires, among other inputs, Xlox and Hox11/13b regulatory inputs. In P. miniata, a first activation of Cdx expression occurs in a ring of cells surrounding the blastopore of the early gastrula embryos (Annunziata et al. 2013) where Hox11/13b is also expressed (Fig. 1(c, d)). This early expression of Cdx represents a common feature between sea star, mouse, and other Chordates. Indeed, the first embryonic territory of expression for Cdx2 in murine embryos is the posterior primitive streak where this gene is involved in axial elongation (Chawengsaksophak et al. 2004). In Xenopus tropicalis, the three orthologs Cad1, Cad2, and Cad3 are first expressed in the early gastrula around the blastopore and later in the posterior embryo, including the gut (Reece-Hoyes et al. 2002); finally, in the amphioxus Branchiostoma floridae, the first expression of Cdx is detected in a ring of cells surrounding the blastopore opening (Osborne et al. 2009). Interestingly, Cdx early activation in the blastopore region has been found also in protostomes such as insects (Lengyel and Iwaki 2002) and priapulids (Martin-Duran and Hejnol 2015). The striking conservation of the early expression of Cdx genes suggests an ancestral role for these TFs in the early stages of gut development in bilaterians that appears to be lost in the sea urchin embryo.
Another interesting difference concerns the expression of the Brachyury (Bra) gene. Bra is expressed around the blastopore throughout bilateria, where, in most cases, it co-expresses with Cdx, FoxA, and Wnt genes (Lengyel and Iwaki 2002). However, after gastrulation, Bra is expressed in the hindgut in ecdysozoans, echinoderms, and hemichordates and in the notochord in cephalochordates and vertebrates (Herrmann and Kispert 1994; Holland et al. 1995; Peterson, et al. 1999a, b; Shoguchi et al. 1999; Tagawa et al. 1998), both tissues undergoing morphogenesis resulting in axial elongation. It has been indeed proposed that Brachyury ancestral function was in the early morphogenetic movements of internalization and cell rearrangement (Holland 2000; McGhee 2000; Wu and Lengyel 1998), rather than in specific tissue differentiation, as also supported by the block in gastrulation in sea urchin after Brachyury perturbation (Gross and McClay 2001; Rast et al. 2002).
Pancreas and pancreatic cells in metazoans
Animals have specialized cell types devoted to the production of digestive enzymes and hormones, which are needed to breakdown food particles and balance energy resources, respectively. In mammals, both food digestion and glucose level are controlled by pancreas, a specialized organ composed of two tissues: the exocrine pancreas, which contains the digestive enzyme-producing acinar cells, and the endocrine pancreas. The endocrine cells are clustered together in islets and embedded in the exocrine tissue.
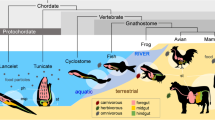
Non-mammalian vertebrates have a primitive pancreas-like organ: in the hagfish, endocrine cells are clustered together but are not embedded within the exocrine tissue (Ostberg et al. 1976; Youson and Al-Mahrouki 1999); in teleosts, endocrine cells are closely associated into a distinct islet-like tissue, but they are not intermingled with the exocrine pancreas like in mammals (Falkmer et al. 1985; Youson and Al-Mahrouki 1999; Youson et al. 2006; Yui and Fujita 1986). Based on the data available on vertebrate pancreatic cells, it seems that the endocrine cells formed an islet-like tissue first, and only after this first event, the exocrine tissue folded and budded out of the gut lumen to associate with the islets to form a distinct organ (the pancreas). In Fig. 2, a reconstruction of the evolutionary history of deuterostome pancreatic cell types based on morphological features (for vertebrates), and similar gene expression (for all other animals) is shown. It is worth mentioning here that, wherever identified as a proper organ, the pancreas arises from within a Pdx/Xlox positive domain.
Evolution of pancreatic cells in deuterostomes. The cartoons show simplified models displaying localization of pancreatic exocrine cells (magenta) and endocrine cells (green). The only echinoderm with available information on pancreatic cells is the sea urchin larva. In the sea urchin larva, exocrine cells are clustered in the upper stomach (s) in a belt-like domain, while endocrine cells producing ILP1 (an insulin-like peptide) are localized close to the exocrine cells and in the intestine (i) in a feeding-dependent fashion. In cephalochordates and urochordates, endocrine and exocrine cells are scattered in the gut lumen and do not show any aggregation. Exocrine cells are localized in the stomach in tunicates only. In the cyclostome hagfish, endocrine cells bud out of the gut to form the first islets containing insulin-producing cells mainly. In gnathostomes, the islets contain all of the pancreatic endocrine hormones, and the exocrine pancreas folds and branches out of the pancreatic duct. In mammals, the endocrine tissue is represented by the islets of Langerhans (green ovals in the figure) imbedded in the acinar cells (represented as light magenta background). Data for this figure come from Al-Mahrouki and Youson 1999; Falkmer et al. 1985; Lecroisey et al. 2015; Olinski et al. 2006; Perillo et al. 2016; Slack 1995; Youson and Al-Mahrouki 1999; and Youson et al. 2006
Interestingly, cells with exocrine and endocrine functions do not have a defined localization in the gut of non-bilaterian animals (Goldberg 2002; Lemaitre and Miguel-Aliaga 2013; Lentz 1966; Wu and Brown 2006). Studies on cephalochordates and tunicates showed that endocrine and exocrine cells do not exhibit any gut specialization and members of the insulin family are produced by gut cells (He et al. 2018; Lecroisey et al. 2015; Olinski et al. 2006; Reinecke and Collet 1998). The only evidence of exocrine cells clustered in a well-defined endodermal domain comes from an early-branched deuterostome, the sea urchin. Work from our lab showed that cells expressing exocrine markers such as digestive enzymes are clustered in a belt-like structure of the sea urchin larval stomach. In close proximity to the exocrine cells, there are also endocrine cells expressing an insulin-like peptide. Based on these observations, one hypothesis is that the endocrine and exocrine pancreatic cells were restricted to a precise gut area in the deuterostome ancestor, and that cephalochordates and urochordates lost this trait. This distribution of pancreatic exocrine-like and endocrine-like cells in non-vertebrate deuterostomes raises more questions. What drove the localization of the exocrine and endocrine cells in the same region of the gut? What is the minimal toolkit of genes and signaling pathways that specified gut cells to a pancreatic fate? How are food intake and energy storage balanced in animals without a pancreas? Is there a conserved gene regulatory module that defines pancreatic cells?
Pancreatic-like exocrine cells in the sea urchin
Three main factors define pancreatic exocrine cells in vertebrates: the production of digestive enzymes, the expression of the basic helix-loop-helix transcriptional factor Ptf1a (pancreatic transcription factor 1) that promotes exocrine cells differentiation and maintenance, and cell specification through Notch signaling. Digestive enzymes have been isolated from adult sea urchin, sea star, and sea cucumbers (Hernandez-Samano et al. 2017; Sun et al. 2015; Trenzado et al. 2012; Wang and Hiebert 2001; Williams 1975; Winter and Neurath 1970). The sea urchin larva also produces several digestive enzymes like β-glucanase, α-amylase (Vacquier 1971a, b; Vacquier et al. 1971), carboxypeptidases, and lipases (Perillo et al. 2016). The most extensive description of digestive cells has been provided in the sea urchin S. purpuratus larva (Perillo et al. 2016), while information on other echinoderm larvae is scarce. In the sea urchin, cells producing digestive enzymes are clustered in the larval upper stomach in a belt-like domain and digestive enzyme gene expression significantly increases during feeding. These same cells of the upper stomach also express the orthologs of vertebrate TFs that define the exocrine pancreas fate. In the mammalian pancreas, Hnf1a activates the transcription of Ptf1a in pancreatic precursors (Haumaitre et al. 2005), and in turns Ptf1a controls exocrine cell maturation and regulates digestive enzymes production (Krapp et al. 1998; Petrucco et al. 1990). In vertebrates, Ptf1a activity alone is required to keep the exocrine cell fate, and Ptf1a low levels promote the expression of endocrine genes and the reprogramming of exocrine to endocrine fate (Hesselson et al. 2011). Similarly, Hnf1 controls Ptf1a endodermal expression in the differentiating sea urchin gut, and Ptf1a induces the expression of genes coding for digestive enzymes, such as Cpa2L, Amy3, and Pnlp2/5 (carboxypeptidase, amylase, and lipase respectively) (Perillo et al. 2016). Ptf1a binds to an extended bipartite DNA sequence in the promoter of mammalian pancreatic digestive enzyme genes (Masui et al. 2007). This sequence consists of an E-box and a TC-box spaced one or two helical DNA-turns apart. Based on the homology with mammalian binding sequences, putative Ptf1a binding sites were found on the promoters of the sea urchin digestive enzymes. Luciferase essays have also showed that the sea urchin Ptf1a is able to bind its mammalian partner proteins to activate transcription of the rat chymotrypsinogen elements (Perillo et al. 2016). This evidence suggests that the sea urchin Ptf1a acts like its mammalian ortholog to control exocrine cells differentiation and activate digestive enzyme production.
Another characteristic of pancreatic cell development is that Notch signaling balances exocrine cells fate by repressing exocrine cells differentiation in gut cells (Esni et al. 2004; Hald et al. 2003; Murtaugh et al. 2003). This role is conserved in sea urchins, where Notch signaling blocks the differentiation toward an exocrine fate by repressing Ptf1a expression in cells close to the belt-like exocrine domain. All the above findings indicate that the minimal toolkit necessary to define pancreatic exocrine cells is present in an early-branched deuterostome. Based on available results, the essential gene regulatory program that specifies for exocrine cells is established in the sea urchin gut (Fig. 2; for or a summary of the above described gene interactions see also the GRN reported in Fig. 4(B)). In both mouse and zebrafish, Ptf1a promotes acinar cell maturation while Notch signaling represses acinar cell differentiation. Ptf1a controls the expression of Mist1 and of the digestive enzymes to maintain the acinar phenotype. All the above similarities between vertebrate and sea urchin GRNs, together with the evidence that the exocrine cells are restricted to the sea urchin larva upper stomach, suggest that the clustering of the exocrine pancreas predates the emergence of the pancreas as a discrete organ.
Pancreatic endocrine-like cells and insulin peptides in the sea urchin
Pancreatic endocrine β-cells produce the hormone insulin that balances nutrient metabolism, organism growth, and cell survival. To date, members of the insulin family in echinoderms have been found in the Lytechinus variegatus and S. purpuratus genomes, but not in other echinoderms. The S. purpuratus genome has two members of the insulin family, ILP1 and ILP2 (insulin-like peptides 1 and 2) (Burke et al. 2006). Ilp1 gene structure is similar to the proto-insulin ancestor, while Ilp2 is divergent. Ilp1 is always expressed by a group of cells in the larva upper stomach, while the protein localization is feeding dependent. When food is available, ILP1 protein is localized in a group of cells in the stomach and in few intestine cells. When food is scarce, both ILP1 gene and protein expression increase drastically in the intestine cells (Perillo and Arnone 2014). ILP1 protein is very similar to pig insulin at the protein sequence level. For instance, ILP1 antigen is also recognized by a pig anti-insulin Ab (Perillo and Arnone 2014), and porcine insulin stimulates larval growth by modulating collagens and histones gene expression (de Pablo et al. 1988).
The ILP1+ cells in the stomach are localized in close proximity to the exocrine cells. This localization suggests that exocrine and endocrine cells became restricted to the same gut region for the first time in the echinoderm lineage. A still unanswered question is what is the gene regulatory module of the endodermal ILP1+ cells. So far, pancreatic endocrine transcriptional factors known to activate a pancreatic endocrine program have been found in sea urchin neurons only. In the gastrula, apical plate and ciliary band neuronal precursors defined by SoxC (Garner et al. 2016) also express Ptf1a and Xlox (Perillo et al. 2018; Wood et al. 2018). In the larva, Xlox expression is restricted to the lateral ganglia that also express Isl (Perillo et al. 2018), the vertebrate ortholog of which is expressed in developing pancreatic endocrine cells and brain (Thor et al. 1991). Considering that pancreatic endocrine cells and neurons share remarkably similar features (Alpert et al. 1988; Le Douarin 1988; Pearse and Polak 1971), it has been proposed by several authors that the pancreatic cells co-opted a neuronal gene program (Arntfield and van der Kooy 2011; Eberhard 2013). Remarkably, a shared origin for endocrine and neuronal cells is supported by the observation that the endocrine adrenomedullary cells that originate from the neural crest can be transformed into neurons when exposed to nerve growth factor in vivo and in vitro (Aloe and Levi-Montalcini 1979; Forander et al. 2001; Ogawa et al. 1984). The evidence that a gene program that predates the pancreas as a discrete organ is present in subsets of sea urchin neurons suggests that gut cells co-opted a pre-existing pancreatic program from an ancestral neuron of a deuterostome ancestor (Perillo et al. 2018).
Comparison of muscle structures involved in digestive systems
The fundamental ability of animals to ingest and transport food along their digestive tract is based on the alternating waves of gastrointestinal muscle contraction and relaxation. Echinoderm embryos are characterized from two different types of gastrointestinal muscles, the circumesophagael muscles that contract in order the food to be ingested (Fig. 3(a, c)), and the myoepithelial cells, which compartmentalize the digestive tract in distinct domains with different digestive functions and defecation (Fig. 3(b, d)). The circumesophagael muscles are mesodermally derived (Andrikou et al. 2013; Burke and Alvarez 1988; Cameron and Davidson 1991; Chia 1977; Crawford and Martin 1998; Crawford and Chia 1978; Gliznutsa and Dautov 2011; Gustafson and Wolpert 1967; Ishimoda-Takagi et al. 1984; Wessel et al. 1990); they appear to be of the smooth muscle type as they are mononucleated and have few dense bodies and a long term contraction-relaxation periodicity and the organization of thick and thin filaments lacks a periodic structure (Burke 1981; Gliznutsa and Dautov 2011; Kaneko et al. 2009; Kungurtzeva and Dautov 2001). In the case of sea urchins and ophiuroids, these muscles also share striated muscle morphological properties since their dense bodies are periodically aligned across the width of the filamentous region that resembles indistinct Z-lines (Burke 1981; Kungurtzeva and Dautov 2001). The myoepithelial cells arise from the endoderm and form three sphincters; two of them (pyloric and anal) morphologically resemble smooth muscles, while the third one (cardiac) has striated muscle features (Burke 1981). Not all echinoderm larvae possess three sphincters. The digestive tract of sea urchin (Figs. 1 and 3(b)) and ophiuroid pluteus larvae is composed from cardiac, pyloric, and anal sphincters, while the bipinnaria larvae of sea stars does not have the pyloric sphincter (Figs. 1 and 3(d)).
Gastrointestinal musculature in echinoderm larvae. DIC sequential images during esophageal muscle contraction (a-a’, c-c’) and immunohistochemistry of gastrointestinal muscles in sea urchin pluteus larva (b) and sea star bipinnaria larva (d). Black arrowheads indicate the position of the esophageal contraction. Fluorescent images are projections of merged confocal stacks. Esophageal muscles and myoepithelial cells (sphincters) are stained with anti-MHC antibody in (b) and phalloidin in (d). Sphincters are indicated with white arrows. Cilia and nervous system are stained green with acetylated tubulin. Nuclei are stained blue with DAPI. Anterior is to the top. as, anal sphincter; cs, cardiac sphincter; e, esophagus; i, intestine; m, mouth; ps, pyloric sphincter; s, stomach
The most extensive description of muscle development has been conducted in the sea urchin S. purpuratus (Andrikou et al. 2013; Burke and Alvarez 1988). The first pair of myoblasts is formed at late gastrula stage (45-50hpf), when one or two mesodermal cells at the oral side of the tip of the archenteron, express Myosin heavy chain (Mhc). These cells are part of the coelomic sacs, which at this developmental stage are seen as a single vesicle that pinches off from the tip of the archenteron. At the same time, the future cardiac and anal sphincters start to be formed. As the embryo develops, more cells are adopting the myoblast fate without being clear whether this is due to a proliferation event or to de novo specification. Later on, at prism stage (55-60hpf), morphogenetic movements start to take place. The paired flap-like coelomic sacs start to extend laterally and two rows of myoblast cells form that surround the stomodeum. At early pluteus stage (65–70 hpf), the connection between the two coelomic pouches closes and processes of the myoblasts from each pouch extend toward the midline of the esophagus. No direct contact between the myoblasts and the esophageal epithelial cells takes place. In each coelomic sac, an average of 7–8 myoblasts and another mean of 7–9 other than myoblasts is seen. At late pluteus stage (72–96 hpf), processes of the myoblasts fuse from both sides to form the muscle bands that encircle the esophagus (Fig. 3(b)). Finally, ventrolateral processes extend from branches of muscle cells situated at the posterior end of the coelomic pouches. The pyloric sphincter is formed and the first synchronized contractions of the gastrointestinal musculature occur.
In sea stars, the esophagael muscle cells of the bipinnaria larvae are derived from the coeloms in a similar manner to the sea urchin larvae (Crawford and Martin 1998). At late gastrula stage (3 dpf), mesenchyme cells start to migrate from the expanded coelomic tip of the archenteron toward the esophagus. Few hours later (4 dpf), the coelomic sacs have almost completely separated from the tip of the archenteron and the myoblasts extend fine filopodia from one end of the cell. By day 5, the presumptive esophageal muscle cells cover one third of the surface of the esophagus with their single processes. Over the next 24 h, the myoblasts start to elongate and by day 7, and they consist of two elongated processes that encircle the esophagus. The boundaries of adjacent cells are separated from their neighbors, with little overlap or contact between them. Branches of muscle fibers run in the longitudinal axis of the esophagus and form lateral processes. At this stage, 12–13 rings of esophageal myofilaments are observed (Fig. 3(d)) and the first contractions are taking place.
The molecular interplay that governs esophageal muscle development has been described in the sea urchin species S. purpuratus (Andrikou et al. 2015) and shares impressive similarities not only with vertebrates but also with tunicates, flies, and nematodes (Andrikou and Arnone 2015; Ciglar and Furlong 2009). Naïve, FoxY-expressing secondary mesenchyme cells, located at the oral lateral side of the vegetal plate, receive an inductive FGF signal from the oral ectoderm at the onset of gastrulation, which triggers the expression of a set of myogenic transcriptional regulators required for the proper esophageal muscle formation. This set of genes includes, among others, three more Fox factors—FoxC, FoxF, and FoxL1—, Myocardin, Twist, SoxE, Schratchx, Six1/2, Tbx6, and Myod2. All these TFs are highly interconnected and occupy different hierarchical levels of the myogenic GRN. FoxC, SoxE, SchratchX, and Six1/2 start to be expressed at the early/mid gastrula stage and provide positive inputs to their downstream targets FoxF and FoxL1, and negative inputs to genes belonging to different mesodermal GRNs, such as Scl, Dachs, Pitx2, and Not. At the end of the myogenic GRN stand, the differentiation drivers Myod2 and Tbx6 which start to be expressed at the late gastrula stage and will in turn activate the muscle differentiation battery (e.g., Mhc) (Fig. 4(c)).
View from the genome of three GRNs operating in the gastrointestinal tract of the sea urchin larva. A summary of the main regulatory gene interactions occurring in all nuclei (view from the genome) during specification/differentiation of the posterior gut (a), the exocrine pancreatic-like cell type (b) and the esophageal muscles (c) is reported. The diagrams are generated using the Biotapestry software (www.biotapestry.org)
Remarkably, the GRN responsible for sphincter formation in sea urchins differs from the one driving esophageal musculature development. For instance, the formation of the cardiac sphincter is not driven by a FGF signal. Embryos, in which FGF is inhibited, are still able to form the cardiac sphincter while the esophageal muscle is absent (Andrikou et al. 2015). Moreover, FoxY, FoxC, FoxF, FoxL1, Six1/2, Tbx6, and MyoD2 morphants retain their cardiac sphincter in most of the cases observed (Andrikou et al. 2015). This indicates that the cardiac sphincter is not a byproduct of the esophageal muscle formation, but it is governed by a distinct, still unknown, endodermal GRN. Moreover, a different transcriptional network controls the pyloric sphincter formation (Fig. 1). A key gene of this GRN is Xlox. Xlox morphants have a malformed gut, which miss the pyloric sphincter—and lack, in the presumptive pyloric sphincter region, the expression of Mhc—and show a reduced digestive capacity with stomach terminal differentiation genes and digestive enzymes being severely downregulated (Annunziata and Arnone 2014; Cole et al. 2009). However, in these embryos, the cardiac sphincter and the esophageal muscle formation remain unaffected suggesting that these three different types of muscle are orchestrated by diverse GRNs. Interestingly, in sea star larvae, although the spatial expression of Xlox is conserved with the one in sea urchins (Annunziata et al. 2013), the pyloric sphincter is absent (Fig. 1). Given the fact that the role of this transcription factor is conserved in vertebrates, where the loss of the orthologous gene Pdx1 also causes a disrupted pyloric sphincter (Offield et al. 1996), it is likely that the function of Xlox in controlling the pyloric sphincter formation has been lost in sea stars. More studies are needed to reveal whether the regulatory landscapes of sphincter development share similarities or not between echinoderms and vertebrates.
Conclusions
The development of a functional gastrointestinal system requires a series of coordinated cellular and molecular events that will result in the formation of a number of specialized cell types. The reconstruction of these molecular events driving cell type specification in the echinoderm and vertebrate gut and gut-associated structures revealed sub-specialization of the TFs involved. One example is the molecular toolkit recruited for the antero-posterior patterning of the gut: the combinatorial activation of TFs in specific cells along the gut defines the identity of gut compartments. Another interesting example is the compact gene regulatory module that controls exocrine pancreas development and function. Finally, the two different types of gastrointestinal muscle cells, esophageal, and myoepithelial sphincters characterized not only from different embryological origins but also different underlying GRNs.
Overall, the regulatory landscape of gut development and compartmentalization is highly conserved among animals, suggesting a deep homology within deuterostomes and likely also within Bilateria. On the other hand, the high level of sub-specialization so far observed in sea urchin larvae, even at the gene regulatory network level, might offer an instrument to understand the evolution of gastrointestinal cell types and to dissect the mechanisms driving cell type differentiation in more complex systems such as the human gut. This could eventually be instrumental to identify causes of gut malformations and dysfunctions and, ultimately, even improve transplantation opportunities through organoid generation.
References
Al-Mahrouki AA, Youson JH (1999) Ultrastructure and immunocytochemistry of the islet organ of osteoglossomorpha (Teleostei). Gen Comp Endocrinol 116:409–421
Aloe L, Levi-Montalcini R (1979) Nerve growth factor-induced transformation of immature chromaffin cells in vivo into sympathetic neurons: effect of antiserum to nerve growth factor. Proc Natl Acad Sci U S A 76:1246–1250
Alpert S, Hanahan D, Teitelman G (1988) Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell 53:295–308
Andrikou C, Arnone MI (2015) Too many ways to make a muscle: evolution of GRNs governing myogenesis. Zool Anz 256:2–13
Andrikou C, Iovene E, Rizzo F, Oliveri P, Arnone MI (2013) Myogenesis in the sea urchin embryo: the molecular fingerprint of the myoblast precursors. EvoDevo 4:33
Andrikou C, Pai CY, Su YH, Arnone MI (2015) Logics and properties of a genetic regulatory program that drives embryonic muscle development in an echinoderm. eLife 4:e07343
Annunziata R, Arnone MI (2014) A dynamic regulatory network explains ParaHox gene control of gut patterning in the sea urchin. Development 141:2462–2472
Annunziata R, Martinez P, Arnone MI (2013) Intact cluster and chordate-like expression of ParaHox genes in a sea star. BMC Biol 11:68
Annunziata R, Perillo M, Andrikou C, Cole AG, Martinez P, Arnone MI (2014) Pattern and process during sea urchin gut morphogenesis: the regulatory landscape. Genesis 52:251–268
Arenas-Mena C, Cameron RA, Davidson EH (2006) Hindgut specification and cell-adhesion functions of Sphox11/13b in the endoderm of the sea urchin embryo. Develop Growth Differ 48:463–472
Arnone MI, Rizzo F, Annunciata R, Cameron RA, Peterson KJ, Martinez P (2006) Genetic organization and embryonic expression of the ParaHox genes in the sea urchin S. purpuratus: insights into the relationship between clustering and colinearity. Dev Biol 300:63–73
Arnone M, Byrne M, Martínez P (2015) Echinodermata. In: Wanninger A (Ed) Evolutionary developmental biology of invertebrates 6: Deuterostomia. Springer-Verlag, Wien, pp 1-58
Arntfield ME, van der Kooy D (2011) β-Cell evolution: how the pancreas borrowed from the brain. Bioessays 33:582–587
Ayanbule F, Belaguli NS, Berger DH (2011) GATA factors in gastrointestinal malignancy. World J Surg 35:1757–1765
Besnard V, Wert SE, Hull WM, Whitsett JA (2004) Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns 5:193–208
Boyle MJ, Seaver EC (2010) Expression of FoxA and GATA transcription factors correlates with regionalized gut development in two lophotrochozoan marine worms: Chaetopterus (Annelida) and Themiste lageniformis (Sipuncula). EvoDevo 1:2
Boyle MJ, Yamaguchi E, Seaver EC (2014) Molecular conservation of metazoan gut formation: evidence from expression of endomesoderm genes in Capitella teleta (Annelida). EvoDevo 5:39
Burke R (1981) Structure of the digestive tract of the pluteus larva of Dendraster excentricus (Echinodermata: Echinoida). Zoomorphology 98:209–225
Burke RD, Alvarez CM (1988) Development of the esophageal muscles in embryos of the sea urchin Strongylocentrotus purpuratus. Cell Tissue Res 252:411–417
Burke RD, Angerer LM, Elphick MR, Humphrey GW, Yaguchi S, Kiyama T, Liang S, Mu X, Agca C, Klein WH, Brandhorst BP, Rowe M, Wilson K, Churcher AM, Taylor JS, Chen N, Murray G, Wang D, Mellott D, Olinski R, Hallböök F, Thorndyke MC (2006) A genomic view of the sea urchin nervous system. Dev Biol 300:434–460
Cameron RA, Davidson EH (1991) Cell type specification during sea urchin development. Trends Genet 7:212–218
Cary GA, Cameron RA, Hinman VF (2019) Genomic resources for the study of echinoderm development and evolution. Methods Cell Biol 151:65–88
Chawengsaksophak K, de Graaff W, Rossant J, Deschamps J, Beck F (2004) Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci U S A 101:7641–7645
Chia F-S (1977) Scanning electron microscopic observations of the mesenchyme cells in the larvae of the starfish Pisaster ochraceus. Acta Zool 58:45–51
Ciglar L, Furlong EE (2009) Conservation and divergence in developmental networks: a view from Drosophila myogenesis. Curr Opin Cell Biol 21:754–760
Cole AG, Rizzo F, Martinez P, Fernandez-Serra M, Arnone MI (2009) Two ParaHox genes, SpLox and SpCdx, interact to partition the posterior endoderm in the formation of a functional gut. Development 136:541–549
Crawford BJ, Chia FS (1978) Coelomic pouch formation in the starfish Pisaster ochraceus (Echinodermata: Asteroidea). J Morphol 157:99–119
Crawford B, Martin C (1998) Ultrastructure and differentiation of the larval esophageal muscle cells of the starfish Pisaster ochraceus. J Morphol 237:1–18
Croce JC, McClay DR (2010) Dynamics of Delta/notch signaling on endomesoderm segregation in the sea urchin embryo. Development 137:83–91
Croce J, Range R, Wu SY, Miranda E, Lhomond G, Peng JC, Lepage T, McClay DR (2011) Wnt6 activates endoderm in the sea urchin gene regulatory network. Development 138:3297–3306
Davidson EH, Rast JP, Oliveri P, Ransick A, Calestani C, Yuh CH, Minokawa T, Amore G, Hinman V, Arenas-Mena C, Otim O, Brown CT, Livi CB, Lee PY, Revilla R, Rust AG, Pan Z, Schilstra MJ, Clarke PJ, Arnone MI, Rowen L, Cameron RA, McClay DR, Hood L, Bolouri H (2002) A genomic regulatory network for development. Science 295:1669–1678
Eberhard D (2013) Neuron and beta-cell evolution: learning about neurons is learning about beta-cells. Bioessays 35:584
Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, Ball DW, Leach SD (2004) Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development 131:4213–4224
Falkmer S, Dafgård E, el-Salhy M, Engström W, Grimelius L, Zetterberg A (1985) Phylogenetical aspects on islet hormone families: a minireview with particular reference to insulin as a growth factor and to the phylogeny of PYY and NPY immunoreactive cells and nerves in the endocrine and exocrine pancreas. Peptides 6(Suppl 3):315–320
Forander P, Broberger C, Stromberg I (2001) Glial-cell-line-derived neurotrophic factor induces nerve fibre formation in primary cultures of adrenal chromaffin cells. Cell Tissue Res 305:43–51
Frobius AC, Seaver EC (2006) ParaHox gene expression in the polychaete annelid Capitella sp. I. Dev Genes Evol 216:81–88
Gao N, White P, Kaestner KH (2009) Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell 16:588–599
Garner S, Zysk I, Byrne G, Kramer M, Moller D, Taylor V, Burke RD (2016) Neurogenesis in sea urchin embryos and the diversity of deuterostome neurogenic mechanisms. Development 143:286–297
Gliznutsa LA, Dautov SS (2011) Cell differentiation during the larval development of the ophiuroid Amphipholis kochii Lütken, 1872 (Echinodermata: Ophiuroidea). Russ J Mar Biol 37:384–400
Goldberg WM (2002) Gastrodermal structure and feeding responses in the scleractinian Mycetophyllia reesi, a coral with novel digestive filaments. Tissue Cell 34:246–261
Gross JM, McClay DR (2001) The role of Brachyury (T) during gastrulation movements in the sea urchin Lytechinus variegatus. Dev Biol 239:132–147
Gustafson T, Wolpert L (1967) Cellular movement and contact in sea urchin morphogenesis. Biol Rev Camb Philos Soc 42:442–498
Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J (2003) Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol 260:426–437
Haumaitre C, Barbacci E, Jenny M, Ott MO, Gradwohl G, Cereghini S (2005) Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A 102:1490–1495
He C, Han T, Liao X, Zhou Y, Wang X, Guan R, Tian T, Li Y, Bi C, Lu N, He Z, Hu B, Zhou Q, Hu Y, Lu Z, Chen J-Y (2018) hagocytic intracellular digestion in amphioxus (Branchiostoma). Proc Biol Sci 285
Herrmann BG, Kispert A (1994) The T genes in embryogenesis. Trends Genet 10:280–286
Hernandez-Samano AC, Guzman-Garcia X, Garcia-Barrientos R, Ascencio-Valle F, Sierra-Beltran A, Guerrero-Legarreta I (2017) Characterization of protease activity from the digestive tract and tentacles of Isostichopus fuscus sea cucumber. ISJ 14:282-294
Hesselson D, Anderson RM, Stainier DY (2011) Suppression of Ptf1a activity induces acinar-to-endocrine conversion. Curr Biol 21:712–717
Hinman VF, Davidson EH (2007) Evolutionary plasticity of developmental gene regulatory network architecture. Proc Natl Acad Sci U S A 104:19404–19409
Hinman VF, Nguyen AT, Cameron RA, Davidson EH (2003) Developmental gene regulatory network architecture across 500 million years of echinoderm evolution. Proc Natl Acad Sci U S A 100:13356–13361
Holland LZ (2000) Body-plan evolution in the Bilateria: early antero-posterior patterning and the deuterostome-protostome dichotomy. Curr Opin Genet Dev 10:434–442
Holland PW, Koschorz B, Holland LZ, Herrmann BG (1995) Conservation of Brachyury (T) genes in amphioxus and vertebrates: developmental and evolutionary implications. Development 121:4283–4291
Hui JH, Raible F, Korchagina N, Dray N, Samain S, Magdelenat G, Jubin C, Segurens B, Balavoine G, Arendt D, Ferrier DE (2009) Features of the ancestral bilaterian inferred from Platynereis dumerilii ParaHox genes. BMC Biol 7:43
Ishimoda-Takagi T, Chino I, Sato H (1984) Evidence for the involvement of muscle tropomyosin in the contractile elements of the coelom-esophagus complex in sea urchin embryos. Dev Biol 105:365–376
Kaneko H, Kawahara Y, Okamoto M, Dan-Sohkawa M (2009) Study on the nature of starfish larval muscle cells in vitro. Zool Sci 14: 287-296
Krapp A, Knöfler M, Ledermann B, Bürki K, Berney C, Zoerkler N, Hagenbüchle O, Wellauer PK (1998) The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev 12:3752–3763
Kudtarkar P, Cameron RA (2017) Echinobase: an expanding resource for echinoderm genomic information. Database 2017:bax074
Kulakova MA, Cook CE, Andreeva TF (2008) ParaHox gene expression in larval and postlarval development of the polychaete Nereis virens (Annelida, Lophotrochozoa). BMC Dev Biol 8:61
Kungurtzeva LA, Dautov SS (2001) Ultrastructure of the digestive tract in the ophiopluteus of Ophiura sarsi. Invertebr Reprod Dev 39:209–220
Le Douarin NM (1988) On the origin of pancreatic endocrine cells. Cell 53:169–171
Lecroisey C, Le Pétillon Y, Escriva H, Lammert E, Laudet V (2015) Identification, evolution and expression of an insulin-like peptide in the cephalochordate Branchiostoma lanceolatum. PLoS One 10:e0119461
Lee PY, Davidson EH (2004) Expression of Spgatae, the Strongylocentrotus purpuratus ortholog of vertebrate GATA4/5/6 factors. Gene Expr Patterns 5:161–165
Lemaitre B, Miguel-Aliaga I (2013) The digestive tract of Drosophila melanogaster. Annu Rev Genet 47:377–404
Lengyel JA, Iwaki DD (2002) It takes guts: the Drosophila hindgut as a model system for organogenesis. Dev Biol 243:1–19
Lentz TL (1966) Intramitochondrial glycogen granules in digestive cells of Hydra. J Cell Biol 29:162–167
Lowe EK, Cuomo C, Arnone MI (2017) Omics approaches to study gene regulatory networks for development in echinoderms. Brief Funct Genomics 16:299–308
Malakhov V (1998) Embryological and histological peculiarities of the order Enoplida, a primitive group of nematodes. Russ J Nematol 6:41-46
Martin-Duran JM, Hejnol A (2015) The study of Priapulus caudatus reveals conserved molecular patterning underlying different gut morphogenesis in the Ecdysozoa. BMC Biol 13:29
Martin-Duran JM, Passamaneck YJ, Martindale MQ, Hejnol A (2016) The developmental basis for the recurrent evolution of deuterostomy and protostomy. Nat Ecol Evol 1:5
Masui T, Long Q, Beres TM, Magnuson MA, MacDonald RJ (2007) Early pancreatic development requires the vertebrate suppressor of hairless (RBPJ) in the PTF1 bHLH complex. Genes Dev 21:2629–2643
McEdward LR, Miner BG (2001) Larval and life-cycle patterns in echinoderms. Can J Zool 79:1125–1170
McGhee JD (2000) Homologous tails? Or tales of homology? BioEssays 22:781–785
McGhee JD (2013) The Caenorhabditis elegans intestine. Wiley Interdiscip Rev Dev Biol 2:347–367
Monaghan AP, Kaestner KH, Grau E, Schutz G (1993) Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development 119:567–578
Murtaugh LC, Stanger BZ, Kwan KM, Melton DA (2003) Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 100:14920–14925
Nezlin LP, Yushin VV (1994) The digestive tract of the echinopluteus of Echinocardium cordatum (Echinodermata, Echinoida): its ultrastructure and innervation. Can J Zool 72:2090–2099
Nielsen C (2017) Evolution of deuterostomy and origin of the chordates. Biol Rev Camb Philos Soc 92:316–325
Nielsen C (2019) Blastopore fate: amphistomy, protostomy or deuterostomy. In: eLS. John Wiley & Sons Ltd, Chichester. http://www.els.net [DOI: 10.1002/9780470015902.a0027481]
Nielsen C, Brunet T, Arendt D (2018) Evolution of the bilaterian mouth and anus. Nat Ecol Evol 2:1358–1376
Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV (1996) PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122:983–995
Ogawa M, Ishikawa T, Irimajiri A (1984) Adrenal chromaffin cells form functional cholinergic synapses in culture. Nature 307:66–68
Olinski RP, Dahlberg C, Thorndyke M, Hallböök F (2006) Three insulin-relaxin-like genes in Ciona intestinalis. Peptides 27:2535–2546
Oliveri P, Walton KD, Davidson EH, McClay DR (2006) Repression of mesodermal fate by foxa, a key endoderm regulator of the sea urchin embryo. Development 133:4173–4181
Osborne PW, Benoit G, Laudet V, Schubert M, Ferrier DE (2009) Differential regulation of ParaHox genes by retinoic acid in the invertebrate chordate amphioxus (Branchiostoma floridae). Dev Biol 327:252–262
Ostberg Y, Van Noorden S, Pearse AG, Thomas NW (1976) Cytochemical, immunofluorescence, and ultrastructural investigations on polypeptide hormone containing cells in the intestinal mucosa of a cyclostome, Myxine glutinosa. Gen Comp Endocrinol 28:213–227
de Pablo F, Chambers SA, Ota A (1988) Insulin-related molecules and insulin effects in the sea urchin embryo. Dev Biol 130:304–310
Pearse AGE, Polak JM (1971) Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut 12:783–788
Perillo M, Arnone MI (2014) Characterization of insulin-like peptides (ILPs) in the sea urchin Strongylocentrotus purpuratus: insights on the evolution of the insulin family. Gen Comp Endocrinol 205:68–79
Perillo M, Wang YJ, Leach SD, Arnone MI (2016) A pancreatic exocrine-like cell regulatory circuit operating in the upper stomach of the sea urchin Strongylocentrotus purpuratus larva. BMC Evol Biol 16:117
Perillo M, Paganos P, Mattiello T, Cocurullo M, Oliveri P, Arnone MI (2018) New neuronal subtypes with a “pre-pancreatic” signature in the sea urchin Stongylocentrotus purpuratus. Front Endocrinol (Lausanne) 9:650
Peter IS, Davidson EH (2010) The endoderm gene regulatory network in sea urchin embryos up to mid-blastula stage. Dev Biol 340:188–199
Peterson KJ, Cameron RA, Tagawa K, Satoh N, Davidson EH (1999a) A comparative molecular approach to mesodermal patterning in basal deuterostomes: the expression pattern of Brachyury in the enteropneust hemichordate Ptychodera flava. Development 126:85
Peterson KJ, Harada Y, Cameron RA, Davidson EH (1999b) Expression Pattern of Brachyury and Not in the Sea Urchin: Comparative Implications for the Origins of Mesoderm in the Basal Deuterostomes. Dev Biol 207:419–431
Petrucco S, Wellauer PK, Hagenbüchle O (1990) The DNA-binding activity of transcription factor PTF1 parallels the synthesis of pancreas-specific mRNAs during mouse development. Mol Cell Biol 10:254–264
Rast JP, Cameron RA, Poustka AJ, Davidson EH (2002) Brachyury target genes in the early sea urchin embryo isolated by differential macroarray screening. Dev Biol 246:191–208
Reece-Hoyes JS, Keenan ID, Isaacs HV (2002) Cloning and expression of the cdx family from the frog Xenopus tropicalis. Dev Dyn 223:134–140
Reinecke M, Collet C (1998) The phylogeny of the insulin-like growth factors. Int Rev Cytol 183:1–94
de Rosa R, Prud'homme B, Balavoine G (2005) Caudal and even-skipped in the annelid Platynereis dumerilii and the ancestry of posterior growth. Evol Dev 7:574–587
Schmidt-Rhaesa A (2007) The evolution of organ systems. Oxford University Press, Oxford
Shashikant T, Khor JM, Ettensohn CA (2018) Global analysis of primary mesenchyme cell cis-regulatory modules by chromatin accessibility profiling. BMC Genomics 19:206
Shoguchi E, Satoh N, Maruyama YK (1999) Pattern of Brachyury gene expression in starfish embryos resembles that of hemichordate embryos but not of sea urchin embryos. Mech Dev 82:185–189
Slack JM (1995) Developmental biology of the pancreas. Development 121:1569–1580
Stainier DY (2002) A glimpse into the molecular entrails of endoderm formation. Genes Dev 16:893–907
Stern CD (2004) Gastrulation: from cells to embryo. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF (1997) Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet 15:106–110
Strathmann RR (1975) Larval feeding in echinoderms. Am Zool 15:717–730
Sun J, Zhang L, Pan Y, Lin C, Wang F, Kan R, Yang H (2015) Feeding behavior and digestive physiology in sea cucumber Apostichopus japonicus. Physiol Behav 139:336–343
Tagawa K, Humphreys T, Satoh N (1998) Novel pattern of Brachyury gene expression in hemichordate embryos. Mech Dev 75:139–143
Thor S, Ericson J, Brännström T, Edlund T (1991) The homeodomain LIM protein Isl-1 is expressed in subsets of neurons and endocrine cells in the adult rat. Neuron 7:881–889
Treadwell AL (1901) The cytogeny of Podarke obscura Verrill. J Morphol 17:399–486
Trenzado CE, Hidalgo F, Villanueva D, Furné M, Díaz-Casado ME, Merino R, Sanz A (2012) Study of the enzymatic digestive profile in three species of Mediterranean sea urchins. Aquaculture 344-349:174–180
Vacquier VD (1971a) The appearance of -1,3-glucanohydrolase activity during the differentiation of the gut of sand dollar plutei. Dev Biol 26:1–10
Vacquier VD (1971b) The effects of glucose and lithium chloride on the appearance of -1,3-glucanohydrolase activity in sand dollar plutei. Dev Biol 26:11–16
Vacquier VD, Korn LJ, Epel D (1971) The appearance of -amylase activity during gut differentiation in sand dollar plutei. Dev Biol 26:393–399
Wang L, Hiebert SW (2001) TEL contacts multiple co-repressors and specifically associates with histone deacetylase-3. Oncogene 20:3716–3725
Wei Z, Angerer RC, Angerer LM (2011) Direct development of neurons within foregut endoderm of sea urchin embryos. Proc Natl Acad Sci U S A 108:9143–9147
Wessel GM, Zhang W, Klein WH (1990) Myosin heavy chain accumulates in dissimilar cell types of the macromere lineage in the sea urchin embryo. Dev Biol 140:447–454
Williams DC (1975) The occurrence and distribution of digestive enzymes in the pyloric caecum of the purple starfish Pisaster ochraceus. Comp Biochem Physiol A Comp Physiol 52:85–90
Winter WP, Neurath H (1970) Purification and properties of a trypsin-like enzyme from the starfish Evasterias trochelii. Biochemistry 9:4673–4679
Wood NJ, Mattiello T, Rowe ML, Ward L, Perillo M, Arnone MI, Elphick MR, Oliveri P (2018) Neuropeptidergic systems in pluteus larvae of the sea urchin Strongylocentrotus purpuratus: neurochemical complexity in a “simple” nervous system. Front Endocrinol (Lausanne) 9:628
Wu Q, Brown MR (2006) Signaling and function of insulin-like peptides in insects. Annu Rev Entomol 51:1–24
Wu LH, Lengyel JA (1998) Role of caudal in hindgut specification and gastrulation suggests homology between Drosophila amnioproctodeal invagination and vertebrate blastopore. Development 125:2433
Yaguchi J, Yaguchi S (2019) Evolution of nitric oxide regulation of gut function. Proc Natl Acad Sci U S A 116:5607–5612
Youson JH, Al-Mahrouki AA (1999) Ontogenetic and phylogenetic development of the endocrine pancreas (islet organ) in fish. Gen Comp Endocrinol 116:303–335
Youson JH, Al-Mahrouki AA, Amemiya Y, Graham LC, Montpetit CJ, Irwin DM (2006) The fish endocrine pancreas: review, new data, and future research directions in ontogeny and phylogeny. Gen Comp Endocrinol 148:105–115
Yui R, Fujita T (1986) Immunocytochemical studies on the pancreatic islets of the ratfish Chimaera monstrosa. Arch Histol Jpn 49:369–377
Zorn AM, Wells JM (2009) Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 25:221–251
Funding
This work was partially supported by the Marie Curie ITN EVONET (project 215781) to MIA (and fellowship to CA), fellowship of the SZN PhD program (to CC, MP and RA) and fellowships POR Campania FSE 2007–2013 Project MODO, Model Organism (to CA, MP and RA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Annunziata, R., Andrikou, C., Perillo, M. et al. Development and evolution of gut structures: from molecules to function. Cell Tissue Res 377, 445–458 (2019). https://doi.org/10.1007/s00441-019-03093-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03093-9