Abstract
In recent years, several studies have found that the disruption of type IA receptor of bone morphogenetic proteins (BMPR1A) could increase bone mass. However, whether disruption of BMPR1A could have an effect on bone quality and bone strength is currently unknown. Osteoblast-targeted conditional knockout (cKO) of BMPRIA by crossing 3.2-kb Col1-CreER™ mice with BMPR1A fx +/+ mice was conducted. Then, in vitro and in vivo studies were employed to examine the effect of BMPR1A knockout on bone quality and bone strength. It was found that the ultimate force and stiffness of the femora decreased significantly in cKO mice when compared to control mice. The content of collagen and mineralization level decreased as the structure of the collagen became disorganized. The morphology of osteocytes in cKO mice was abnormal as well. The expression level of osteocalcin, a marker for the terminal differentiation of osteoblasts, decreased in cKO mice. This data indicated that the differentiation of osteoblasts in cKO mice was impaired. Immunohistochemistry examination revealed deregulated expression of dickkopf 1(DKK1) in osteocytes in cKO mice. Adding DKK1 to the culture medium reversed these effects. In conclusion, even though disruption of BMPR1A could increase bone mass, it also impairs bone quality and bone strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone mass is affected by two main processes: bone formation and bone resorption. These processes involve three main types of cells in the bone: osteoblasts, osteoclasts, and osteocytes. Osteoblasts are the main cells responsible for bone formation. They have also been proven to play a pivotal role in regulating the genesis and function of osteoclasts. The function of osteoblasts is thought to be regulated by various hormones and by locally acting growth factors, among which bone morphogenetic proteins (BMPs) have been proven to be one of the most important factors (Bandyopadhyay et al. 2006; Danesh et al. 2009; Kumar et al. 2011; Nakao et al. 2009; Okamoto et al. 2006; Wu et al. 2003).
There are two types of receptors for BMPs. Type II receptors are constitutively active kinases that transphosphorylate type I receptors act upon during ligand binding. Type I receptors activate intracellular substrates such as Smad proteins and thus determine the specificity of intracellular signals (Miyazono et al. 2010; Zeng et al. 2010). Different types of type I receptors have different effects on the bone remodeling process (Bragdon et al. 2011; Kaps et al. 2004; Lavery et al. 2008). A transgenic mouse line that expresses a dominant-negative form type IB receptor for BMPs (BMPR1B) showed dramatic impairment in postnatal bone formation along with an upregulation of BMPR1B, resulting in enhanced osteogenesis (Singhatanadgit et al. 2008; Yi et al. 2000; Zhao et al. 2002). The constitutive activation of activin receptor-like kinase-2 (ALK2), another type of BMP type I receptor, leads to ectopic endochondral bone formation and joint fusion. Furthermore, ALK2 has been proven to be connected with a congenital disorder, causing the progressive and widespread postnatal ossification of soft tissues called fibrodysplasia ossificans progressiva. These findings indicate that BMPR1B and ALK2 are involved in controlling bone mass positively (Yu et al. 2008). In contrast, disruption of type IA receptor of BMPs (BMPR1A) in osteoblasts leads to dramatically increased bone mass (Kamiya et al. 2008a, b; Kamiya et al. 2010; Mishina et al. 2004). Further studies have shown that the in vivo systemic administration of a soluble BMPR1A fusion protein (mBMPR1A–mFc) increases bone mineral density, cortical thickness, trabecular bone volume, while decreasing trabecular separation, making it a promising factor for the possible treatment of low bone volume disorders, such as osteoporosis (Baud'huin et al. 2012).
Bone quality is controlled by the intrinsic properties of bone material and structure, of which material properties refer to the degree of mineralization, crystallinity, and collagen characteristics, and structural properties refer to the diameter and thickness of the cortices, the porosity of the cortical shell, and trabecular bone mass (Davison et al. 2006). Bone strength can be defined as the ability of bone to resist fracture, which depends on bone mass and bone quality (Davison et al. 2006). While it has been proved that disruption of BMPR1A-mediated signaling pathway could increase trabecular bone mass, its effect on bone quality and bone strength is currently unknown. In the present study, BMPR1A was disrupted in osteoblasts and then its effects on bone quality and bone strength were assessed and explored a possible mechanism for this effect.
Methods
Animals
The Ethical Committee of the Daping Hospital, Third Military Medical University, P. R. China, have reviewed and approved the experimental protocol. Mice with the genotype of BMPR1A fx +/+ were mated with 3.2-kb Col1-CreER™ mice to produce offspring mice with the genotype 3.2-kb Col1-CreER™; BMPR1A fx +/+. Tamoxifen (TM; Sigma-Aldrich, St. Louis, MO, USA) was then injected to turn off expression of BMPR1A. Three-day-old 3.2-kb Col1-CreER™; BMPR1A fx +/+ and male mice were intraperitoneally injected with a TM dose of 75 mg/kg × 4 days (once a day, lasted 4 days). Wild-type male littermates were used as controls and were subjected to the same TM injection procedure as the cKO mice. All mice cited above were at 4 weeks of age. Both the BMPR1A fx +/+ mice and the 3.2-kb Col1-CreER™ mice were obtained from Dr. Jianquan Feng (Department of Biomedical Sciences, Baylor College of Dentistry, Texas A&M Health Science Center) (Jing et al. 2013).
Radiographic imaging and tissue preparation
Four weeks after birth, the mice were deeply anesthetized, and radiographic images of their entire skeletons were obtained using a Fixation MX-20 system (Faxitron, Wheeling, Illinois). The mice were then sacrificed with an overdose of anesthetic. Thetibiae and femora were removed and fixed overnight. Some of the samples were embedded in plastic, and 8-μm undecalcified sections were cut for Von Kossa staining. The remaining samples were decalcified in 10% ethylenediaminetetraacetic acid. Once they were adequately decalcified, the samples were tissue-processed, embedded in paraffin, and sectioned at a thickness of 4 μm coronally. The sections were de-paraffinized and rehydrated and then used for hematoxylin and eosin (H&E) staining, immunohistochemistry, Sirius Red staining, Masson staining and TRAP staining.
Mechanical testing
The tibiae were used for mechanical strength in a three-point bending test by using a BOSE ElectroForce ELF 3200 computer-controlled testing machine, which has a force resolution of 0.05 N (Bao et al. 2017; Chen et al. 2015). During the test, each tibia was placed horizontally on two lower supporters that were 6.5 mm apart, with the anterior surface facing upward. The pressing force was applied vertically to the mid-shaft of the bone. Each bone was compressed at a speed of 0.05 mm/s until breaking, and force-displacement data were collected every 0.01 s. Based on the data, a force-displacement curve was created, and the ultimate force (UF; N) was defined as the bending force at the breaking point. The stiffness was calculated from the slope of the linear portion of the curve.
MicroCT examination
The tibiae were dissected and subjected to three-dimensional microCT analysis by using a viva CT 40 (Scanco Medical, Bassersdorf, Switzerland), following the procedural recommendations by the American Society for Bone and Mineral Research (Bao et al. 2017; Chen et al. 2015). The scanning medium was ethanol; the X-ray tube potential was 45 kVp, and the voxel size was 10 μm3. Images were reconstructed and analyzed with EVS Beam software with a global threshold of 1400 Hounsfield units. Quantitative morphometric data were based on the region of interest as follows: the trabecular bone region starting from the growth plate reference level and extending 44 slices (0.8 mm) distally and cortical bone starting from the mid-diaphysis and extending 22 slices (0.4 mm) proximally. The total bone volume fraction (the percentage of bone volume to total volume, BV/TV), mean trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N) were calculated for trabecular bone, whereas the average cortical thickness (Ct.Th) was quantified from cortical bone.
Von Kossa staining
Von Kossa staining was employed to monitor the mineralization ability of the bone. Prepared sections were stained with Silver Nitrate solution, then exposed to strong light until the mineralized bone turned black.This usually takes 30 min–1 h. Then, the slides were placedinto the hazardous waste container followed by the aspiration of silver nitrate from the slides. After rinsing with distilled water, they were quickly dipped into 5% Sodium Thiosulfate for10 s. Then, Van Gieson Working Solution was applied for 5 min. Collect the used solution to the Waste Base & Acid Container, do not pour it into the sink. The slides were then dehydrated and rinsed in 95% alcohol for 2 min, 100% alcohol for 3 min (twice), xylene for 2 min (twice), and then cover-slipped with Permount (Bao et al. 2017; Chen et al. 2015).
Immunohistochemical staining
Immunohistochemistry was performed as previously described (Bao et al. 2017; Chen et al. 2015; Jia et al. 2013). The primary antibodies used were rabbit anti-goat, osterix (Osx, 1:300), mouse anti-rat alkaline phosphatase (ALP, 1:200), osteocalcin (OCN, 1:300), DKK1(1:300), and β-catenin(1:300). The antibodies were procured from Santa Cruz Biotechnology, Inc., USA. The biotinylated goat anti-mouse, rabbit anti-goat, and goat anti-rabbit IgG were acquired from Boster (Wuhan, China). To obtain the percentage of cells expressing a given marker protein, photomicrographic images of each section were captured with an Olympus microscope and digital camera under × 200 magnification. The number of specific antigen-positive cells was counted from five random-picked fields. The mean and standard deviation of the percentage of positive cells were calculated for each group and were used for the statistical analysis.
H&E and TRAP staining
H&E and TRAP staining were performed as described in a previous report (Chen et al. 2015; Jia et al. 2013). Each section was then photographed under × 200 magnification, and the numbers of osteoblasts and TRAP-positive osteoclasts were counted from five random picked fields. The mean number and standard deviation of the cells were calculated and used for the statistical analyses. These parameters comply with the guidelines of the nomenclature committee of the American Society of Bone and Mineral Research (Chen et al. 2015).
Masson staining and Sirius red staining for collagen
For the Masson staining, prepared sections were stained with Weigert’s iron hematoxylin working solution for 10 min, followed by Biebrich scarlet-acid fuchsin solution for 10 min. The sections were then differentiated in phosphomolybdic–phosphotungstic acid solution for 10 min and directly transferred (without rinsing) to aniline blue solution and stained for 5 min. After briefly rinsing in distilled water, they were differentiated in 1% acetic acid solution for 2 min, then rinsed in 95% alcohol for 2 min, 100% alcohol for 3 min (twice), xylene for 2 min (twice), and coverslipped with Permount. Collagen was stained blue in color (Chen et al. 2015).
Sirius Red staining was used to demonstrate differences in collagen structure as previously reported (Bao et al. 2017). The prepared sections were stained with Weigert’s hematoxylin for 8 min, and then slides were washed for 10 min in running tap water. Subsequently, the slides were stained with picro-Sirius red for 1 h, followed by washing in acidified water. The water was removed, and the slides were dehydrated and rinsed in 95% alcohol for 2 min, 100% alcohol for 3 min (twice), xylene for 2 min (twice), and cover-slipped with Permount. The collagen morphology (structure) was then observed under a polarized light microscope.
Scanning electron microscopy
The tibiae samples were rubbed with sandpaper, polished three times with Metadi supreme polycrystalline diamond suspensions, and kept in a vacuum system overnight. The next day, the samples were coated with carbonate and scanned with a FEI/Philips XL30 Field-Emission Environmental Scanning Electron Microscope.
In vitro study
Isolation of osteoblasts
Osteoblasts were isolated from the long bones of 4-week-old 3.2-kb Col1-CreERTM; BMPR1A fx +/+ mice and control mice, according to the protocols reported previously (Bao et al. 2017; Chen et al. 2015). Briefly, the tibiae and femora were removed; the epiphyses were severed, and the bone marrow was flushed out. Then, the diaphyses were sectioned into small pieces with size of approximately 1–2 mm3. The bone pieces were then incubated with 4 mL of collagenase solution at 37 °C in a shaking water bath for 2 h to remove all remaining soft tissue and adhering cells and then transferred into medium containing 10% fetal bovine serum (FBS) to inhibit further collagenase activity, rinsed three times with the medium, and transferred into 25-cm2 flasks at a density of approximately 20–30 fragments per flask. The culture medium was replaced three times per week.
To explore the role of DKK1 in the biological function of osteoblasts, 30 ng/ml recombinant mouse DKK1 (R&D Systerms) was added to the medium, and the cells were cultured for an additional 3 days. Then, the following parameters were examined. Cells not cultured with DKK1 were used as controls.
Real-time PCR
Three mice from each group were sampled in 4 weeks after the TM injection. The whole tibia were dissected, and the samples were immediately shock-frozen in liquid nitrogen and homogenized. The RNA was then isolated using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Real-time PCR using the SYBR green detection method was performed to examine the expression levels of receptor activator of nuclear factor-kappaB ligand (RANKL), osteoprotegerin (OPG) and collagen I, OSX, ALP, and OCN. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a control, and the expression of a given gene was expressed as a proportion relative to the mean value of GAPDH. The relative expression ratio of RANKL vs OPG was calculated and used for the statistical analysis. The primers for the real-time PCR are listed in Table 1.
Mineralization induction and alizarin red staining
Alizarin red staining was employed to monitor the mineralization ability of the isolated osteoblasts. Briefly, primary osteoblasts were cultured for 3 days. Then osteogenic differentiation medium (DMEM supplemented with 10% FBS, 100 nM dexamethasone, 10 mM β-glycerophosphate, 250 mM ascorbic acid, 1% penicillin, and 1% streptomycin) was added to the plates. After 14 days of culture, the cells were stained with 0.005% Alizarin Red S in 0.2N KOH for 15 min to observe mineralization.
Statistical analysis
All data were expressed as the mean ± standard deviation. Statistical significance was evaluated by one-way ANOVA by using SPSS 11.0 software. Data were considered significant at P < 0.05.
Results
Increased osteoblast numbers and decreased osteoclast numbers contribute to increase of bone mass in BMPR1A knockout mice
After disruption of BMPR1A, bone density increased significantly in BMPR1A knockout mice as found by X-ray examination, while HE staining revealed that trabecular bone mass increased dramatically in BMPR1A knockout mice (Fig. 1a–c). These were confirmed by micro-CT examination that trabecular bone mass increased significantly in the BMPR1A knockout mice, as indicated by increased BV/TV, Tb.N, Tb.Th, Ct.Th, and decreased Tb.Sp (Fig. 1d–i).
General description of BMPR1A KO mice. a X-ray radiography of whole skeletons of BMPR1A KO mice and control mice. b, c H&E staining of tibia of BMPR1A KO mice and control mice. d–e Longitudinal microCT image of tibial metaphysis in BMPR1A KO mice and control mice. f–g Longitudinal microCT image of tibial cortical bone in BMPR1A KO mice and control mice. h–l Histomorphometric analysis of tibiae in BMPR1A KO and control mice. BV/TV bone volume per tissue volume, Tb.N, trabecular number, Tb.Th, trabecular thickness, Tb.Sp trabecular separation, Ct.Th cortical thickness. All mice cited above were at 4 weeks of age (cKO means BMPR1A KO mice). The values are expressed as the means ± SDs. (n = 6/group, **p < 0.01, *p < 0.05; Scale bars 500 μm in b and c)
The number of osteoblasts and osteoclasts plays an important role in controlling the bone mass. The effects of the BMPR1A knockout on the number of osteoblasts and osteoclasts then were studied. It was found that the number of OSX and ALP-positive osteoblasts in the tibia of cKO mice was greater than that of the control mice (Fig. 2a–i). Meanwhile, the TRAP-stained slides indicated that the number of osteoclasts in the cKO mice was lower than that in the control mice (Fig. 2k–m). Furthermore, the downregulation of RNAKL and the increased expression level of OPG mediated by osteoblasts contributed to these changes (Fig. 2n–p).
BMPR1A knockout in osteoblasts affects the number of osteoblasts. IHC staining of OSX (a, b, e, f, i) and ALP (c, d, g, h, j) on paraffin tibial sections of BMPR1A KO mice and control mice. k–m TRAP staining of tibiae of BMPR1A KO mice and control mice and the quantitative statistics of the number osteoclasts (arrows: osteoclasts). n–p The ratio of RANKL:OPG mRNA expression levels in osteoblasts isolated from BMPR1A KO mice and control mice. All mice cited above were at 4 weeks of age. The values are expressed as the means ± SDs. (**P < 0.01; n = 6/group; Scale bars 50 μm in e–h, 100 μm in a–d and k–l, black box area: the typical IHC-positive cells position)
Disruption of BMPR1A in osteoblasts impairs bone quality and bone strength of long bone in mice
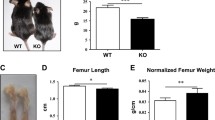
After BMPR1A was knocked out in osteoblasts, massive bone mass formed in bone marrow cavity of long bones. However, three-bending test three-point bending test revealed that the ultimate force and stiffness decreased significantly in cKO mice. When compared to control mice, the data indicated that bone strength decreased significantly in cKO mice (Fig. 3a, b).
BMPR1A knockout in osteoblasts impaired the bone strength and affects the bone collagen synthesis The ultimate force (a) and stiffness (b) of tibia in BMPR1A KO mice and control mice. c, d Masson staining images of tibiae in BMPR1A KO and control mice. e, f Sirius Red staining in BMPR1A KO and control mice. All mice cited above were at 4 weeks of age. The values are expressed as the means ± SDs. (**P < 0.01; n = 6/group; Scale bars 100 μm)
Masson staining revealed that the collagen content in the cKO mice was slightly lower than that of the control mice (Fig. 3c–d), while the Sirius Red staining demonstrated that the collagen in the cKO mice was not well organized when compared with control mice (Fig. 3e–f).
As for the mineralization, Von Kossa staining revealed that the mineralization levels in the BMPR1A knockout mice were lower than those in the control mice (Fig. 4a–b). In vitro, mineralization inducing showed that the mineralization level decreased in osteoblasts isolated from BMPR1A knockout mice (Fig. 4c–e). This was further confirmed by scanning electron microscopy (Fig. 4f, g). Additionally, scanning electron microscopy revealed that the osteocytes in BMPR1A knockout mice showed fewer dendrites (Fig. 4h, i).
Decreased bone mineralization in BMPR1A KO mice. a, b Von Kossa staining of undecalcified tibial diaphysis of tibiae in BMPR1A KO and control mice. c–e Alizarin red staining of osteoblasts isolated from BMPR1A KO mice, BMPR1A KO mice together with recombinant mouse DKK1 protein, and control mice. f–i Scanning electron microscopy images of osteocytes and osteoblasts in BMPR1A KO and control mice. All mice cited above were at 4 weeks of age. (Scale bars 500 μm in a and b; 100 μm in f and g; 5 μm in h and i)
The osteoblastic BMPR1A knockout impairs the terminal differentiation of osteoblasts
Osterix and alkaline phosphatase are two markers for the early differentiation of osteoblasts (Liu et al. 2013; Martineau et al. 2014; Nishikawa et al. 2015; Uchihashi et al. 2013). We found that the expression level of osterix and alkaline phosphatase were increased in long bones of cKO mice (Fig. 2a–j). In contrast, the expression level of osteocalcin, a marker for the terminal differentiation of osteoblasts (Martineau et al. 2014; Nishikawa et al. 2015; Uchihashi et al. 2013), was decreased in cKO mice when compared with control mice (Fig. 5a–e). During terminal differentiation, osteoblasts secrete collagen, which induced the mineralization process. However, not only was collagen secreted, but the mineralization in cKO mice was also impaired. These findings were further confirmed by real-time PCR (Fig. 5f). This partly explained the impaired terminal differentiation of the osteoblasts.
The exprssion of osteoblast differentiation markers in BMPR1A KO mice and control mice. a–e Anti-OCN (the terminal differentiation markers of osteoblasts). IHC analysis on paraffin tibial sections of BMPR1A KO mice and control mice revealed that the number of OCN positive osteoblasts (arrows) was decreased in BMPR1A KO mice. f The OSX, ALP, OCN mRNA expression levels in BMPR1A KO mice and control mice. All mice cited above were at 4 weeks of age. The values are expressed as the means ± SDs. (*P < 0.05, **P < 0.01; n = 6/group; Scale bars 50 μm in (c, d), and 100 μm in (a, b), black box area: the typical IHC-positive cells position)
Next, we explored the possible mechanisms involved in the impairment of the terminal differentiation of osteoblasts in cKO mice. It was found that dickkopf 1 expression was decreased in cKO mice’s osteocytes compared with the control mice, while the expression of β-catenin was increased in BMPR1A knockout mice (Fig. 6a–j). After adding of dickkopf 1 into the culture medium, it was found that the expression level of collagen I (Fig. 6k) and mineralization level were almost restored to the level of control mice (Fig. 4c–e).
Mechanisms for the impaired in BMPR1A KO mice. Anti-DKK1 (a, b, e, f, i) and anti-β-catenin (c, d, g, h, j) IHC analysis of paraffin tibial sections from BMPR1A KO and control mice (arrows: DKK1 positive cells in b and β-catenin positive cells in d); (k) The collagen1 mRNA expression levels in BMPR1A KO mice, BMPR1A KO mice together with recombinant mouse DKK1 protein and control mice; All mice cited above were at 4 weeks of age. The values are expressed as the means ± SDs. (**P < 0.01; n = 6/group; Scale bars 50 μm in (e–h) and 100 μm in (a–d), black box area: the typical IHC-positive cells position)
Discussion
Several studies have demonstrated that disrupting BMPR1A in osteoblasts or blocking the BMPR1A-regulated signaling pathway could increase bone mass, which has a potential application in the treatment of osteoporosis and bone fracture (Baud'huin et al. 2012; Kamiya et al. 2008a, b; Kamiya et al. 2010; Mishina et al. 2004). The current study further confirmed that the bone mass was strongly increased in cKO mice. However, it was found that after disruption of BMPR1A in osteoblasts, the content of collagen decreased slightly; the structure of collagen became disorganized, and the mineralization level decreased (see before). In addition, the morphology of osteocytes in cKO mice became abnormal. These data indicated that bone quality was impaired after BMPR1A deletion in osteoblasts. Bone strength can be defined as the ability of the bone to resist fracture, which depends on bone mass and on bone quality. The bone strength decreased as confirmed by three-bending mechanical test in our study. The data indicated that the effect of impaired bone quality on bone strength might be greater than that of increased bone mass in cKO mice, thus contributing to the decreased bone strength.
Osteoblasts play an important role in bone formation and also play a pivotal role in regulating osteoclast genesis and function (Glass et al. Glass II et al. 2005; Holmen et al. 2005; Zuo et al. 2012). During the early stage of bone formation, osteoblasts secrete osteoid, forming the scaffolding for new bone. Then, osteoblasts secrete noncollagenous organic proteins to induce the terminal differentiation of osteoblasts and mineralization of the osteoid (Kawasaki et al. 2009; Liu et al. 2013; Nishikawa et al. 2015; Uchihashi et al. 2013). After BMPR1A knockout in osteoblasts in the our study, the early differentiation of osteoblasts was not disturbed, and the expression levels of early differentiation markers, such as ALP and OSX, were upregulated. In contrast, the OCN (a terminal differentiation marker) expression levels were downregulated, and osteoblasts terminal differentiation was impaired, as indicated by the decreased mineralization level, disorganized collagen arrangement, and downregulated osteocalcin expression.
DKK1 is a potent inhibitor of Wnt/β-catenin signaling and also plays an important role in terminal osteoblast differentiation (Li et al. 2005; van der Horst et al. 2005). Then, possible mechanisms were explored. Our results revealed an upregulated expression of β-catenin in osteoblasts and downregulated expression of Dickkopf 1 in osteocytes in BMPR1A knockout mice, which are consistent with the finding of Kamiya, et al. (Kamiya et al. 2010). The upregulated expression of β-catenin in osteoblasts along with downregulated expression of dickkopf 1 in osteocytes were thought to be the main mechanisms for increasing bone mass in BMPR1A knockout mice (Kamiya et al. 2010). However, downregulated expression of dickkopf 1 might have an adverse effect on the terminal differentiation of osteoblasts (Chen et al. 2015). It had been found that Wnt signaling must be downregulated by increased dickkopf 1 and dickkopf 2 expression levels to promote mineralized matrix formation (Feng et al. 2006; Kawasaki et al. 2009), and the Wnt/β-catenin signaling should be finely tuned, otherwise, terminal differentiation of osteoblast and mineralized matrix formation will be impaired, resulting in decreased bone strength (Bao et al. 2017). Our data found that adding dickkopf 1 to the culture medium could reverse the decreased expression level of collagen I and the hindered mineralization level in osteoblasts isolated from BMPR1A knockout mice. These data indicated that DKK1 played a role in impaired bone quality in BMPR1A knockout mice. But the interactions between BMP and Wnt signaling in osteoblasts are still unknown. While Liu etc. found that the cell proliferation was enhanced, cell differentiation and mineralization were inhibited when RANKL/OPG was significantly decreased (Liu et al. 2016). In our study the RANKL/OPG was significantly decreased in cKO mice, so some of the osteoblastic phenotypes observed in vivo here (reduced differentiation) may be secondary to defective osteoclast activity. However, these two possible mechanistic speculations require testing by further studies.
In summary, our study found that disruption of BMPR1A could impair bone quality and bone strength although it could increase bone mass. Downregulation of dickkopf 1 played a role in this process. These adverse effects should be addressed before the adoption of any therapeutic clinical application involving adjustment of the BMPR1A signaling pathway.
References
Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ (2006) Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet 2:e216
Bao Q, Chen S, Qin H, Feng J, Liu H, Liu D, Li A, Shen Y, Zhong X, Li J, Zong Z (2017) Constitutive beta-catenin activation in osteoblasts impairs terminal osteoblast differentiation and bone quality. Exp Cell Res 350:123–131
Baud'huin M, Solban N, Cornwall-Brady M, Sako D, Kawamoto Y, Liharska K, Lath D, Bouxsein ML, Underwood KW, Ucran J, Kumar R, Pobre E, Grinberg A, Seehra J, Canalis E, Pearsall RS, Croucher PI (2012) A soluble bone morphogenetic protein type IA receptor increases bone mass and bone strength. Proc Natl Acad Sci U S A 109:12207–12212
Bragdon B, Thinakaran S, Moseychuk O, Gurski L, Bonor J, Price C, Wang L, Beamer WG, Nohe A (2011) Casein kinase 2 regulates in vivo bone formation through its interaction with bone morphogenetic protein receptor type Ia. Bone 49:944–954
Chen S, Feng J, Bao Q, Li A, Zhang B, Shen Y, Zhao Y, Guo Q, Jing J, Lin S, Zong Z (2015) Adverse effects of osteocytic constitutive activation of ss-catenin on bone strength and bone growth. J Bone Miner Res Off J Am Soc Bone Miner Res 30:1184–1194
Danesh SM, Villasenor A, Chong D, Soukup C, Cleaver O (2009) BMP and BMP receptor expression during murine organogenesis. Gene Expression Patterns: GEP 9:255–265
Davison KS, Siminoski K, Adachi JD, Hanley DA, Goltzman D, Hodsman AB, Josse R, Kaiser S, Olszynski WP, Papaioannou A, Ste-Marie LG, Kendler DL, Tenenhouse A, Brown JP (2006) Bone strength: the whole is greater than the sum of its parts. Semin Arthritis Rheum 36:22–31
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315
Glass DA II, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764
Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO (2005) Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem 280:21162–21168
Jia M, Chen S, Zhang B, Liang H, Feng J, Zong Z (2013) Effects of constitutive beta-catenin activation on vertebral bone growth and remodeling at different postnatal stages in mice. PLoS One 8:e74093
Jing J, Ren Y, Zong Z, Liu C, Kamiya N, Mishina Y, Liu Y, Zhou X, Feng JQ (2013) BMP receptor 1A determines the cell fate of the postnatal growth plate. Int J Biol Sci 9:895–906
Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y (2008a) Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res Off J Am Soc Bone Miner Res 23:2007–2017
Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y (2008b) BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development 135:3801–3811
Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, Mishina Y (2010) Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res Off J Am Soc Bone Miner Res 25:200–210
Kaps C, Hoffmann A, Zilberman Y, Pelled G, Haupl T, Sittinger M, Burmester G, Gazit D, Gross G (2004) Distinct roles of BMP receptors type IA and IB in osteo−/chondrogenic differentiation in mesenchymal progenitors (C3H10T1/2). BioFactors 20:71–84
Kawasaki K, Buchanan AV, Weiss KM (2009) Biomineralization in humans: making the hard choices in life. Annu Rev Genet 43:119–142
Kumar J, Swanberg M, McGuigan F, Callreus M, Gerdhem P, Akesson K (2011) LRP4 association to bone properties and fracture and interaction with genes in the Wnt- and BMP signaling pathways. Bone 49:343–348
Lavery K, Swain P, Falb D, Alaoui-Ismaili MH (2008) BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem 283:20948–20958
Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, Harris SE, Rowe DW, Ke HZ, Wu D (2005) Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet 37:945–952
Liu F, Fang F, Yuan H, Yang D, Chen Y, Williams L, Goldstein SA, Krebsbach PH, Guan JL (2013) Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Miner Res Off J Am Soc Bone Miner Res 28:2414–2430
Liu HJ, Yan H, Yan J, Li H, Chen L, Han LR, Yang XF (2016) Substance P promotes the proliferation, but inhibits differentiation and mineralization of osteoblasts from rats with spinal cord injury via RANKL/OPG system. PLoS One 11:e0165063
Martineau, C., Kevorkova, O., Brissette, L., Moreau, R. (2014) Scavenger receptor class B, type I (Scarb1) deficiency promotes osteoblastogenesis but stunts terminal osteocyte differentiation. Physiol Reports 2. 2(10): e12117. https://doi.org/10.14814/phy2.12117
Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG, Hanks MC, Amling M, Pinero GJ, Harada S, Behringer RR (2004) Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem 279:27560–27566
Miyazono K, Kamiya Y, Morikawa M (2010) Bone morphogenetic protein receptors and signal transduction. J Biochem 147:35–51
Nakao Y, Koike T, Ohta Y, Manaka T, Imai Y, Takaoka K (2009) Parathyroid hormone enhances bone morphogenetic protein activity by increasing intracellular 3′, 5′-cyclic adenosine monophosphate accumulation in osteoblastic MC3T3-E1 cells. Bone 44:872–877
Nishikawa Y, Akiyama Y, Yamamoto K, Kobayashi M, Watanabe E, Watanabe N, Shimizu N, Mikami Y, Komiyama K (2015) Osteocytes up-regulate the terminal differentiation of pre-osteoblasts via gap junctions. Biochem Biophys Res Commun 456:1–6
Okamoto M, Murai J, Yoshikawa H, Tsumaki N (2006) Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res Off J Am Soc Bone Miner Res 21:1022–1033
Singhatanadgit W, Salih V, Olsen I (2008) RNA interference of the BMPR-IB gene blocks BMP-2-induced osteogenic gene expression in human bone cells. Cell Biol Int 32:1362–1370
Uchihashi K, Aoki S, Matsunobu A, Toda S (2013) Osteoblast migration into type I collagen gel and differentiation to osteocyte-like cells within a self-produced mineralized matrix: a novel system for analyzing differentiation from osteoblast to osteocyte. Bone 52:102–110
van der Horst G, van der Werf SM, Farih-Sips H, van Bezooijen RL, Lowik CW, Karperien M (2005) Downregulation of Wnt signaling by increased expression of Dickkopf-1 and -2 is a prerequisite for late-stage osteoblast differentiation of KS483 cells. J Bone Miner Res Off J Am Soc Bone Miner Res 20:1867–1877
Wu XB, Li Y, Schneider A, Yu W, Rajendren G, Iqbal J, Yamamoto M, Alam M, Brunet LJ, Blair HC, Zaidi M, Abe E (2003) Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J Clin Invest 112:924–934
Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM (2000) The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 127:621–630
Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD (2008) BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med 14:1363–1369
Zeng S, Chen J, Shen H (2010) Controlling of bone morphogenetic protein signaling. Cell Signal 22:888–893
Zhao M, Harris SE, Horn D, Geng Z, Nishimura R, Mundy GR, Chen D (2002) Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation. J Cell Biol 157:1049–1060
Zuo C, Huang Y, Bajis R, Sahih M, Li YP, Dai K, Zhang X (2012) Osteoblastogenesis regulation signals in bone remodeling. Osteoporos Int 23:1653–1663
Funding
This work was funded by National Science Foundation of China (81271935).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bao, Q., Li, A., Chen, S. et al. Disruption of bone morphogenetic protein type IA receptor in osteoblasts impairs bone quality and bone strength in mice. Cell Tissue Res 374, 263–273 (2018). https://doi.org/10.1007/s00441-018-2873-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2873-3