Abstract
Receptor cells of the olfactory epithelium (OE) and vomeronasal organ (VNO) project axons to glomeruli in the main olfactory bulb (MOB) and accessory olfactory bulb (AOB), respectively and undergo continuous turnover throughout life. Alpha1–2 fucose (α1–2Fuc) glycan mediates neurite outgrowth and synaptic plasticity and plays important roles in the formation of the olfactory system during development. We previously confirmed the localization of α1–2Fuc glycan in the olfactory system of 3- to 4-month-old mice but whether such localization persists throughout life remains unknown. Here, the MOB, AOB, OE and VNO of 1-, 3- and 8-month-old mice were histochemically examined using Ulex europaeus agglutinin-I (UEA-I) that specifically binds to α1–2Fuc glycan. Binding sites for UEA-I in the MOB were similar among all age groups but the ratio of UEA-I-positive glomeruli significantly decreased with aging. The frequency of UEA-I-positive receptor cells in the OE of the two older groups was also significantly lower than that of 1-month-old mice. On the other hand, UEA-I binding in the AOB and VNO did not significantly differ among all three groups. These findings suggest that the primary pathway of the main olfactory system requires the role of α1–2Fuc glycan in young mice rather than old mice, while the vomeronasal pathway equally requires this glycan in both young and old mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The olfactory epithelium (OE) in the nasal cavity is the peripheral receptor organ of the main olfactory system and it is composed of basal, receptor, and supporting cells. Receptor cells of the OE project axons to the glomeruli in the main olfactory bulb (MOB). The basal cells are precursors that differentiate into receptor cells and mature while moving from the basal region to the surface of the epithelium (Graziadei and Monti Graziadei 1979). The vomeronasal system detects specific chemicals such as pheromones and receptor cells in the epithelium of the vomeronasal organ (VNO) project axons to the glomeruli in the accessory olfactory bulb (AOB). Because the neurogenesis of precursor cells proceeds in both olfactory and vomeronasal epithelia even in adults, these receptor cells undergo continuous turnover throughout life (Graziadei and Monti Graziadei 1979).
Glycosylation affects the functional properties of proteins and lipids and regulates some cellular functions. Neuronal functions and morphological changes, including neurite outgrowth and synaptic plasticity, are mediated by α1–2 fucose (α1–2Fuc) glycan (Pohle et al. 1987; Krug et al. 1991, 1994; Matthies et al. 1996; Lorenzini et al. 1997; Kalovidouris et al. 2005; Murrey et al. 2006). Murrey et al. (2009) reported that a deficiency of α1–2-specific fucosyltransferase 1 constricts development of the mouse olfactory bulb and that ratios of MOB glomeruli expressing α1–2Fuc glycan are high and low in 3-day-old mice and adults, respectively. Therefore, α1–2Fuc glycan might play important roles in the development of the olfactory system.
We previously detailed the localization of α1–2Fuc glycan in the olfactory system of 3- to 4-month-old (adult) mice (Kondoh et al. 2017). In terms of primary pathways in these mice, α1–2Fuc glycan is found in some immature receptor cells of the OE and several glomeruli of the MOB and in most mature vomeronasal receptor cells and all glomeruli in the entire, or anterior-half region of AOB (Kondoh et al. 2017). These findings indicate that α1–2Fuc glycan functionally differs between the main olfactory and vomeronasal pathways. However, the localization of α1-2Fuc glycan in the olfactory system throughout life remains unknown. The present study aims to histochemically determine the age-related changes of α1–2Fuc glycan in the main olfactory and vomeronasal pathways using Ulex europaeus agglutinin-I (UEA-I) that binds to α1–2Fuc glycan.
Materials and methods
Animals
Male ICR mice (Japan SLC, Shizuoka, Japan) were maintained under a 12-h light:12-h dark cycle at a controlled ambient temperature of 24 °C ± 2 °C. One- (the OE and brain, n = 4; the VNO, n = 3), three- (the OE and brain, n = 5; the VNO, n = 3) and eight- (the OE and brain, n = 5; the VNO, n = 3) month-old mice were anesthetized during the light cycle using an intraperitoneal injection of pentobarbital (0.20 mg/g body weight) and sacrificed by cardiac perfusion with Bouin fixative. This study proceeded according to the Regulations on the Management and Operation of Animal Experiments and the Animal Care and Use Committee of Obihiro University of Agriculture and Veterinary Medicine approved the experimental protocol (Approval numbers 27-20 and 28-36).
Lectin histochemistry
The OE, VNO and brain including the MOB and AOB were removed from mice and histochemically processed as described (Kondoh et al. 2017). Briefly, the OE and brain specimens were embedded in paraffin, sliced sagittally into 5-μm-thick sections and then deparaffinized. The VNO was cut frontally into 5-μm-thick sections. Sections were incubated with 0.3% H2O2 in methanol and 2.5% bovine serum albumin, followed by 20 μg/mL of biotinylated UEA-I (B-1065; Vector Laboratories, Burlingame, CA, USA) at 4 °C overnight. The sections were then immersed in avidin-biotin-peroxidase complex (PK-6100; Vector) for 30 min and visualized using Tris-HCl buffer containing 0.006% H2O2 and 0.02% 3,3′-diaminobenzidine tetrahydrochloride. Control sections were stained after UEA-I was absorbed with 0.5 M L-fucose and no specific stainings were found in controls.
Quantitation of UEA-I-positive MOB glomeruli and OE receptor cells
The binding was assessed using a Microphot-FX (Nikon, Tokyo, Japan) equipped with a Digital Sight DS-5 M camera (Nikon). Three slices of the olfactory bulbs in the range containing the complete layer structures of the AOB and three slices of the OE within the ethmoturbinals (II) near the brain at the intervals of 100 μm were assessed per mouse. Grayscale images of the MOB were inversed and measured using ImageJ (National Institutes of Health) software. We determined the lower intensity threshold for detection of UEA-I-positive AOB glomeruli and third and two-thirds parts of this threshold were respectively applied for detection of UEA-I-positive and intensely positive glomeruli in the MOB. The numbers of total glomeruli, UEA-I-positive glomeruli and intensely positive glomeruli in the MOB were counted and estimated ratios are shown as means ± SEM. The MOB derived from three specimens (a 1-month-old and two 8-month-old mice) were histologically damaged and excluded from the statistics of quantitation of UEA-I-positive MOB glomeruli. The number of UEA-I-positive olfactory receptor cells in an area of 250 μm of the OE was counted and the numbers per millimeter epithelium were estimated and shown as mean ± SEM. Pairwise comparisons between two ages proceeded using Mann–Whitney U tests.
Results
UEA-I reaction in the MOB
In the MOB of 3-month-old mice, UEA-I reacted to the olfactory nerve layer, some glomeruli and some neuronal fibers in the external to internal plexiform layers, as well as many fibers in the granular cell layer, lateral olfactory tract and rostral migratory stream, as described (Kondoh et al. 2017). These UEA-I-positive sites were similar among all age groups (Fig. 1) but the number of glomeruli that were positive for UEA-I varied according to age as summarized in Table S1 and described below.
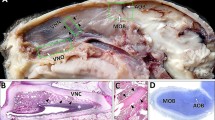
UEA-I binding in sagittal section of whole olfactory bulbs from one- (a) and eight- (b) month-old mice. Left and upper sides are rostral and dorsal, respectively. Solid and dashed boxes, region that is magnified in Figs. 2 and 3, respectively. *Glomerular layer of accessory olfactory bulb (AOB). GL glomerular layer of main olfactory bulb, ONL olfactory nerve layer of main olfactory bulb. Bars = 500 μm
Glomeruli intensely positive for UEA-I were found mainly at the dorsal posterior and ventral posterior ends of the MOB glomerular layer in all groups and the ratio of UEA-I-positive MOB glomeruli decreased with advancing age (Fig. 2). Among three groups, the ratio was the highest (positive, 74.2% ± 8.7%; intensely positive, 28.0% ± 5.4%) and lowest (positive, 9.7% ± 2.0%; intensely positive, 4.5% ± 0.9%) in 1- and 8-month-old mice, respectively (p < 0.05, n = 3–5; Fig. 2d). Regions of UAE-I positivity in the olfactory nerve layer were also reduced with aging (Fig. 1) but the frequency of UEA-I positive fibers in the other layers appeared similar among all three groups of mice.
UEA-I binding in glomeruli of main olfactory bulb of one- (a), three- (b) and eight- (c) month-old mice. Arrows and arrowheads, intensely and weakly positive glomeruli, respectively. *Negative glomeruli. Bars = 100 μm. d Age-dependent decrease in ratio of UEA-I-positive glomeruli. *p < 0.05; Mann–Whitney U test, n = 3–5
UEA-I reaction in the AOB
Ulex europaeus agglutinin-I reacted to the entire vomeronasal nerve layer and all (or the anterior half of) the glomeruli in the AOB of mice at all tested age groups, as described (Kondoh et al. 2017). The UEA-I binding pattern did not differ among them (Fig. 3).
UEA-I reaction in the OE
Receptor cells positive for UEA-I mainly located at the basal region of the OE in 3-month-old mice (Fig. 4b), as described (Kondoh et al. 2017), whereas those in 1-month-old mice were scattered throughout the epithelium (Fig. 4a). Ulex europaeus agglutinin-I-positive cells were sparse in the OE of 8-month-old mice (Fig. 4c). Among the three groups, the frequency of UEA-I-positive receptor cells was significantly higher in 1-month-old, than in 3- and 8-month-old mice (433 ± 14 vs. 185 ± 10 and 157 ± 16 cells/mm epithelium; p < 0.05, n = 4–5; Fig. 4d). Several cells at the surface of the OE were also positive for UEA-I at all ages (Fig. 4a, b, c).
UEA-I binding in olfactory epithelium of one- (a), three- (b) and eight- (c) month-old mice. Arrows and arrowheads indicate cells positive for UEA-I at the basal region and surface of olfactory epithelium, respectively. Bars = 20 μm. d Age-dependent decrease in frequency of UEA-I-positive cells per millimeter epithelium. *p < 0.05; Mann–Whitney U test, n = 4–5
UEA-I reaction in the VNO
Ulex europaeus agglutinin-I reacted to the cell membranes of most receptor cells in the vomeronasal sensory epithelium at all tested age groups, as described (Kondoh et al. 2017). The UEA-I binding pattern did not differ among them (Fig. 5).
Discussion
Alpha1–2Fuc glycan in neurons is associated with their functions and morphological changes (Pohle et al. 1987; Krug et al. 1991, 1994; Matthies et al. 1996; Lorenzini et al. 1997; Kalovidouris et al. 2005; Murrey et al. 2006) and it is required for normal development of the olfactory bulb (Murrey et al. 2009). The amount of α1–2Fuc glycan in the olfactory bulb of adult mice varies in a diurnal manner (Kondoh et al. 2014), indicating that this glycan plays important roles in neuronal function not only during development and growth but also after reaching maturity. Our previous study of 3- to 4-month-old mice (Kondoh et al. 2017) showed that glycoconjugates bind to α1–2Fuc glycan within the primary and secondary pathways of the main olfactory system, in local circuits of the MOB and within the primary pathway of the vomeronasal system. The present study showed that α1–2Fuc glycan localization in the olfactory system is synchronized at all ages, although the frequency of MOB glomeruli and olfactory receptor cells containing this glycan varied according to age, indicating that the roles of this glycan in the olfactory system persist throughout all tested ages. In addition, our preliminary observations implicated that the UEA-I reactivity in the MOB and AOB of 28-month-old mice is similar to that of 8-month-old mice (Fig. S1).
Immature receptor cells in the OE are positive for UEA-I, whereas most mature cells are not (Kondoh et al. 2017), indicating that α1–2Fuc glycan is associated with dendrite and neurite outgrowth from immature neurons within the primary pathway of the main olfactory system. The present study showed that the frequency of receptor cells in the OE and the ratio of MOB glomeruli containing α1–2Fuc glycan, that seem to receive axons from immature receptor cells in the OE, declines with age. The proliferation of basal cells (Weiler and Farbman 1997; Kondo et al. 2010) and the death rate of mature neurons (Kondo et al. 2010) in the rodent OE decrease with advancing age, whereas the time course of neuronal differentiation of immature cells is similar regardless of age (Kondo et al. 2010). Therefore, the lifespan of receptor cells in the OE is longer, turnover decreases with aging and the rate of immature receptor cells that extend axons to glomeruli in the MOB seems to decrease in aged mice. The present findings might reflect an age-related reduction of the number of immature neurons in the OE.
The findings of UEA-I binding in the mouse olfactory system vary (Lundh et al. 1989; Ducray et al. 1999; Lipscomb et al. 2002, 2003; Salazar et al. 2001; Salazar and Sánchez Quinteiro 2003; Murrey et al. 2009; Barrios et al. 2014; Kondoh et al. 2014, 2017). We previously suggested that this diversity does not depend on sex, mouse strain, method of section preparation, UEA-I batch and fixative or detection method, although cutting angles might affect the identification of UEA-I binding sites (Kondoh et al. 2017). In addition to one report indicating a much higher ratio of UEA-I-positive glomeruli in the MOB of a 3-day-old, than adult mice (Murrey et al. 2009), the present findings support the notion that the UEA-I reactivity of the glomeruli in the MOB and receptor cells in the OE differs among age groups.
In contrast to the main olfactory system, all (or the anterior half of) glomeruli in the AOB of all age groups were positive for UEA-I and most of the receptor cells in the VNO were also positive for UEA-I throughout all tested ages. The present results support our previous notion that α1–2Fuc glycan might be associated with neuronal function in addition to neurite outgrowth within the primary pathway of the vomeronasal system, unlike the main olfactory system (Kondoh et al. 2017).
Alpha1-2Fuc glycan mediates neurite outgrowth and synaptic plasticity (Pohle et al. 1987; Krug et al. 1991, 1994; Matthies et al. 1996; Lorenzini et al. 1997; Kalovidouris et al. 2005; Murrey et al. 2006) and is associated with the development of the MOB (Murrey et al. 2009). The present UEA-I histochemistry revealed an age-dependent decrease in the numbers of glomeruli and receptor cells containing α1–2Fuc glycan in the main olfactory system but not vomeronasal system. It is suggested that the primary pathway of the main olfactory system requires the role of α1–2Fuc glycan in young mice rather than old mice, while the vomeronasal pathway equally requires this glycan in both young and old mice.
References
Barrios AW, Núñez G, Sánchez Quinteiro P, Salazar I (2014) Anatomy, histochemistry, and immunohistochemistry of the olfactory subsystems in mice. Front Neuroanat 8:63
Ducray A, Propper A, Kastner A (1999) Detection of alpha-L fucose containing carbohydrates in mouse immature olfactory neurons. Neurosci Lett 274:17–20
Graziadei PPC, Monti Graziadei GA (1979) Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol 8:1–18
Kalovidouris SA, Gama CI, Lee LW, Hsieh-Wilson LC (2005) A role for fucose α(1–2) galactose carbohydrates in neuronal growth. J Am Chem Soc 127:1340–1341
Kondo K, Suzukawa K, Sakamoto T, Watanabe K, Kanaya K, Ushio M, Yamaguchi T, Nibu K, Kaga K, Yamasoba T (2010) Age-related changes in cell dynamics of the postnatal mouse olfactory neuroepithelium: cell proliferation, neuronal differentiation, and cell death. J Comp Neurol 518:1962–1975
Kondoh D, Tateno H, Hirabayashi J, Yasumoto Y, Nakao R, Oishi K (2014) Molecular clock regulates daily α1–2-fucosylation of the neural cell adhesion molecule (NCAM) within mouse secondary olfactory neurons. J Biol Chem 289:36158–36165
Kondoh D, Kamikawa A, Sasaki M, Kitamura N (2017) Localization of α1–2 fucose glycan in the mouse olfactory pathway. Cells Tissues Organs 203:20–28
Krug M, Jork R, Reymann K, Wagner M, Matthies H (1991) The amnesic substance 2-deoxy-D-galactose suppresses the maintenance of hippocampal LTP. Brain Res 540:237–242
Krug M, Wagner M, Staak S, Smalla KH (1994) Fucose and fucose-containing sugar epitopes enhance hippocampal long-term potentiation in the freely moving rat. Brain Res 643:130–135
Lipscomb BW, Treloar HB, Greer CA (2002) Novel microglomerular structures in the olfactory bulb of mice. J Neurosci 22:766–774
Lipscomb BW, Treloar HB, Klenoff J, Greer CA (2003) Cell surface carbohydrates and glomerular targeting of olfactory sensory neuron axons in the mouse. J Comp Neurol 467:22–31
Lorenzini CG, Baldi E, Bucherelli C, Sacchetti B, Tassoni G (1997) 2-deoxy-D-galactose effects on passive avoidance memorization in the rat. Neurobiol Learn Mem 68:317–324
Lundh B, Brockstedt U, Kristensson K (1989) Lectin-binding pattern of neuroepithelial and respiratory epithelial cells in the mouse nasal cavity. Histochem J 21:33–43
Matthies H, Staak S, Krug M (1996) Fucose and fucosyllactose enhance in vitro hippocampal long-term potentiation. Brain Res 725:276–280
Murrey HE, Gama CI, Kalovidouris SA, Luo WI, Driggers EM, Porton B, Hsieh-Wilson LC (2006) Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proc Natl Acad Sci U S A 103:21–26
Murrey HE, Ficarro SB, Krishnamurthy C, Domino SE, Peters EC, Hsieh-Wilson LC (2009) Identification of the plasticity-relevant fucose-α(1–2)-galactose proteome from the mouse olfactory bulb. Biochemistry 48:7261–7270
Pohle W, Acosta L, Rüthrich H, Krug M, Matthies H (1987) Incorporation of [3H]fucose in rat hippocampal structures after conditioning by perforant path stimulation and after LTP-producing tetanization. Brain Res 410:245–256
Salazar I, Sánchez Quinteiro P (2003) Differential development of binding sites for four lectins in the vomeronasal system of juvenile mouse: from the sensory transduction site to the first relay stage. Brain Res 979:15–26
Salazar I, Sánchez Quinteiro P, Lombardero M, Cifuentes JM (2001) Histochemical identification of carbohydrate moieties in the accessory olfactory bulb of the mouse using a panel of lectins. Chem Senses 26:645–652
Weiler E, Farbman AI (1997) Proliferation in the rat olfactory epithelium: age-dependent changes. J Neurosci 17:3610–3622
Acknowledgements
We thank the staff of the Laboratory Animal Center, Obihiro University of Agriculture and Veterinary Medicine, for maintaining the animals.
Funding
Grant-in-Aid for Young Scientists (B) KAKENHI (15K20841) to D.K. from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the Regulations on the Management and Operation of Animal Experiments and the Animal Care and Use Committee of Obihiro University of Agriculture and Veterinary Medicine approved the experimental protocol (Approval numbers 27-20 and 28-36).
Rights and permissions
About this article
Cite this article
Kondoh, D., Sasaki, M. & Kitamura, N. Age-dependent decrease in glomeruli and receptor cells containing α1–2 fucose glycan in the mouse main olfactory system but not in the vomeronasal system. Cell Tissue Res 373, 361–366 (2018). https://doi.org/10.1007/s00441-018-2819-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2819-9