Abstract
Enteroendocrine cells are endocrine-like cells found in the luminal epithelia of the digestive tract. These cells have been described in most animal phyla. In echinoderms, the cells have been described mainly in organisms of the class Asteroidea (sea stars) and Holothuroidea (sea cucumbers). Here, we describe what is known about the enteroendocrine cells of the Echinodermata, including the cell types, their distribution in the digestive tract, their neuropeptide content and their regeneration and compare them to what has been found in other animal species, mainly in vertebrates. We also discuss the newly described view of enteroendocrine cells as chemical sensors of the intestinal lumen and provide some histological evidence that similar functions might be found within the echinoderms. Finally, we describe the temporal regeneration of the enteroendocrine cells in the holothurian intestine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enteroendocrine cells (EECs) are endocrine cells found within the digestive tract of animal species. These cells have been described in most, if not all, metazoans, where a digestive tract is present. They comprise what has been called a “diffuse endocrine system” that, in mammals, is thought to be the largest endocrine system of the body. The cells are thought to be involved in multiple functions that include the detection and response to environmental, microbial, nutrient and other factors. Their key localization in the luminal mucosa, at the interface between the intestinal lumen and the internal body system, reinforces their importance in transducing information from the environment that lies outside the body epithelial boundaries to the tissues of the organism.
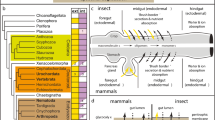
EECs have been best studied in vertebrate animals (Fig. 1a). They have been described as small granulated cells that are scattered among the cells of the luminal epithelium of the gastrointestinal tract (Fawcett 1994; Furness 2006; Latorre et al. 2016). They have an oval or pyramidal structure, lying on the basal lamina and many of them extending to the luminal surface where they have microvilli that come into contact with the luminal content. Their cell bodies contain numerous secretory granules that have been found to contain multiple types of amine and peptide hormones. These granules are usually localized toward the basal end of the cells and, when released, their contents are thought to enter the adjacent circulatory vessels and carried to their target cells in different body organs.
Models of enteroendocrine cells (EECs). EECs are portrayed as polarized cells in the mucosal membrane of the digestive tract that have their apical end projecting into the luminal space while basally they are attached to the basal lamina. a The “classical” view of these cells is that they are endocrine-like cells that release their contents into the adjacent vascular vessels. b The “emergent” model is that they have basal extensions or “neuropods” that contact directly the nervous system acting as neurosensors
There have been various attempts to classify EECs into subtypes. For example, a subtype of EEC that contained biological amines usually found in chromaffin cells (catecholamines and/or serotonin) was classified as enterochromaffin cells. Although they were initially considered to be different from peptide-containing EEC, further experimental work has shown that a large number of enterochromaffin cells also expressed peptide hormones (Diwakarla et al. 2017; Fothergill and Furness 2018). Other classification attempts relied on the type of neuropeptide hormone that the cells expressed but recent research has shown that multiple peptides could be expressed by single EECs. This was made evident by the application of single-cell sequencing to the EECs, showing that there are at least eight clusters of mature EECs that can be identified in the mammalian small intestine and that key hormones were expressed by cells in different clusters (Haber et al. 2017). These new findings have made the EEC classification systems defined by a single hormone or by a letter code obsolete.
In the last few years, investigators’ perception of EECs has taken a new twist. Traditionally, EECs have been perceived as being “classical endocrine” cells releasing their peptide or neuroactive contents in a paracrine or endocrine mode. However, recent experiments in mammals have shown that, at least some EECs have axon-like basal processes, named neuropods, that form synapse-like connections with nerves (Bohórquez et al. 2011, 2015; Liddle 2019) (Fig. 1b). This has led to a new view of, at least some EECs, being epithelial sensory transducers equivalent to the olfactory cells in the nose or to taste cells in taste buds. In this way, these EECs contact directly the fiber of a sensory neuron that takes the information directly to the brainstem (Bohórquez et al. 2015). Hence, the use of the terminology “gut epithelial sensors” to highlight the sensory/electrical transducer nature of the EECs (Kaelberer and Bohórquez 2018).
Peptide-like immunoreactivity in echinoderm EECs
As for other animal species, EECs have been described within the digestive tract of echinoderms. (Table 1 lists the various EECs that have been described, the method of detection, the specie, and the reference). The vast majority of EEC descriptions have been done using immunohistological techniques, mainly with antibodies against specific neuropeptides. This implies that most of the earlier reports identify what has been named as “peptide-like immunoreactivity” in view that the antibody could be recognizing a similar but not identical antigen and that there is no certainty that the peptide being recognized is the “real” peptide.
To our knowledge, the first EEC descriptions in the phylum Echinodermata were done by Martinez and colleagues (Martínez et al. 1989). The cells were found in the pyloric caeca of a sea star (Marthasterias glacialis) using electron microscopy and immunohistochemistry. EECs were described within the luminal epithelium of this organ using histological stains (Grimelius silver impregnation). They were shown to have the typical traits of EECs (Figs. 2, 3, 4 and 5 show typical echinoderm EECs): a thin oval morphology that extends from the basal lamina to the lumen, an elongated nucleus in a central position and large numbers of secretory granules that are usually electron-dense. A long apical cytoplasmic process extended to the lumen and the basal region was in direct contact with nerve fibers. Some of these cells were found to express neuropeptide-like immunoreactivity to somastotatin, glucagon and pancreatic polypeptide.
Anatomy of echinoderm intestinal system showing the localization of enteroendocrine cells. a Diagram of transverse section of intestinal system. b Higher magnification showing the localization of two enteroendocrine cells within the luminal epithelium. c Typical morphology of an enteroendocrine cell showing the elongated body extending from the lumen to the basal lamina. ce coelomic epithelium, cm circular muscle, ic internal connective tissue, le luminal epithelium, lm longitudinal muscle. Bar = 25 μm
In situ hybridization for corazonin neuropeptide. a, b Transverse section of the pyloric duct of the sea star Asterias rubens showing the mRNA in some enteroendocrine cells in the mucosal layer. Cells expressing the mRNA for the peptide are marked with arrowheads in low (a) and high (b) magnification. lu lumen, me mesentery, mu mucosal layer, vml visceral muscle layer. From Tian et al. (2017). Bar = a 90 μm and b 20 μm
Electron microscopy of enteroendocrine cells in the sea star Marthasterias glacialis. a Diagram of accompanying electron micrograph. b Enteroendocrine cell (e) contacting nerve terminals (asterisks). c Higher magnification of nerve processes. From Martínez et al. (1989). Bar = 1.5 μm
The pyloric caeca is an organ specific to the digestive tract of asteroids but in later studies, Martinez and other investigators showed the presence of EECs in other regions of the digestive system (Martínez et al. 1993, 1994, 1996). In addition, they expanded the number of neuropeptides that could be found within the neuroendocrine cells and fine-tuned the description of the asteroid EEC system. Thus, EECs were found throughout the digestive system of the sea star, including the cardiac and pyloric stomach, the intestine and the pyloric and rectal caeca (Martínez et al. 1993, 1996). Immunoreactivity to alpha-MSH, PYY, adrenomedullin and FMRFamide was also detected in EECs (Martínez et al. 1993, 1996). However, the definitive result was the finding of S1 immunoreactivity in EECs, since this was the first neuropeptide isolated from echinoderms (in what will be named the SALMFamide peptide family) and as such the first neuropeptide where the observed immunoreactivity in the EEC could be directly correlated to the peptide sequence (Elphick et al. 1991). Moreover, these findings were confirmed by immunocytochemical studies in the digestive system of a second starfish species, Asterias rubens (Moore and Thorndyke 1993; Newman et al. 1995).
Neuropeptide-containing EECs have also been described within the luminal epithelia of other echinoderm classes. In the sea cucumber Holothuria glaberrima, our group has shown that cells immunoreactive to GFSKLYFamide, a holothurian neuropeptide of the SALMFamide family, are found in the small and large intestine (Díaz-Miranda et al. 1995) (Fig. 3). Immunoreactivity to a different peptide, calbindin, has also been documented in the intestine of H. glaberrima (Díaz-Balzac et al. 2016). Another neuropeptide that has been isolated and characterized from holothurian tissues NGIWamide has also been documented in the EECs of the sea cucumber Apostichopus japonicus (Inoue et al. 1999). Thus, in holothurians, similar to sea stars, EECs have been described that expressed holothurian-isolated neuropeptides and these cells can be found along most of the digestive tract including esophagus, stomach, small and large intestines and rectum (García-Arrarás et al. 2001).
Echinoderm EECs have been immune-labeled with other non-peptide markers. In this respect, they have been shown to express immunoreactivity to C-terminal amidating enzymes (PAM) and the nitric oxide synthetase enzyme in the digestive system of asteroids (Martínez et al. 1993, 1994) and to Pax6 and Nurr1 transcription factors in the digestive system of holothurians (Díaz-Balzac et al. 2014).
The genomic era of neuropeptide characterization
In the not so distant past, EECs were recognized by the expression of peptide-like immunoreactivity; however, in most of these cases, the specific nature of the immunoreactivity (the peptide sequence) remained uncertain. In recent years, the situation has reversed. The sequencing of the sea urchin genome and the study of mRNA transcriptomes caused a major shift in the study of the echinoderm neuroendocrine system. While previously most of the studies depended on the purification and characterization of neuropeptides using protein isolation technologies or on the identification of peptide-like immunoreactivity using anti-sera, the genomic and transcriptomic studies suddenly provided the sequence to multiple putative neuropeptides that now needed to be characterized in relation to their cellular/tissue localization (Burke et al. 2006; Rowe and Elphick 2012). Thus, in situ hybridization was used to determine the expression of putative neuroactive peptides in EECs (see Fig. 4 as an example). In addition, a few studies managed to bridge the gap in knowledge and used immunohistochemistry and mRNA in situ hybridization to reveal the expression by EECs of putative peptides encoded by the genes characterized from the genomic data. These studies mainly led by Elphick and colleagues are summarized in Table 1.
Nonetheless, while genomic or transcriptomic studies have been done in members of most echinoderm classes (Rowe and Elphick 2012; Rowe et al. 2014; Semmens et al. 2016; Zandawala et al. 2017; Kim et al. 2018; Suwansa-ard et al. 2018) and new putative neuropeptides have been described, the characterization of EECs expressing these peptides has been limited to members of the Asteroidea and the Holothuroidea. To the best of our knowledge, no neuropeptide-expressing cell has been described in sea urchins, ophiuroids, or crinoids.
Echinoderm EECs share many characteristics of vertebrate EECs
It is important to highlight that in addition to their elongated morphology that extends from the basal lamina to the lumen and their vesicular content, echinoderm EECs share many other similarities with vertebrate EECs. First, similar to vertebrates the peptides expressed within the EECs are the same peptides expressed by neurons of the central and peripheral nervous system. For example, in vertebrates, EECs might express PYY or CCK, while in echinoderms, EECs express the same peptides that are expressed in the echinoderm neurons, such as SALMFamides or corazonin-type peptides. This makes the functional characterization of the EECs very difficult because sometimes neuronal fibers containing the neuropeptide can be found within the digestive tract and even within the adjacent subepithelial plexus that underlies the luminal epithelium. Thus, an effect elicited by extrinsic neuropeptide addition cannot be clearly ascribed to the nervous system or to the EECs.
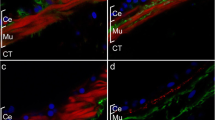
Second, there are different populations of EECs. In vertebrates, as explained earlier, the neuropeptide content has been used to identify different cell populations. In echinoderms, of the many peptide markers that have been identified, some show specific distributions that suggest different subpopulations. In our laboratory, we put this to the test by labeling cells with anti-GFSKLYFamide and with anti-calbindin. These markers identify two different subpopulations of EECs. They show somewhat different morphologies, where those expressing GFSKLYamide usually have an extension that enters the connective tissue. In addition, their localization within the epithelial lumen differs: the nuclei of those expressing GFSKLYamide are located at about 12 μm from the basal lamina, similar to those of most other luminal cells, while the nuclei of those expressing calbindin are located at about 30 μm, much closer to the cells apical end (unpublished observations) (Fig. 3). Thus, in sea cucumbers, at least two populations of EECs are present.
Third, the number of EECs is relatively small in comparison with the number of other cells in the luminal epithelium. In experiments done in our laboratory, the number of EECs labeled with either GFSKLYFamide or calbindin in a particular region of the digestive tract of a holothurian lies between 4 and 7% (unpublished observations).
Fourth, they are found along the entire length of the digestive tract. Although their numbers might differ, EECs can be found along the entire digestive tract and cells in different locations might express the same or different neuropeptides. Even within a region of the digestive tract, the EEC populations might be heterogeneously distributed. For example, cells expressing ArPPLN1-type peptides in the digestive system of A. rubens can be found throughout the cardiac stomach but in the pyloric stomach they are mainly found within floor region and are concentrated on the oral side of the pyloric ducts and of the pyloric caeca (Lin et al. 2017).
There are, however, some differences between echinoderm and vertebrate EECs. The main one lies in that while EECs expressing serotonin have been widely described in vertebrates (Bordi et al. 2000; Gershon 2013), no echinoderm serotonergic EEC has been described.
EECs as “gut epithelial sensors”
It is interesting that some echinoderm EECs perfectly fit the description as epithelial sensory components of the nervous system (Bohórquez et al. 2015; Liddle 2019). In the initial description of echinoderm EECs, there is already the description of how EECs usually have nerve terminals closely associated with their basal end (Fig. 5). The contact between the nerve fibers and the endocrine cells is very close where both membranes are seen in close association, contrary to what had been described in mammals that the nerves do not cross the basal lamina. It took almost three decades to finally show that nerve fibers in mammals do cross the luminal epithelia basal lamina and synapse with the EECs.
One of the findings in holothurians is that some of the neuroendocrine-like cells extend long neurite-like fibers that can be followed into the submucosa or connective tissue and in some cases even to the muscle layer in the mesothelium (Fig. 6) (García-Arrarás et al. 2001).
GFSKLYFamide expressing cell in the luminal epithelium of the sea cucumber Holothuria glaberrima. The cell body (arrow) is labeled in the cytoplasm and a long axon-like process extends from it that runs through the connective tissue and enters the muscle layer (arrowhead). CE coelomic epithelium, IC internal connective tissue, LE luminal epithelium. Bar = 50 μm
Neuroendocrine cell regeneration
Echinoderms are well known for their regenerative abilities. In fact, in recent years, regeneration of the sea cucumber digestive system has been well studied by our group. We described that EECs are found within the regenerating intestine and the timing of their reappearance (García-Arrarás et al. 1999; Tossas et al. 2014). Our data show that not only the intestinal EECs are regenerated but that they appear very early in the formation of the luminal epithelia (Fig. 7). Soon after the luminal epithelia forms, the first EECs can be observed (Tossas et al. 2014). However, the results suggest that not all neuroendocrine cell types appear simultaneously but that different subpopulations appear at different regeneration stages, being those labeled with the anti-calbindin antibody the first to appear, followed by those expressing GFSKLYamide and the latest population to appear are those labeled with the anti-Pax6 marker.
Enteroendocrine cell regeneration in H. glaberrima intestine. a Diagram of regenerating intestine at different stages showing the appearance of enteroendocrine cells expressing calbindin (red), GFSKLFYamide (blue) and Pax6 (green) immunoreactivity. Immunoreactive cells are shown at b 10 days of regeneration for calbindin, c 14 days for GFSKLYFamide and d 21 days for Pax6. (Modified from Tossas et al. 2014). Bar = 25 μm
Concluding remarks
In summary, extensive evidence is available that describes EECs in echinoderms. At present, most of the information is restricted to a small number of species from two classes of the Echinodermata: the Holothuroidea and the Asteroidea. Future experiments will surely extend these findings to other species and other classes. This will provide an extended view of comparative findings that will help to discern the commonalities of EECs in echinoderms. Similarly, future studies should provide information on the number and different types of EECs found in the digestive tract and a cohesive view of how these cell types are related to those of other deuterostomes.
Future studies should also address the origin of the EECs. While it is well established that most of the echinoderm nervous system originates from the embryonic ectoderm (Mashanov et al. 2007; Hinman and Burke 2018), the origin of the EECs is assumed to be the same as in its vertebrate relatives. Thus, EECs are thought to be of endodermal origin and it has been assumed that they are formed during intestinal homeostasis and regeneration in the same way as they are in mammals, that is, from a multipotent stem cell in the luminal epithelium. In view of the phylogenetic distance of echinoderms and mammals, it might be interesting to determine if this assumption is correct.
Arguably, one of the least explored characteristics of the EECs is their function. Although the neuroactive molecules that are found within echinoderm EECs have been well described and continue to be expanded, what are now necessary are studies on the functional aspects of these cells and their contents. The effects of neuropeptides on gut motility have been reported in sea cucumbers (CCK, GFSKLYFamide, NGIWYamide) and starfish (S1, S2, NGFFYamide, ArGnRH, ArCRZ, ArPPLN1-type peptide and ArPPLN2-type peptide). However, as mentioned above, the presence of the same neuropeptide content in both EECs and nerve fibers increases the difficulty in studying EEC function, making it impossible to ascertain a specific role to the EECs versus the enteric nerves. Additional limitations are found in the lack of genetic/molecular tools, such as those used in vertebrates (Sinagoga et al. 2018; Kaelberer et al. 2018), to identify EEC function. Nonetheless, we expect that in the not so distant future, similar tools will be made available to pursue the functional analysis of echinoderm cells. This should include EECs, endocrine cells and neurons associated with the digestive tract.
References
Bohórquez DV, Chandra R, Samsa L, Vigna S, Liddle R (2011) Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol 42:3–13
Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA (2015) Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 125(2):782–786
Bordi C, D’Adda T, Axxoni C, Ferraro G (2000) Classification of gastric endocrine cells at the light and electron microscopical levels. Microsc Res Tech 48(5):258–271
Burke RD, Angerer LM, Elphick MR, Humphrey GW, Yaguchi S, Kiyama T, Liang S, Mu X, Agca C, Klein WH, Brandhorst BP, Rowe M, Wilson K, Churcher AM, Taylor JS, Chen N, Murray G, Wang D, Mellot D, Olinski R, Hallböök F, Thorndyke MC (2006) A genomic view of the sea urchin nervous system. Dev Biol 300(1):434–460
Cai W, Kim CH, Go HJ, Egertova M, Zampronio CG, Jones AM, Park NG, Elphick MR (2018) Biochemical, anatomical, and pharmacological characterization of calcitonin-type neuropeptides in starfish: discovery of an ancient role as muscle relaxants. Front Neurosci 12:382
Díaz-Balzac CA, Vázquez-Figueroa LD, García-Arrarás JE (2014) Novel markers identify nervous system components of the holothurian nervous system. Invertebr Neurosci 14(2):113–125
Díaz-Balzac CA, Lázaro-Peña MI, Vázquez-Figueroa LD, Díaz-Balzac RJ, García-Arrarás JE (2016) Holothurian nervous system diversity revealed by neuroanatomical of the sea urchin genome analysis. PLoS One 11(3):e0151129
Díaz-Miranda L, Blanco RE, García-Arrarás JE (1995) Localization of the heptapeptide GFSKLYFamide in the sea cucumber Holothuria glaberrima (Echinodermata): a light and electron microscopy study. J Comp Neurol 352:626–640
Diwakarla S, Fothegill LJ, Fakhry J, Callaghan J, Furness JB (2017) Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol Motil 29(6). https://doi.org/10.1111/nmo.13101
Elphick MR, Price DA, Lee TD, Thorndyke MC (1991) The SALMFamides: a new family of neuropeptides isolated from and echinoderm. Proc R Soc Lond 243:121–127
Fawcett DW (1994) Bloom and Fawcett, a textbook of histology. Chapman& Hall, NY
Fothergill LJ, Furness JB (2018) Diversity of enteroendocrine cells investigated at cellular and subcellular levels: the need for a new classification. Histochem Cell Biol 150:693–702
Furness JB (2006) The enteric nervous system. Blackwell Publishing, Massachusetts
García-Arrarás JE, Díaz-Miranda L, Torres II, File S, Jiménez LB, Rivera-Bermudez K, Arroyo EJ, Cruz W (1999) Regeneration of the enteric nervous system in the sea cucumber Holothuria glaberrima. J Comp Neurol 406:461–475
García-Arrarás JE, Rojas-Soto M, Jiménez LB, Díaz-Miranda L (2001) The enteric nervous system of echinoderms: unexpected complexity revealed by neurochemical analysis. J Exp Biol 204:865–873
Gershon MD (2013) 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 20(1):14–21
Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shin HN, Yilmaz O, Xavier RJ, Regev A (2017) A single-cell survey of the small intestinal epithelium. Nature 551:333–339
Hinman VF, Burke RD (2018) Embryonic neurogenesis in echinoderms. Wiley Interdiscip Rev Dev Biol 7(4):e316
Inoue M, Birenheide R, Koisumi O, Kobayakawa Y, Muneoka Y, Motokawa T (1999) Localization of the neuropeptide NGIWYamide in the holothurian nervous system and its effects on muscular contraction. Proc R Soc B Biol Sci 266:993–1000
Kaelberer MM, Bohórquez DV (2018) The now and then of gut-brain signaling. Brain Res 1693:192–196
Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, Bohórquez DV (2018) A gut-brain neural circuit for nutrient sensory transduction. Science 361(6408):eaat5236
Kim CH, Go HJ, Oh Y, Jo YH, Elphick MR, Park NG (2018) Transcriptomics reveals tissue/organ-specific differences in gene expression in the starfish Patiria pectinifera. Mar Gen 37:92–96
Latorre R, Sternini C, De Giorgio R, Greenwood-Van Meerveld B (2016) Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil 28(5):620–630
Liddle RA (2019) Neuropods. Cell Mol Gastrol Hepatol In press. https://doi.org/10.1016/j.jcmgh.2019.01.006
Lin M, Egertová M, Zampronio CG, Jones AM, Elphick MR (2017) Pedal peptide/orcokinin-type neuropeptide signaling in a deuterostome: the anatomy and pharmacology of starfish myorelaxant peptide in Asterias rubens. J Comp Neurol 525(18):3890–3917
Lin M, Egertová M, Zampronio CG, Jones AM, Elphick MR (2018) Functional characterization of a second pedal peptide/orcokinin-type neuropeptide signaling system in the starfish Asterias rubens. J Comp Neurol 526:858–876
Martínez A, Villaro AC, Sesma P (1989) Microscopic study of the pyloric caeca of the starfish Marthasterias glacialis (Echinodermata): finding of endocrine cells. J Morphol 202:151–164
Martínez A, López J, Montuenga LM, Sesma P (1993) Regulatory peptides in the gut endocrine cells and nerves in the starfish Marthasterias glacialis. Cell Tissue Res 271:375–380
Martínez A, Riveros-Moreno V, Polak JM, Moncada S, Sesma P (1994) Nitric oxide (NO) synthase immunoreactivity in the starfish Marthasterias glacialis. Cell Tissue Res 275:599–603
Martínez A, Unsworth EJ, Cuttitta F (1996) Adrenomedullin-like immunoreactivity in the nervous system of the starfish Marthasterias glacialis. Cell Tissue Res 283:169–172
Mashanov VS, Zueva OR, Heinzeller T, Aschauer B, Dolmatov IY (2007) Developmental origin of the adult nervous system in a holothurian: an attempt to unravel the enigma of neurogenesis in echinoderms. Evol Dev 9(3):244–256
Moore SJ, Thorndyke MC (1993) Immunocytochemical mapping of the novel echinoderm neuropeptide SALMFamide 1 (S1) in the starfish Asterias rubens. Cell Tissue Res 274:605–618
Newman SJ, Elphick MR, Thorndyke MC (1995) Tissue distribution of the SALMFamide neuropeptides S1 and S2 in the starfish Asterias rubens using novel monoclonal and polyclonal antibodies. I. Nervous and locomotory systems. Proc R Soc Lond B 261:139–143
Rowe ML, Elphick MR (2012) The neuropeptide transcriptome of a model echinoderm, the sea urchin Strongylocentrotus purpuratus. Gen Comp Endocrinol 179:331–344
Rowe ML, Achhala S, Elphick MR (2014) Neuropeptides and polypeptide hormones in echinoderms: new insights from analysis of the transcriptome fo the sea cucumber Apostichopus japonicus (2014). Gen Comp Endocrinol 197:43–55
Semmens DC, Mirabeau O, Moghul I, Pancholi MR, Wurm Y, Elphick MR (2016) Transcriptomic identification of starfish neuropeptide precursors yields new insights into neuropeptide evolution. Open Biol 6:150224
Sinagoga KL, McCauley HA, Múnera JO, Reynolds NA, Enriquez JR, Watson C, Yang HC, Helmrathe MA, Wells JM (2018) Deriving functional human enteroendocrine cells from pluripotent stem cells. Development 145(19):dev165795
Suwansa-ard S, Chaiyamoon A, Talarovicova A, Tinikul R, Tinikul Y, Poomtong T, Elphick MR, Cummins SF, Sobhon P (2018) Transcriptomic discovery and comparative analysis of neuropeptide precursors in sea cucumber (Holothuroidea). Peptides 99:231–240
Tian S, Egertová M, Elphick MR (2017) Functional characterization of paralogous gonadotropin-releasing hormone-type and corazonin-type neuropeptides in an echinoderm. Front Endocrinol 8:1–24
Tinoco AB, Semmens DC, Patching EC, Gunner EF, Egertová M, Elphick MR (2018) Characterization of NGFFYamide signaling in starfish reveals roles in regulation of feeding behavior and locomotory systems. Front Endocrinol 9:507
Tossas K, Qi-Huang S, Cuyar E, García-Arrarás JE (2014) Temporal and spatial analysis of enteric system regeneration in the sea cucumber Holothuria glaberrima. Regeneration 1(3):10–26
Yañez-Guerra LA, Delroisse J, Barreiro-Iglesias A, Slade SE, Scrivens JH, Elphick MR (2018) Discovery and functional characterisation of a luqin-type neuropeptide signaling system in a deuterostoms. Sci Rep 8:7220
Zandawala M, Moghul I, Yañez-Guerra LA, Delroisse J, Abulkassimova N, Hugall AF, O’Hara TD, Elphick MR (2017) Discovery of novel representatives of bilaterian neuropeptide families and reconstruction of neuropeptide precursor evolution in ophiuorid echinoderms. Open Biol 7:170129
Acknowledgments
We would like to thank Ms. Griselle Valentin for editorial help and the preparation of the figures.
Funding
This project was funded by NIH (Grant R15NS01686). We also acknowledge partial support from NIH R21AG057974 and the University of Puerto Rico.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Most data have been previously published and are available in the scientific literature. In the case of unpublished data applicable, international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
García-Arrarás, J.E., Lefebre-Rivera, M. & Qi-Huang, S. Enteroendocrine cells in the Echinodermata. Cell Tissue Res 377, 459–467 (2019). https://doi.org/10.1007/s00441-019-03053-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03053-3