Abstract
Despite the common structure of vertebrates, the development of the vertebral column differs widely between teleosts and tetrapods in several respects, including the ossification of the centrum and the function of the notochord. In contrast to tetrapods, vertebral development in teleosts is not fully understood, particularly for large fish with highly ossified bones. We therefore examined the histology and gene expression profile of vertebral development in fugu, Takifugu rubripes, a model organism for genomic research. Ossification of the fugu centrum is carried out by outer osteoblasts expressing col1a1, col2a1, and sparc, and the growing centra completely divide the notochord into double cone-shaped segments that function as intercentral joints. In this process, the notochord basal cells produce a thick notochord sheath exhibiting Alcian-blue–reactive cartilaginous properties and composing the intercentral ligament in cooperation with the external ligament connective tissue. Synthesis of the matrix by the basal cells was ascertained by an in vitro test. Expression of twist2 indicates that this connective tissue is descended from the embryonic sclerotome. Notochord basal cells express sox9, ihhb, shh, and col2a1a, suggesting that the signaling system involved in chondrocyte proliferation and matrix production also functions in notochord cells for notochord sheath formation. We further found that the notochord expression of both ntla and shh is maintained in the fugu vertebral column, whereas it is turned off after embryogenesis in zebrafish. Thus, our results demonstrate that, in contrast to zebrafish, a dynamic morphogenesis and molecular network continues to function in fugu until the establishment of the adult vertebral column.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vertebral column, which is composed of a repetitive series of bony centra (vertebral body) and joint tissues, is an organ evolved specifically in the vertebrates, discriminating them from invertebrates (Fleming et al. 2015; Mansfield et al. 2015). In teleosts, the molecular developmental biology of the vertebral column has been intensively studied by using zebrafish and medaka as small model organisms (Bensimon-Brito et al. 2012; Fleming et al. 2004; Inohaya et al. 2007; Renn et al. 2013; Willems et al. 2012). Morphological characterization of the vertebral column of several non-model large fish, including perch and salmon, has also been reported (Nordvik et al. 2005; Schmitz 1995). These studies have demonstrated that the anatomy and development of the vertebral column differs significantly between teleosts and tetrapods in several respects, despite the common structure of vertebrates.
For example, although the tetrapod centrum forms by endochondral ossification, in which cartilaginous primordia are formed and then replaced by the bones, the centrum of teleosts forms by membranous ossification, which involves direct ossification of the collagenous matrix (Christ et al. 2000; Fleming et al. 2004; Inohaya et al. 2007). In zebrafish, initial membranous ossification of the centrum is carried out by notochord cells, which secrete bone matrix into the notochord sheath (Fleming et al. 2004). In medaka, on the other hand, sclerotome-derived osteoblasts and external ligament connective tissue covering the notochord sheath produce the bone matrix of the centrum (Inohaya et al. 2010). Thus, variation in the membranous ossification process exists among teleosts.
In addition, the intercentral joint of teleosts consists primarily of the notochord; in stark contrast, the joint of tetrapods, the intercentral disk, is formed by chondrogenic sclerotome-derived cells (Inohaya et al. 2010; Schmitz 1995). These data also indicate that the fate of the sclerotome differs significantly between tetrapods and teleosts; the sclerotome of tetrapods forms the cartilaginous primordia of the centrum and intercentral disk, whereas the sclerotome of the teleosts differentiates into osteoblasts and external ligament connective tissue surrounding the notochord sheath (Inohaya et al. 2010).
At present, however, the data are not sufficient to illuminate the cellular and molecular bases of teleost vertebral column development. In a recent study with RNA-seq analysis, we found that various developmental regulatory genes involved in embryonic notochord and sclerotome development, such as ntl, twist2, and shh, are expressed in the post-larval vertebral column of the marine teleost flounder (Paralichthys olivaceus), suggesting that these genes continue to function in the vertebral column until the adult stage (Ibaraki et al. 2015). Most teleosts, including flounder, are much larger than zebrafish and medaka, and their centrum is more heavily ossified than that of the smaller fish (Grotmol et al. 2003; Schmitz 1995). Considering that teleosts evolved in the ocean, we hypothesized that features that would enhance our understanding of vertebral column development in teleosts could be more readily identified in larger marine fish, such as fugu (Takifugu rubripes), as examined here.
Fugu is a model organism for genomic research (Aparicio et al. 2002) and is an ideal species for research on vertebral column development at the molecular level because of the availability of its genome information and its large body size (approximately 70 cm maximum). To investigate the process of centrum and intercentral joint formation and its interaction with the notochord, we have examined, in this study, the histology and mRNA expression profile of fugu vertebral development. As expected, we have found a dynamic morphogenesis and molecular network that underlies adult-type vertebral column development in fugu and that has not been reported for zebrafish, including the segmentation of the notochord by the centrum into a double cone-shaped chamber that functions as an intervertebral joint and expression of ntla, shh, col2a1a, and sox9 by the notochord cells in the vertebral column.
Materials and methods
Animals
Fugu embryos were obtained as described previously (Uji et al. 2011). After hatching, larvae were cultured in 500-l plastic tanks and supplied with circulating filtered seawater maintained at 20 °C. Larvae were fed rotifers at 10–25 days post-fertilization (dpf), Artemia nauplii at 20–40 dpf, and then a formulated diet. After being anesthetized with tricaine, fish were fixed in 4 % paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for skeletal staining and Bouin’s fixative for hematoxylin-eosin (H-E) staining and section in situ hybridization (SISH). Fish maintenance and experiments were approved by the University Committee of Animal Experiments.
Skeletal, H-E, and von Kossa staining
Samples fixed in 4 % PFA were bleached by using 1 % H2O2/1 % KOH. For cartilage staining, samples were soaked overnight in 0.1 % Alcian blue 8GX (Sigma, St. Louis, MO, USA) dissolved in 1 % HCl/70 % ethanol. Next, the samples were washed with 1 % HCl/70 % ethanol solution and soaked in 80 % glycerol in PBS. For bone staining, samples were treated with Alizarin red S solution (Sigma) prepared according to the manufacturer’s directions and soaked in 80 % glycerol/1 % KOH.
Samples fixed in Bouin’s fixative were embedded in paraffin and cut into 8-μm sections. After removal of the paraffin, the sections were subjected to H-E staining.
For Alcian blue and von Kossa double staining, samples fixed with PFA were processed to 8-μm paraffin sections. After removal of the paraffin, sections were first incubated in 0.1 % Alcian blue 8GX in 1 % HCl/70 % ethanol for 15 min and then treated with von Kossa staining by using the Calcium Stain Kit (ScyTek Laboratories, Logan, UT, USA). von Kossa staining was performed according to the manufacturer’s instructions.
Reverse transcription plus polymerase chain reaction
Gill, liver, muscle, skin, vertebral column, and brain were dissected from anesthetized 150-dpf fugu of approximately 13 cm in body length, and total RNA was extracted by using Sepasol-RNA Super (Nacalai Tesque, Kyoto, Japan) according to the manufacturer’s directions. The RNA was treated with DNase I (Takara, Tokyo, Japan) to digest contaminating DNA, after which the RNA was ethanol-precipitated and dissolved to give a concentration of 1 μg/μl in RNase-free water (Takara). Total RNA (1 μg) was reverse-transcribed to first-strand cDNA by using Moloney murine leukemia virus (M-MLV) reverse transcriptase, ReverTra Ace (Toyobo, Tokyo, Japan), in a total volume of 20 μl.
Polymerase chain reaction (PCR) primers were prepared for the following genes: fugu no tail a (ntla), twist family bHLH transcription factor 2 (tsist2), transcription factor Cbfa1 (runx2), SRY (sex-determining region Y)-box 9 (sox9), sonic hedgehog (shh), Indian hedgehog b (ihhb), collagen type I alpha 1 (col1a1), collagen type II alpha 1 (col2a1), secreted protein acidic cysteine-rich (sparc, also called osteonectin), and actin beta-like 2 (β-actin). The gene symbols, GenBank accession numbers, PCR product sizes, and primer sequences are listed in Table 1. Aliquots of 1 μl cDNA were used for each 20-μl PCR with Taq DNA polymerase (Takara). PCR conditions were as follows: 40 cycles of 95 °C for 30 s, 55 °C for 30 s, and 75 °C for 60 s. PCR products were electrophoresed on a 2 % agarose gel (Nacalai), stained with ethidium bromide, and photographed.
In situ hybridization
To prepare templates for synthesizing probes for SISH, cDNA fragments of the genes listed in Table 1 were PCR-amplified by using fugu embryonic cDNA prepared as described previously (Suzuki et al. 2002). The PCR products were cloned into pGEM-T Easy Vector (Promega, Madison, WI, USA), and digoxigenin (DIG)-labeled antisense riboprobes were synthesized by using a DIG RNA Labeling kit (Roche Diagnostics, Mannheim, Germany) with an appropriate RNA polymerase. SISH was performed as described previously (Kurokawa and Suzuki 2002).
Tissue culture
The vertebral column was dissected from 13-cm fugu. After the vertebral column had been cut at the intercentral joint by using a scalpel, notochord tissue was collected, minced into approximately 0.5-mm pieces, and then plated on a Col-1–coated Chamber Slide II (Iwaki, Funahashi, Japan) with DMEM containing 10 % fetal bovine serum and penicillin/streptomycin. After 6 days of culture, the tissues were fixed with 4 % PFA and stained with Alcian blue 8GX in 1 % HCl/70 % ethanol.
Photography
Photomicrographs were taken by using a Leica DFC500 or 450C Digital Camera (Leica, Wetzlar, Germany) attached to a Leica DM2500 microscope. Images were subsequently processed by using Photshop CS5 (Adobe Systems, San Jose, CA, USA).
Results
Structure of adult fugu vertebral column
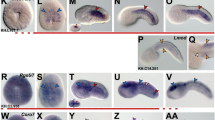
We observed fugu skeletal formation at the larval, juvenile, and adult stages histologically (Fig. 1a–g). The terminology of vertebral components used here principally follows that described previously for perch, Perca flavescens (Schmitz 1995). The vertebral column of fugu examined here is composed of 22 centra, with non-ossified intervertebral ligaments between the centra (Fig. 1h, i). The neural and hemal arches extend from the centra and are attached to the spine (Fig. 1i). Dissection of the vertebral column showed that the centrum is highly ossified up to the central region, and a double cone-shaped chamber, consisting of the notochord segmented by adjacent centra, exists between the centra (Fig. 1j, k). Thus, the vertebral column of adult fugu consists of a repetitive pattern of segmented centra and intercentral joints composed of a segmented notochord.
Structure of fugu vertebral column. a–g Lateral view of fugu at stages of skeletal observation (dpf days post-fertilization, BL body length). h Alizarin red staining in whole mount. i Vertebral column stained with Alizarin red. j Horizontal fracture of vertebral column. k Hematoxylin-eosin (H-E)-stained section. l Higher magnification of segmented part of notochord. m Higher magnification of intercentral ligament. Space between notochord sheath and basal cells is formed by an artifact, and they originally attach closely as shown in Fig. 3g. n, o Alcian blue staining of vertebral column sections adjacent to l and m, respectively (bc notochord basal cell, ct centrum, ee elastic externa, el external ligament, elc external ligament connective tissue, ha hemal arch, hs hemal spine, il intercentral ligament, m bone matrix, na neural arch, no notochord, ns neural spine, nsh notochord sheath, nst notochord string, vc vacuolated cell)
The notochord forms two types of extracellular matrix: an outer notochord sheath and an inner notochord string (Fig. 1k–m). The notochord sheath consists of non-ossified tissue with reactivity to Alcian blue covering the inner surface of the vertebral column (Fig. 1n, o). The notochord string comprises fibrous tissue with strong affinity for eosin (Fig. 1l). The notochord sheath forms intercentral ligaments with the elastic externa, external ligament, and outer elastic connective tissue (Fig. 1m; see also below). The notochord string runs through the median line of the notochord chamber, connecting the inner side by the adjacent centra (Fig. 1i, j). Thus, both the notochord sheath and notochord string function to connect the adjacent centra. A layer of notochord basal cells is attached to the inner surface of the notochord sheath, and vacuolated cells fill the notochord chamber (Fig. 1l, m).
Early skeletogenesis
The early phase of larval vertebral skeletogenesis was observed by whole-mount staining of cartilage and bone (Fig. 2). Fugu larvae hatch at 3 dpf at 20 °C (Uji et al. 2011). No skeletal elements have developed by the time of hatching (data not shown). At 10 dpf, auditory capsules attached to the notochord, pharyngeal arches, and pectoral fins began cartilaginous skeletogenesis, but vertebral column formation had not yet begun (Fig. 2a, b). The vertebral elements that first appeared at 15–20 dpf were the anterior-most neural arches extending dorsally from the notochord to surround the spinal cord (Fig. 2e, i). These arches formed by membranous ossification without the formation of cartilage (Fig. 2c–g). Next, the centrum began to form from the anterior-most elements by membranous ossification in order to ring the notochord from the point of contact with the neural arch (Fig. 2e, j, k). All vertebrae, 22 in total, had appeared by 40 dpf (Fig. 1h), and intercentral ligaments (which do not react with Alizarin red) formed between the adjacent centra (Fig. 2f, g, h). Concomitantly, the hemal arches and neural and hemal spines also formed by membranous ossification (Fig. 1j, k). Thus, the entire fugu vertebral structure formed by membranous ossification without the production of any cartilage.
Early development of cartilaginous and bony skeleton in fugu. a–h Cartilaginous and ossified skeletons visualized by Alcian blue and Alizarin red staining in whole mount. d, g Higher magnification views of rostral end of notochord and attached cartilage (dorsal view). i–k Higher magnification views of anterior vertebral column stained with Alizarin red at initial phase of skeletogenesis (ac auditory capsule, af anal fin, b basioccipital bone, cc caudal fin cartilage, cf caudal fin, ct centrum, df dorsal fin, ha hemal arch, il intercentral ligament, no notochord, na neural arch, pa pharyngeal arch, pf pectoral fin, pr proximal radial, sc spinal cord)
Ossification of centrum and segmentation of notochord
We then histologically followed the formation of the vertebral column through the establishment of the adult form (Fig. 3). The bone matrix of the centrum, which is strongly eosinophilic and stained by von Kossa, began to accumulate between 10 and 24 dpf (Fig. 3e, g, l, m), and synthesis of bone matrix was active at the anterior and posterior part of the centra (Fig. 3b, n, o). It then formed highly branched trabeculae (Fig. 3h, k). By 400 dpf, highly ossified adult-type centra were established (Fig. 1i, j). Double-staining with von Kossa and Alcian blue clearly showed that bone matrix accumulation of the centrum began along the outer surface of the notochord sheath, which was reactive with Alcian blue (Fig. 3l, m). During further growth of centrum, calcium deposition took place only at the outer surface of the notochord sheath (Fig. 3l, m).
Histology of notochord, centra, and intercentral ligaments in developing vertebral column. e, f, k Horizontal sections; all others are sagittal sections. a, c Vertebral column stained with Alizarin red in lateral view. b, d Horizontal fractures. e–k H-E-stained sections; Alcian blue staining was also carried out (f). i–o Double-staining with von Kossa and Alcian blue to discriminate notochord sheath and calcium deposition of bone matrix (green arrows positions at which notochord segmented by centra, bc notochord basal cells, ct centrum, ee elastic externa, el external ligament, elc external ligament connective tissue, ha hemal arch, hs hemal spine, il intercentral ligament, ij intercentral joint [encircled], m bone matrix, me melanophore, no notochord, na neural arch, nsh notochord sheath, nst notochord string, ns neural spine, sc spinal cord, vc notochord vacuolated cells)
The notochord, which was a simple tube-like structure at 10 dpf (Figs. 2a, 3e), began to segment by 24 dpf, forming the pre-pattern of the periodic segments of the centra and intercentral ligaments; the notochord expanded at the intercentral ligaments and contracted by the accumulating bone matrix of the centra (Fig. 3g). The notochord contracted further by the inward growth of the centra (Fig. 3a, h) and was finally completely segmented by the centra at 62 dpf (Fig. 3d, k). The segmented notochord formed double cone-shaped chambers between the adjacent centra, producing the intercentral joints (Fig. 3k; see also below).
Formation of notochord sheath, notochord string, and intercentral ligaments
At 10 dpf, the notochord consisted of an outer thin notochord sheath (stainable by Alcian blue) and inner vacuolated cells (Fig. 3e, f). The notochord sheath grew into a thick acellular layer covering the inner surface of the bone matrix of the centrum by 40 dpf (Fig. 3j, n, o). The notochord sheath grew extremely thick between the fringes of adjacent centra and composed the intercentral ligaments in combination with the elastic externa, elastic ligaments, and external elastic connective tissue (Fig. 3i, o). The external ligament connective tissue consisted of densely packed fibroblastic cells and was attached to the elastic ligaments and the peripheral region of the bone matrix of the centrum (Fig. 3i, o). The elastic ligament was a thin matrix continuing to the centrum bone matrix, and the elastic externa consisted of highly eosinophilic dense matrix (Fig. 3i). The notochord basal cells covering the inner surface of the notochord sheath became visible at 24 dpf (Fig. 3g) and were thick at the intercentral ligaments (Fig. 3i, o).
The eosinophilic matrix began to accumulate in the notochord chamber at the contracting portion (Fig. 3h, j) and finally formed the fibrous notochord string connecting the adjacent centra at 32–62 dpf (Fig. 3k). The notochord string was more eosinophilic than the bone matrix (Fig. 3k). These data thus demonstrate that, during segmentation by the centra, the notochord undergoes dynamic morphogenesis, including the formation of the thick notochord sheath and elastic externa that compose the intervertebral ligaments, the notochord string connecting adjacent centra, and a large chamber filled with vacuolated cells.
The diameter of the notochord was approximately 200 μm at 10 dpf, and by 400 dpf, the maximum diameter of the segmented notochord was approximately 5 mm, indicating that the notochord continued to grow during vertebral column formation. The size of the vacuolated notochord cells did not change and was approximately 80 μm (Fig. 3e, k).
Gene expression in vertebral column
Using RT-PCR, we examined the tissue expression of nine genes related to notochord and skeletal formation in 150-dpf fugu: ntla, shh, ihhb, twist2, runx2, sox9, col2a1a, col1a1, and sparc (Table 1). All of these genes were expressed in the vertebral column, and the expression of ntla and ihhb was specific to the vertebral column (Fig. 4a). The genes shh, twist2, runx2, col2a1a, col1a1, and sparc were also expressed in the gill and skin, both of which have skeletal elements (Fig. 4a).
Expression of ntla, shh, ihhb, twist2, runx2, sox9, col2a1, col1a1, and sparc in the vertebral column of 40-dpf fugu (for an explanation of gene names, see Table 1). a Reverse transcription plus polymerase chain reaction (PCR) analysis of tissue expression (arrowhead in twist2 position of expected PCR product). Production of multiple PCR bands for col2a1a is presumably attributable to alternative splicing in the region selected for amplification. b–m mRNA localization by section in situ hybridization (SISH). All sections are sagittal in orientation (arrows SISH signals in osteoblasts covering the bone matrix of the centrum, bc notochord basal cell, elc external ligament connective tissue, no notochord, vc notochord vacuolated cell)
Using SISH, we determined those cells in the vertebral column expressing each of the nine genes examined by PCR. Both basal and vacuolated cells in the notochord expressed ntla (Fig. 4b, c). Of note, the vacuolated cells expressed only ntla among the nine genes examined, whereas the basal cells expressed shh, ihhb, runx2, sox9, col2a1a, and sparc, in addition to ntla (Fig. 4b–e, g–j, m). Thus, of the nine genes examined, the notochord basal cells expressed all except twist2 and col1a1.
The cells covering the outer surface of the bone matrix of the centrum expressed col1a1 and sparc, indicating that these cells are osteoblasts (Fig. 4k–m). The osteoblasts also expressed col2a1a (Fig. 4i). The external ligament connective tissue expressed twist2, runx2, sox9, col2a1a, col1a1, and sparc (Fig. 4f, g, j, l, m).
Matrix synthesis by notochord basal cells
Histologically, we observed that the cytoplasm of notochord basal cells in the proximity of intervertebral ligament included Alcian-blue-positive substances (Fig. 5a). To verify this observation, we collected notochord explants from the vertebral column of 150-dpf fugu and cultured them in vitro (Fig. 5b, c). The notochord basal cells formed aggregations, and an Alcian-blue-positive substance was produced in the cell aggregates (Fig. 5d). We could detect ihh expression in the cultured cells, indicating that major components of the cell aggregate were the notochord basal cells (Fig. 5e). Thus, the matrix production observed in vitro was reproduced by the in vitro culture experiment. These data suggest that the notochord basal cells synthesize and secrete extracellular matrix components, including cartilaginous proteoglycan, to form the notochord sheath.
Matrix synthesis by notochord basal cells and in vitro culture of notochord cells. a Production of Alcian-blue–positive matrix by notochord basal cells in proximity of intervertebral ligament (bc notochord basal cell, ij intercentral joint [encircled], m bone matrix, nsh notochord sheath). b Notochord tissue collected from 150-dpf fugu vertebral column (arrowhead thickened notochord basal cells at the intervertebral ligament). c Culture notochord cells forming a cellular aggregate. d Alcian blue staining of cultured notochord cells. Alcian-blue–positive matrix is synthesized in the cell aggregate (bc notochord basal cell, vc notochord vacuolated cell). e Expression of ihh in cultured notochord
The data regarding the developmental process and tissue mRNA expression in vertebral column are summarized in Fig. 6.
Summary of developmental process and gene expression profile in fugu vertebral column. a Representation of developmental process of vertebral column, highlighting the segmentation of the notochord by growing centra. b Histology of the vertebral column indicating tissue mRNA expression. c List of signal-positive (+) and signal-negative (−) cells in the SISH (ct centrum, ha hemal arch, hs hemal spine, il intercentral ligament, no notochord, na neural arch, ns neural spine, nst notochord string)
Discussion
Centrum and intervertebral joint formation
In medaka and zebrafish, the notochord is not completely segmented by centra, even in adult fish, and there is no notochord string (Bird and Mabee 2003; Haga et al. 2009; Inohaya et al. 2007). This adult vertebral column structure is similar to that of 24– to 40-dpf fugu larvae. In fugu, we observed that the centra continued to grow by active bone matrix formation and completely divided the notochord into double cone-shaped segments, forming intervertebral joints. During this process, the notochord cells formed a thick notochord sheath covering the inner surface of the centra and fibrous notochord string. The notochord sheath formed intervertebral ligaments connecting the edges of adjacent centra, and the notochord string also connected the adjacent centra from the inside. Thus, in fugu, the centra and notochord continue coordinated dynamic morphogenesis to establish a repetitive series of segmented centra and intervertebral joints until the adult form of the vertebral column is established (Fig. 6a). With regard to the origin of the bone matrix that occupies the center of centra, the centra is formed as highly branched trabeculae and is equipped with a space for osteoblasts between trabeculae (Figs. 1l, 3k); we suppose that such a highly branched structure allows the inward growth of the centrum to segment the notochord into intervertebral joints.
A notochord string is also found in the notochord chamber of perch (Schmitz 1995). We therefore hypothesize that, in large fish, the highly ossified centra, thick notochord sheath, and fibrous notochord string function to give physical strength to the vertebral column, and that the filling of the notochord chamber with vacuolated cells helps reduce the weight of the vertebral column.
Centrum ossification
Reports concerning the ossification of the centra in teleosts indicate that the process takes place by membranous ossification, unlike in tetrapods (Fleming et al. 2004; Inohaya et al. 2007; Schmitz 1995). We ascertained that the centra and all other vertebral elements develop by membranous ossification in fugu. In zebrafish, membranous ossification of the centra is reportedly initiated by the notochord cells, which supply bone matrix to the notochord sheath (Fleming et al. 2001). On the other hand, in medaka, osteoblasts and external ligament connective tissue cells located on the outer surface of the notochord sheath produce the centrum bone matrix (Inohaya et al. 2007). Col1 and Sparc, which form a complex, are major components of the bone matrix (Ricard-Blum 2011; Termine et al. 1981). In fugu, we have found that the notochord cells do not express col1a1, and that osteoblasts expressing both col1a1 and sparc are located on the outer surface of the centrum (Fig. 4k, m). Histologically, we have shown that the notochord sheath synthesized by the notochord cells is not ossified tissue but rather has cartilaginous properties, and that ossification of the bone matrix takes place along the outer surface of the notochord sheath (Figs. 3l–o, 6b, c). These data suggest that, in fugu, the notochord cells do not contribute to bone matrix production and instead indicate that outer osteoblasts are responsible for matrix production and centrum ossification. The process of centrum ossification in fugu is thus more similar to that in medaka than that in zebrafish. The control of centrum growth by outer osteoblasts is understandable for large fish that form highly developed centra, such as fugu.
The osteoblasts of zebrafish and gar (Lepisosteus oculatus) are reported to express not only col1a1, but also col2a1, which is specific for chondrocytes in tetrapods (Eames et al. 2012; Ricard-Blum 2011). Similarly, in the present study, we have found that fugu centrum osteoblasts express col2a1, in addition to col1a1 (Fig. 6b, c). Therefore, the production of Col2 in addition to Col1 as the bone matrix seems to be a general feature of teleosts.
External ligament connective tissue
The structure of the fugu intervertebral ligament observed in the present study, consisting of the notochord sheath, elastic externa, elastic ligament, and outer elastic connective tissue, is similar to that of perch (Schmitz 1995). Regarding the fate of embryonic sclerotome cells, these cells in medaka have been demonstrated to differentiate into the external ligament connective tissue cells and osteoblasts that form the centrum (Inohaya et al. 2007). We have found that twist2, a marker of sclerotome cells (Gitelman 1997; Yasutake et al. 2004), is expressed by the external ligament connective tissue (Fig. 6b, c), suggesting that this tissue descends from the sclerotome in fugu, as suggested for medaka (Inohaya et al. 2007).
We have further found that the fugu external ligament connective tissue expresses matrix protein genes, including col1a1, col2a1a, and sparc (Fig. 6b, c), suggesting that the external ligament connective tissue participates in bone matrix production together with osteoblasts, as suggested for medaka (Inohaya et al. 2007). In addition, runx2, a master transcription factor for osteoblast differentiation (Komori et al. 1997), is expressed in this tissue, indicating that the external ligament connective tissue cells have osteoblastic properties for bone matrix production, similar to osteoblasts.
Function of notochord cells
The notochord sheath of lamprey reportedly contains Col2 fibers (Eikenberry et al. 1984). In mice, the notochord cells secrete Col2 as a component of the notochord sheath (Smits and Lefebvre 2003). Post-embryonic notochord cells are classified into two types: the basal cells, which line the inner surface of the notochord sheath, and the inner vacuolated cells (Schmitz 1995). In agreement with data from lamprey and mouse studies, we have found that fugu notochord basal cells express col2a1 (Fig. 6b, c). In addition, we have observed that the notochord sheath contains cartilaginous proteoglycan that is reactive with Alcian blue (Fig. 1n, o), and in tests in vitro, cultured notochord basal cells synthesize this proteoglycan (Fig. 5d), suggesting that fugu notochord basal cells secrete Col2 and proteoglycan to produce the notochord sheath. We therefore conclude that the synthesis of Col2 for notochord sheath formation is a conserved function of notochord cells in vertebrates, from the cyclostomes to the lineages of both teleosts and mammals.
We have also found that fugu notochord basal cells co-express sparc and col2a1 (Fig. 6b, c). In mammals, even though Sparc is a major component of bone matrix in complex with Col1, Sparc is also synthesized by chondrocytes together with Col2 under certain physiologic conditions (Nakamura et al. 1996). Therefore, in fugu, Sparc and Col2 synthesized by notochord basal cells might form the matrix in the notochord sheath.
In mammals, the transcription factor Sox9 regulates Col2 expression in chondrocytes, and Ihh signaling stimulates the proliferation of chondrocytes (Lefebvre et al. 1997; St-Jacques et al. 1999). In addition, Shh reportedly stimulates Sox9 and Col2a1 expression in mesenchymal stem cells; thus, Sox9, Ihh, and Shh are upstream regulators of cartilaginous skeletal development in tetrapods (Warzecha et al. 2006). For chondrocytes, Runx2 functions to control their proliferation by inducing Ihh expression (Yoshida et al. 2004). In the present study, we have found that fugu notochord basal cells express ihhb, shh, sox9, runx2, and col2a1 (Fig. 6b, c), suggesting that the system that regulates Col2 synthesis in tetrapod chondrocytes also functions in the notochord basal cells of fugu for notochord sheath synthesis. Thus, the notochord basal cells of fugu, unlike those of zebrafish, are not osteoblastic but rather are unique cells that have a cartilaginous property and that function specifically in notochord sheath synthesis.
Expression of ntla and shh by notochord cells
The transcription factor ntla plays an essential role in notochord development and is expressed by embryonic notochord cells in all chordates. In zebrafish, ntla expression ceases after embryogenesis (Schulte-Merker et al. 1992). Expression of shh in the notochord induces sclerotome formation in the somite and ceases after embryogenesis in zebrafish (Krauss et al. 1993). Thus, the expression of both ntla and shh in the notochord ceases after embryogenesis in zebrafish. In contrast, in the present study, we have found that the expression of both ntla and shh is maintained in the fugu notochord during the development of the vertebral column; ntla is expressed by all notochord cells, and shh is expressed by notochord basal cells (Fig. 6b, c). We have observed the expression of both ntla and shh at least up to 150 dpf (data not shown). Thus, our data suggest that, unlike zebrafish, ntla and shh continue to function in the notochord cells until the adult stage in fugu. Using a transgenic line, Haga et al. (2009) have reported that, in zebrafish, the shhb (previously named tiggy-winkle hedgehog) promoter drives gfp expression in the intervertebral joint, although in situ hybridization (ISH) does not detect a signal for shhb from the vertebral column. Therefore, shhb might be expressed in the intervertebral joint, also in zebrafish, at a level lower than that of ISH sensitivity.
The zebrafish genes for which transcription is directly controlled by ntla have been identified (Morley et al. 2009). Several genes that we have found to be expressed by fugu notochord basal cells, including shh, ihhb, sox9, col2a1a, and sparc, are not included among the genes identified by Morley et al. (2009). As such, the genes downstream of ntla that are involved in vertebral column development in fugu remain unidentified. However, our data indicate that the notochord cells retain some role in vertebral column development and growth regulated by ntla from the embryonic to adult stages.
Comparison with tetrapod vertebral development
Our research on fugu demonstrates that the fate of the sclerotome in teleosts (external ligament connective tissue cells that synthesize bone matrix) differs from that in tetrapods (chondrocytes of the centrum and intercentral disk primordia), as suggested for medaka (Inohaya et al. 2007). In the present study, we have found that, in fugu, the external ligament connective tissue, which descends from the sclerotome, expresses both col2a1a and sox9 (Fig. 6b, c). Therefore, the regulation of Col2 synthesis by Sox9, which is typical of tetrapod chondrocytes, is conserved in the sclerotome-derived cells that form the centra by membranous ossification in teleosts. We also speculate that specialization of sclerotome-derived cells to osteoblastic cells differentiated from chondrocytes took place in the teleost lineage after the branching of the tetrapod lineage, because in ancestral fish, including the Chondrichthyes and Cyclostomata, the vertebral column is cartilaginous (Fleming et al. 2015).
We have found that fugu notochord cells synthesize Col2 as a matrix for the notochord sheath, as shown in tetrapods (Smits and Lefebvre 2003). In tetrapods, the notochord degenerates into a rudimentary tissue, the inner nucleus pulposus, in the intervertebral disk (O'Rahilly and Müller 1994). In contrast, in fugu, the notochord keeps growing and synthesizing extracellular matrix components to produce the thick notochord sheath until the adult stage is reached, and the notochord basal cells maintain some functions initiated during the embryonic stage via the ntl and shh signaling network.
Thus, in fugu, the notochord plays multiple important roles in vertebral development, including segmentation, imparting physiologic strength and flexion, and reducing the weight of the vertebral column. The profile of vertebral development described here for fugu possibly represents the original vertebral column developmental system in large teleosts that evolved in the ocean and might provide clues for future studies of the evolutionary diversity of vertebral column development in teleosts and tetrapods.
References
Aparicio S, Chapman J, Stupka E, Putnam N, Chia JM, Dehal P, Christoffels A, Rash S, Hoon S, Smit A, Gelpke MD, Roach J, Oh T, Ho IY, Wong M, Detter C, Verhoef F, Predki P, Tay A, Lucas S, Richardson P, Smith SF, Clark MS, Edwards YJ, Doggett N, Zharkikh A, Tavtigian SV, Pruss D, Barnstead M, Evans C, Baden H, Powell J, Glusman G, Rowen L, Hood L, Tan YH, Elgar G, Hawkins T, Venkatesh B, Rokhsar D, Brenner S (2002) Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science 297:1301–1310
Bensimon-Brito A, Cardeira J, Cancela ML, Huysseune A, Witten PE (2012) Distinct patterns of notochord mineralization in zebrafish coincide with the localization of Osteocalcin isoform 1 during early vertebral centra formation. BMC Dev Biol 12:28
Bird NC, Mabee PM (2003) Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev Dyn 228:337–357
Christ B, Huang R, Wilting J (2000) The development of the avian vertebral column. Anat Embryol (Berl) 202:179–194
Eames BF, Amores A, Yan YL, Postlethwait JH (2012) Evolution of the osteoblast: skeletogenesis in gar and zebrafish. BMC Evol Biol 12:27
Eikenberry EF, Childs B, Sheren SB, Parry DA, Craig AS, Brodsky B (1984) Crystalline fibril structure of type II collagen in lamprey notochord sheath. J Mol Biol 176:261–277
Fleming A, Keynes RJ, Tannahill D (2001) The role of the notochord in vertebral column formation. J Anat 199:177–180
Fleming A, Keynes R, Tannahill D (2004) A central role for the notochord in vertebral patterning. Development 131:873–880
Fleming A, Kishida MG, Kimmel CB, Keynes RJ (2015) Building the backbone: the development and evolution of vertebral patterning. Development 142:1733–1744
Gitelman I (1997) Twist protein in mouse embryogenesis. Dev Biol 189:205–214
Grotmol S, Kryvi H, Nordvik K, Totland GK (2003) Notochord segmentation may lay down the pathway for the development of the vertebral bodies in the Atlantic salmon. Anat Embryol (Berl) 207:263–272
Haga Y, Dominique VJ 3rd, Du SJ (2009) Analyzing notochord segmentation and intervertebral disc formation using the twhh:gfp transgenic zebrafish model. Transgenic Res 18:669–683
Ibaraki H, Wu X, Uji S, Yokoi H, Sakai Y, Suzuki T (2015) Transcriptome analysis of vertebral bone in the flounder, Paralichthys olivaceus (Teleostei, Pleuronectiformes), using Illumina sequencing. Mar Genomics 24:269–276
Inohaya K, Takano Y, Kudo A (2007) The teleost intervertebral region acts as a growth center of the centrum: in vivo visualization of osteoblasts and their progenitors in transgenic fish. Dev Dyn 236:3031–3046
Inohaya K, Takano Y, Kudo A (2010) Production of Wnt4b by floor plate cells is essential for the segmental patterning of the vertebral column in medaka. Development 137:1807–1813
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764
Krauss S, Concordet JP, Ingham PW (1993) A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75:1431–1444
Kurokawa T, Suzuki T (2002) Development of neuropeptide Y-related peptides in the digestive organs during the larval stage of Japanese flounder, Paralichthys olivaceus. Gen Comp Endocrinol 126:30–38
Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B (1997) SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol 17:2336–2346
Mansfield JH, Haller E, Holland ND, Brent AE (2015) Development of somites and their derivatives in amphioxus, and implications for the evolution of vertebrate somites. EvoDevo 6:21
Morley RH, Lachani K, Keefe D, Gilchrist MJ, Flicek P, Smith JC, Wardle FC (2009) A gene regulatory network directed by zebrafish No tail accounts for its roles in mesoderm formation. Proc Natl Acad Sci U S A 106:3829–3834
Nakamura S, Kamihagi K, Satakeda H, Katayama M, Pan H, Okamoto H, Noshiro M, Takahashi K, Yoshihara Y, Shimmei M, Okada Y, Kato Y (1996) Enhancement of SPARC (osteonectin) synthesis in arthritic cartilage. Increased levels in synovial fluids from patients with rheumatoid arthritis and regulation by growth factors and cytokines in chondrocyte cultures. Arthritis Rheum 39:539–551
Nordvik K, Kryvi H, Totland GK, Grotmol S (2005) The salmon vertebral body develops through mineralization of two preformed tissues that are encompassed by two layers of bone. J Anat 206:103–114
O'Rahilly R, Müller F (1994)Neurulation in the normal human embryo.Ciba Found Symp 181:70-89
Renn J, Buttner A, To TT, Chan SJ, Winkler C (2013) A col10a1:nlGFP transgenic line displays putative osteoblast precursors at the medaka notochordal sheath prior to mineralization. Dev Biol 381:134–143
Ricard-Blum S (2011) The collagen family. Cold Spring Harb Perspect Biol 3:a004978
Schmitz RJ (1995) Ultrastructure and function of cellular components of the intercentral joint in the percoid vertebral column. J Morphol 226:1–24
Schulte-Merker S, Ho RK, Herrmann BG, Nusslein-Volhard C (1992) The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development 116:1021–1032
Smits P, Lefebvre V (2003) Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development 130:1135–1148
St-Jacques B, Hammerschmidt M, McMahon AP (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13:2072–2086
Suzuki T, Kurokawa T, Hashimoto H, Sugiyama M (2002) cDNA sequence and tissue expression of Fugu rubripes prion protein-like: a candidate for the teleost orthologue of tetrapod PrPs. Biochem Biophys Res Commun 294:912–917
Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR (1981) Osteonectin, a bone-specific protein linking mineral to collagen. Cell 26:99–105
Uji S, Kurokawa T, Hashimoto H, Kasuya T, Suzuki T (2011) Embryogenic staging of fugu, Takifugu rubripes, and expression profiles of aldh1a2, aldh1a3 and cyp26a1. Dev Growth Differ 53:715–725
Warzecha J, Gottig S, Bruning C, Lindhorst E, Arabmothlagh M, Kurth A (2006) Sonic hedgehog protein promotes proliferation and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in vitro. J Orthop Sci 11:491–496
Willems B, Buttner A, Huysseune A, Renn J, Witten PE, Winkler C (2012) Conditional ablation of osteoblasts in medaka. Dev Biol 364:128–137
Yasutake J, Inohaya K, Kudo A (2004) Twist functions in vertebral column formation in medaka, Oryzias latipes. Mech Dev 121:883–894
Yoshida CA, Yamamoto H, Fujita T, Furuichi T, Ito K, Inoue K, Yamana K, Zanma A, Takada K, Ito Y, Komori T (2004) Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev 18:952–963
Author information
Authors and Affiliations
Corresponding author
Additional information
Takamasa Kaneko and Tohru Suzuki contributed equally to this work.
This work was supported by grants from the Japan Ministry of Education, Culture, Sports, Science and Technology (grant nos.: Kiban-B: 26292110 to T.S. and Kiban-C:15 K07571 and Wakate-B:26850127 to H.Y.).
Rights and permissions
About this article
Cite this article
Kaneko, T., Freeha, K., Wu, X. et al. Role of notochord cells and sclerotome-derived cells in vertebral column development in fugu, Takifugu rubripes: histological and gene expression analyses. Cell Tissue Res 366, 37–49 (2016). https://doi.org/10.1007/s00441-016-2404-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2404-z