Abstract
For the development of uterine receptivity, many morphological and molecular changes occur in the apical surface of luminal uterine epithelial cells (UECs) including an increase in vesicular activity. Vesicular movements for exocytosis and endocytosis are dependent on microtubules; however, changes in microtubules in UECs during early pregnancy have received little attention. β-tubulin, one of the main component of microtubules, is distributed throughout the cytoplasm of UECs on day 1 (non-receptive) of pregnancy in the rat. On day 5.5, β-tubulin is concentrated above the nuclei and by day 6 (receptive), β-tubulin is concentrated in a band-like fashion above the nucleus. Western blot analysis of isolated UECs found two bands (50 and 34 kDa) for β-tubulin in UECs during early pregnancy. The intensity of the 34 kDa band was significantly higher on day 6 compared to day 1. The increase in the 34 kDa band may be due to higher proteolytic activity associated with microtubule polymerisation during the receptive state. Transmission electron microscopy showed fragmented microtubules at the time of receptivity in UECs. This is the first study to show that microtubules are reorganised during uterine receptivity. This re-organisation likely facilitates vesicular movement and promotes the reorganisation of the apical plasma membrane for uterine receptivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During uterine receptivity, the apical plasma membrane of the luminal uterine epithelial cells (UECs) undergoes a morphological and molecular re-organisation to become receptive to the blastocyst (Murphy 1994; Png and Murphy 2000). This involves the loss of long evenly spaced microvilli, the actin terminal web becomes disorganised and non-adhesive proteins are lost. These changes have been linked to aspects of epithelial-mesenchymal transition (Denker 1990). At this time, the apical surface instead has adhesive proteins, uterodomes, an increase in plasma membrane cholesterol and an increase in intramembranous particles (Murphy and Martin 1985, 1987, Murphy 1993, 1994, 2004; Aplin 1997; Moore et al. 2016). Collectively these changes are now termed “the plasma membrane transformation” (Murphy 2004). Also, during the receptive phase, on day 5.5 (apposition stage) of pregnancy in the rat, there is a large number of vesicles present in the apical cytoplasm, which dissipate after receptivity. These vesicles are thought to be involved in this re-organisation of the apical plasma membrane during uterine receptivity (Parr 1982).
The movement of vesicles is dependent upon the presence of microtubules; however, the organisation of microtubules in UECs during early pregnancy has not been examined (Lunam and Murphy 1983; Sheetz et al. 1987). A previous study examined the morphological effects of the microtubule inhibitor colchicine on UECs using low-power transmission electron microscopy (TEM). This study showed a reduction in microvillar length but microtubules themselves were not examined (Lunam and Murphy 1983). This maybe because microtubules are difficult to visualise with routine TEM protocols and technology at the time had limited ability to resolve microtubules. Thus, the present study optimised a new method more suitable to visualise microtubules in the TEM in UECs during early pregnancy.
Microtubules are long cytoskeletal filaments made of α- and β-tubulin in a tubular heterodimer (Mandelkow et al. 1977; Lodish et al. 2000). Microtubules function as transport intermediates to facilitate movement of vesicles within the cell to precise target areas (Cole and Lippincott-Schwartz 1995). These vesicles are composed of a vesicular membrane and vesicle content plus are involved in both exocytic and endocytic pathways (Cole and Lippincott-Schwartz 1995; Yeaman et al. 1999). These vesicle pathways are directed by motor proteins that travel along microtubules. The vesicular transport direction is signalled by SNARE proteins and various other microtubule-associated proteins. These proteins maintain microtubule dynamics and organisation within a cell to accommodate their changing needs (Kraemer et al. 1999; Tai et al. 1999; Pelham 2001; Müsch 2004; Jolly and Gelfand 2010).
In epithelial cells, microtubule asymmetry allows efficient polarised vesicle targeting. Microtubules are polarised with the minus-ends directed for exocytosis and plus-ends directed for endocytosis. The minus ends of the microtubules are slow growing and the plus ends are fast growing and this polarity facilitates directional vesicular movement (Kraemer et al. 1999; Tai et al. 1999). During uterine receptivity, exocytic and endocytic activity are at their peak and are thought to enable rapid remodelling of the apical surface (Parr 1980, 1982; Murphy and Martin 1987; Ng et al. 2013). Thus it is hypothesised that this will be reflected by a corresponding reorganisation of microtubules in UECs during uterine receptivity.
Materials and methods
Animals and mating
This study used female virgin Wistar rats aged 10–12 weeks. All procedures were approved by The University of Sydney Animal Ethics Committee. Rats were housed in plastic cages at 21 °C under a 12 h light-dark cycle and were provided with free access to food and water. Pro-oestrus female rats were mated overnight with males of proven fertility. The presence of sperm in a vaginal smear the following morning indicated successful mating and this was designated day 1 of pregnancy. Uterine tissue was collected from five rats each from days 1, 3, 5.5, 6 and 7 of pregnancy. In total, 25 rats were used for this study.
Tissue collection
Rats were administered 20 mg/kg of sodium pentobarbitone (Vibac Animal Health, NSW, Australia) intraperitoneally and the uterine horns were collected under deep anaesthesia, before euthanasia. The uterine horns were then used for immunofluorescence, Western blotting and TEM.
Immunofluorescence analysis
Uterine horns (5 mm pieces) were coated with Tissue-Tek OCT (Sakura Finetek, CA, USA), snap frozen in supercooled isopentane (BDH Laboratory supplies, Poole Dorset, England, UK) and stored under liquid nitrogen until use. Frozen sections (7 μm) were cut using a Leica CM 3050 cryostat (Leica, Heerbrugg, Switzerland) and air-dried on gelatine-chrome alum-coated slides.
Sections were fixed in 4% formaldehyde in 0.1 M phosphate buffer for 10 min at room temperature, washed with PBS and blocked with 1% bovine serum albumin (BSA; Sigma Aldrich, MO, USA) in PBS for 30 min at room temperature. Sections were incubated overnight at 4 °C with mouse monoclonal anti-β-tubulin antibody (10 μg/ml; T8328; Sigma Aldrich) diluted in PBS/1% BSA. Concurrently, control sections were incubated with non-immune IgG (Sigma Aldrich) at the same concentration as the primary antibody. Sections were washed with PBS and incubated with FITC-conjugated goat anti-mouse IgG (3 μg/ml; 115-095-166; Jackson ImmunoResearch, PA, USA) for 30 min at room temperature. Sections were washed in PBS and mounted using Vectashield with DAPI (Vector Laboratories, CA, USA) and coverslipped (No. 1 thickness).
A ZEISS Axio Imager deconvolution Microscope (Carl Zeiss, Australasia) with a ZEISS AxioCam HR digital monochrome CCD camera (Carl Zeiss, Australasia) was used to acquire images through ZEN 2013 (Blue edition) software (Carl Zeiss, Australasia).
Isolation of rat luminal uterine epithelial cells
UECs were isolated as previously described (Kaneko et al. 2008) and immediately placed into lysis buffer (50 mM Tris–HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 0.1% SDS, 0.5% Deoxycholic acid, 1% Igepal and 1% protease inhibitor cocktail; Sigma Mammalian Cell lysis kit, Sigma Aldrich) with 10% PhosSTOP phosphatase inhibitor (Roche, NSW, Australia). The isolated cells were homogenised using a 23-gauge needle and a 1-ml syringe (Livingstone International, Rosebery, NSW, Australia) and centrifuged at 8000×g at 4 °C for 3 min. The supernatant was collected and frozen immediately in liquid nitrogen and stored at − 80 °C until use.
Western blot analysis
Protein concentrations were determined using the BCA assay (Micro BCA™ protein assay kit; Thermo Fisher Scientific, MA, USA) and CLARIOstar microplate reader (BMG labtech Durham, NC, USA) according to the manufacturer’s instructions. Protein samples (20 μg) and sample buffer (8% glycerol, 50 mM Tris–HCl, pH 6.8, 1.6% SDS, 0.024% bromophenol blue, 4% β-2-mercaptoethanol) were heated at 95 °C for 5 min prior to loading onto a 10% TGX FastCast SDS-polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA, USA; cat# 161-0183). Proteins were separated through electrophoresis at 200 V for 40 min then transferred to a polyvinylidene difluoride (PVDF) membrane (Immunobilon™ transfer membrane; Millipore, Bedford, MA, USA) at 100 V for 1 h 30 min. Membranes were blocked with 5% skim milk in TBS-t (10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20) for 1 h at room temperature with constant agitation then incubated with mouse monoclonal anti-β-tubulin antibody (1 μg/ml; T8328; Sigma Aldrich) diluted in 1% skim milk in TBS-t overnight at 4 °C on a rocking platform. The membranes were washed 3 × 10 min with TBS-t and subsequently incubated for 2 h with HRP-conjugated sheep anti-mouse IgG antibody (1 μg/ml; GE Healthcare, OH, USA) at room temperature with constant agitation. Protein bands were detected with the use of Immobilon Western HRP Substrate (Merck Millipore, MA, USA) and captured using a CCD camera and the Bio-Rad ChemiDoc MP System (Bio-Rad). Membranes were then incubated in stripping buffer (62.5 mM Tris–HCl (pH 6.7), 2% SDS and 100 mM β-mercaptoethanol) at 60 °C for 45 min and reprobed with mouse monoclonal anti-β-actin antibody (0.4 μg/ml; A1978; Sigma Aldrich) and HRP-conjugated sheep anti-mouse IgG antibody to ensure equal loading.
Densitometry analysis
Protein band intensities were quantified using the Volume Analysis Tool with local background subtraction in Bio-Rad Image Lab 4.0 software (Bio-Rad) and normalised to β-actin from the same lane. Statistical analysis was performed on normalised intensities with GraphPad Prism Software (Version 6.04, GraphPad Software, Inc., CA, USA). Variations in band intensity as a function of day of pregnancy (day 1, 3, 5.5, 6 and 7) were analysed using one-way analysis of variance (ANOVA). For multiple comparisons, Tukey’s post-hoc test was applied (reporting multiplicity adjusted P values) to determine which pairs of means were significantly different. Probability values of P < 0.05 were considered significant. All graphs were generated using GraphPad Prism Software and demonstrate the mean ± SEM.
Transmission electron microscopy
Uteri were cut into 5 mm pieces and were directly fixed in Karnovsky’s fixative (2.5% glutaraldehyde (ProSciTech, Australia), 2% paraformaldehyde (ProSciTech, Australia) in 0.1 M Sorenson’s phosphate buffer (PB, pH 7.4)) for 45 min at room temperature. The tissue was further cut into 0.5–1 mm slices under a droplet of fixative and returned to fresh fixative for another 45 min. The tissue was washed in 0.1 M PB then washed again with 0.1 M maleate buffer (MB). Next, the tissue was post fixed with 1% tannic acid in 0.1 M MB. Tissue was rinsed in 0.1 M MB, then dH20 and further fixed with 1% uranyl acetate in dH20. Tissue was rinsed again in dH20 and dehydrated with a graded series of ethanol then infiltrated with Spurr’s resin (SPI supplies, Leicestershire, England, UK). Uterine slices were embedded in fresh Spurr’s resin in BEEM® capsules (ProSciTech, Australia) and polymerised at 60 °C for 24 h. Two blocks per animal were cut at 60–70 nm using a Leica Ultracut S ultramicrotome (Leica, Heerbrugg, Switzerland) and mounted onto 400-mesh copper grids. Sections were post-stained with 2% uranyl acetate in dH20 for 10 min and then by Reynold’s lead citrate for 10 min. Sections were examined in a Jeol 1400 TEM (Jeol Ltd., Japan) at 100 kV. Microtubule visualisation was confirmed by measuring the filaments diameter, which was consistently around 20 nm.
Results
β-Tubulin is present in luminal UECs during early pregnancy
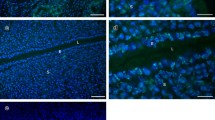
Immunofluorescence was used to localise β-tubulin in rat luminal UECs on days 1, 3, 5.5, 6 and 7. On days 1 and 3 of pregnancy, β-tubulin was evenly distributed throughout the cytoplasm of the cell (Fig. 1a, b). On day 5.5 of pregnancy, β-tubulin becomes more concentrated in the apical cytoplasmic portion of UECs (Fig. 1c). By day 6, β-tubulin becomes concentrated in a band-like fashion located above the nucleus in UECs (Fig. 1d). On day 7, β-tubulin is concentrated in the apical cytoplasm (Fig. 1e). Non-immune controls were performed with all immunofluorescence protocols and showed no staining in UECs. A representative image of day 7 non-immune control is shown (Fig. 1f).
β-tubulin localisation in rat uterus. a, b Days 1 and 3 of pregnancy show diffuse cytoplasmic β-tubulin localisation in UECs. c On day 5.5 of pregnancy, β-tubulin is concentrated to the apical cytoplasm. d Day 6, β-tubulin is concentrated in a band-like fashion restricted above the nucleus in UECs. e On day 7 of pregnancy, β-tubulin is in the apical region of the cytoplasm. f Non-immune control shows no staining in UECs, day 7 is shown as a representative example. All scale bars are 20 μm. (L lumen, E epithelium and S stroma)
β-Tubulin abundance during early pregnancy
Western blot analysis showed β-tubulin was present in isolated UECs on days 1, 3, 5.5, 6 and 7 of pregnancy (Fig. 2). Two bands were observed with molecular weights of 50 and 34 kDa. UEC lysate from days 1, 3, 5.5, 6 and 7 all showed the known 50 kDa band as well as a 34 kDa band; β-actin was used as a loading control (Fig. 2a). There was no significant difference in abundance of the 50 kDa band between days of pregnancy (Fig. 2b). Densitrometric analysis revealed a significant increase in the 34 kDa band on day 6 compared to day 1 of pregnancy (one-way ANOVA, degrees of freedom = 3.435, n = 4, p = 0.0196) (Fig. 2c).
Western blot analysis of β-tubulin in isolated UECs. a β-tubulin is present at 50 and 34 kDa in isolated UECs on days 1, 3, 5.5, 6 and 7 of pregnancy. β-actin was used as a loading control. b Densitometric and statistical analysis (one-way ANOVA) found no significant difference between the days for the 50 kDa β-tubulin band. c Statistical analysis through one-way ANOVA found a significant increase on day 6 compared to day 1 (*p < 0.05) of pregnancy for the 34 kDa fragment. b, c Error bar is the mean ± S.E.M, n = 4
Transmission electron microscopy of microtubules in UECs during early pregnancy
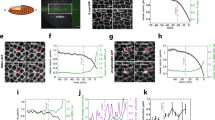
A new TEM protocol was optimised to visualise microtubule distribution in UECs on days 1, 5.5 and 6 of early pregnancy (Figs 3, 4 and 5). This protocol is different from standard TEM protocols where osmium is used as a counter stain, staining all lipids. By omitting osmium and using tannic acid and uranyl acetate on block, it was easier to visualise microtubules under the TEM. On day 1 of pregnancy, microtubules were randomly distributed throughout the cytoplasm of the UECs. The microtubules were straight and rod-like (Fig. 3a–c). On day 5.5 of pregnancy, microtubules were readily found in the apical portion of the cytoplasm above the nucleus and associated with the large number of vesicles that were also present in this region. Microtubules were observed to be fragmented, bent and aligned perpendicular to the apical plasma membrane (Fig. 4a–c). On day 6 of pregnancy, microtubules remained in the apical portion of the cytoplasm above the nucleus and associated with a large number of vesicles. Microtubules were seen in fragmented sizes that were aligned perpendicular to the apical plasma membrane, with bends in their morphology (Fig. 5a–c).
Transmission electron microscopy of microtubules in UECs on day 5.5 of early pregnancy. a–c On day 5.5 of pregnancy, microtubules (arrows) with a diameter of ~20 nm were concentrated above the nucleus and closely associated with a number of apical vesicles (V) near the apical plasma membrane (APM). Microtubules showed bends in their morphology. All scale bars are 200 nm
Transmission electron microscopy of microtubules in UECs on day 6 of early pregnancy. a–c On day 6 of pregnancy, microtubules (arrows) with a diameter of ~20 nm were concentrated above the nucleus and closely associated with a number of vesicles (V). Microtubules showed bends in their morphology. All scale bars are 200 nm
Further observations
Caged vesicles were highlighted within the cytoplasm of UECs (Fig. 6a). Further examination is required to determine if these are either coat protein vesicle I or II (COPI/II) or clathrin-coated vesicles (CCV). Centrosomes were also highlighted by this new staining protocol in the apical portion of UECs (Fig. 6b).
Transmission electron microscopy of microtubules in UECs during early pregnancy. a Caged vesicles (arrow) possibly COPI/II or CCV were highlighted within the cytoplasm and near vesicles (V) in UECs. b Centrosomes were highlighted in the cytoplasm near the apical plasma membrane (APM) of UECs with this TEM protocol throughout all days of pregnancy. All scale bars are 200 nm
Discussion
This is the first study to demonstrate a change in microtubule organisation in UECs during early pregnancy. On day 1 of pregnancy (non-receptive), β-tubulin is diffusely cytoplasmic in UECs; microtubules were found throughout the cytoplasm and maintained a very straight rod-like appearance (Fig. 7a). This is consistent with previous studies in small intestine luminal epithelium and hepatic cells that show a similar distribution of microtubules to the non-receptive UECs (Slautterback 1963; Yoon et al. 1998; Oriolo et al. 2007). At the beginning of uterine receptivity on day 5.5 of pregnancy (apposition), β-tubulin becomes more concentrated in the apical cytoplasmic region where there is also a peak in the number of apical vesicles present (Parr 1982; Murphy and Martin 1987). By day 6 of pregnancy, during adhesion, β-tubulin becomes concentrated in a band-like fashion above the nucleus of UECs. This apical cytoplasmic area is where the apical vesicles reside, thus suggesting microtubule re-organisation is associated with these vesicles and play an active role in apical vesicle transport. This reorganisation of microtubules might be facilitating the removal of anti-adhesive proteins (via endocytosis) and their replacement with adhesive proteins (via exocytosis) at the time of uterine receptivity.
Schematic diagram of the changing organisation of microtubules on days 1 and 6 of pregnancy in UECs. a On day 1 of pregnancy, microtubules (MT) are straight, rod-like and distributed throughout the entire UECs, with no polarity. b On day 6 of pregnancy, microtubules are fragmented, highly associated with vesicles (V) and are polarised
The change in membrane composition for uterine receptivity is thought to be due to a combination of both endocytosis and exocytosis that occurs in UECs (Parr 1980; Murphy 1993). Microtubules are known to play an active role in vesicle transport (Schnapp et al. 1985; Allen et al. 1985; Welte 2004). Also reorganisation of microtubules with the aid of molecular motor proteins allows efficient polarised vesicle targeting. Molecular motor proteins such as kinesin and dynein provide active movement of cytoplasmic vesicles in bidirectional transport in polarised epithelia (Kraemer et al. 1999; Tai et al. 1999). This mechanism may also contribute to the change in apical surface proteins that lead to receptivity in UECs.
Microtubules are polymers that continuously undergo stochastic periods of growth (polymerisation) and shrinkage (depolymerisation) known as dynamic instability, thereby remodelling their organisation within a cell (Mitchison and Kirschner 1984; Horio and Murata 2014). During polymerisation, these microtubules are physically impacted by fluctuating forces of motor proteins that lead to bending and curvature of microtubules, a phenomenon that can be morphologically observed (Bicek et al. 2009; Shekhar et al. 2013). During uterine receptivity on days 5.5 and 6 of pregnancy, bent and curved microtubules were observed suggesting that vesicle transport during receptivity is highly reliant on motor protein activities working with microtubules.
Maintaining cellular function requires microtubules to assemble themselves into a variety of higher-order arrays that undergo continual transformation to meet the changing needs of the cell (Baas et al. 2005; Roll-Mecak and McNally 2010; Sharp and Ross 2012). Regulatory proteins that bind to and modify the dynamic behaviours of individual microtubules mediate this plasticity. An important class of regulatory proteins known as microtubule-severing enzymes (such as spastin and katanin) work by cutting microtubules into short fragments (Wacławek and Włoga 2011; Sharp and Ross 2012). The present study observed short fragments of microtubules under the TEM during uterine receptivity particularly on day 6 of pregnancy, suggesting that the microtubules in UECs may be highly regulated by these microtubule severing enzymes. This severing mechanism permits new microtubule growth and in addition releases microtubules from nucleation sites and allows microtubule transport from one domain of the cell to another (Baas et al. 2005; Roll-Mecak and McNally 2010).
The results of Western blot analysis showed that there was a significant increase in the 34 kDa band on day 6 compared to day 1 of pregnancy. A 34 kDa band has previously been described when β-tubulin was digested with proteases, including Chymotrypsin, Pronase, Papain, Protease K, Subtilisin and V-8 protease. These proteases were found to cleave β-tubulin into 34 and 21 kDa fragments as seen in Western blot analysis (Sackett and Wolff 1986; Serrano et al. 1986, 1988). The increase in cleaved microtubules in UECs suggests that such enzymes may be digesting β-tubulin and enhancing microtubule polymerisation at the time of receptivity (Sackett and Wolff 1986). This 34 kDa proteolytic product along with the short microtubules observed under the TEM during uterine receptivity suggests microtubule severing proteins and tubulin proteases may be playing a role in reorganising the microtubules in UECs to facilitate bidirectional vesicle transport. Short microtubule fragments are known to undergo transport events in axons while long microtubules are immotile (Baas et al. 2006). These short microtubules are easily selected by molecular motor proteins for rapid transport of vesicles containing proteins and thus may be essential for uterine receptivity.
The short microtubule fragments seen during uterine receptivity were orientated perpendicularly towards the apical surface of the plasma membrane (Fig. 7b). This suggests that fragmented microtubules are maintaining an apico-basal polarity, similar to non-fragmented microtubules in other polarised cells (Müsch 2004; Muroyama and Lechler 2017). In other epithelial cells, the negative end is directed towards the apical surface of the cell originating from the apically located centrosomes with the positive end directed basally. This polarity along with the asymmetry of microtubules in epithelial cells allows efficient polarised trafficking of apical vesicles in a bidirectional fashion (Kraemer et al. 1999; Tai et al. 1999). These observations suggest that exocytic activity travels from the positive to negative end of microtubules allowing apical vesicles to fuse with the apical surface and exit as extracellular vesicles. In contrast, endocytic activity travels in the opposite direction where it may be involved in removing non-receptive proteins from the UECs surface (Lodish et al. 2000; Ng et al. 2013; Noordstra and Akhmanova 2017).
In conclusion, this study established a TEM method that highlights microtubules in cells that have dense membranous organelles. This method can also be applied to examine other cellular structures that are normally overshadowed by dense membranous organelles. The present study also demonstrated that microtubules are reorganised during uterine receptivity, which is likely to promote bidirectional vesicle transport. This may facilitate the observed rapid turnover of the apical plasma membrane in UECs, which is essential in establishing a receptive surface for blastocyst attachment and implantation.
References
Allen RD, Weiss DG, Hayden JH et al (1985) Gliding movement of and bidirectional transport along single native microtubules from squid axoplasm: evidence for an active role of microtubules in cytoplasmic transport. J Cell Biol 100:1736–1752
Aplin JD (1997) Adhesion molecules in implantation. Rev Reprod 2:84–93. https://doi.org/10.1530/ror.0.0020084
Baas PW, Karabay A, Qiang L (2005) Microtubules cut and run. Trends Cell Biol 15:518–524. https://doi.org/10.1016/j.tcb.2005.08.004
Baas PW, Vidya Nadar C, Myers KA (2006) Axonal transport of microtubules: the long and short of it. Traffic 7:490–498. https://doi.org/10.1111/j.1600-0854.2006.00392.x
Bicek AD, Tüzel E, Demtchouk A et al (2009) Anterograde microtubule transport drives microtubule bending in LLC-PK1 epithelial cells. Mol Biol Cell 20:2943–2953. https://doi.org/10.1091/mbc.E08-09-0909
Cole NB, Lippincott-Schwartz J (1995) Organization of organelles and membrane traffic by microtubules. Curr Opin Cell Biol 7:55–64. https://doi.org/10.1016/0955-0674(95)80045-X
Denker HW (1990) Trophoblast-endometrial interactions at embryo implantation: a cell biological paradox. Troph Res 4:3–29
Horio T, Murata T (2014) The role of dynamic instability in microtubule organization. Front Plant Sci 5:511. https://doi.org/10.3389/fpls.2014.00511
Jolly AL, Gelfand VI (2010) Cytoplasmic microtubule sliding. Commun Integr Biol 3:589–591. https://doi.org/10.4161/cib.3.6.13212
Kraemer J, Schmitz F, Drenckhahn D (1999) Cytoplasmic dynein and dynactin as likely candidates for microtubule-dependent apical targeting of pancreatic zymogen granules. Eur J Cell Biol 78:265–277. https://doi.org/10.1016/S0171-9335(99)80060-0
Lodish H, Berk A, Zipursky S L, Matsudaira P (2000) Molecular cell biology, 4th edition
Lunam CA, Murphy CR (1983) Alterations in microvilli of uterine epithelial cells after colchicine treatment. Z Mikrosk Anat Forsch 97:1005–1008
Mandelkow E, Thomas J, Cohen C (1977) Microtubule structure at low resolution by x-ray diffraction. Proc Natl Acad Sci U S A 74:3370–3374
Mitchison T, Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312:237–242. https://doi.org/10.1038/312237a0
Moore CL, Cheng D, Shami GJ, Murphy CR (2016) Correlated light and electron microscopy observations of the uterine epithelial cell actin cytoskeleton using fluorescently labeled resin-embedded sections. Micron 84:61–66. https://doi.org/10.1016/j.micron.2016.02.010
Muroyama A, Lechler T (2017) Microtubule organization, dynamics and functions in differentiated cells. Development 144:3012–3021. https://doi.org/10.1242/dev.153171
Murphy C (1994) Plasma membrane transformation: a common response of uterine epithelial cells during the peri-implantation period. Cell Biol Int 18:1115–1128. https://doi.org/10.1006/cbir.1994.1038
Murphy CR (1993) The plasma membrane of uterine epithelial cells: structure and Histochemistry. Prog Histochem Cytochem 27:1–66. https://doi.org/10.1016/S0079-6336(11)80004-5
Murphy CR (2004) Uterine receptivity and the plasma membrane transformation. Cell Res 14:259–267. https://doi.org/10.1038/sj.cr.7290227
Murphy CR, Martin B (1985) Cholesterol in the plasma membrane of uterine epithelial cells: a freeze-fracture cytochemical study with digitonin. J Cell Sci 78:163–172
Murphy CR, Martin B (1987) Digitonin cytochemistry reveals cholesterol-rich vesicles in uterine epithelial cells. Acta Histochem 81:143–147. https://doi.org/10.1016/S0065-1281(87)80003-X
Müsch A (2004) Microtubule organization and function in epithelial cells. Traffic 5:1–9. https://doi.org/10.1111/j.1600-0854.2003.00149.x
Ng YH, Rome S, Jalabert A et al (2013) Endometrial Exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One 8:e58502. https://doi.org/10.1371/journal.pone.0058502
Noordstra I, Akhmanova A (2017) Linking cortical microtubule attachment and exocytosis. F1000Research 6:469. Doi: https://doi.org/10.12688/f1000research.10729.1
Oriolo AS, Wald FA, Ramsauer VP, Salas PJI (2007) Intermediate filaments: a role in epithelial polarity. Exp Cell Res 313:2255–2264. https://doi.org/10.1016/j.yexcr.2007.02.030
Parr MB (1980) Endocytosis at the basal and lateral membranes of rat uterine epithelial cells during early pregnancy. J Reprod Fertil 60:95–99
Parr MM (1982) Apical vesicles in the rat uterine epithelium during early pregnancy: a morphometric study. Biol Reprod 26:915–924
Pelham HR (2001) SNAREs and the specificity of membrane fusion. Trends Cell Biol 11:99–101
Png FY, Murphy CR (2000) Closure of the uterine lumen and the plasma membrane transformation do not require blastocyst implantation. Eur J Morphol 38:122–127. https://doi.org/10.1076/0924-3860(200004)38:2;1-F;FT122
Roll-Mecak A, McNally FJ (2010) Microtubule-severing enzymes. Curr Opin Cell Biol 22:96–103. https://doi.org/10.1016/j.ceb.2009.11.001
Sackett DL, Wolff J (1986) Proteolysis of tubulin and the substructure of the tubulin dimer. J Biol Chem 261:9070–9076
Schnapp BJ, Vale RD, Sheetz MP, Reese TS (1985) Single microtubules from squid axoplasm support bidirectional movement of organelles. Cell 40:455–462. https://doi.org/10.1016/0092-8674(85)90160-6
Serrano L, Wandosell F, De J et al (1988) Effect of specific proteolytic cleavages on tubulin polymer formation. Biochem J 252:683–691
Serrano L, Wandosell F, de la Torre J, Avila J (1986) Proteolytic modification of tubulin. Methods Enzymol 134:179–190. https://doi.org/10.1016/0076-6879(86)34087-4
Sharp DJ, Ross JL (2012) Microtubule-severing enzymes at the cutting edge. J Cell Sci 125:2561–2569. https://doi.org/10.1242/jcs.101139
Sheetz MP, Vale R, Schnapp B et al (1987) Movements of vesicles on microtubules. Ann N Y Acad Sci 493:409–416. https://doi.org/10.1111/j.1749-6632.1987.tb27227.x
Shekhar N, Neelam S, Wu J et al (2013) Fluctuating motor forces bend growing microtubules. Cell Mol Bioeng 6:120–129. https://doi.org/10.1007/s12195-013-0281-z
Slautterback DB (1963) Cytoplasmic microtubules. I Hydra J Cell Biol 18:367–388. https://doi.org/10.1083/jcb.18.2.367
Tai AW, Chuang JZ, Bode C et al (1999) Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell 97:877–887
Wacławek E, Włoga D (2011) Microtubule severing proteins—structure and regulation of activity. Postepy Biochem 62
Welte MA (2004) Bidirectional transport along microtubules. Curr Biol 14:R525–R537. https://doi.org/10.1016/j.cub.2004.06.045
Yeaman C, Grindstaff KK, Nelson WJ (1999) New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev 79:73–98. https://doi.org/10.1002/(sici)1097-4644(19960616)61:4<514::aid-jcb4>3.0.co
Yoon Y, Torok N, Krueger E et al (1998) Ethanol-induced alterations of the microtubule cytoskeleton in hepatocytes. Am J Phys 274:G757–G766
Acknowledgements
The authors acknowledge the support and facilities of the Bosch Institute Advanced Microscopy Facility, The University of Sydney and the assistance provided by Dr. Louise Cole. The authors also acknowledge the support and facilities provided by the Bosch Institute Molecular Biology Facility and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Australian Centre for Microscopy & Microanalysis, The University of Sydney. Financial support was provided by the Australian Research Council, The Ann Macintosh Foundation of the Discipline of Anatomy and Histology and the Murphy Laboratory.
Author information
Authors and Affiliations
Contributions
S.N.K. designed the study, performed all experiments, analysed data and took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Kalam, S.N., Dowland, S., Lindsay, L. et al. Microtubules are reorganised and fragmented for uterine receptivity. Cell Tissue Res 374, 667–677 (2018). https://doi.org/10.1007/s00441-018-2887-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2887-x