Abstract
Mitochondria acquire the majority of their proteins from the cytosol in a process that is mediated by intricate multimeric machineries designed to allow proteins to cross and/or to insert themselves into the two mitochondrial membranes. Ongoing studies carried out in yeast over the past few decades have led to the discovery of numerous protein components that constitute several mitochondrial translocases. One of these complexes, the mitochondrial TIM23, is the major translocase for matrix proteins and is the focus of this review. The components of the TIM23 complex are categorized into four functional types. The first type plays the role of receptor for preproteins in the intermembrane space. The second type forms the actual channel that allows proteins to cross the inner mitochondrial membrane. The third species functions as part of the motor that mediates the final steps of import across the inner membrane. Additional components play regulatory roles orchestrating the action of this myriad of subunits. Recent studies provide new insights into the function of the mammalian TIM23 complex and the role that it plays under pathological conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The odyssey of nuclear-encoded but mitochondrially localized proteins to their final destination begins upon their translation in the cytosol. Most mitochondrial proteins are translocated posttranslationally across the mitochondrial outer membrane, but some also undergo cotranslational import (Knox et al. 1998; Lesnik et al. 2014; Yogev et al. 2007). Proteins that localize to the matrix need to cross both the outer and inner mitochondrial membranes. This process is mediated by two machineries acting in a consecutive concerted manner, the TOM (translocase of the outer mitochondrial membrane) and the TIM23 (translocase of the inner mitochondrial membrane) complexes.
The TOM complex is the main entry pore for most of the proteins that are destined for the mitochondria (Dukanovic and Rapaport 2011). Once a precursor protein is synthesized in the cytosol, it is escorted to the mitochondrial outer membrane by cytosolic chaperones. The translocation event commences upon interaction with the receptor subunits of the outer-membrane TOM complex. These receptor subunits also recognize the escorting chaperones thereby forming a receptor-precursor-chaperone complex. Further binding of ATP to this complex causes the dissociation of the precursor protein from the chaperone and its insertion into the outer-membrane import channel (Komiya et al. 1997; Young et al. 2003). Once the precursor protein has crossed the outer membrane, it binds components of the TOM complex located on the trans side of the membrane. This facilitates passage of the precursor proteins across the outer membrane and into the inter-membrane space (IMS; Bolliger et al. 1995; Mayer et al. 1995) in which additional translocases assist the polypeptide in reaching its final destination.
Precursor proteins that utilize the TIM23 complex cross the inner membrane and eventually reach their correct destination. These include proteins that are translocated into the matrix, a number of inner membrane proteins, and a few IMS proteins, and even some outer membrane proteins have recently been shown to be processed by this translocase (Sinzel et al. 2016; Song et al. 2014; Wenz et al. 2014). In this review, we elaborate on our present knowledge about the function of the TIM23 complex and the specific roles of its constituent components and cofactors.
The TIM23 complex
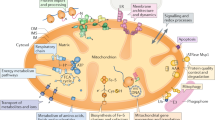
TIM23 is a hetero-oligomeric complex that is anchored to the mitochondrial inner membrane. It consists of a membrane-embedded core complex and an import motor, which is largely exposed to the matrix side of the inner membrane. The integral membrane protein sub-complex constitutes the import channel and consists of three essential subunits: Tim23 (Dekker et al. 1993), Tim17 (Maarse et al. 1994), and Tim50 (Geissler et al. 2002; Mokranjac et al. 2003a; Yamamoto et al. 2002), plus the non-essential Mgr2 (Gebert et al. 2012) and Tim21 (Chacinska et al. 2005; Mokranjac et al. 2005). The import motor (also called the PAM or presequence translocase-associated motor complex) is responsible for completing protein translocation across the inner membrane in a process that derives energy from ATP hydrolysis. It consists of several components: Tim44 (Horst et al. 1993; Maarse et al. 1992), the 70-kDa mitochondrial heat shock protein (mtHsp70; Kang et al. 1990; Schneider et al. 1994), Tim16 (also called Pam16; Frazier et al. 2004; Kozany et al. 2004), Tim14 (also called Pam18; Li et al. 2004; Mokranjac et al. 2003b; Truscott et al. 2003), Pam17 (van der Laan et al. 2005), and Mge1 (Laloraya et al. 1994; Voos et al. 1994; Fig. 1a).
TIM23 (translocase of the inner mitochondrial membrane) complex. a Various members of the TIM23 complex have been identified in yeast. The TIM23 complex consists of integral membrane proteins (purple) that participate in the initial import from the TOM (translocase of the outer mitochondrial membrane) complex to the TIM23 complex and partial import across the inner membrane (IMS inter-membrane space, IM inner membrane, Mge1 cochaperone). Complete import across the inner membrane and into the matrix requires the associated import motor (blue) and additional energy, which is supplied by ATP and utilized by mtHsp70 (70-kDa mitochondrial heat shock protein). b Human TIM23 complex. Human homologs of various complex members have been identified and studied. Almost all members are associated with diseases (red). Members of the complex that have not been studied in humans are indicated by question marks (PD Parkinson’s disease, AD Alzheimer’s disease, DCMA dilated cardio myopathy with ataxia, EVEN-PLUS an autosomal recessive syndrome characterized by epiphysis and vertebral dysplasia and numerous other abnormalities)
The TIM23 complex is extremely complex and dynamic, reflecting the elaborate pathway that the precursor protein must go through once it enters the inter-membrane space. As a first step, the precursor is recognized by the receptor subunits of TIM23 in the IMS. Then, it is transferred to the import channel found in the inner membrane. Once in the import pore, the precursor is sorted either to the inner membrane or the matrix in a process that is not fully understood. For matrix-destined proteins, the import motor takes over and actively translocates the protein into the matrix. Finally, regardless of the precursor protein’s final destination, the targeting signal is cleaved in the matrix, and the precursor reaches its mature form.
Over the past few years, a large amount of information has been accumulated regarding the structural organization of the complex, in yeast and its homologs in mammals. Here, we review the latest discoveries regarding the structure and function of the TIM23 complex and the way that various components of the system are involved in disease and pathological conditions.
Tim50 and Tim23 act as receptors for incoming precursor proteins
Tim23 and Tim50 are both essential proteins of the TIM23 complex in yeast. Tim50 exposes, to the IMS, a large hydrophilic domain that is anchored in the membrane by one transmembrane helix (Geissler et al. 2002; Mokranjac et al. 2003a; Yamamoto et al. 2002). Tim23 consists of two main domains: its N-terminal half is hydrophilic and is exposed to the IMS, whereas its C-terminal half is predicted to span the inner membrane with four trans-membrane segments (TM1-4; Dekker et al. 1993). Tim50 has been found to keep the translocation channel closed in a presequence-regulated mode (Meinecke et al. 2006). In the IMS, Tim23 and Tim50 bind each other, and this interaction has been determined to be essential for protein translocation by the TIM23 complex (Gevorkyan-Airapetov et al. 2009; Tamura et al. 2009). Both proteins also bind the incoming precursor protein, and together, they act as receptors for the imported presequence (Bauer et al. 1996; Geissler et al. 2002; Marom et al. 2011; Mokranjac et al. 2009; Schulz et al. 2011). Tim50 has been shown to bind precursor proteins while still passing through the TOM complex. Hence, Tim50 is the first component of the TIM23 complex that is expected to interact with the precursor protein and mediates the transfer of the incoming protein from the TOM complex to the TIM23 complex (Chacinska et al. 2003; Geissler et al. 2002; Mokranjac et al. 2003a, 2009; Waegemann et al. 2015; Yamamoto et al. 2002). Tim50 binds the precursor at two different binding sites that communicate with each other. The C-terminal binding site seems to transfer the precursor protein to the Tim50 core domain, and from there, the precursor protein is transferred to the inner membrane channel (Rahman et al. 2014).
The hydrophilic N-terminal half of Tim23 is intrinsically unstructured (de la Cruz et al. 2010; Gevorkyan-Airapetov et al. 2009), implying that it is extremely flexible and should be able to bind various partner proteins. Indeed, in addition to Tim50 and the incoming precursor protein, the N-terminal domain also forms dimers (Alder et al. 2008b; Bauer et al. 1996) and binds Tom22 and Tim21 at several contact sites in its soluble domain (Bajaj et al. 2014; Tamura et al. 2009). Furthermore, its extreme N-terminus has been shown to span the outer membrane once a precursor protein is in transit (Popov-Celeketic et al. 2008), thereby enabling a tight connection between the two mitochondrial membranes. Tim50 also interacts with various partner proteins, among them Tom22 and Tim21 (Chacinska et al. 2003; Lytovchenko et al. 2013; Shiota et al. 2011; Tamura et al. 2009; Waegemann et al. 2015). This demonstrates that the handover of the precursor protein to the Tim23 channel is complicated and involves cooperation of several different components of both the TOM and TIM23 complexes.
In short, the IMS domains of both Tim23 and Tim50 serve as active components of the complex in the IMS side. Tim50 is the first component of the complex to interact with the incoming precursor protein and, together with Tim23, directs the precursor to the import channel. Tim23 serves as a hub that binds various subunits of the complexes thereby keeping the components in close proximity to each other, while enabling the flexibility that is needed for the transfer of the protein from the TOM complex to the import channel.
Crossing the inner membrane
Once the precursor has reached the import channel, it next has to cross the inner membrane. This process is driven by three main forces. The first is the membrane potential across the inner membrane. A second diving force is the increasing affinities of the precursor proteins to the components on the trans side (i.e., Tim44) of the complex compared with its affinities to the components in the cis side (i.e., Tim23 and Tim50; Marom et al. 2011). Finally, the third driving force is the motor function that is carried out by the ATP hydrolyzing mtHsp70 chaperone and its associated subunits.
The precursor crosses the inner membrane via the voltage-gated channel of TIM23. The pore has been found to be ∼13 Å wide and can therefore accommodate only one α-helix in transit (Schwartz and Matouschek 1999; Truscott et al. 2001). Early on, Tim23 was shown to constitute an integral part of the import channel itself. Tim17 is an additional essential core protein of the TIM23 complex, and its membrane domain is homologous to Tim23 at the primary sequence level (Kubrich et al. 1994; Sirrenberg et al. 1996). It is predicted to span the inner membrane of the mitochondrion with four trans-membrane helices and exposes short N-terminal and C-terminal segments to the IMS (Kubrich et al. 1994). Tim17 was initially thought to constitute part of the import channel. However, the Tim23 C-terminal membrane domain was eventually shown to be sufficient to form a pore, which lacked the pre-sequence sensitivity (Truscott et al. 2003). In mitoplasts prepared from wild-type mitochondria, the TIM23 complex possesses a twin-pore structure, whereas in mitoplasts prepared from Tim17-depleted mitochondria, the twin-pore collapses into a single pore. Hence, Tim17 seems to regulate the formation of the correct twin-pore structure, and this function is attributed to its C-terminal segment (Martinez-Caballero et al. 2007). The N-terminal segment of Tim17 has been found to regulate voltage gating of the channel (Martinez-Caballero et al. 2007).
Recent work has revealed that TMs 1 and 2 of Tim23 are found in close proximity to the substrate during its import suggesting that they comprise part of the import channel (Alder et al. 2008a; Pareek et al. 2013). TMs 1 and 2 have also been found to mediate the interaction of Tim23 with Tim17 and the dimerization of Tim23 through the GxxxG motifs present throughout these helices (Alder et al. 2008b; Demishtein-Zohary et al. 2015; Pareek et al. 2013). Furthermore, we have determined that the destabilization of GxxxG interactions in these helices causes Tim44 to dissociate from the TIM23 complex (Demishtein-Zohary et al. 2015).
Taken together, the data suggest the following sequence of events. Once the precursor has reached the import channel, it is bound by the channel-forming Tim23 subunit, while Tim17 regulates the formation of the correct channel structure and presequence sensitivity. The membrane potential and the increasing affinities of the precursor protein to Tim44 in trans are the main forces that drive the precursor across the inner membrane and to emerge in the matrix.
Final steps of translocation into the matrix
Final translocation across the inner membrane into the matrix is facilitated by the translocation motor and fueled by ATP hydrolysis. Tim44 acts as an anchor that recruits mtHsp70 and other members of the motor to the TIM23 complex (Kronidou et al. 1994). MtHsp70 contains an ATPase domain and a peptide-binding pocket. Upon binding ATP, the peptide-binding pocket of mtHsp70 opens thus allowing substrate binding. ATP hydrolysis to ADP causes the binding pocket to lock on the bound substrate, concomitant with the release of mtHsp70 from Tim44 and the further insertion of the precursor into the matrix (Liu et al. 2003; Slutsky-Leiderman et al. 2007). Release of the peptide from mtHsp70 is enabled once ADP is removed and exchanged with ATP (Bukau et al. 2006). The membrane-associated J-protein complex, Tim14/Tim16, and the cochaperone, Mge1, modulate the function of mtHsp70. Tim14 stimulates ATP hydrolysis to ADP thereby causing the binding pocket to close (Mokranjac et al. 2003b; Truscott et al. 2003). Tim16 strongly interacts with Tim14 and seems to regulate the ability of the latter to stimulate the ATPase function of mtHsp70 (Li et al. 2004). Mge1 is a nucleotide exchange factor that replaces ADP with ATP, enabling the release of the bound peptide and the recycling of mtHsp70 (Miao et al. 1997).
The interface between the import motor and the membrane-embedded channel complex has been mapped to a number of different interaction points. First, Tim44 has been found to bind Tim23 (Ting et al. 2014) and Tim17 (Banerjee et al. 2015). A tertiary interaction seems to occur between Tim23, Tim17, and Tim44 and enables the emerging precursor protein to immediately bind mtHsp70 for further translocation into the matrix. A second interface is found between Tim44 and Tim16 (D’Silva et al. 2008; Schilke et al. 2012). An additional interaction site has been shown to exist between Tim17 and Tim14 (Chacinska et al. 2005). Tim14 and Tim16 form a tight complex, the destabilization of which causes Tim14 to dissociate from the TIM23 supercomplex (D’Silva et al. 2008). Hence, Tim16 also recruits Tim14 to the membrane-embedded complex. Pam17 has also been shown to bind Tim17, further stabilizing the membrane-embedded complex (Ting et al. 2014). In addition, the first loop of Tim23 has also been determined to be important for the recruitment of PAM members (Pareek et al. 2013).
Thus, the import motor is bound to the membrane-embedded complex via a number of different subunit interactions to ensure its presence upon emergence of the precursor from the import channel and to allow non-interrupted import across the inner membrane into the matrix.
Regulation of the function of the complex
Once the precursor enters the inner membrane channel, it is then either sorted to the inner membrane or fully transferred to the import motor for its final translocation to the matrix. The way that this decision is made remains to be elucidated. Several non-essential components including Tim21, Mgr2, and Pam17 have been suggested to regulate this process.
The N-terminal domain of Tim21 spans the inner membrane of the mitochondria with one predicted trans-membrane helix, and its C-terminal domain exposes a hydrophilic domain to the IMS (Chacinska et al. 2005; Mokranjac et al. 2005). Tim21 has been shown to connect the TIM23 complex to the TOM complex via interaction with Tom22 (Albrecht et al. 2006; Chacinska et al. 2005, 2010; Mokranjac et al. 2005; Tamura et al. 2009). It has been found to play a role in forming the TOM-TIM23-precursor complex; however, it can be exchanged by a new Tim21 molecule, once this complex is formed (Ieva et al. 2014), demonstrating the dynamic nature of its interaction with the core of the TIM23 complex. Tim21 has also been shown to couple the respiratory-chain super-complexes (complex III and IV) to the TIM23 complex (van der Laan et al. 2006; Wiedemann et al. 2007). Indeed, it has been found to bind both complexes independently, again, highlighting its highly dynamic character.
Mgr2, a second regulator of the complex, is predicted to consist of two transmembrane helices. It has been proposed to connect Tim21 to the core TIM23 complex because, in its absence, Tim21 binds only the respiratory complexes (Gebert et al. 2012). Recent work has shown Mgr2 to regulate and control the lateral release of inner membrane proteins from the TIM23 complex to the inner membrane (Ieva et al. 2014). In its absence, the lateral release of the protein to the inner membrane is enhanced, whereas over-expression of Mgr2 delays the lateral release of the protein to the inner membrane.
Pam17, a third regulatory protein of the complex, is considered to be a part of the import motor and to participate in the initial transfer across the inner membrane (Schiller 2009; van der Laan et al. 2005). Data suggest that this protein modulates the activity of the complex in a manner antagonistic to that of Tim21 (Popov-Celeketic et al. 2008); however, the exact mode of action has yet to be clarified.
Human TIM23 under pathological conditions
In recent years, the human TIM23 complex has attracted considerable attention. Several human orthologs of yeast TIM23 subunits have been studied, mostly in the context of their involvement in a variety of pathological conditions, as summarized in Table 1 (see also Fig. 1b).
An indication that defects in import machinery components can lead to pathological situations was first demonstrated with the human deafness dystonia syndrome, which was shown to be caused by mutations in human Tim8 (DDP1). Tim8, together with Tim13, is involved in the import of Tim23 into mitochondria, providing indirect evidence that import defects can cause mitochondrial diseases (Roesch et al. 2002; Rothbauer et al. 2001). Surprisingly, the biogenesis of Tim23 is not affected by reduced levels of DDP1; however, alterations in mitochondrial morphology have been observed (Engl et al. 2012).
Although no specific disease has been directly linked to Tim23, homozygous knockout mice are not viable, indicating that, similar to the situation in yeast, Tim23 is also essential in mice (Ahting et al. 2009). Moreover, heterozygous mice, with a 50 % reduction of the protein levels, exhibit a reduced lifespan, neurological phenotypes, and signs of premature aging.
Despite the structural conservation between the yeast and human TIM23 complexes, a number of interesting differences have been observed between these translocases. Whereas in yeast, only one gene of Tim17 exists, two paralogs have been described in humans, namely Tim17a and Tim17b, the latter of which exists as two isoforms, namely Tim17b1 and Tim17b2 (Bauer et al. 1999). A recent study of the human TIM23 complex has demonstrated that the three Tim17 variants (Tim17a, Tim17b1, and Tim17b2) are present in three distinct forms of the human TIM23 complex: human translocase B1, human translocase B2, and human translocase A, in which Tim17b1, Tim17b2, and Tim17a are integrated, respectively (Sinha et al. 2014). Tim17a has been found to serve as a suppressor of mitochondrial DNA instability in human cells and, in this way, may prevent disorders connected with mitochondrial DNA loss (Iacovino et al. 2009). Furthermore, high expression of Tim17a has been observed to be associated with breast cancer (Salhab et al. 2010; Xu et al. 2010; Yang et al. 2016).
Another study demonstrating differences between the human and yeast complexes was reported for Tim50. Human Tim50 was shown to possess phosphatase activity in vivo, the role of which is unknown (Guo et al. 2004). However, in yeast, the active site residues of the motif are not conserved, and no evidence for phosphatase activity has been found (Geissler et al. 2002). In cells, the loss of Tim50 causes mitochondrial permeabilization and dysfunction followed by cell death (Guo et al. 2004). Furthermore, like human Tim17a, the expression of Tim50 levels are increased in breast cancer, and the suppression of its expression inhibits the ability of cancer cells to proliferate (Gao et al. 2015). Recently, a homozygous point mutation in hTim50 (G372S) has been linked to 3-methylglutaconic aciduria disorder, characterized by epilepsy, microcephaly, developmental delay, and visual deficit spastic quadriplegia (Serajee and Huq 2015).
Comparison of the human import motor with that of yeast has shown hTim44 to be loosely associated with the inner membrane. A single proline to glutamine point mutation in hTim44 has been found to be correlated with oncocytic thyroid carcinoma (Bonora et al. 2006). In addition, Tim44 has been revealed to be upregulated in hyperglycemic states in mice with diabetes (Wada and Kanwar 1998). Indeed, a transgenic mouse overexpressing Tim44 exhibits improved insulin sensitivity and is protected from type 2 diabetes and obesity (Wang et al. 2015).
Human Tim16 is termed Magmas, and its expression has been demonstrated to increase in prostate cancer cells (Jubinsky et al. 2005). Additionally, a homozygous missense mutation (N76D) in MAGMAS correlates with severe skeletal dysplasia (Mehawej et al. 2014). Human mitochondria contain two different Tim14 homologs: DnaJC19 (JC19) and DnaJC15 (JC15). Both human B translocases interact with the JC19 DnaJ protein, whereas translocase A interacts with the JC15 DnaJ protein (Sinha et al. 2014). A point mutation in JC19 resulting in defective splicing and loss of the full-length DNAJC19 transcript has been found to be associated with dilated cardio-myopathy with ataxia (DCMA) syndrome, an inherited condition represented by heart problems, movement difficulties, and impairment in multiple body systems (Davey et al. 2006).
Finally, mortalin, the human mitochondrial Hsp70 protein, is involved in numerous cellular processes and has been demonstrated to be involved in a large number of pathological conditions. In yeast, three homologs of mtHsp70 are present in the mitochondria. The most important is the product of the Ssc1 gene, which serves as a motor component and as a major protein-folding machine (Horst et al. 1997). In human mitochondria, only one homolog exists. In addition to playing an active role in the import of proteins into the mitochondria and in the folding of proteins in the matrix, mortalin has been detected in the cytosol and the nucleus. In the cytosol, mortalin has been shown to bind and inactivate p53, a tumor suppressor protein, leading to the uncontrolled proliferation of the cells. Indeed, the upregulation of mortalin has been shown to be linked to tumorogenesis in several studies (Ando et al. 2014; Lu et al. 2011a, 2011b; Wadhwa et al. 2006). Altered expression of mortalin has been found to be connected with Parkinson’s (Burbulla et al. 2014; De Mena et al. 2009; Jin et al. 2006) and Alzheimer’s (Park et al. 2014) diseases. Notably, three mutations, namely R126W, A476T, and P509S, have been associated with Parkinson’s disease (Burbulla et al. 2010; De Mena et al. 2009); these point mutations have been identified in heterozygous patients and are absent among the control group. However, the contribution of these mutations in mortalin for the development of early onset Pakinsons’ disease is not clear (Freimann et al. 2013). Recently, two separate single homozygous point mutations in mortalin have also been found to be associated with the EVEN-PLUS syndrome, an autosomal recessive syndrome characterized by epiphysis and vertebral dysplasia and numerous other abnormalities (Royer-Bertrand et al. 2015). In these patients, the mutations in mortalin are homozygous. Interestingly, one of these mutations, R126W maps to the same amino acid whose mutation has been associated with Parkinson’s disease. This highlights the great importance of the fine tuning of the protein level in vivo: if one normal copy of the protein exists, its function is partly impaired, resulting in early onset Parkinson’s disease, whereas two dysfunctional copies cause severe congenital malformations and skeletal dysplasia.
Concluding remarks
Intense research over the past few decades has led to a general understanding of the way that proteins undergo translocation into mitochondria. Most of the subunits of the large TIM23 translocation supercomplex have been identified, and their specific functions are slowly being elucidated. Linkage of many diseases to mutations in the various components of the TIM23 complex highlights the great importance of each individual component and the necessity for the correct cooperation and balance between the subunits. With the exception of Tim23, all the essential Tim components in yeast have been directly linked to human diseases (Table 1). Point mutations have been associated with diseases with regard to all the essential Tim components, except for Tim23 and Tim17. Since these proteins are essential, their mutation probably leads to embryonic lethality. However, we cannot exclude the possibility that point mutations in these proteins resulting in their partial function might be connected with diseases in the future.
References
Ahting U, Floss T, Uez N, Schneider-Lohmar I, Becker L, Kling E, Iuso A, Bender A, de Angelis MH, Gailus-Durner V, Fuchs H, Meitinger T, Wurst W, Prokisch H, Klopstock T (2009) Neurological phenotype and reduced lifespan in heterozygous Tim23 knockout mice, the first mouse model of defective mitochondrial import. Biochim Biophys Acta 1787:371–376
Albrecht R, Rehling P, Chacinska A, Brix J, Cadamuro SA, Volkmer R, Guiard B, Pfanner N, Zeth K (2006) The Tim21 binding domain connects the preprotein translocases of both mitochondrial membranes. EMBO Rep 7:1233–1238
Alder NN, Jensen RE, Johnson AE (2008a) Fluorescence mapping of mitochondrial TIM23 complex reveals a water-facing, substrate-interacting helix surface. Cell 134:439–450
Alder NN, Sutherland J, Buhring AI, Jensen RE, Johnson AE (2008b) Quaternary structure of the mitochondrial TIM23 complex reveals dynamic association between Tim23p and other subunits. Mol Biol Cell 19:159–170
Ando K, Oki E, Zhao Y, Ikawa-Yoshida A, Kitao H, Saeki H, Kimura Y, Ida S, Morita M, Kusumoto T, Maehara Y (2014) Mortalin is a prognostic factor of gastric cancer with normal p53 function. Gastric Cancer 17:255–262
Bajaj R, Jaremko L, Jaremko M, Becker S, Zweckstetter M (2014) Molecular basis of the dynamic structure of the TIM23 complex in the mitochondrial intermembrane space. Structure 22:1501–1511
Banerjee R, Gladkova C, Mapa K, Witte G, Mokranjac D (2015) Protein translocation channel of mitochondrial inner membrane and matrix-exposed import motor communicate via two-domain coupling protein. Elife 4:e11897
Bauer MF, Sirrenberg C, Neupert W, Brunner M (1996) Role of Tim23 as voltage sensor and presequence receptor in protein import into mitochondria. Cell 87:33–41
Bauer MF, Gempel K, Reichert AS, Rappold GA, Lichtner P, Gerbitz KD, Neupert W, Brunner M, Hofmann S (1999) Genetic and structural characterization of the human mitochondrial inner membrane translocase. J Mol Biol 289:69–82
Bolliger L, Junne T, Schatz G, Lithgow T (1995) Acidic receptor domains on both sides of the outer membrane mediate translocation of precursor proteins into yeast mitochondria. EMBO J 14:6318–6326
Bonora E, Evangelisti C, Bonichon F, Tallini G, Romeo G (2006) Novel germline variants identified in the inner mitochondrial membrane transporter TIMM44 and their role in predisposition to oncocytic thyroid carcinomas. Br J Cancer 95:1529–1536
Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125:443–451
Burbulla LF, Schelling C, Kato H, Rapaport D, Woitalla D, Schiesling C, Schulte C, Sharma M, Illig T, Bauer P, Jung S, Nordheim A, Schols L, Riess O, Kruger R (2010) Dissecting the role of the mitochondrial chaperone mortalin in Parkinson’s disease: functional impact of disease-related variants on mitochondrial homeostasis. Hum Mol Genet 19:4437–4452
Burbulla LF, Fitzgerald JC, Stegen K, Westermeier J, Thost AK, Kato H, Mokranjac D, Sauerwald J, Martins LM, Woitalla D, Rapaport D, Riess O, Proikas-Cezanne T, Rasse TM, Kruger R (2014) Mitochondrial proteolytic stress induced by loss of mortalin function is rescued by Parkin and PINK1. Cell Death Dis 5:e1180
Chacinska A, Rehling P, Guiard B, Frazier AE, Schulze-Specking A, Pfanner N, Voos W, Meisinger C (2003) Mitochondrial translocation contact sites: separation of dynamic and stabilizing elements in formation of a TOM-TIM-preprotein supercomplex. EMBO J 22:5370–5381
Chacinska A, Lind M, Frazier AE, Dudek J, Meisinger C, Geissler A, Sickmann A, Meyer HE, Truscott KN, Guiard B, Pfanner N, Rehling P (2005) Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell 120:817–829
Chacinska A, van der Laan M, Mehnert CS, Guiard B, Mick DU, Hutu DP, Truscott KN, Wiedemann N, Meisinger C, Pfanner N, Rehling P (2010) Distinct forms of mitochondrial TOM-TIM supercomplexes define signal-dependent states of preprotein sorting. Mol Cell Biol 30:307–318
D’Silva PR, Schilke B, Hayashi M, Craig EA (2008) Interaction of the J-protein heterodimer Pam18/Pam16 of the mitochondrial import motor with the translocon of the inner membrane. Mol Biol Cell 19:424–432
Davey KM, Parboosingh JS, McLeod DR, Chan A, Casey R, Ferreira P, Snyder FF, Bridge PJ, Bernier FP (2006) Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. J Med Genet 43:385–393
de la Cruz L, Bajaj R, Becker S, Zweckstetter M (2010) The intermembrane space domain of Tim23 is intrinsically disordered with a distinct binding region for presequences. Protein Sci 19:2045–2054
De Mena L, Coto E, Sanchez-Ferrero E, Ribacoba R, Guisasola LM, Salvador C, Blazquez M, Alvarez V (2009) Mutational screening of the mortalin gene (HSPA9) in Parkinson’s disease. J Neural Transm (Vienna) 116:1289–1293
Dekker PJ, Keil P, Rassow J, Maarse AC, Pfanner N, Meijer M (1993) Identification of MIM23, a putative component of the protein import machinery of the mitochondrial inner membrane. FEBS Lett 330:66–70
Demishtein-Zohary K, Marom M, Neupert W, Mokranjac D, Azem A (2015) GxxxG motifs hold the TIM23 complex together. FEBS J 282:2178–2186
Dukanovic J, Rapaport D (2011) Multiple pathways in the integration of proteins into the mitochondrial outer membrane. Biochim Biophys Acta 1808:971–980
Engl G, Florian S, Tranebjaerg L, Rapaport D (2012) Alterations in expression levels of deafness dystonia protein 1 affect mitochondrial morphology. Hum Mol Genet 21:287–299
Frazier AE, Dudek J, Guiard B, Voos W, Li Y, Lind M, Meisinger C, Geissler A, Sickmann A, Meyer HE, Bilanchone V, Cumsky MG, Truscott KN, Pfanner N, Rehling P (2004) Pam16 has an essential role in the mitochondrial protein import motor. Nat Struct Mol Biol 11:226–233
Freimann K, Zschiedrich K, Bruggemann N, Grunewald A, Pawlack H, Hagenah J, Lohmann K, Klein C, Westenberger A (2013) Mortalin mutations are not a frequent cause of early-onset Parkinson disease. Neurobiol Aging 34:e2619–e2620
Gao SP, Sun HF, Jiang HL, Li LD, Hu X, Xu XE, Jin W (2015) Loss of TIM50 suppresses proliferation and induces apoptosis in breast cancer. Tumour Biol 37:1279–1287
Gebert M, Schrempp SG, Mehnert CS, Heisswolf AK, Oeljeklaus S, Ieva R, Bohnert M, von der Malsburg K, Wiese S, Kleinschroth T, Hunte C, Meyer HE, Haferkamp I, Guiard B, Warscheid B, Pfanner N, van der Laan M (2012) Mgr2 promotes coupling of the mitochondrial presequence translocase to partner complexes. J Cell Biol 197:595–604
Geissler A, Chacinska A, Truscott KN, Wiedemann N, Brandner K, Sickmann A, Meyer HE, Meisinger C, Pfanner N, Rehling P (2002) The mitochondrial presequence translocase: an essential role of Tim50 in directing preproteins to the import channel. Cell 111:507–518
Gevorkyan-Airapetov L, Zohary K, Popov-Celeketic D, Mapa K, Hell K, Neupert W, Azem A, Mokranjac D (2009) Interaction of Tim23 with Tim50 is essential for protein translocation by the mitochondrial TIM23 complex. J Biol Chem 284:4865–4872
Guo Y, Cheong N, Zhang Z, De Rose R, Deng Y, Farber SA, Fernandes-Alnemri T, Alnemri ES (2004) Tim50, a component of the mitochondrial translocator, regulates mitochondrial integrity and cell death. J Biol Chem 279:24813–24825
Horst M, Jeno P, Kronidou NG, Bolliger L, Oppliger W, Scherer P, Manning-Krieg U, Jascur T, Schatz G (1993) Protein import into yeast mitochondria: the inner membrane import site protein ISP45 is the MPI1 gene product. EMBO J 12:3035–3041
Horst M, Oppliger W, Rospert S, Schonfeld HJ, Schatz G, Azem A (1997) Sequential action of two hsp70 complexes during protein import into mitochondria. EMBO J 16:1842–1849
Iacovino M, Granycome C, Sembongi H, Bokori-Brown M, Butow RA, Holt IJ, Bateman JM (2009) The conserved translocase Tim17 prevents mitochondrial DNA loss. Hum Mol Genet 18:65–74
Ieva R, Schrempp SG, Opalinski L, Wollweber F, Hoss P, Heisswolf AK, Gebert M, Zhang Y, Guiard B, Rospert S, Becker T, Chacinska A, Pfanner N, van der Laan M (2014) Mgr2 functions as lateral gatekeeper for preprotein sorting in the mitochondrial inner membrane. Mol Cell 56:641–652
Jin J, Hulette C, Wang Y, Zhang T, Pan C, Wadhwa R, Zhang J (2006) Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: relevance to Parkinson disease. Mol Cell Proteomics 5:1193–1204
Jubinsky PT, Short MK, Mutema G, Morris RE, Ciraolo GM, Li M (2005) Magmas expression in neoplastic human prostate. J Mol Histol 36:69–75
Kang PJ, Ostermann J, Shilling J, Neupert W, Craig EA, Pfanner N (1990) Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature 348:137–143
Knox C, Sass E, Neupert W, Pines O (1998) Import into mitochondria, folding and retrograde movement of fumarase in yeast. J Biol Chem 273:25587–25593
Komiya T, Rospert S, Schatz G, Mihara K (1997) Binding of mitochondrial precursor proteins to the cytoplasmic domains of the import receptors Tom70 and Tom20 is determined by cytoplasmic chaperones. EMBO J 16:4267–4275
Kozany C, Mokranjac D, Sichting M, Neupert W, Hell K (2004) The J domain-related cochaperone Tim16 is a constituent of the mitochondrial TIM23 preprotein translocase. Nat Struct Mol Biol 11:234–241
Kronidou NG, Oppliger W, Bolliger L, Hannavy K, Glick BS, Schatz G, Horst M (1994) Dynamic interaction between Isp45 and mitochondrial hsp70 in the protein import system of the yeast mitochondrial inner membrane. Proc Natl Acad Sci U S A 91:12818–12822
Kubrich M, Keil P, Rassow J, Dekker PJ, Blom J, Meijer M, Pfanner N (1994) The polytopic mitochondrial inner membrane proteins MIM17 and MIM23 operate at the same preprotein import site. FEBS Lett 349:222–228
Laloraya S, Gambill BD, Craig EA (1994) A role for a eukaryotic GrpE-related protein, Mge1p, in protein translocation. Proc Natl Acad Sci U S A 91:6481–6485
Lesnik C, Cohen Y, Atir-Lande A, Schuldiner M, Arava Y (2014) OM14 is a mitochondrial receptor for cytosolic ribosomes that supports co-translational import into mitochondria. Nat Commun 5:5711 [Erratum: Nat Commun 6:6813]
Li Y, Dudek J, Guiard B, Pfanner N, Rehling P, Voos W (2004) The presequence translocase-associated protein import motor of mitochondria. Pam16 functions in an antagonistic manner to Pam18. J Biol Chem 279:38047–38054
Liu Q, D’Silva P, Walter W, Marszalek J, Craig EA (2003) Regulated cycling of mitochondrial Hsp70 at the protein import channel. Science 300:139–141
Lu WJ, Lee NP, Kaul SC, Lan F, Poon RT, Wadhwa R, Luk JM (2011a) Induction of mutant p53-dependent apoptosis in human hepatocellular carcinoma by targeting stress protein mortalin. Int J Cancer 129:1806–1814
Lu WJ, Lee NP, Kaul SC, Lan F, Poon RT, Wadhwa R, Luk JM (2011b) Mortalin-p53 interaction in cancer cells is stress dependent and constitutes a selective target for cancer therapy. Cell Death Differ 18:1046–1056
Lytovchenko O, Melin J, Schulz C, Kilisch M, Hutu DP, Rehling P (2013) Signal recognition initiates reorganization of the presequence translocase during protein import. EMBO J 32:886–898
Maarse AC, Blom J, Grivell LA, Meijer M (1992) MPI1, an essential gene encoding a mitochondrial membrane protein, is possibly involved in protein import into yeast mitochondria. EMBO J 11:3619–3628
Maarse AC, Blom J, Keil P, Pfanner N, Meijer M (1994) Identification of the essential yeast protein MIM17, an integral mitochondrial inner membrane protein involved in protein import. FEBS Lett 349:215–221
Marom M, Dayan D, Demishtein-Zohary K, Mokranjac D, Neupert W, Azem A (2011) Direct interaction of mitochondrial targeting presequences with purified components of the TIM23 protein complex. J Biol Chem 286:43809–43815
Martinez-Caballero S, Grigoriev SM, Herrmann JM, Campo ML, Kinnally KW (2007) Tim17p regulates the twin pore structure and voltage gating of the mitochondrial protein import complex TIM23. J Biol Chem 282:3584–3593
Mayer A, Neupert W, Lill R (1995) Mitochondrial protein import: reversible binding of the presequence at the trans side of the outer membrane drives partial translocation and unfolding. Cell 80:127–137
Mehawej C, Delahodde A, Legeai-Mallet L, Delague V, Kaci N, Desvignes JP, Kibar Z, Capo-Chichi JM, Chouery E, Munnich A, Cormier-Daire V, Megarbane A (2014) The impairment of MAGMAS function in human is responsible for a severe skeletal dysplasia. PLoS Genet 10:e1004311
Meinecke M, Wagner R, Kovermann P, Guiard B, Mick DU, Hutu DP, Voos W, Truscott KN, Chacinska A, Pfanner N, Rehling P (2006) Tim50 maintains the permeability barrier of the mitochondrial inner membrane. Science 312:1523–1526
Miao B, Davis JE, Craig EA (1997) Mge1 functions as a nucleotide release factor for Ssc1, a mitochondrial Hsp70 of Saccharomyces cerevisiae. J Mol Biol 265:541–552
Mokranjac D, Paschen SA, Kozany C, Prokisch H, Hoppins SC, Nargang FE, Neupert W, Hell K (2003a) Tim50, a novel component of the TIM23 preprotein translocase of mitochondria. EMBO J 22:816–825
Mokranjac D, Sichting M, Neupert W, Hell K (2003b) Tim14, a novel key component of the import motor of the TIM23 protein translocase of mitochondria. EMBO J 22:4945–4956
Mokranjac D, Popov-Celeketic D, Hell K, Neupert W (2005) Role of Tim21 in mitochondrial translocation contact sites. J Biol Chem 280:23437–23440
Mokranjac D, Sichting M, Popov-Celeketic D, Mapa K, Gevorkyan-Airapetov L, Zohary K, Hell K, Azem A, Neupert W (2009) Role of Tim50 in the transfer of precursor proteins from the outer to the inner membrane of mitochondria. Mol Biol Cell 20:1400–1407
Pareek G, Krishnamoorthy V, D’Silva P (2013) Molecular insights revealing interaction of Tim23 and channel subunits of presequence translocase. Mol Cell Biol 33:4641–4659
Park SJ, Shin JH, Jeong JI, Song JH, Jo YK, Kim ES, Lee EH, Hwang JJ, Lee EK, Chung SJ, Koh JY, Jo DG, Cho DH (2014) Down-regulation of mortalin exacerbates Abeta-mediated mitochondrial fragmentation and dysfunction. J Biol Chem 289:2195–2204
Popov-Celeketic D, Mapa K, Neupert W, Mokranjac D (2008) Active remodelling of the TIM23 complex during translocation of preproteins into mitochondria. EMBO J 27:1469–1480
Rahman B, Kawano S, Yunoki-Esaki K, Anzai T, Endo T (2014) NMR analyses on the interactions of the yeast Tim50 C-terminal region with the presequence and Tim50 core domain. FEBS Lett 588:678–684
Roesch K, Curran SP, Tranebjaerg L, Koehler CM (2002) Human deafness dystonia syndrome is caused by a defect in assembly of the DDP1/TIMM8a-TIMM13 complex. Hum Mol Genet 11:477–486
Rothbauer U, Hofmann S, Muhlenbein N, Paschen SA, Gerbitz KD, Neupert W, Brunner M, Bauer MF (2001) Role of the deafness dystonia peptide 1 (DDP1) in import of human Tim23 into the inner membrane of mitochondria. J Biol Chem 276:37327–37334
Royer-Bertrand B, Castillo-Taucher S, Moreno-Salinas R, Cho TJ, Chae JH, Choi M, Kim OH, Dikoglu E, Campos-Xavier B, Girardi E, Superti-Furga G, Bonafe L, Rivolta C, Unger S, Superti-Furga A (2015) Mutations in the heat-shock protein A9 (HSPA9) gene cause the EVEN-PLUS syndrome of congenital malformations and skeletal dysplasia. Sci Rep 5:17154
Salhab M, Patani N, Jiang W, Mokbel K (2010) High TIMM17A expression is associated with adverse pathological and clinical outcomes in human breast cancer. Breast Cancer 19:153–160
Schilke BA, Hayashi M, Craig EA (2012) Genetic analysis of complex interactions among components of the mitochondrial import motor and translocon in Saccharomyces cerevisiae. Genetics 190:1341–1353
Schiller D (2009) Pam17 and Tim44 act sequentially in protein import into the mitochondrial matrix. Int J Biochem Cell Biol 41:2343–2349
Schneider HC, Berthold J, Bauer MF, Dietmeier K, Guiard B, Brunner M, Neupert W (1994) Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature 371:768–774
Schulz C, Lytovchenko O, Melin J, Chacinska A, Guiard B, Neumann P, Ficner R, Jahn O, Schmidt B, Rehling P (2011) Tim50’s presequence receptor domain is essential for signal driven transport across the TIM23 complex. J Cell Biol 195:643–656
Schwartz MP, Matouschek A (1999) The dimensions of the protein import channels in the outer and inner mitochondrial membranes. Proc Natl Acad Sci U S A 96:13086–13090
Serajee F, Huq A (2015) A distinct type of 3-methylglutaconic aciduria due to a mutation in the translocase of inner mitochondrial membrane 50 (TIMM50) gene. ASHG Meeting 2015 Abstract no. 2299, https://ep70.eventpilotadmin.com/web/page.php?page=IntHtml&project=ASHG15&id=150121703
Shiota T, Mabuchi H, Tanaka-Yamano S, Yamano K, Endo T (2011) In vivo protein-interaction mapping of a mitochondrial translocator protein Tom22 at work. Proc Natl Acad Sci U S A 108:15179–15183
Sinha D, Srivastava S, Krishna L, D’Silva P (2014) Unraveling the intricate organization of mammalian mitochondrial presequence translocases: existence of multiple translocases for maintenance of mitochondrial function. Mol Cell Biol 34:1757–1775
Sinzel M, Tan T, Wendling P, Kalbacher H, Ozbalci C, Chelius X, Westermann B, Brugger B, Rapaport D, Dimmer KS (2016) Mcp3 is a novel mitochondrial outer membrane protein that follows a unique IMP-dependent biogenesis pathway. EMBO Rep 17:965–981
Sirrenberg C, Bauer MF, Guiard B, Neupert W, Brunner M (1996) Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22. Nature 384:582–585
Slutsky-Leiderman O, Marom M, Iosefson O, Levy R, Maoz S, Azem A (2007) The interplay between components of the mitochondrial protein translocation motor studied using purified components. J Biol Chem 282:33935–33942
Song J, Tamura Y, Yoshihisa T, Endo T (2014) A novel import route for an N-anchor mitochondrial outer membrane protein aided by the TIM23 complex. EMBO Rep 15:670–677
Tamura Y, Harada Y, Shiota T, Yamano K, Watanabe K, Yokota M, Yamamoto H, Sesaki H, Endo T (2009) Tim23-Tim50 pair coordinates functions of translocators and motor proteins in mitochondrial protein import. J Cell Biol 184:129–141
Ting SY, Schilke BA, Hayashi M, Craig EA (2014) Architecture of the TIM23 inner mitochondrial translocon and interactions with the matrix import motor. J Biol Chem 289:28689–28696
Truscott KN, Kovermann P, Geissler A, Merlin A, Meijer M, Driessen AJ, Rassow J, Pfanner N, Wagner R (2001) A presequence- and voltage-sensitive channel of the mitochondrial preprotein translocase formed by Tim23. Nat Struct Biol 8:1074–1082
Truscott KN, Voos W, Frazier AE, Lind M, Li Y, Geissler A, Dudek J, Muller H, Sickmann A, Meyer HE, Meisinger C, Guiard B, Rehling P, Pfanner N (2003) A J-protein is an essential subunit of the presequence translocase-associated protein import motor of mitochondria. J Cell Biol 163:707–713
van der Laan M, Chacinska A, Lind M, Perschil I, Sickmann A, Meyer HE, Guiard B, Meisinger C, Pfanner N, Rehling P (2005) Pam17 is required for architecture and translocation activity of the mitochondrial protein import motor. Mol Cell Biol 25:7449–7458
van der Laan M, Rissler M, Rehling P (2006) Mitochondrial preprotein translocases as dynamic molecular machines. FEMS Yeast Res 6:849–861
Voos W, Gambill BD, Laloraya S, Ang D, Craig EA, Pfanner N (1994) Mitochondrial GrpE is present in a complex with hsp70 and preproteins in transit across membranes. Mol Cell Biol 14:6627–6634
Wada J, Kanwar YS (1998) Characterization of mammalian translocase of inner mitochondrial membrane (Tim44) isolated from diabetic newborn mouse kidney. Proc Natl Acad Sci U S A 95:144–149
Wadhwa R, Takano S, Kaur K, Deocaris CC, Pereira-Smith OM, Reddel RR, Kaul SC (2006) Upregulation of mortalin/mthsp70/Grp75 contributes to human carcinogenesis. Int J Cancer 118:2973–2980
Waegemann K, Popov-Celeketic D, Neupert W, Azem A, Mokranjac D (2015) Cooperation of TOM and TIM23 complexes during translocation of proteins into mitochondria. J Mol Biol 427:1075–1084
Wang Y, Katayama A, Terami T, Han X, Nunoue T, Zhang D, Teshigawara S, Eguchi J, Nakatsuka A, Murakami K, Ogawa D, Furuta Y, Makino H, Wada J (2015) Translocase of inner mitochondrial membrane 44 alters the mitochondrial fusion and fission dynamics and protects from type 2 diabetes. Metabolism 64:677–688
Wenz LS, Opalinski L, Schuler MH, Ellenrieder L, Ieva R, Bottinger L, Qiu J, van der Laan M, Wiedemann N, Guiard B, Pfanner N, Becker T (2014) The presequence pathway is involved in protein sorting to the mitochondrial outer membrane. EMBO Rep 15:678–685
Wiedemann N, van der Laan M, Hutu DP, Rehling P, Pfanner N (2007) Sorting switch of mitochondrial presequence translocase involves coupling of motor module to respiratory chain. J Cell Biol 179:1115–1122
Xu X, Qiao M, Zhang Y, Jiang Y, Wei P, Yao J, Gu B, Wang Y, Lu J, Wang Z, Tang Z, Sun Y, Wu W, Shi Q (2010) Quantitative proteomics study of breast cancer cell lines isolated from a single patient: discovery of TIMM17A as a marker for breast cancer. Proteomics 10:1374–1390
Yamamoto H, Esaki M, Kanamori T, Tamura Y, Nishikawa S, Endo T (2002) Tim50 is a subunit of the TIM23 complex that links protein translocation across the outer and inner mitochondrial membranes. Cell 111:519–528
Yang X, Si Y, Tao T, Martin TA, Cheng S, Yu H, Li J, He J, Jiang WG (2016) The impact of TIMM17A on aggressiveness of human breast cancer cells. Anticancer Res 36:1237–1241
Yogev O, Karniely S, Pines O (2007) Translation-coupled translocation of yeast fumarase into mitochondria in vivo. J Biol Chem 282:29222–29229
Young JC, Hoogenraad NJ, Hartl FU (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112:41–50
Acknowledgments
We thank Dana Dayan and Dr. Celeste Weiss for useful comments and discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
A.A. is supported by the Israel Science Foundation (ISF-1507/13) and the DFG trilateral project (Reference number SCHO 754/5-2).
Rights and permissions
About this article
Cite this article
Demishtein-Zohary, K., Azem, A. The TIM23 mitochondrial protein import complex: function and dysfunction. Cell Tissue Res 367, 33–41 (2017). https://doi.org/10.1007/s00441-016-2486-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2486-7