Abstract
Bone morphogenetic protein 2 (BMP-2) has a critical function in bone and cartilage development and in repairing damaged organs and tissue. However, clinical use of BMP-2 at doses of 0.5–1 mg/ml for orthopedics has been associated with severe postoperative swelling requiring emergency surgical intervention. We determined whether a high concentration of BMP-2 induces inflammatory responses in macrophages and the suppression of osteogenesis in hMSCs. We obtained human periodontal ligament stem cells and bone marrow stem cells from the maxilla, i.e., human mesenchymal stem cells (hMSCs), from the periodontal ligament of extracted third molar teeth and from the bone marrow of the maxilla, respectively. Osteogenic differentiation was measured by alkaline phosphatase activity and alizarin red S staining. Proteins were assessed by flow cytometry, enzyme-linked immunosorbent assay, Western blot and immunocytochemistry. Changes of gene expression were measured by reverse transcription plus the polymerase chain reaction (RT-PCR) and real-time PCR. A high BMP-2 concentration inhibited the early stages of osteogenesis in hMSCs. Co-culturing THP-1 cells (human monocytic cells) with hMSCs reduced the late stages of osteogenesis compared with those seen in hMSCs alone. In addition, high-dose BMP-2 induced the expression of inflammatory cytokines in THP-1 cells and the expression of the anti-inflammatory cytokine tumor-necrosis-factor-α-inducible gene 6 protein (TSG-6) in hMSCs. Consistent with the anti-inflammatory effects of hMSCs when co-cultured with THP-1 cells, interleukin-1β expression was downregulated by TSG-6 treatment of THP-1 cells. Our findings suggest that a high BMP-2 concentration triggers inflammation that causes inflammatory cytokine release from THP-1 cells, leading to the suppression of osteogenesis, whereas TSG-6 secreted by hMSCs suppresses inflammatory reactions through p38 and ERK in the mitogen-activated protein kinase pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue regeneration with stem cells is a promising field in regenerative medicine. Among candidate stem cells for tissue regeneration, mesenchymal stem cells (MSCs) have potential clinical applications. Tissue defects in the oral cavity are challenging because of the complex composition and multiple cell types in this area. After a diseased tooth or jawbone is removed, primarily the jawbone must be regenerated (G.T. Huang et al. 2009). Among MSCs of dental origin, periodontal ligament stem cells (PDLSCs), which are obtained from extracted third molars, have been identified and characterized as a multipotent stem cell that can be used for hard tissue regeneration (Seo et al. 2004; Vasandan et al. 2014). Another candidate for bone regeneration is represented by bone marrow stem cells (BMSCs) from marrow stromal cells (Akintoye et al. 2006; G.T. Huang et al. 2009).
Human bone morphogenetic protein-2 (BMP-2), which has been approved by the United States Food and Drug Administration (FDA) for use in bone regeneration and repair, is used at 0.5-1 mg/ml in autogenous bone grafts, spinal fusion, segmental defects, open tibia fractures and oral maxillofacial reconstruction (Boyne et al. 2005; Fiorellini et al. 2005; Swiontkowski et al. 2006; Tumialan et al. 2008; Wei et al. 2012). Unlike an in vitro study of treatment with 50-200 ng/ml BMP-2 (Guan et al. 2015), the clinical doses of BMP-2 are extremely high. In the first clinical reports of 1 mg/ml BMP-2 use, several adverse effects were reported (K.B. Lee et al. 2011a; Perri et al. 2007; Shields et al. 2006; Smucker et al. 2006; Zara et al. 2011). High BMP-2 concentrations increase osteoclast activation with transient bone resorption (Toth et al. 2009). A frequently reported side-effect of BMP-2 is inflammatory swelling. Patients undergoing anterior cervical spinal fusion generally exhibit severe soft tissue swelling and require surgery to explore and drain a swollen anterior neck with transplantation of rhBMP-2 sponges (Vaidya et al. 2007). Previous research has shown that BMP-2 triggers the chemotaxis of monocytes, macrophages and lymphocytes (G.T. Lee et al. 2010; Simoes Sato et al. 2014; Talati et al. 2014). During inflammation and bone formation, inflammatory cytokines triggered by immune cells not only perpetuate inflammation but also activate bone resorption in osteoporosis, rheumatoid arthritis and other bone diseases (Mundy 2007; Redlich and Smolen 2012). The inflammatory mechanisms acting between immune cells and hMSCs in bone formation have been investigated in vivo. With high doses of BMP-2, infiltrated immune cells, inducing inflammatory cytokines, have been found to be increased near the transplanted area that formed aberrant bone (Shen et al. 2013; Zara et al. 2011). To reduce life-threatening episodes and increase bone formation, the optimum BMP-2 concentration needs to be determined and the inflammatory environment induced by BMP-2 needs to be controlled. Moreover, the establishment of these conditions will elucidate the interactions between immune cells and hMSCs.
Recent reports have demonstrated that hMSCs regulate inflammation (Castro-Manrreza et al. 2014; Kim and Hematti 2009; Liu et al. 2016; Nazarov et al. 2013; Nemeth, et al. 2009). In addition to their immunomodulatory characteristics, hMSCs also have the capacity to differentiate into cell types such as adipocytes, osteoblasts and neurons (Kim et al. 2012). hMSCs secrete the anti-inflammatory protein, tumor necrosis factor-α (TNF-α)-inducible gene 6 protein (TSG-6), which attenuates inflammatory reactions. TSG-6 suppresses macrophages from expressing inflammatory cytokines in response to mitogen-activated protein kinase (MAPK) signaling (R.H. Lee et al. 2009a, 2014). However, the role of hMSCs on BMP-2-induced inflammation is not known.
Therefore, we constructed a local inflammatory environment induced by a high BMP-2 concentration by co-culturing macrophages and hMSCs in order to study the role of hMSCs in an inflammatory environment. We hypothesized that a high concentration BMP-2 causes inflammatory reactions of THP-1 cells, leading to the suppression of the osteogenesis of hMSCs. Moreover, TSG-6 secreted by hMSCs suppresses BMP-2-induced inflammation. Overall, we identified the role of hMSCs on high BMP-2 concentrations, inducing TSG-6 to downregulate inflammatory reactions through the p38 and MEK/MAPK pathway.
Materials and methods
Cell culture conditions
hPDLSCs from impacted third molars of humans (n = 8, age 17–29) and BMSCs from human maxilla (n = 4, age 20-26) were collected at the Department of Oral and Maxillofacial Surgery, Seoul National University Dental Hospital. This protocol was approved by the Institutional Review Board of the Seoul National University School of Dentistry (IRB no. S-D20080009). Collected PDLSCs and BMSCs were digested separately in 3 mg/ml type 1 collagenase (BioBasic, Toronto, ON, Canada) and 4 mg/ml dispase II (Gibco BRL, Long Island, N.Y., USA) for 1 h with shaking at 37 °C in a 5 % CO2 incubator as previously described (Seo et al. 2004). Cultures were in grown in alpha minimum essential medium (α-MEM) supplemented with 100 μM L-ascorbic acid, 2 mM L-glutamine, 100 U/ml antibiotics-antimycotics (all from Gibco) and 15 % fetal bovine serum (FBS; Equitech-Bio, Kerrville, Tex., USA). PDLSCs and BMSCs from the second to sixth passages were used for experiments. A human monocyte cell line, THP-1, was provided by the Korean Cell Line Bank (KCLB) and cultured in Roswell Park Memorial Institute medium (RPMI 1640 medium) supplemented with 100 U/ml antibiotics-antimycotics (both from Gibco) and 10 % FBS (Equitech-Bio).
Co-culture of macrophages and MSCs
THP-1 cells were activated with 50 nM phorbol 12-myristate 13-acetate (PMA) for 3 days, followed with 1000 or 5000 ng/ml recombinant human BMP-2 (rhBMP-2; Daewoong Pharmaceutical, Seongnam, Korea) for 24 h. THP-1 cells were stimulated for 3 days in RPMI 1640 medium with PMA and equal numbers of MSCs were seeded for 1 day and co-cultured in growth medium. MSCs and THP-1 cells were cultured alone as controls. Recombinant human TSG-6 protein (R&D Systems, Minneapolis, Minn., USA) was added to THP-1 cells at a concentration of 10 or 100 ng/ml.
Osteogenic differentiation
To induce osteogenic differentiation, MSCs with or without THP-1 cells were cultured in MSC growth medium until 80-90 % confluent. Co-cultured cells were changed to differentiation medium consisting of α-MEM supplemented with 10 % FBS, 10 nM dexamethasone (Sigma-Aldrich, St. Louis, Mo., USA), 5 mM glycerol phosphate (Sigma-Aldrich), 100 U/ml antibiotics-antimycotics (Gibco) and 100 μM L-ascorbic acid. After 5 days of incubation in the osteogenic medium with BMP-2, a QuantiChrom Alkaline Phosphatase (ALP) Assay Kit (BioAssay Systems, Hayward, Calif., USA) was used to detect ALP activity following the manufacturer’s directions. Absorbance was measured at 405 nm with a microplate reader (Bio-Rad). An ALP staining kit (Sigma-Aldrich) was also used to detect ALP in MSCs after 5 days in osteogenic medium. After incubation in differentiation medium for 10 days, BMSCs and PDLSCs were stained with 40 mM alizarin red S solution (pH 4.2) to detect calcium deposits. Stained alizarin red particles were quantified by means of a solution of 20 % methanol and 10 % acetic acid and detected with a spectrophotometer (Fluostar Optima; BMG LABTECH, Ortenberg, Germany) at 450 nm.
Gene expression analysis
Total RNA was isolated from cells with an RNA Mini Kit (Ambion, Carlsbad, Calif., USA) and reverse-transcribed with a SuperScript III First-Strand Synthesis System kit (Invitrogen, Carlsbad, Calif., USA). The reverse transcription plus polymerase chain reaction (RT-PCR) was performed with the primers listed in Table S1 (Hong et al. 2009). Real-time RT-PCR was performed on a Real-time PCR System 7500 (Applied Biosystems, Foster City, Calif., USA) to quantify mRNA expression. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Hs99999905_m1) was used as an endogenous control. The following human-specific primers and probes (all from Applied Biosystems) were used: TNF-α (Hs01113624_g1), interleukin (IL)-1β (Hs00174097_m1) and TSG-6 (TNFAIP6; Hs01113602_m1). Expression was quantified by the ∆∆CT method.
Flow cytometry analysis
Activated THP-1 cells were subjected to flow cytometry to detect macrophage phenotypes showing CD11b and CD14 expression. Approximately 5 × 105 cells were activated with PMA for 3 days, followed with BMP-2 for 24 h. Activated cells were double-stained with allophycocyanin7 (APC-7)-conjugated mouse anti-human CD11b (BD Bioscience, Franklin Lakes, N.J., USA) and fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD14 (BD Bioscience). Stained cells were detected and analyzed with a FACS Aria IIII (BD Bioscience).
Enzyme-linked immunosorbent assay
To identify cytokines released from THP-1 cells with or without MSCs, the cultured cells were starved in serum-free medium overnight. After overnight starvation, cells were activated with 1000 and 5000 ng/ml BMP-2 for 24 h, supernatants containing inflammatory-related products induced by BMP-2 without FBS were collected and enzyme-linked immunosorbent assay (ELISA) was performed to determine TNF-α and IL-1β levels. A human TSG-6 ELISA kit was used for the quantitative measurement of human TSG-6 in supernatants (RayBiotech, Norcross, Ga., USA). The absorbance of each sample was measured with a microplate reader (Fluostar Optima, BMG LABTECH) at 450 nm.
Immunocytochemistry
A total of 105 THP-1 cells were plated and cultured in 2-well chamber slides with 50 nM PMA in RPMI 1640 medium for 3 days. Stimulated THP-1 cells were incubated without FBS overnight. Starved THP-1 cells were treated with 5000 ng/ml BMP-2 and 10-100 ng/ml TSG-6 for 3 h. After fixation, blocked cells were incubated with anti-nuclear factor kappa-B (NF-kB) p65 antibody (Abcam, Cambridge, UK) in blocking solution at 4 °C overnight. The cells were then incubated in anti-rabbit IgG secondary antibody (Abcam) for 1 h. Mounting medium containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, Calif., USA) was used to stain nuclei. Fluorescent imaging was performed on a confocal laser scanning microscope (FV300, Olympus America, Center Valley, Pa., USA).
Western blotting analysis
PMA-stimulated THP-1 cells were cultured and treated with 5000 ng/ml BMP-2 and 10 ng/ml TSG-6 for 10, 30, or 60 min. Proteins from THP-1 cells were collected in cell extraction buffer (Invitrogen) with protease inhibitors and 1 mM phenylmethane sulfonyl fluoride. Proteins on membranes were detected with NF-κB, phosphorylated (p)-NF-κB, Erk1/2, p-Erk1/2, JNK (c-Jun N-terminal kinase), p-JNK, p38, and p-p38 primary antibodies (1:1000, Cell Signaling Technologies, Boston, Mass., USA) followed by horseradish peroxidase (HRP)-linked secondary antibody. As a control, we used β-actin (1:1000) antibody. The immunoblots were visualized with an HRP chemiluminescent detection kit (SurModics, Eden Prairie, Mnn., USA) and measured with a MicroChemi analyzer (DNR Bio-image Analyzer).
Statistical analysis
Statistical analysis was performed with a one-way analysis of variance followed by Tukey’s HSD test with SPSS 22 (SPSS, Chicago, Ill., USA). ELISA data are presented as the means ± SE from quadruple replicates and others from triplicate replicates. A statistically significant difference between data was assigned if P < 0.05.
Results
High concentrations of BMP-2 inhibit early osteogenic differentiation of MSCs
In the early stages of osteogenic differentiation, hPDLSCs and hBMSCs had low levels of ALP activity in the presence of 1000 or 5000 ng/ml BMP-2 on day 5 (Fig. 1a). This pattern was identical to that of ALP staining (Fig. 1b). Both BMP-2 concentrations suppressed early osteogenic differentiation in hPDLSCs and hBMSCs. However, hMSCs co-cultured with THP-1 cells had higher levels of ALP activity in the early stages than did hMSCs alone.
Osteoblastic differentiation of mesenchymal stem cells (MSCs) is inhibited by a high bone morphogenetic protein 2 (BMP-2) concentration. a Alkaline phosphatase (ALP) activity from dental MSCs cultured alone or with human monocytic cells (THP-1). THP-1 cells co-cultured with bone marrow stem cells (BMSC)/periodontal ligament stem cells (PDLSC) or monocultured THP-1 cells were incubated in osteogenic medium with 1000 ng/ml or 5000 ng/ml BMP-2 for 5 days (NC negative control with no osteogenic differentiation). Each bar represents the mean ± SE of three independent experiments. *P < 0.05 compared with the control group, n = 3. b ALP protein was detected on day 5 by an ALP staining kit
Co-culturing MSCs with THP-1 cells inhibits late osteogenic differentiation
The late stages of osteogenic differentiation were evaluated by alizarin red S staining (Fig. 2a, b). The inhibitory effect of THP-1 cells on osteogenic differentiation was overcome by BMP-2 in the late stages of differentiation. However, co-cultured hMSCs and THP-1 cells underwent less osteogenic differentiation in the late stages than did MSCs alone. Consequently, the initially inhibitory effect of BMP-2 concentrations changed to an inductive osteogenic signal in the late stages.
Co-culturing THP-1 cells with MSCs inhibits late osteogenic differentiation of dental MSCs. a Calcium deposits from osteogenic differentiation of both BMSCs and PDLSCs with or without THP-1 cells were stained with alizarin red S solution at day 10 (NC negative control with no osteogenic differentiation). b Stained calcium deposits were destained and quantified with 20 % methanol and 10 % acetic acid. Each bar represents the mean ± SE of three independent experiments *P < 0.05 compared with the control group, n = 3
Characteristics of THP-1 cells in BMP-2-induced inflammation
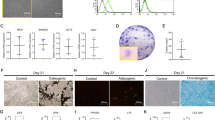
To determine the response to BMP-2 of each cell, the gene expression of BMP-related receptors on THP-1 cells, PDLSCs and BMSCs was determined by RT-PCR. The BMP receptors related to signaling consist the type I receptors ALK2, ALK3 and ALK6 and the type II receptors Act-RIIA, Act-RIIB and BMP-RII. The gene expression of BMP-related receptors differed among THP-1 cells, PDLSCs and BMSCs (Fig. 3a). The activation of THP-1 cells was examined via flow cytometry analysis. CD11b and CD14 expressed on activated macrophages from monocytes were double-stained to examine the activation of THP-1 cells. In the absence of stimulation by PMA, THP-1 cells were not activated (Fig. 3b). Surface expression of activated macrophage markers, namely CD14 and CD68, on activated THP-1 cells changed in response to BMP-2 treatment. CD14 and CD68 were overexpressed at 5000 ng/ml BMP-2. BMP-2 at a concentration of 5000 ng/ml also activated the inflammatory stage of THP-1 cells (Fig. 3c). As shown in Fig. 3d, 5000 ng/ml BMP-2 induced an inflammatory response in THP-1 cells. THP-1 cells highly expressed TNF-α and IL-1β in the presence of 5000 ng/ml BMP-2.
Characteristics of THP-1 cells during BMP-2-induced inflammation. a Reverse transcription plus the polymerase chain reaction (RT-PCR) showed that MSCs and phorbol 12-myristate 13-acetate (PMA)-stimulated THP-1 cells had different levels of BMP receptors, although some similarities were apparent. b–b’’’ Flow cytometric analysis of CD14 and CD11b expression in THP-1 cells. Results for THP-1 activated with 50 nM PMA for 3 days followed by BMP-2 for 1 day were obtained by using fluorescein isothiocyanate (FITC)-conjugated anti-human CD14 and allophycocyanin (APC)-conjugated anti-human CD11b as surface markers of mature macrophages. The number of CD14 and CD11b double-positive cells was increased by BMP-2 treatment, regardless of dose. c, c’ RT-PCR for macrophage markers (CD14 and CD68) on THP-1 cells after treatment with various BMP-2 concentrations. Gene expression was normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) per sample. d, d’ Gene expression determined by RT-PCR. Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) gene expression relative to GAPDH were measured after culture with various BMP-2 concentrations for 24 h (n = 3). Each bar represents the mean ± SE. *P < 0.05 compared with the control group
MSCs inhibit inflammatory cytokine expression in THP-1 cells
To examine the immunosuppressive effects of hMSCs on BMP-2-induced inflammation, pro-inflammatory gene expression and protein accumulation in response to BMP-2 treatment were determined by real-time RT-PCR (Fig. 4b, c) and ELISA (Fig. 4d, e) following the experimental scheme shown in Fig. 4a. With a 24-h BMP-2 treatment, 5000 ng/ml BMP-2 was required for PMA-stimulated THP-1 cells to release pro-inflammatory cytokines. Stimulated THP-1 cells increased expression of the TNF-α and IL-1β genes in the presence of 5000 ng/ml BMP-2. Co-culturing THP-1 cells in the presence of PDLSCs or BMSCs decreased levels of inflammatory cytokines IL-1β and TNF-α. PDLSCs significantly suppressed the gene expression of inflammatory cytokine IL-1β to a greater extent than did BMSCs. The suppression of TNF-α genes on co-culturing THP-1 cells with PDLSCs was similar to the suppression in BMSCs (Fig. 4b, c). The levels of TNF-α and IL-1β proteins followed a pattern similar to gene expression in response to BMP-2 treatment (Fig. 4d, e). This result demonstrated that PMA-stimulated THP-1 cells secreted excessive levels of inflammatory cytokines in the presence of 5000 ng/ml BMP-2, thereby worsening the local inflammatory environment. In contrast, TNF-α and IL-1β expression induced by PMA was significantly suppressed by co-culture with MSCs.
MSCs suppress the expression of inflammatory cytokines in the presence of various BMP-2 concentrations. a Experimental scheme. Samples were collected for enzyme-linked immunosorbent assay (ELISA) and real-time PCR. b, c Real-time RT-PCR (n = 3) normalized to GAPDH expression showed that the expression of IL-1β and TNF-α was blocked by 24-h co-culture of MSCs with PMA-stimulated THP-1 cells (RQ relative quantification). d, e ELISA assay (n = 4) for IL-1β and TNF-α in supernatants following BMP-2 treatment for 24 h. Each bar represents the mean ± SE. *P < 0.05 compared with the control group
TSG-6 secreted by MSCs in response to BMP-2 interferes with inflammatory reactions of THP-1 cells through MAPK signaling
In the presence of 5000 ng/ml BMP-2, both PDLSCs and BMSCs secreted a significant amount of TSG-6 as shown by real-time RT-PCR (Fig. 5a). PDLSCs and BMSCs treated with 5000 ng/ml BMP-2 secreted significant protein levels of TSG-6 (Fig. 5b). Furthermore, PDLSCs and BMSCs co-cultured with THP-1 cells in 5000 ng/ml BMP-2 also showed increased protein expression of TSG-6. TSG-6 lowered IL-1β expression by BMP-2-treated THP-1 cells. Moreover, a BMP-2 concentration of 5000 ng/ml increased IL-1β expression, which was significantly inhibited by exogenous TSG-6 (Fig. 5c, d). TNF-α expression did not change in response to TSG-6 induced by 5000 ng/ml BMP-2 (data not shown). Treatment with 100 ng/ml TSG-6 for 3 h with BMP-2 induction blocked NF-κB translocation to the nucleus in THP-1 cells (Fig. 5e). Western blotting revealed that TSG-6 blocked NF-κB phosphorylation (Fig. 5f).
Tumor-necrosis-factor-α-inducible gene 6 protein (TSG-6) secreted by MSCs in response to BMP-2 induction interferes with the inflammatory reactions of THP-1 cells through nuclear factor kappa-B (NF-κB) signaling. a Real-time RT-PCR revealed that PDLSCs and BMSCs in the presence or absence of THP-1 cells secreted TSG-6 in response to BMP-2 treatment for 24 h, as normalized to GAPDH. b Quantitative measurement of human TSG-6 was performed with an ELISA kit. Levels of TSG-6 were measured upon BMP-2 treatment for 24 h. c, d Real-time analysis and ELISA showed that TSG-6 inhibited IL-1β expression in THP-1 cells. THP-1 cells were exposed to 50 nM PMA for 3 days and with 1000 ng/ml or 5000 ng/ml BMP-2 and 10 ng/ml TSG-6 for 24 h. e–e’’’ Immunofluorescence analysis of NF-κB. THP-1 cells were pre-stimulated with 50 nM PMA and then exposed to BMP-2 for 3 h in the presence of 10 or 100 ng/ml TSG-6. NF-κB translocation into the nucleus (red circle) was decreased by TSG-6 treatment. Magnification ×200. Bars 50 μm. f Western blots were performed to measure NF-κB phosphorylation. THP-1 cells were stimulated with 50 nM PMA and treated with 5000 ng/ml BMP-2 or 10 ng/ml TSG-6 for 10, 30, or 60 min. Proteins were collected and the ratio of phospho-NF-κB to total NF-κB was determined by immunoblotting. The immunoblot data were normalized to β-actin. Each bar represents the mean ± SE. *P < 0.05 compared with the control group, n = 3
The effect of TSG-6 on downstream NF-κB signaling in THP-1 cells showed that extracellular signal-regulated kinase 1/2 (ERK1/2; Fig. 6a) and p38 signaling (Fig. 6b) were blocked at 60 min, whereas TSG-6 did not affect JNK signaling during BMP-2 treatment (Fig. 6c). Moreover, TSG-6 treatment for 60 min inhibited JNK signaling. This result indicates that TSG-6 secreted by MSCs blocks NF-κB translocation and IL-1β secretion through NF-κB/Erk1/2/p-38 signaling in THP-1 cells.
TSG-6 inhibits p38 and extracellular signal-regulated kinase 1/2 (Erk1/2) signaling in THP-1 cells activated by a high BMP-2 concentration. THP-1 cells were treated with 5000 ng/ml BMP-2 and/or 10 ng/ml TSG-6 for 10, 30, or 60 min. Immunoblotting data were analyzed by the ratio of phospho-ERK1/2 (p-Erk1/2), phospho-p38 (p-p38), and phospho-c-Jun N-terminal kinase (p-JNK) to total ERK1/2 (t-Erk1/2), total p38 (t-p38), and total JNK protein (t-JNK), respectively. a ERK1/2 signaling was activated by BMP-2 at 60 min, whereas TSG-6 treatment inhibited ERK1/2 signaling. b Moreover, p38 signaling was inhibited by TSG-6 treatment at high BMP-2 concentrations. c JNK signaling had no significant influence on protein levels. Each bar represents the mean ± SE. *P < 0.05 compared with the control group, n = 3
Discussion
This study was designed to investigate the immunosuppressive effects of MSCs on immune reactions triggered by macrophages at high BMP-2 concentrations. In current clinical and experimental applications of BMP-2, the inflammatory response induced by high concentrations of exogenous BMP-2 may decrease initial bone formation. Our study suggests that high BMP-2 concentrations boost the initial local inflammatory reaction by releasing inflammatory cytokines, whereas MSCs reduce the inflammatory reaction through TSG-6.
Dense infiltration of inflammatory cells was observed around the grafted collagen sponge with 20 μg/ml of BMP-2 on the dorsal side of the mouse (Fig. S1). Bone remodeling/regeneration is a consequence of the balancing mechanism between osteoblasts and osteoclasts (Manolagas and Jilka 1995; Teitelbaum 2000). Monocytic macrophages were selected as candidate immune cells in this study because they are derived from a hematopoietic lineage related to osteoclasts. Monocytes from blood vessels differentiate and activate into macrophages after infiltrating blood vessels in inflammatory sites and can then differentiate into osteoclasts (Haynes et al. 2001; Hume et al. 2002). Similarly, we observed that high doses of BMP-2 triggered the infiltration and activation of macrophages. Our in vitro experimental scheme mimicked the in vivo clinical applications. In our research, a monocytic macrophage cell line, THP-1, was activated with PMA in order to convert them into activated inflammatory cells (for details of THP-1 cells, see Figs. S2, S3, S4). At the same time, osteogenic medium was applied to MSCs to drive differentiation into osteoblasts or THP-1 cells. A previous study had shown that BMP-2 secretion was increased in co-cultured MSCs and monocytes/macrophages in the early stages of osteogenic differentiation (Pirraco et al. 2013). ALP is an early osteogenic marker; however, some researchers have also found ALP to be an indicator of inflammation. ALP is induced during acute and chronic inflammation in rodent and human models (Krötzsch et al. 2005; Takabayashi et al. 2014). In our experiment, THP-1 cells were influenced by an inflammatory condition, namely PMA activation, which might affect early ALP activity. Our co-cultured MSCs and macrophages exhibited enhanced early osteogenic differentiation (ALPase activity) relative to MSCs alone. However, a high concentration of exogenous BMP-2 inhibited the early stages of osteogenesis in both MSCs alone and MSCs co-cultured with THP-1 cells. Previous studies have demonstrated that the inflammatory environment inhibits osteoblastic differentiation (R.L. Huang et al. 2014b). In the present study, alizarin red S staining indicated that the late stages of osteogenic differentiation were suppressed in MSCs co-cultured with macrophages. In addition, osteogenic differentiation induced by high BMP-2 concentrations was reversed, resulting in higher calcium deposits in the late stages of osteogenesis. This reversal in the late stages of osteogenic induction can be explained by the discontinued PMA treatment of THP-1 cells. Therefore, the effects of inflammatory macrophages were reduced in late osteogenesis in our experiments. Reduced inflammatory effects increased the osteogenesis of hMSCs. This is a possible explanation of the contrasting effects of BMP-2 on the early and late stages of osteogenesis.
Clinical disorders of osteoporosis involving inflammatory conditions include rheumatoid arthritis, cystic fibrosis and periodontitis (Redlich and Smolen 2012; Shead et al. 2010). Our results indicate that an inappropriately high BMP-2 concentration stimulates macrophages to secrete inflammatory cytokines and delays osteogenic differentiation in MSCs. This implies that the reduced bone formation seen during the clinical use of high BMP-2 concentrations is caused by increased inflammatory cytokines. BMP-2 is thought to be responsible for inducing a pro-inflammatory environment. The inflammatory characteristics of rhBMP-2 have been observed in vitro and in vivo in the form of soft tissue swelling and inflammatory cytokine release in a rodent model (K.B. Lee et al. 2011b, 2012). We also found that monocytic macrophages express the genes for type I and type II BMP receptors. PDLSCs and BMSCs also express BMP receptors at differing levels, suggesting that BMP-2 affects downstream signaling heterogeneously in immune cells and MSCs. Our results show that BMP-2-induced macrophages cause the secretion of several inflammatory cytokines. In MSCs, however, BMP-2 participates in both Sma- and Mad-related family (SMAD) and MAPK signaling to increase the expression of osteogenic-related genes. In BMP-2-induced inflammation, MSCs differentiate into osteoblasts to a lesser degree, supporting previous studies showing that TNF-α and IL-1β can inhibit MAPK signaling to reduce osteogenic gene expression in murine MSCs (R.L. Huang et al. 2014a; G.T. Lee et al. 2010).
In addition to osteoblast differentiation, another MSC function is immunomodulation (Gibon et al. 2016; Ma et al. 2014; Prockop and Oh 2012). In disease models, MSCs suppress inflammatory responses through several mechanisms (R.H. Lee et al. 2009b, 2014; D.E. Lee et al. 2016; Manning et al. 2015; Wang et al. 2012). Our results suggest that MSCs secrete TSG-6 in response to BMP-2. This result indicates that the administeration of a high BMP-2 concentration to MSCs triggers an anti-inflammatory reaction that affects macrophages. In MSC and macrophage co-cultures, we observed the inhibited macrophage secretion of inflammatory cytokines, such as IL-1β and TNF-α. PDLSCs suppressed inflammation more effectively at a higher BMP-2 concentration than did BMSCs. Less IL-1β was produced upon treatment of the macrophages with TSG-6; this was consistent with results from the macrophage and MSC co-cultures. Previous studies have shown that TSG-6 from MSCs regulates macrophages through NF-κB signaling triggered by lipopolysaccharide (LPS) or zymosan-induction (Choi et al. 2011; R.L. Huang et al. 2014a; Sullivan et al. 2014). We found that, at a high BMP-2 concentration, macrophages induced NF-κB translocation into the nucleus, similar to that seen in LPS- or zymosan-induced inflammation. On TSG-6 treatment, NF-κB phosphorylation was inhibited in THP-1 cells at a high BMP-2 concentration. Although the inhibition of inflammation by TSG-6 has been tested in other studies, little agreement is evident regarding the manner in which inflammatory cytokines and osteogenic stimulation are differentially activated during NF-κB signaling in MSCs (R.L. Huang et al. 2014a; R.H. Lee et al. 2014; Mahoney et al. 2008).
In conclusion, the release of inflammatory cytokines by PMA-activated macrophages is increased by a high BMP-2 concentration. TSG-6 secreted by MSCs suppresses inflammatory reactions related to high BMP-2 concentrations through MAPK signaling in macrophages. The differing tendencies of PDLSCs and BMSCs in the presence of BMP-2 should be studied in the context of oral maxillofacial reconstruction therapy. By determining the anti-inflammatory effects of TSG-6 from dental-tissue-derived MSCs, we can expand the use of MSCs in clinical trials, especially in bone-related diseases, by establishing optimal therapeutic BMP-2 concentrations.
References
Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG (2006) Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone 38:758–768
Boyne PJ, Lilly LC, Marx RE, Moy PK, Nevins M, Spagnoli DB, Triplett RG (2005) De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg 63:1693–1707
Castro-Manrreza ME, Mayani H, Monroy-Garcia A, Flores-Figueroa E, Chavez-Rueda K, Legorreta-Haquet V, Santiago-Osorio E, Montesinos JJ (2014) Human mesenchymal stromal cells from adult and neonatal sources: a comparative in vitro analysis of their immunosuppressive properties against T cells. Stem Cells Dev 23:1217–1232
Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ (2011) Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 118:330–338
Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, Spagnoli D, Toljanic J, Jones A, Nevins M (2005) Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol 76:605–613
Gibon E, Lu L, Goodman SB (2016) Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther 7:44
Guan J, Zhang J, Zhu Z, Niu X, Guo S, Wang Y, Zhang C (2015) Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells. Stem Cell Res Ther 6:5
Haynes DR, Crotti TN, Loric M, Bain GI, Atkins GJ, Findlay DM (2001) Osteoprotegerin and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclast formation by cells in the human rheumatoid arthritic joint. Rheumatology (Oxford) 40:623–630
Hong JH, Lee GT, Lee JH, Kwon SJ, Park SH, Kim SJ, Kim IY (2009) Effect of bone morphogenetic protein-6 on macrophages. Immunology 128:e442–e450
Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792–806
Huang RL, Yuan Y, Tu J, Zou GM, Li Q (2014a) Opposing TNF-alpha/IL-1beta- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis 5:e1187
Huang RL, Yuan Y, Zou GM, Liu G, Tu J, Li Q (2014b) LPS-stimulated inflammatory environment inhibits BMP-2-induced osteoblastic differentiation through crosstalk between TLR4/MyD88/NF-kappaB and BMP/Smad signaling. Stem Cells Dev 23:277–289
Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T (2002) The mononuclear phagocyte system revisited. J Leukoc Biol 72:621–627
Kim BC, Bae H, Kwon IK, Lee EJ, Park JH, Khademhosseini A, Hwang YS (2012) Osteoblastic/cementoblastic and neural differentiation of dental stem cells and their applications to tissue engineering and regenerative medicine. Tissue Eng Part B Rev 18:235–244
Kim J, Hematti P (2009) Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 37:1445–1453
Krötzsch E, Salgado RM, Caba D, Lichtinger A, Padilla L, Di Silvio M (2005) Alkaline phosphatase activity is related to acute inflammation and collagen turnover during acute and chronic wound healing. Wound Repair Regen 13:A28–A48
Lee DE, Ayoub N, Agrawal DK (2016) Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther 7:37
Lee GT, Kwon SJ, Lee JH, Jeon SS, Jang KT, Choi HY, Lee HM, Kim WJ, Kim SJ, Kim IY (2010) Induction of interleukin-6 expression by bone morphogenetic protein-6 in macrophages requires both SMAD and p38 signaling pathways. J Biol Chem 285:39401–39408
Lee KB, Murray SS, Taghavi CE, Song KJ, Brochmann EJ, Johnson JS, Keorochana G, Liao JC, Wang JC (2011a) Bone morphogenetic protein-binding peptide reduces the inflammatory response to recombinant human bone morphogenetic protein-2 and recombinant human bone morphogenetic protein-7 in a rodent model of soft-tissue inflammation. Spine J 11:568–576
Lee KB, Taghavi CE, Song KJ, Sintuu C, Yoo JH, Keorochana G, Tzeng ST, Fei Z, Liao JC, Wang JC (2011b) Inflammatory characteristics of rhBMP-2 in vitro and in an in vivo rodent model. Spine 36:E149–E154
Lee KB, Taghavi CE, Murray SS, Song KJ, Keorochana G, Wang JC (2012) BMP induced inflammation: a comparison of rhBMP-7 and rhBMP-2. J Orthop Res 30:1985–1994
Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ (2009a) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5:54–63
Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ (2009b) The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood 113:816–826
Lee RH, Yu JM, Foskett AM, Peltier G, Reneau JC, Bazhanov N, Oh JY, Prockop DJ (2014) TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci U S A 111:16766–16771
Liu M, Zeng X, Wang J, Fu Z, Wang J, Liu M, Ren D, Yu B, Zheng L, Hu X, Shi W, Xu J (2016) Immunomodulation by mesenchymal stem cells in treating human autoimmune disease-associated lung fibrosis. Stem Cell Res Ther 7:63
Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y (2014) Immunobiology of mesenchymal stem cells. Cell Death Differ 21:216–225
Mahoney DJ, Mikecz K, Ali T, Mabilleau G, Benayahu D, Plaas A, Milner CM, Day AJ, Sabokbar A (2008) TSG-6 regulates bone remodeling through inhibition of osteoblastogenesis and osteoclast activation. J Biol Chem 283:25952–25962
Manning CN, Martel C, Sakiyama-Elbert SE, Silva MJ, Shah S, Gelberman RH, Thomopoulos S (2015) Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Res Ther 6:74
Manolagas SC, Jilka RL (1995) Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 332:305–311
Mundy GR (2007) Osteoporosis and inflammation. Nutr Rev 65:S147–S151
Nazarov C, Lo Surdo J, Bauer SR, Wei CH (2013) Assessment of immunosuppressive activity of human mesenchymal stem cells using murine antigen specific CD4 and CD8 T cells in vitro. Stem Cell Res Ther 4:128
Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15:42–49
Perri B, Cooper M, Lauryssen C, Anand N (2007) Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J 7:235–239
Pirraco RP, Reis RL, Marques AP (2013) Effect of monocytes/macrophages on the early osteogenic differentiation of hBMSCs. J Tissue Eng Regen Med 7:392–400
Prockop DJ, Oh JY (2012) Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem 113:1460–1469
Redlich K, Smolen JS (2012) Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov 11:234–250
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155
Shead EF, Haworth CS, Barker H, Bilton D, Compston JE (2010) Osteoclast function, bone turnover and inflammatory cytokines during infective exacerbations of cystic fibrosis. J Cyst Fibros 9:93–98
Shen J, James AW, Zara JN, Asatrian G, Khadarian K, Zhang JB, Ho S, Kim HJ, Ting K, Soo C (2013) BMP2-induced inflammation can be suppressed by the osteoinductive growth factor NELL-1. Tissue Eng Part A 19:2390–2401
Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, Shields CB (2006) Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine 31:542–547
Simoes Sato AY, Bub GL, Campos AH (2014) BMP-2 and -4 produced by vascular smooth muscle cells from atherosclerotic lesions induce monocyte chemotaxis through direct BMPRII activation. Atherosclerosis 235:45–55
Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG (2006) Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine 31:2813–2819
Sullivan CB, Porter RM, Evans CH, Ritter T, Shaw G, Barry F, Murphy JM (2014) TNFalpha and IL-1beta influence the differentiation and migration of murine MSCs independently of the NF-kappaB pathway. Stem Cell Res Ther 5:104
Swiontkowski MF, Aro HT, Donell S, Esterhai JL, Goulet J, Jones A, Kregor PJ, Nordsletten L, Paiement G, Patel A (2006) Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am 88:1258–1265
Takabayashi H, Shinohara M, Mao M, Phaosawasdi P, El-Zaatari M, Zhang M, Ji T, Eaton KA, Dang D, Kao J, Todisco A (2014) Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology 147:e397
Talati M, West J, Zaynagetdinov R, Hong CC, Han W, Blackwell T, Robinson L, Blackwell TS, Lane K (2014) BMP pathway regulation of and by macrophages. PLoS One 9:e94119
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289:1504–1508
Toth JM, Boden SD, Burkus JK, Badura JM, Peckham SM, McKay WF (2009) Short-term osteoclastic activity induced by locally high concentrations of recombinant human bone morphogenetic protein-2 in a cancellous bone environment. Spine 34:539–550
Tumialan LM, Pan J, Rodts GE, Mummaneni PV (2008) The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenetic protein-2: a review of 200 patients. J Neurosurg Spine 8:529–535
Vaidya R, Carp J, Sethi A, Bartol S, Craig J, Les CM (2007) Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J 16:1257–1265
Vasandan AB, Shankar SR, Prasad P, Sowmya Jahnavi V, Bhonde RR, Jyothi Prasanna S (2014) Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. J Cell Mol Med 18:344–354
Wang N, Shao Y, Mei Y, Zhang L, Li Q, Li D, Shi S, Hong Q, Lin H, Chen X (2012) Novel mechanism for mesenchymal stem cells in attenuating peritoneal adhesion: accumulating in the lung and secreting tumor necrosis factor alpha-stimulating gene-6. Stem Cell Res Ther 3:51
Wei S, Cai X, Huang J, Xu F, Liu X, Wang Q (2012) Recombinant human BMP-2 for the treatment of open tibial fractures. Orthopedics 35:e847–e854
Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung J, Kwak J, Wu BM, Ting K, Soo C (2011) High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A 17:1389–1399
Acknowledgements
This research was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI12C0763). BMP-2 was kindly donated by the Daewoong Pharmaceutical Company without any conditions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOCX 14 kb)
Fig. S1
High concentrations of BMP-2 trigger the infiltration of immune cells from blood vessels. a Collagen sponges (8 mm in diameter, 1 mm in thickness) as carriers were transplanted with 1, 5, 10, and 20 μg/ml BMP-2 onto the dorsum of mouse. b After 24 h, inflammatory cells were identified on the swollen soft tissue near the implanted collagen sponge with BMP-2 in hematoxylin and eosin staining. c To detect infiltrated activated macrophages, immunohistochemistry was performed via staining with F4/80 antibody (Abcam 100790, 1:100). Red arrows indicate activated macrophages. (GIF 153 kb)
Fig. S2
Morphology of THP-1 cells. Without PMA stimulation, THP-1 cells alone cannot be stimulated with BMP-2 treatment. (GIF 59 kb)
Fig. S3
Flow cytometry data of THP-1 cells stimulated with BMP-2. After 3 days of 50 nM PMA stimulation, THP-1 cells were treated with BMP-2 for 24 h. THP-1 cells double-stained with CD11b and CD14 were increased in a BMP-2 dose dependent manner. Each experiment was repeated three times. (GIF 75 kb)
Fig. S4
Antibody array of THP-1 cells with or without PDLSC/BMSCs. a Array map (POS positive control spot, NEG negative control spot, BLANK blank spot). b THP-1 cells were activated with 50 nM PMA for 3 days and triggered with 5000 ng/ml BMP-2 for 24 hours. Inflammatory cytokine expression was enhanced upon BMP-2 induction. Co-culturing MSCs and THP-1 cells resulted in lower levels of inflammatory cytokines than in THP-1 cells alone (arrows anti-inflammatory cytokines, such as IL-10 and TGF-β1). (GIF 95 kb)
Rights and permissions
About this article
Cite this article
Um, S., Kim, H.Y., Lee, JH. et al. TSG-6 secreted by mesenchymal stem cells suppresses immune reactions influenced by BMP-2 through p38 and MEK mitogen-activated protein kinase pathway. Cell Tissue Res 368, 551–561 (2017). https://doi.org/10.1007/s00441-017-2581-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2581-4