Abstract
Our aim is to investigate the cytobiological effects of autologous platelet-rich fibrin (PRF) on dental pulp stem cells (DPSCs) and to explore the ectopic and orthotopic possibilities of dental pulp revascularization and pulp-dentin complex regeneration along the root canal cavities of the tooth by using a novel tissue-engineered transplant composed of cell-sheet fragments of DPSCs and PRF granules. Canine DPSCs were isolated and characterized by assaying their colony-forming ability and by determining their cell surface markers and osteogenic/adipogenic differentiation potential. The biological effects of autologous PRF on DPSCs, including cell proliferation, alkaline phosphatase (ALP) activity and odonto-/osteogenic gene expression, were then investigated and quantified. A novel transplant consisting of cell-sheet fragments of DPSCs and PRF granules was adopted to regenerate pulp-dentin-like tissues in the root canal, both subcutaneously in nude mice and in the roots of canines. PRF promoted the proliferation of DPSCs in a dose- and time-dependent manner and induced the differentiation of DPSCs to odonto-/osteoblastic fates by increasing the expression of the Alp, Dspp, Dmp1 and Bsp genes. Transplantation of the DPSC/PRF construct led both to a favorable regeneration of homogeneous and compact pulp-like tissues with abundantly distributed blood capillaries and to the deposition of regenerated dentin along the intracanal walls at 8 weeks post-operation. Thus, the application of DPSC/PRF tissue constructs might serve as a potential therapy in regenerative endodontics for pulp revitalization or revascularization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulp exposure, infection and necrosis can be caused by dental trauma and caries, both of which are prevalent dental problems. Under these circumstances, the dental pulp should typically not be preserved and endodontic treatment is the most common clinical treatment. Although the methods for root canal treatment are constantly being researched and improved and the success rate continues to increase, some problems still exist. One potential problem is that the teeth receiving the root canal therapy will become brittle because of the lack of nutrition from the dental pulp blood and can be easily fractured over time. Furthermore, some dentin is lost during the preparation of the root canal; this will thin the root canal wall and thus reduce its resistance capability. Hence, regenerative endodontics, which attempts to fill the canal with vital tissue to help the pulp/dentin be revitalized or revascularized and thus to restore its function to the original state, has gained much attention over the past decade (Kim et al. 2010).
Researchers have applied the concept of tissue engineering technology to test whether pulp/dentin can be regenerated in the root canal space (Nakashima and Huang 2013). One key classification of these studies is whether they are based on the use of exogenous cells, that is, whether they are cell-based or cell-free (Kim et al. 2010). Although a chemotaxis-based approach by utilizing cell homing rather than cell delivery for pulp-like tissue regeneration has been proposed in an ectopic model (Kim et al. 2010), many other cell-free approaches have not demonstrated the regeneration of pulp and dentin in canals from which the pulp tissue is completely removed (Lin et al. 2013; Wang et al. 2010). The stem-cell-based approach has demonstrated that pulp/dentin tissues can be regenerated in the emptied root canal space with blood supply (Hargreaves et al. 2008; Huang et al. 2010b; Iohara et al. 2011; Murray et al. 2007; Nakashima and Huang 2013; Rosa et al. 2013; Sloan and Smith 2007). To identify a suitable cell population for this goal, many odontogenic stem cells, including dental pulp stem cells (DPSCs; Gronthos et al. 2000), periodontal ligament stem cells (PDLSCs; Seo et al. 2004), stem cells from human exfoliated deciduous teeth (SHED; Cordeiro et al. 2008), and stem cells from apical papilla (SCAP; Sonoyama et al. 2008) and some non-odontogenic stem cells, including adipose-derived stem cells (ADSCs; Hung et al. 2011), bone marrow mesenchymal stem cells (BMSCs; Li et al. 2007; Yu et al. 2007), embryonic stem cells (ESCs; Ohazama et al. 2004), neural crest cells (NCCs; Jiang et al. 2008) and even hair follicle stem cells (HFSCs; Wu et al. 2009), have been selected for screening. Studies have demonstrated that all odontogenic stem cells have a certain degree of multipotency in vitro and form pulp-dentin complexes combined with scaffold materials in vivo, except for PDLSCs, which tend to form bone-like tissues in vivo. In contrast, non-odontogenic stem cells other than BMSCs have not yet been confirmed to have the potential for tooth regeneration. Among these cell types, postnatal DPSCs have the most potential as stem cells for endodontic tissue regeneration (Gronthos et al. 2002; Murray et al. 2007; Nakashima et al. 2004, 2009). The transplantation of DPSCs with tooth slices or scaffold materials in vivo has demonstrated that DPSCs can differentiate into odontoblasts and further form the structure of pulp-dentin complexes (Gronthos et al. 2002; Huang et al. 2006a, b). However, other investigators have emphasized that these types of pulp-dentin complex tissues are incomplete because of a lack of some growth factors that are essential for cell differentiation (Iohara et al. 2004; Liu et al. 2004). Thus, the independent transplantation of DPSCs is more likely to form osteoid dentin or calcified tissues instead of a pulp-dentin complex (Yang et al. 2009; Zhang et al. 2006).
As one of the key ingredients required for pulp regeneration, an ideal scaffold must provide space for cells and growth factors, have an excellent biological compatibility, be easy to shape and be biodegradable with no toxic byproducts (Prescott et al. 2008). Although hydroxyapatite/tricalcium phosphate (HA/TCP) ceramic powder (Gronthos et al. 2000, 2002; Miura et al. 2003) and a copolymer of poly-D, L-lactide and glycolide (PLG; Huang et al. 2008, 2010b) have often been used as scaffolds for pulp regeneration, they are exogenous and have certain limitations in terms of their degradation rates. Platelet-rich fibrin (PRF) is a second-generation platelet concentrate. The main components of PRF are collagen fibrins that can be degraded over time, as in a blood clot. A significant number of platelets embedded in the PRF framework are activated during fibrin remodeling and the α-chain is then degranulated and releases multiple growth factors in a natural ratio and at a natural rate. In addition, PRF contains the majority of the types of leukocytes in the blood and these leukocytes can take up active roles in anti-inflammatory and immune-regulatory activity (Dohan Ehrenfest et al. 2009). Based on these properties, we consider that PRF meets the requirements for two key aspects of the three characteristics that are necessary for tissue-engineered pulp-dentin complexes by serving as an ideal scaffold and a persistent source of multiple growth factors on a natural scale, whereas the possible effects of PRF on DPSCs, which have the potential for odontoblast-like cell differentiation, are still currently unknown. In addition, the three-dimensional (3D) structure of PRF might also be optimal for stem cell delivery and for the formation of pulp-like connective tissues but this still requires confirmation in further experiments.
Based on these prominent features, we investigated the biological effects of various concentrations of PRF on DPSCs in vitro. Once we identified the optimal ratio of the seed cells and compound growth factors, a cell transplant system consisting of cell-sheet fragments of DPSCs and PRF granules was designed to regenerate pulp-like tissues in the root canal space both subcutaneously in nude mice and in the root canals of canines. As part of our series of studies on pulp-dentin and periodontal tissue regeneration with stem cell/PRF constructs (Zhao et al. 2013), the present study might provide new insights into the regeneration of pulp/dentin tissue and benefit patients with revascularized or revitalized teeth.
Materials and methods
Isolation and characterization of DPSCs
Canine DPSCs were isolated and cultured according to previously reported protocols with slight modifications (Gronthos et al. 2000; Huang et al. 2006b). Briefly, three canines (approximately 5 months old) were obtained from the Animal Center, Fourth Military Medical University. Following general anesthesia (xylazine hydrochloride injections [0.1 ml/kg injection] supplemented with pentobarbital sodium [30 mg/kg injection]) and local anesthesia (Primacaine: 4 % articaine with 1:100,000 tartaric acid epinephrine), pulp tissue was obtained from the upper first molar on the right by using a barbed broach and was immersed into an ice-cold phosphate-buffered saline (PBS; Hyclone, Road Logan, Utah, USA) solution containing 100 U/ml penicillin (Sigma-Aldrich, St. Louis, Mo., USA) and 100 μg/ml streptomycin (Sigma-Aldrich) for transfer to the laboratory. After removal of approximately 2 mm from the apical region, the remainder of the pulp was washed several times with PBS and then minced into small cubes (0.5 mm3). The tissue cubes were digested with a solution of 3 mg/ml collagenase (type I) supplemented with 4 mg/ml dispase (both from Sigma-Aldrich) in 2 ml α-minimum essential medium (α-MEM, Hyclone) for 30 min in a cell incubator. Then, the cells were passed through a 70-μm strainer to obtain single-cell suspensions. The cells were plated into 60-mm plates (Nunc, Thermo, Denmark) and cultured in α-MEM supplemented with 15 % fetal bovine serum (FBS; Hyclone), 0.292 mg/ml glutamine (Hyclone), 100 units/ml penicillin/streptomycin (Hyclone) and 50 mg/ml ascorbic acid (Sigma-Aldrich) at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air to obtain passage 0 (P0) single-cell-derived clones. Cell cultures at P3-P5 were used for the following in vitro study.
For colony-forming unit fibroblast assays, the canine DPSCs (at P3) were seeded at a density of 1 × 103 cells/well onto 90-mm dishes and cultured in α-MEM supplemented with 10 % FBS for 8 days. The medium was changed every 3 days. The cells were fixed, stained with 0.1 % toluidine blue for 15 min and then observed by phase-contrast microscopy (IX70, Olympus, Tokyo, Japan). Only the aggregates containing more than 50 cells were scored as colonies.
Canine DPSCs (at P3) were used for flow cytometry analysis of cell surface markers, by using a previously reported procedure (Dissanayaka et al. 2011; Zhao et al. 2013). A cell suspension with a concentration of 1 × 106 cells/ml was used for the identification of several cell markers, according to the manufacturer’s instructions, including STRO-1 (Santa Cruz Biotechnology, Santa Cruz, Calif., USA), CD146 (Abcam, Cambridge, Mass., USA), CD90 (Abcam), CD29 (Abcam), CD45 (Abcam) and CD34 (Santa Cruz Biotechnology). The analysis was performed with a Vantage Cell Sorter (Becton & Dickinson, Mountain View, USA) and the data were analyzed by using the Mod-Fit 2.0 cell cycle analysis program (Becton & Dickinson).
The multi-directional differentiation ability of canine DPSCs (at P3) was detected by using mineral nodules and a lipid-formation assay in vitro (each cell line in four wells, which were randomly divided for the various inductions). Briefly, the cells were seeded into 6-well plates (Nunc) at a concentration of 2 × 105 cells/well. When they reached 80 % confluence, the osteogenic medium (α-MEM containing 10 % FBS, 50 mg/ml ascorbic acid, 10 nM dexamethasone, and 10 mM β-glycerophosphate) or adipogenic medium (α-MEM containing 10 % FBS, 100 nM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, and 50 mM indomethacin) was then changed and refreshed at 3-day intervals to induce differentiation. After 3 weeks of osteogenic induction or 5 weeks of adipogenic induction, the cells were stained with alizarin red (pH 4; Sigma-Aldrich) or fresh Oil Red O solution (Sigma-Aldrich) for 15 min. The mineral nodules and lipid areas were then observed. All images were captured (600D, Tokyo, Japan).
Preparation, light microscopy and scanning electron microscopy of PRF
Venous blood (20 ml from each canine) was collected from the jugular vein for PRF preparation by using a previously reported protocol (Dohan et al. 2006; Zhao et al. 2013). Briefly, the blood was centrifuged at 400g for 10 min without anticoagulation and then compressed with a sterile gauze to obtain a fibrin membrane. The PRF membrane was fixed and the morphology was observed by using both light microscopy (BX50; Olympus Optical, Tokyo, Japan) and scanning electron microscopy (S-4800; Hitachi, Tokyo, Japan).
Construction of DPSCs/PRF
The canine DPSCs (at P3) obtained in the previous section were placed into six-well plates at a density of 1 × 104 cells/well in complete medium until they reached subconfluence. Vitamin C was then added at a concentration of 48 μg/ml for a further incubation until the edge of the cell sheet became slightly rolled up; this is thought to indicate cell-sheet maturation. A region of the cell sheet was saved for ultrastructural observation and the remaining fraction was used to construct the DPSCs/PRF. Vein blood was collected from the jugular vein of the donor canine for PRF preparation, as previously described and these PRFs were then cut into small pieces (0.5 × 0.5 × 0.5 mm) and planted into the dish containing the cell sheet for one additional week. Both the cell sheet and the DPSCs/PRF were fixed with glutaraldehyde at 4 °C for 1 h, dehydrated in a graded ethanol series and then observed and captured by scanning electron microscopy (S-4800; Hitachi, Tokyo, Japan).
Effects of PRF on DPSCs
The canine DPSCs (at P3) were co-cultured with increasing concentrations of PRF membrane in six-well plates (Nunc) at a density of 1 × 104 cells/well. Based on previous experiments for the study of PRF application in vitro and in vivo (Zhao et al. 2013), one PRF membrane extracted from 10 ml of blood was defined as the standard amount of PRF and the DPSCs were then divided into three experimental groups, each of which was treated with a different “concentration” of PRF (1/8, 2/8, or 3/8 of the standard amount of PRF extracted from 10 ml of blood); cells without PRF served as the control (control group).
Cells from the various groups were cultured in α-MEM containing 10 % FBS for 6 h for cell adhesion and a predetermined amount of PRF was then added. Half of the medium was changed on the third day and the medium was subsequently completely changed every 3 days. On each day of the 7-day culture period, the PRF membranes were removed, and the cells were digested and suspended in 3 ml medium for cell counting. The results from the various groups were statistically compared. The alkaline phosphatase (ALP) activity on days 7 and 14 and the mRNA expression levels of Alp, dentin sialophosphoprotein (Dspp), dentin matrix protein 1 (Dmp1) and bone sialoprotein (Bsp) on days 7, 14 and 21 were used to determine the effects of PRF on cell differentiation, by using methods described in a previous study (Zhao et al. 2013). Briefly, the cells were initially cultured in α-MEM containing 10 % FBS for 48 h. The medium was then changed to calcification medium (α-MEM containing 10 % FBS, 50 mg/ml ascorbic acid, 10 nM dexamethasone, and 10 mM β-glycerophosphate) and the PRF was added simultaneously. Half of the medium was changed on the third day and the medium was then completely changed every 3 days. Two milliliters of ALP-staining solution (a mixture of 10 ml buffer, 33 μl BCIP [5-bromo-4-chloro-3-indolylphosphate] and 66 μl NBT [nitro blue tetrazolium chloride; Beyotime Institute of Biotechnology, Nantong, China]) was added to each well and incubated for 30 min at room temperature in the dark. Representative images were captured and the integrated optical density (IOD) of the blue stain of the cytoplasm, which is considered an indicator of ALP activity, was analyzed by using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, Md., USA). For real-time polymerase chain reaction (PCR) analysis, the total cellular RNA was isolated and the relative gene expression levels of Alp, Bsp, Dspp and Dmp1 were evaluated (Takara RNA PCR kit AMV; Takara Shuzo, Tokyo, Japan). The reaction product was quantified by using a relative quantification tool (CFX Manager; Bio-Rad) with GAPDH (glyceraldehyde-phosphate dehydrogenase) as a reference gene. The primer sequences for Gapdh, Alp, Bsp, Dspp and Dmp1 (Sango Biotech, Shanghai, China) are listed in Table 1.
Transplantation of DPSC/PRF construct into ectopic transplantation model
The effects of the DPSC/PRF construct on the regeneration of dental-pulp-like tissue were evaluated by using animal models, including both nude mice and canines (Fig. 1). Freshly extracted human teeth were used for the root fragment preparations (Huang et al. 2010b). The radicular portions were cut into sections approximately 6–7 mm long and the root canals were then prepared and enlarged to 1–3 mm in diameter. One end of the canal was sealed with mineral trioxide aggregate (MTA) cement (Dentsply Endodontics, Tulsa, Okla., USA) of approximately 2-mm thickness. Thus, the available space in the canal was approximately 4–5 mm long. The root fragments were wrapped with sterile PBS-soaked gauze, placed in an incubator to cure the MTA and then immersed in 17 % ethylenediamine tetraacetic acid (EDTA) for 10 min and 19 % citric acid at room temperature for 1 min to remove the smear layer. Next, betadine was used for 30 min and 5.25 % NaOCl for 10–15 min for disinfection and sterilization. Finally, the root fragments were rinsed with and immersed in PBS containing antibiotics and then incubated for another 7 days to ensure that they were not contaminated.
Scheme for dental-pulp-like tissue regeneration strategy in animal models. The preparation of cell-sheet fragments involved the ex vivo expansion of the dental pulp stem cells (DPSCs) and the harvest of the cell sheets and their fragments. Platelet-rich fibrin (PRF) was prepared from blood drawn from the jugular vein and the membrane was cut into granules for DPSC/PRF construct engineering. The DPSC/PRF construct, the cell sheet fragments, or the PRF granules were transplanted into human root fragments in nude mice and into the canals of premolars in canines to evaluate the regeneration of dental-pulp-like tissues in the various groups
The canine DPSCs (at P3) were cultured into cell sheets and cut into fragments (0.5 × 0.5 mm), whereas autologous PRF was cut into small granules (0.5 × 0.5 × 0.5 mm). The transplanted DPSC/PRF construct for the subsequent ectopic or orthotopic pulp-like tissue regeneration was obtained by combining cell-sheet fragments of DPSCs with PRF granules at the ratio that we had selected from the previous section.
Forty-eight tooth fragments were transplanted subcutaneously into the dorsal regions of 24 nude mice (six-week-old males; Fourth Military Medical University Animal Center, Xi’an, China), with two transplants per subject, according to the grouping of the inserted grafts: Group I, the DPSC/PRF construct; Group II, cell-sheet fragments of the DPSCs only; Group III, PRF granules only; and Group IV, no graft, which was considered the control group. The mice were anesthetized with 1 % sodium pentobarbital and two longitudinal incisions were made on the dorsal side of each nude mouse for two tooth-fragment transplantations. Because of the various sizes of the root canals and the diverse healing abilities of the nude mice, the sizes of the transplanted grafts were normalized by filling the canals. Thus, the transplanted graft volumes per unit area of the root canal among the different experimental groups could be considered relatively equal. The wounds were sutured tightly to obtain primary closure. At 8 weeks post-surgery, the mice were killed and the tooth fragments were removed for histological analysis.
Transplantation of autologous DPSC/PRF construct into the canine model
Three canines (six- to eight-month-old males), obtained from the Animal Center (Fourth Military Medical University), were involved in the present study. The pulp tissue of the upper left first molar of each canine was used to isolate DPSCs according to the above-mentioned method. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Research Ethics Committee of the Fourth Military Medical University (Xi’an, China) and every effort was made to avoid animal suffering at each stage of the experiment.
The obtained DPSCs (at P3) were labeled with 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich) at a concentration of 10 μM for 24 h and then cultured in standard medium containing 48 μg/ml vitamin C for cell sheet formation. The mature cell sheets were stripped and cut into cell-sheet fragments that were approximately 0.5 × 0.5 mm in size. At the same time, approximately 20–30 ml of blood was collected from the jugular vein of the cell donor canines for PRF preparation, as described previously; the PRF was then formed into a membrane and cut into small pieces (approximately 0.5 × 0.5 × 0.5 mm) in a sterile dish. The canine DPSC/PRF construct for the following pulp-like tissue regeneration was obtained by combining cell-sheet fragments with PRF granules at the ratio that we had selected previously.
The experimental model of pulp-like tissue regeneration was established based on a previously reported procedure with slight modifications (Ishizaka et al. 2012). Briefly, the pulp chambers of all of the double-rooted premolars (six upper premolars and four lower double-rooted premolars for each canine) were exposed by using a no. 3 round carbide bur in a high-speed handpiece and the pulp tissues were completely removed by using barbed broaches under general anesthesia and local anesthesia (as previously described). Each root was assumed to be one sample, for a total of 60 samples. The apical foramina of the experimental samples were then enlarged to a diameter of 0.7 mm using a no. 70 K-file (MANI) without additional root canal preparation. After irrigation with 5 ml physiological saline, the root canals were dried with sterile paper points. The 60 canals of the three canines were randomly divided into three groups according to the transplantation graft that was used: Group I, the DPSC/PRF construct; Group II, cell-sheet fragments of DPSCs only; and Group III, PRF granules only. The maxillary central and lateral incisors were used as blank controls (Group IV), with their pulp tissues being removed and root foramina being enlarged as previously described but being filled only with natural blood clots and without any exogenous transplanted grafts. The single-root teeth, the first premolars, were used as positive controls for observing the normal development of the canine dentin-pulp tissues. Given the different shape and bulk of each canal, difficulties were encountered in ensuring that the quantity of cells was identical. Therefore, the transplantation grafts were placed into the root canals by using a root-canal paste carrier to ensure that the grafts contacted the apical tissue up until 1 mm below the root canal orifice. Thus, the quantity of transplanted grafts was normalized to ensure that the transplanted graft volume per unit area of the root canal among the different experimental groups was relatively equal. Following the siphoning of the fluid driven from the grafts, the coronal portions of the root canal were sealed three times with 1–2 mm calcium hydroxide (Dycal), 1 mm glass ionomer (Fuji IX) and composite (Z350; 3 M Dental Products; St Paul, Minn., USA). Eight weeks after transplantation, the canines were killed and the teeth were extracted for histological analysis after separation from the root furcation.
Histological staining
The human tooth root fragments and the canine premolars and incisors were fixed in 4 % paraformaldehyde at 4 °C for 7 days and then demineralized in a 15 % solution of EDTA (Hongyan, Tianjin, China) at pH 6.8. The teeth were embedded in paraffin and sectioned parallel to the long axis of each tooth at a thickness of 5 μm. Three sections were stained from each sample and were subjected to hematoxylin-eosin (HE) staining, Masson staining and immunostaining against BrdU (Sigma-Aldrich) and DSPP (Bioss, Beijing, China). The detailed protocols are briefly described as follows. For modified Masson’s staining, deparaffinized sections were re-fixed in Bouin’s solution for 1.5 h at 60 °C to improve staining quality and then rinsed with running tap water for 5–10 min to remove the yellow color. For HE staining, sections were stained in hematoxylin iron working solution for 8 min, differentiated in hydrochloric-acid-ethanol for 2 min and washed in running tap water for 10 min. After being stained with Ponceau fuchsin acid solution for 4 min and washed in distilled water, the sections were differentiated in 1 % phosphomolybdic acid solution for 5 min and then transferred directly (without rinsing) to aniline blue solution and stained for 5 min. The sections were then rinsed briefly in distilled water and differentiated in 1 % acetic acid solution for 1 min, followed by a rapid dehydration step through 95 % ethyl alcohol and absolute ethyl alcohol (to remove the Biebrich scarlet-acid fuchsin staining) and a clearing step in xylene. To trace the transplanted DPSCs, immunohistochemical staining of BrdU was performed by using an integrated retrieval method, which used 0.1 % Triton X-100/0.1 % citrate buffer and trypsin to enhance permeability, 0.1 mol/l cold HCl to remove the histones from the chromosomes, 2 mol/l HCl to denature the double-stranded DNA and Na2B4O7 to reconstruct the alkaline environment. The primary antibody was a mouse polyclonal to BrdU (diluted 1:500; Sigma-Aldrich). For DSPP staining, the deparaffinized sections were digested by a transparent fatty acid enzyme and then protease, each for 30 min at 37 °C and then immersed in 3 % H2O2 for 10 min to quench endogenous peroxidase activity. After incubation with normal goat serum to block non-specific binding, the sections were incubated with primary antibody (rabbit anti-canine DSPP, diluted 1:200 to 1:500) overnight at 4 °C. After three washes in PBS, bound antibodies were reacted with biotinylated goat anti-rabbit IgG secondary antibody (ZSGB-Bio; Beijing, China) for 15 min at 37 °C. The sections were then developed with the ABC reagent (ZSGB-Bio) by using the diaminobenzidine chromogen for 7 min. Finally, the sections were stained with hematoxylin for 10 s and differentiated with hydrochloric-acid-alcohol (three times) and ammonia for 1 min. The histometric observations were performed by using a projection microscope (BX50, Olympus Optical, Japan) and the images were captured (DP25, Olympus) and analyzed. All data were collected and analyzed by the same investigator to reduce bias and error.
Statistical analysis
The in vitro experiments were conducted in triplicate and repeated three times on separate occasions. The results are expressed as the means ± standard deviation and were compared by using one-way analysis of variance (ANOVA) in combination with the Newman-Keuls post-hoc test. The results were statistically analyzed by using the software SPSS 17.0 (SPSS; USA) and a level of P < 0.05 was accepted as statistically significant.
Results
Isolation and identification of DPSCs
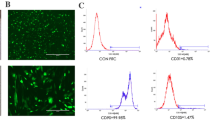
The adherent cells derived from single-cell suspensions demonstrated a shuttle-like morphology and proliferated and grew into colonies within 5 days (Fig. 2a). The mesenchymal stem cell (MSC) properties of these putative stem cells were characterized by assaying their colony-forming ability, characterizing their cell surface markers and evaluating their osteogenic/adipogenic differentiation potential. The cells possessed a good capacity to form colonies when plated at low densities (Fig. 2b, c). The detection of surface molecule expression revealed that the DPSCs were positive for mesenchymal-associated markers, such as STRO-1, CD146, CD90 and CD29 (Fig. 2d–g) and negative for hematopoietic markers, such as CD34 and CD45 (Fig. 2h, i). After 3 weeks of osteogenic induction, cultured DPSCs had formed extensive amounts of alizarin-red-positive mineral deposits, demonstrating their osteogenic potential (Fig. 2j). Meanwhile, after 5 weeks of adipogenic induction, Oil-Red-O-positive cells were observed, providing convincing evidence of their adipogenic differentiation ability (Fig. 2k). The above results suggested that we had successfully obtained DPSCs from pulp tissues.
Isolation and identification of DPSCs. a Adherent cells derived from a single-cell suspension proliferated into a cluster within 5 days. Bar 50 μm. b, c Passaged cells still performed at a high capacity, with colony-forming units forming, after being plated at a low density. Bar 200 μm. d–i Cytometric flow analyses indicated that the DPSCs were positive for the mesenchymal-associated markers STRO-1, CD146, CD90 and CD29 and negative for the hematopoietic markers CD34 and CD45. j, k Representative figures showing the multi-directional differentiation ability of the DPSCs. j After 3 weeks of osteogenic induction, mineralized nodules were formed that stained with alizarin red. Bar 200 μm. k After 5 weeks of adipogenic induction, lipid vacuoles were observed stained with Oil Red O. Bar 100 μm
Microstructure and ultrastructure of PRF, of DPSC cell sheets and of DPSCs/PRF
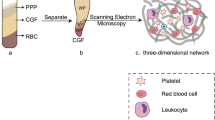
PRF, a fibrin clot, was located in the middle of the tube after the blood was centrifuged (Fig. 3a). When the plasma was removed, the PRF presented a resistant and translucent membrane. The microstructure and ultrastructure of the PRF obtained from the canines were similar to those of PRF obtained from humans, as reported previously (Zhao et al. 2013), that is, the majority of the leukocytes accumulated in the limited junctional area between the red corpuscles and the PRF clot, whereas almost no cells were observed in the upper PRF area (Fig. 3b). Ultrastructural observation revealed that the PRF was a three-dimensional network structure that consisted of fibrin fibers assembled from multiple fibers and fibrillae (Fig. 3c) and that the red blood cells, platelets and leukocytes were distributed in the lower area (Fig. 3d). The cell sheet was composed of multiple layers of cells that were well extended and closely arranged with the same polarity and amount of extracellular matrix (Fig. 3e). After 1 week of co-culture, layers of cells firmly adhered to the PRF structure to form an integral construct of DPSCs/PRF (Fig. 3f).
Structural observations of PRF, of a cell sheet of DPSCs and of the construct of DPSCs/PRF. a PRF forms a fibrin clot after the blood is centrifuged. b Panoramic image of the microstructure of the PRF membrane. Hematoxylin and eosin staining. Magnification: ×40. c–f Ultrastructure of PRF and DPSCs/PRF. Scanning electron microscopy. c Overwhelming majority of PRF is composed of fibrin fibers with a few fibrillae with smaller diameters. Bar 2 μm. d Functional components, including plentiful platelets and leukocytes, were embedded in the lower region of the PRF, together with some red blood cells (RBC). Bar 10 μm. e Large number of cells arranged closely with the same polarity to form a cell sheet. Bar 20 μm. f Cell sheet and PRF adhered firmly to form an integral construct of DPSCs/PRF. Bar 20 μm
Effect of PRF on DPSCs
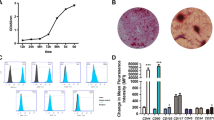
For cell proliferation, the cell numbers in the 2/8 and 3/8 PRF groups were much higher than those in the control group from the fourth day (P < 0.01), although no significant difference was seen between these two test groups (P > 0.05). In contrast, the cell numbers in the 1/8 PRF group showed no significant difference from that of the control (P > 0.05) and exhibited only a very small increase throughout the initial 6-day incubation period (Fig. 4a).
Effect of PRF on DPSCs. a Proliferation assessment of the DPSCs when cultured with a series of doses of PRF (1/8, 2/8, or 3/8 PRF) for 7 days (one PRF membrane was defined as that extracted from 10 ml of blood). The results are expressed as the means ± standard deviation of the cell numbers, which, in the 2/8 and 3/8 PRF groups, were significantly higher compared with that in the control group on the fourth day (d day). ** P < 0.01. b, c Alkaline phosphatase (ALP) activities of the DPSCs in the various experimental groups during a 14-day culture period under in vitro osteogenic induction. b Representative images for the ALP staining of the DPSCs co-cultured with various doses of PRF (1/8, 2/8, or 3/8 PRF) were taken. Bar 500 μm. c ALP activity was analysed via the integrated optical density (IOD) of representative images. Data are representative of means ± standard deviation. * P < 0.05; ** P < 0.01; *** P < 0.001. d–o Relative gene expression levels of Alp (d–f), Dspp (g–i), Dmp1 (j–l), and Bsp (m–o) from DPSCs after being co-cultured with or without various doses (1/8, 2/8, or 3/8) of PRF for 7 days in osteoinductive supplements at various time points (7, 14, and 21 days). Results are expressed as the means ± standard deviation. a P < 0.05, vs control group. b P < 0.05, vs 1/8 PRF group. c P < 0.05, vs 2/8 PRF group
For cell differentiation, the ALP activities of the DPSCs were detected by staining (Fig. 4b). The mean IOD of the images was then determined by using Image-Pro Plus 6.0 software at days 7 and 14 for statistical analysis (Fig. 4c). The PRF enhanced the ALP activity of the DPSCs in a dose-dependent manner at day 7 (P < 0.05; Fig. 4c), with continuing increases over time, even when the PRF was removed. Among the tested groups, the 3/8 PRF group displayed the highest value at each time point (P < 0.05; Fig. 4c). Real-time PCR analyses were used to evaluate the expression of typical odontogenic/osteogenic genes, including Alp, Dspp, Dmp1 and Bsp, in the canine DPSCs when co-cultured with PRF for 7 days. The various genes showed different expression trends. Specifically, PRF significantly increased the expression of Alp and Dspp throughout the experimental period. This effect was significant for Alp at day 7 (P < 0.05; Fig. 4d–f) and for Dspp at days 7 and 14 (P < 0.05; Fig. 4g–i) but it was not significant over time. Interestingly, both the 2/8 and 3/8 PRF significantly increased the expression of Dmp1 (P < 0.05) but the 1/8 PRF only moderately upregulated Dmp1 expression at 21 days (P < 0.05; Fig. 4j–l) and even slightly downregulated its expression at 7 days. As an index of osteogenic differentiation, Bsp expression was inhibited to variable degrees at 7 and 14 days compared with the control group (P < 0.05); however, this effect was not found at day 21 (Fig. 4m–o). In sum, PRF promoted the odonto-/osteoblastic differentiation of canine DPSCs.
Effects of DPSC/PRF construct on pulp regeneration
Based on the above-mentioned results that the 3/8 PRF group (with 3/8 PRF extracted from 10 ml of blood) showed the strongest effects of increasing proliferation and odonto-/osteoblastic differentiation for one piece of DPSC membrane cultured in standard six-well plates at a density of 1 × 104 cells/well, the DPSC/PRF transplants for the following ectopic or orthotopic pulp regeneration experiment were constructed by combining cell-sheet fragments of DPSCs with PRF granules at a ratio of eight pieces of cell sheets obtained from standard six-well plates combined with PRF derived from 30 ml of blood.
Pulp regeneration after ectopic transplantation
All of the tested animals were clinically healed at 8 weeks post-surgery, without any inflammation of the surgical sites or root fragment exposure (Fig. 5). Their histologic variables were observed and compared among the different groups. The emptied canals in Group I were filled with well-organized vascularized pulp-like tissues, presenting significant numbers of cells and the establishment of an extracellular matrix and some newly formed capillaries were present, whereas most of the PRF had degraded (Fig. 5a–c). A continuous layer of mineralized tissues (dentin-like tissues) with variable thickness and a few embedded cells were observed between the regenerated pulp and canal dentinal walls; this layer formed processes and was tightly connected to the regenerated dentin (Fig. 6a). In Group II, an uneven layer of newly mineralized tissue was observed underneath the MTA cement and along part of the intracanal (Fig. 5d–f) but this tissue was much thinner than that in Group I. The human dentinal tubules extended processes into the newly formed tissues without gaps (Fig. 6b). The canal space was filled with a significant number of transplanted cells and showed the establishment of an extracellular matrix. In addition, HE and Masson staining showed that the regenerated pulp tissues were rich in collagenous fibers but lacked new blood capillaries (Fig. 5e, f). In Group III, the majority of the PRF had degraded and some homing cells grew into the canal and the space among the PRF granules (Fig. 5g, h). However, neither functional pulp-like structure nor blood capillaries could readily be observed, as evidenced by the Masson’s tri-chrome staining and almost no dentin-like tissues were found along the intracanal walls (Fig. 5h, i), except for an extremely thin layer of slightly mineralized tissues with many cells embedded (Fig. 6c). For Group IV, which did not have any transplanted materials in the root canals, less than half of the canal space was filled with loose connective tissues, with no new capillaries or dentin-like tissues (Figs. 5j–l, 6d).
Representative panoramic (a, d, g, j) and local images of pulp regeneration after ectopic transplantation. a–c In Group I, the canals were filled with well-organized and vascularized pulp-like tissues and a continuous layer of dentin-like tissues was deposited along the internal root canal walls (rP regenerated pulp-like tissues, D dentin, rDD regenerated dentin on dentin walls, PRF platelet-rich fibrin). d–f In Group II, the regenerated pulp-like tissues contained fewer new capillaries and the dentin-like tissue was observed on the surface of the mineral trioxide aggregate cement and along part of the internal canal walls, with uneven thickness. g–i In Group III, the newly formed pulp-like tissues consisted of some PRF granules and homing cells but lacked a functional structure and capillaries. j–l In Group IV, the newly formed connective tissues were disordered and loosely arranged. Boxed areas in a, d, g, j are shown at higher magnification in b, e, h, k. Sections were stained with hematoxylin-eosin (a, b, d, e, g, h, j, k) and Masson’s tri-chrome staining (c, f, i, l). Bar 100 μm
Higher magnification images of pulp-like tissue regeneration in nude mouse model. a In Group I, dentin-like tissues (red arrow) deposited along human root fragments lack typical dentinal tubule structure but are connected with dentinal tubules of human tooth tightly. Odontoblast-like cells (yellow arrows) are fewer and without polarity (D dentin, rDD regenerated dentin on dentin walls, Od odontobalsts). b In Group II, the pulp-dentin-like tissue was similar to that of Group I but demonstrated a more obvious connection between the dentinal tubules and the regenerated dentin-like tissues. c In Group III, an extremely thin layer of mineralized tissues with many cells was deposited on the dentin walls. d In the blank control root canals, no pulp-dentin-like tissues were found in the canals. Bar 50 μm
Effects of pulp regeneration in canines
In Group I, at 8 weeks after the transplantation of the DPSC/PRF construct, the emptied canal space was filled with homogeneous and compact pulp-like tissues containing abundant and evenly distributed blood capillaries (Fig. 7a–c) but some undegraded PRF was still embedded in the pulp-like tissues. In this group, the canal dentinal walls were thickened by newly formed mineralized tissue that resembled dentin-like tissues. Notably, the root length and the thickness of the root canal walls were increased by the deposition of dentin-like tissues and the apical canal appeared obliterated by calcified tissues (Fig. 7a). However, higher magnification revealed that the dentin-like tissue was different from that of a natural tooth to some degree, with some cells embedded in the dentin-like tissues and some atypical dentinal tubules that showed the specific angle of primary dentinal tubules (Fig. 8a). The odontoblast-like cells were similar to those of a natural tooth but were fewer in number and lacked the representative cell polarity of odontoblasts (Fig. 8a). In Group II, the histological results demonstrated that the pulp-like tissue was composed of a significant number of cells and a profuse extracellular matrix; however, the structure was similar to that of Group I (Fig. 7d). Capillaries and collagen were also easily observable in the canal space (Fig. 7e, f). The dentin-like tissues in the representative sample of this group were continuous, with a uniform thickness, which was thinner than those of Group I (Fig. 7e, f). Although the continued root development of the treated teeth could be observed from the deposition of mineralized tissues on the root surface and the dentin-like tissues in the canals, the apical foramen had not yet closed (Fig. 7d). Higher magnification observations revealed that the pulp-dentin-like tissue was similar to that of Group I, with embedded cells and dentinal tubules, whereas the odontoblast-like cells had a more disordered arrangement (Fig. 8b). Compared with Groups I and II, the structure of the pulp-like tissues in Group III was much looser and more irregular, with many undegraded PRF granules (Fig. 7g). Some cells with active synthesis function were diffusely distributed and had secreted significant amounts of blue-stained collagen. Without exogenous transplanted cells, a few blood capillaries were observed and might have been derived from the host homing cells (Fig. 7h). A moderate layer of mineralized tissue with a variable thickness was deposited onto the primary dentin walls with obvious incremental lines (Fig. 7h, i). However, compared with Group I, the regenerated dentin-like tissues were almost parallel with the primary dentinal tubule. Although a layer of odontoblast-like cells was present beneath the dentin-like tissues, these cells showed little of the polarized morphology possessed by odontoblasts (Fig. 8c). The blank control root canals, which did not receive any transplanted grafts, were filled with some deformed connective tissue containing many red cells but did not show the regular histologic structure of blood capillaries (Fig. 7j, k). The sample from this group involved incisors with a narrower canal space and secondary dentin had obviously been deposited pre-surgery (Fig. 7k, l). Nonetheless, a thin layer of newly formed mineralized tissues was seen in some regions with variable thicknesses (Fig. 8d).
Representative panoramic (a, d, g, j, k) and local images of pulp-like tissue regeneration in the canine model. a–c In Group I, ideal tissue regeneration was indicated by the formation of homogeneous and compact pulp-like tissues containing abundant and evenly distributed capillaries; the canal dentinal walls were thickened by the apposition of newly generated dentin-like tissue; the apical canal appeared to be obliterated by calcified tissues (rP regenerated pulp-like tissues, D dentin, rDD regenerated dentin on dentin walls, BV blood capillaries, PRF platelet-rich fibrin). d–f In Group II, the newborn pulp-like tissues were acceptable but less dense than those in Group I and the dentin-like mineralized tissues were thicker than those in Group I. g–i In Group III, the structure of the regenerated pulp-like tissues was much looser and irregular with many undegraded PRF granules, whereas the root canal walls were thickened by the deposition of dentin-like tissues. j–l In the control group (Group IV, lacking any transplanted graft), only some deformed connective tissues in the canals and a large amount of red cells were found (sD secondary dentin). m–o With regard to the normal pulp from naturally developing teeth, the pulp consisted of regularly arranged connective tissues with abundant blood capillaries and a typical odontoblast layer (P pulp, Od odontoblasts). Boxed areas in a, d, g, j, m are shown at higher magnification in b, e, h, k, n. Sections were stained with hematoxylin-eosin (a, b, d, e, g, h, j, k, m, n) and Masson’s tri-chrome staining (c, f, i, l, o). Bar 100 μm
Higher magnification images of pulp-like tissue regeneration in the canine model. a In Group I, the generated dentin-like tissue was irregular with nontypical dentinal tubules (red arrows) and embedded cells. The odontoblast-like cells (yellow arrows) were similar to those of natural tooth but were fewer in number and lacked the representative cell polarity that odontoblasts possess (D dentin, rDD regenerated dentin on dentin walls, Od odontobalsts, red arrows regenerated dentinal tubules, yellow arrows odontoblasts). b In Group II, the pulp-dentin-like tissue was similar to that of Group I, whereas the odontoblast-like cells had a more disordered arrangement. c In Group III, dentinal tubules of the regenerated dentin-like tissues were almost in parallel with those of primary dentin and the odontoblast-like cells hardly showed polarized morphology that odontoblasts possess. d In the blank control root canals, a thin layer of newborn mineralized tissues occurs in some regions with variable thicknesses (D dentin, sD secondary dentin). e Typical structure of normal dentin-pulp complex. Bar 50 μm
Dspp (the complex of Dsp and Dpp) is a non-collagenous protein that plays a critical role in tissue mineralization. Following immunohistochemical staining for Dspp, immunoreactive cells were predominantly distributed in the newly formed pulp-like tissues, especially the cells aligned with newly formed dentin-like tissues (Fig. 9). However, the expression intensity was slightly different in the various groups, with stronger intensity in Groups I (Fig. 9a) and II (Fig. 9b) and weaker intensity in Groups III (Fig. 9c) and IV (Fig. 9d) compared with normal teeth (Fig. 9e). Higher magnification observations of BrdU immunohistochemical staining revealed that many immunoreactive cells were present in the newly formed pulp-like tissues in both Groups I (Fig. 9f) and II (Fig. 9g). In addition, some BrdU-negative cells were present that probably originated from the host homing cells involved in the newly formed pulp-like tissues. Interestingly, the transplanted cells were involved in the formation of blood capillaries and the majority of the vascular endothelial cells were BrdU-positive in Group I but BrdU-negative in Group II. For Groups III (Fig. 9h) and IV (Fig. 9i) and for normal teeth (Fig. 9j), no BrdU-positive cells were found bcause of the absence of transplanted DPSCs in these two groups
Representative images of the immunohistochemical staining of DSPP (a–e) and BrdU (f–j) for the various groups. DSPP-immunoreactive cells were predominantly distributed in the odontoblast layer (a–c, e). Many BrdU-immunoreactive cells were found in Group I and played a dominant role in the formation of capillary walls (f). The number of BrdU-positive cells was much smaller in Group II (g) and no such cells were found in Groups III (h) and IV (i) or in normal teeth (j) that lacked transplanted cells. Bar 50 μm
Discussion
For pulp regeneration, several issues are considered to be necessary for regenerated pulp identification, including vascularized pulp-like tissues, deposition of new dentin on the existing dentin surface and the formation of a layer of odontoblast-like cells (Huang et al. 2010b). Tissue engineering techniques have been used for pulp regeneration and mainly refer to two approaches: those that are cell-based and those that are cell-free (Huang and Garcia-Godoy 2014). However, few reports demonstrate that pulp-dentin-like tissues can be regenerated by using a cell-free approach (Huang and Garcia-Godoy 2014). In contrast, the cell-based method has been used to regenerate pulp/dentin tissues (Huang et al. 2010b; Iohara et al. 2011; Ishizaka et al. 2013). Much exploration has been undertaken with regard to the maximization of the differentiation potential toward the target tissue and thus to ways of optimizing regeneration. In the present study, PRF was found to promote the proliferation of DPSCs in vitro and similar results were found in previous studies that used several other types of adult stem cells (Dohan Ehrenfest et al. 2010; He et al. 2009; Huang et al. 2010a; Tsai et al. 2009), including our own research on PDLSCs (Zhao et al. 2013); this proliferation-promoting effect was observed because of the multiple natural growth factors released by PRF. Different doses of PRF might induce the differentiation of DPSCs into osteo-/odontoblasts in different ways. In summary, the high dose of PRF, namely 3/8 PRF, significantly increased the gene expression of Alp and Dspp during early phases and facilitated the expression of Bsp at later stages; all of these genes are expressed in both bones and teeth and in both osteoblasts and odontoblasts (Gu et al. 2000; Gundberg 2000; Qin et al. 2002; Shiba et al. 2003; Sumita et al. 2006). However, as a specific protein of hard tissues (Almushayt et al. 2006; He et al. 2003; Narayanan et al. 2003), Dmp1 was upregulated by 3/8 PRF throughout the experimental period, suggesting that 3/8 PRF strongly regulated mineralization. Although the specificity of each marker remains a matter of discussion, the four markers together allow the characterization of the cascade of early events related to mineralization, odontogenesis or osteogenesis. These data are at least consistent with previous studies conducted in vitro (Huang et al. 2010a; Lee et al. 2011). We further provided evidence that, during co-culturing, the functionally active DPSCs and the PRF fibrin form an integral structure over time and that this structure resembles that of PDLSC/PRF, as we reported in a previous study (Zhao et al. 2013), suggesting that this strategy for constructing a tissue-engineered transplant is feasible and favorable. Subsequently, the effect of this newly formed DPSC/PRF transplant on pulp-like tissue regeneration has been validated by using in vivo experiments both in nude mice with root fragments and in canines with endodontically treated root canals, all of which might be more persuasive than either tooth slices or root fragments (Goncalves et al. 2007; Huang et al. 2010b).
Histological and immunohistochemistry staining has revealed increased pulp-like tissue regeneration in the DPSC/PRF group compared with the pure DPSCs or PRF transplant groups, with more compact and structurally normal pulp-like tissues, more newly formed vasculature, thicker newly generated dentin-like tissues and a more obvious odontoblast-like cell arrangement. The histological results for the nude mice and canines are consistent. The successful application of this newly formed construct might be attributable to its components, namely DPSCs and PRF and to the interaction between them. DPSCs have been shown to have the ability to form dentin after being placed subcutaneously in mice (Gronthos et al. 2000) and the use of cell-sheet fragments enhances the cell density (Fujita et al. 2009) and preserves the extracellular matrix because of the lack of enzymatic digestion; the use of these fragments is beneficial for cellular signal transduction and intercellular communication (Kelm and Fussenegger 2010). The most important reason for the successful application is the significant amount of growth factors that are slowly released by the PRF, which promote the proliferation and osteogenic/odontogenic differentiation of DPSCs. The in vivo experiment indicates that some BrdU-positive cells occur in both Groups I (DPSC/PRF) and II (DPSCs), which implies that the transplanted DPSCs indeed participate in the regeneration of pulp-like tissues. However, the amount of BrdU-positive cells in Group I is much higher than that in Group II, which again shows that PRF promotes the proliferation and differentiation of transplanted DPSCs, with both the transplanted cells and their daughter cells being involved in tissue regeneration, particularly angiogenesis. Transforming growth factor-β (TGF-β) has been demonstrated to be one of the promoters of odontoblast development and other growth factors, including platelet-derived growth factor (PDGF), endothelial growth factor (EGF) and insulin-like growth factor-1, have been shown to regulate the proliferation and differentiation of odontoblast precursors (Onishi et al. 1999; Shiba et al. 1998). A previous study revealed that the delivery of multiple cytokines, including basic fibroblastic growth factor, vascular endothelial growth factor (VEGF) and PDGF, alone or in combination with nerve growth factor and bone morphogenetic protein 7 promotes this effect (Kim et al. 2010). In addition, the effect of leukocytes trapped in the PRF is especially noteworthy. Inflammation in the root canal and periapical region is known to be able to seriously affect pulp regeneration (Kawashima 2012; Kim et al. 2010). Therefore, the concentrated leukocytes in the PRF might play a significant role in anti-inflammatory and immune-regulatory activity. Notably, in the group containing PRF only (Group III), i.e., the cell-free group, we also observed a layer of newly generated mineralized tissues, although they were oriented almost vertically to the primary dentinal tubules. This phenomenon might be attributed to the seed cells derived from the homing cells and adjacent cells. PRF promoted the proliferation and differentiation of these cells for the performance of tissue regeneration, but the number of cells was too limited to regenerate favorable pulp-dentin-like tissues. Hence, this type of mineralized tissue was more likely to be tertiary dentin. According to a previous study, the requirement for generating new dentin was the differentiation of stem cells into odontoblast-like cells, and then each cell extended a process to the dentinal tubules and produced extracellular matrix (Huang et al. 2010b). In the present study, we hypothesize that the proliferation of exogenous cells transplanted into the canal space is greatly increased by multiple growth factors released by PRF. Meanwhile, the stem cells close to the dentin walls are guided by signals released from the dentin and differentiate into odontoblast-like cells and the growth factors in PRF significantly facilitate this process. Subsequently, the odontoblast-like cells secrete a matrix and form new dentin-like tissues. The primary dentin and the newly formed dentin-like tissues can be easily demarcated by the obvious incremental line between them. However, the newly generated dentin-like tissues are quite different from the natural tooth in structure, with irregular dentinal tubules and many inlaid cells. This might be explained as follows. During development, each dentinal tubule is produced by one odontoblast. However, this one-to-one relationship no longer exists because of the lack of developmental signaling from the ameloblasts and their membranes (G.T. Huang and Garcia-Godoy 2014); some odontoblasts fill more than 1 dentinal tubule, whereas some dentinal tubules are never occupied (Huang and Garcia-Godoy 2014). In addition, once the transplanted cells are activated and secrete a mineralized matrix too quickly to mineralize, some of the cells might become embedded in the matrix. Under these circumstances, the regeneration of a regular and organized new dentin is impossible.
Based on the results for the immunohistochemical staining of BrdU, the participation of homing hematopoietic cells and adjacent cells has been confirmed in the pulp-like tissue regeneration and is characterized by a significant number of BrdU-negative endogenous cells in each group. The adjacent cells are primarily periodontal/periapical tissues. According to previous studies, the MSCs in the periapical alveolar bone are more likely to differentiate into osteoblasts (Matsubara et al. 2005), whereas the stem cells in PDL tissues are more likely to differentiate into cementogenic or osteogenic cells (Chadipiralla et al. 2010; Seo et al. 2004). Once these cells are activated to participate in the regeneration procedure, they probably form osteoid dentin or cementum-like tissues. Indeed, such tissues were initially reported by Thibodeau et al. (2007) and subsequently confirmed by many animal studies (da Silva et al. 2010; Thibodeau et al. 2007; Wang et al. 2010; Yamauchi et al. 2011a, 2011b; Zhu et al. 2013). These studies found that the newly formed tissues in root canals after revitalization procedures of immature teeth with necrotic pulps are tissues resembling bone, cementum, or periodontal ligaments. Recently, two human cases yielded similar results (Becerra et al. 2014; Shimizu et al. 2013).
Angiogenesis is thought to be a key factor in pulp regeneration, because only the blood vessels are generated in the canal space, leading to the long-term stability of the newly formed tissues (Huang et al. 2010b). In this study, the blood capillaries appeared most favorable in Group I, where they were similar to those of normal pulp. Interestingly, the majority of endothelial cells in the newly formed blood capillaries in this group were BrdU-positive, indicating that these cells were transplanted cells or their daughter cells. However, the proportion of BrdU-positive cells in Group II was much lower. This phenomenon might be attributable to the amount of VEGF that was released by the PRF, which might have induced the stem cells to differentiate into epithelial cells and then promote the growth of new vessels. A similar effect of growth factors on angiogenesis in pulp has been investigated previously (Kim et al. 2010) and was consistent with this study. However, the experimental period was only 2 months. Therefore, in most cases, the generated dental-pulp-like tissues appeared to be incomplete. The experimental period should therefore be prolonged to obtain more powerful evidence for evaluating the effect of this type of construct. In addition, the American Dental Association recommends that evoking pulp bleeding in immature permanent teeth is an important step for regenerative endodontics (Mao et al. 2012) and this has been established to regenerate pulp-like tissues. However, such results have not been found in the present study. One possible reason for this discrepancy is that evoking blood flow into canals is an unstable procedure and can be influenced by many complicated factors, including the stage of tooth development, teeth category, operation and the viability of apical tissues. We should be aware of the uncertainty of the biological responses taking place in the root canals. Thus, the evoking procedure is not always an efficient method for pulp revitalization.
Some challenges remain in the application of DPSCs for dental pulp regeneration, including ethical and commercial issues and the source of seed cells, because pulp regeneration should not be accomplished at the expense of another healthy tooth for the isolation of DPSCs. However, for those patients with wisdom teeth or whose teeth have to be extracted for orthodontic purposes, autologous DPSCs could be obtained from the extracted fresh teeth. Meanwhile, the use of PRF could reduce the cost of dental pulp regeneration by avoiding the application of commercial exogenous growth factors. Thus, the present investigation paves the way for further studies to regenerate dental pulp in clinical settings by utilizing autologous or allogeneic cells in combination with autologous PRF.
In conclusion, this study provides new insight into dental pulp regeneration with DPSC/PRF constructs consisting of cell-sheet fragments of DPSCs and PRF granules at specific ratios. The results indicate that PRF not only provides a well-organized scaffold for cell adhesion and migration but also supplies necessary growth factors for DPSC proliferation and differentiation. Intracanal transplantation of the DPSC/PRF constructs could effectively promote the regeneration of dentin-pulp-like structures both ectopically and orthotopically.
References
Almushayt A, Narayanan K, Zaki AE, George A (2006) Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther 13:611–620
Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM (2014) Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod 40:133–139
Chadipiralla K, Yochim JM, Bahuleyan B, Huang CY, Garcia-Godoy F, Murray PE, Stelnicki EJ (2010) Osteogenic differentiation of stem cells derived from human periodontal ligaments and pulp of human exfoliated deciduous teeth. Cell Tissue Res 340:323–333
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nor JE (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962–969
da Silva LA, Nelson-Filho P, da Silva RA, Flores DS, Heilborn C, Johnson JD, Cohenca N (2010) Revascularization and periapical repair after endodontic treatment using apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing in dogs’ teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:779–787
Dissanayaka WL, Zhu X, Zhang C, Jin L (2011) Characterization of dental pulp stem cells isolated from canine premolars. J Endod 37:1074–1080
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I. Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:e37–e44
Dohan Ehrenfest DM, Rasmusson L, Albrektsson T (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol 27:158–167
Dohan Ehrenfest DM, Doglioli P, de Peppo GM, Del Corso M, Charrier JB (2010) Choukroun’s platelet-rich fibrin (PRF) stimulates in vitro proliferation and differentiation of human oral bone mesenchymal stem cell in a dose-dependent way. Arch Oral Biol 55:185–194
Fujita H, Shimizu K, Nagamori E (2009) Application of a cell sheet-polymer film complex with temperature sensitivity for increased mechanical strength and cell alignment capability. Biotechnol Bioeng 103:370–377
Goncalves SB, Dong Z, Bramante CM, Holland GR, Smith AJ, Nor JE (2007) Tooth slice-based models for the study of human dental pulp angiogenesis. J Endod 33:811–814
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81:531–535
Gu K, Chang S, Ritchie HH, Clarkson BH, Rutherford RB (2000) Molecular cloning of a human dentin sialophosphoprotein gene. Eur J Oral Sci 108:35–42
Gundberg CM (2000) Biochemical markers of bone formation. Clin Lab Med 20:489–501
Hargreaves KM, Geisler T, Henry M, Wang Y (2008) Regeneration potential of the young permanent tooth: what does the future hold? Pediatr Dent 30:253–260
He G, Dahl T, Veis A, George A (2003) Dentin matrix protein 1 initiates hydroxyapatite formation in vitro. Connect Tissue Res 44 (Suppl 1):240–245
He L, Lin Y, Hu X, Zhang Y, Wu H (2009) A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:707–713
Huang GT, Garcia-Godoy F (2014) Missing concepts in de novo pulp regeneration. J Dent Res 93:717–724
Huang GT, Shagramanova K, Chan SW (2006a) Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod 32:1066–1073
Huang GT, Sonoyama W, Chen J, Park SH (2006b) In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res 324:225–236
Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34:645–651
Huang FM, Yang SF, Zhao JH, Chang YC (2010a) Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod 36:1628–1632
Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S (2010b) Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng A 16:605–615
Hung CN, Mar K, Chang HC, Chiang YL, Hu HY, Lai CC, Chu RM, Ma CM (2011) A comparison between adipose tissue and dental pulp as sources of MSCs for tooth regeneration. Biomaterials 32:6995–7005
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A (2004) Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res 83:590–595
Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, Ito M, Matsushita K, Nakamura H, Nakashima M (2011) Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng A 17:1911–1920
Ishizaka R, Iohara K, Murakami M, Fukuta O, Nakashima M (2012) Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 33:2109–2118
Ishizaka R, Hayashi Y, Iohara K, Sugiyama M, Murakami M, Yamamoto T, Fukuta O, Nakashima M (2013) Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials 34:1888–1897
Jiang HB, Tian WD, Liu LK, Xu Y (2008) In vitro odontoblast-like cell differentiation of cranial neural crest cells induced by fibroblast growth factor 8 and dentin non-collagen proteins. Cell Biol Int 32:671–678
Kawashima N (2012) Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? Arch Oral Biol 57:1439–1458
Kelm JM, Fussenegger M (2010) Scaffold-free cell delivery for use in regenerative medicine. Adv Drug Deliv Rev 62:753–764
Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, Fu SY, Koch PD, Mao JJ (2010) Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng A 16:3023–3031
Lee UL, Jeon SH, Park JY, Choung PH (2011) Effect of platelet-rich plasma on dental stem cells derived from human impacted third molars. Regen Med 6:67–79
Li ZY, Chen L, Liu L, Lin YF, Li SW, Tian WD (2007) Odontogenic potential of bone marrow mesenchymal stem cells. J Oral Maxillofac Surg 65:494–500
Lin LM, Ricucci D, Huang GT (2013) Regeneration of the dentine-pulp complex with revitalization/revascularization therapy: challenges and hopes. Int Endod J 47:713–724
Liu H, Li W, Gao C, Kumagai Y, Blacher RW, DenBesten PK (2004) Dentonin, a fragment of MEPE, enhanced dental pulp stem cell proliferation. J Dent Res 83:496–499
Mao JJ, Kim SG, Zhou J, Ye L, Cho S, Suzuki T, Fu SY, Yang R, Zhou X (2012) Regenerative endodontics: barriers and strategies for clinical translation. Dent Clin N Am 56:639–649
Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, Nishimura M, Saito M, Nakagawa K, Yamanaka K, Miyazaki K, Shimizu M, Bhawal UK, Tsuji K, Nakamura K, Kato Y (2005) Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res 20:399–409
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100:5807–5812
Murray PE, Garcia-Godoy F, Hargreaves KM (2007) Regenerative endodontics: a review of current status and a call for action. J Endod 33:377–390
Nakashima M, Huang GT (2013) Pulp and dentin regeneration. In: Huang GT, Thesleff I (eds) Stem cells in craniofacial development and regeneration. Wiley-Blackwell, Hoboken
Nakashima M, Iohara K, Ishikawa M, Ito M, Tomokiyo A, Tanaka T, Akamine A (2004) Stimulation of reparative dentin formation by ex vivo gene therapy using dental pulp stem cells electrotransfected with growth/differentiation factor 11 (Gdf11). Hum Gene Ther 15:1045–1053
Nakashima M, Iohara K, Sugiyama M (2009) Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev 20:435–440
Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A (2003) Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem 278:17500–17508
Ohazama A, Modino SA, Miletich I, Sharpe PT (2004) Stem-cell-based tissue engineering of murine teeth. J Dent Res 83:518–522
Onishi T, Kinoshita S, Shintani S, Sobue S, Ooshima T (1999) Stimulation of proliferation and differentiation of dog dental pulp cells in serum-free culture medium by insulin-like growth factor. Arch Oral Biol 44:361–371
Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, John AS, George A (2008) In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod 34:421–426
Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, Nagai N, Butler WT (2002) The expression of dentin sialophosphoprotein gene in bone. J Dent Res 81:392–394
Rosa V, Zhang Z, Grande RH, Nor JE (2013) Dental pulp tissue engineering in full-length human root canals. J Dent Res 92:970–975
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155
Shiba H, Fujita T, Doi N, Nakamura S, Nakanishi K, Takemoto T, Hino T, Noshiro M, Kawamoto T, Kurihara H, Kato Y (1998) Differential effects of various growth factors and cytokines on the syntheses of DNA, type I collagen, laminin, fibronectin, osteonectin/secreted protein, acidic and rich in cysteine (SPARC), and alkaline phosphatase by human pulp cells in culture. J Cell Physiol 174:194–205
Shiba H, Nakanishi K, Rashid F, Mizuno N, Hino T, Ogawa T, Kurihara H (2003) Proliferative ability and alkaline phosphatase activity with in vivo cellular aging in human pulp cells. J Endod 29:9–11
Shimizu E, Ricucci D, Albert J, Alobaid AS, Gibbs JL, Huang GT, Lin LM (2013) Clinical, radiographic, and histological observation of a human immature permanent tooth with chronic apical abscess after revitalization treatment. J Endod 39:1078–1083
Sloan AJ, Smith AJ (2007) Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis 13:151–157
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166–171
Sumita Y, Honda MJ, Ohara T, Tsuchiya S, Sagara H, Kagami H, Ueda M (2006) Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials 27:3238–3248
Thibodeau B, Teixeira F, Yamauchi M, Caplan DJ, Trope M (2007) Pulp revascularization of immature dog teeth with apical periodontitis. J Endod 33:680–689
Tsai C-H, Shen S-Y, Zhao J-H, Chang Y-C (2009) Platelet-rich fibrin modulates cel proliferation of human periodontally related cells in vitro. J Dent Sci 4:130–135
Wang X, Thibodeau B, Trope M, Lin LM, Huang GT (2010) Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod 36:56–63
Wu G, Deng ZH, Fan XJ, Ma ZF, Sun YJ, Ma DD, Wu JJ, Shi JN, Jin Y (2009) Odontogenic potential of mesenchymal cells from hair follicle dermal papilla. Stem Cells Dev 18:583–589
Yamauchi N, Nagaoka H, Yamauchi S, Teixeira FB, Miguez P, Yamauchi M (2011a) Immunohistological characterization of newly formed tissues after regenerative procedure in immature dog teeth. J Endod 37:1636–1641
Yamauchi N, Yamauchi S, Nagaoka H, Duggan D, Zhong S, Lee SM, Teixeira FB, Yamauchi M (2011b) Tissue engineering strategies for immature teeth with apical periodontitis. J Endod 37:390–397
Yang X, Walboomers XF, van den Beucken JJ, Bian Z, Fan M, Jansen JA (2009) Hard tissue formation of STRO-1-selected rat dental pulp stem cells in vivo. Tissue Eng A 15:367–375
Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y (2007) Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell 99:465–474
Zhang W, Walboomers XF, van Kuppevelt TH, Daamen WF, Bian Z, Jansen JA (2006) The performance of human dental pulp stem cells on different three-dimensional scaffold materials. Biomaterials 27:5658–5668
Zhao YH, Zhang M, Liu NX, Lv X, Zhang J, Chen FM, Chen YJ (2013) The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials 34:5506–5520
Zhu W, Zhu X, Huang GT, Cheung GS, Dissanayaka WL, Zhang C (2013) Regeneration of dental pulp tissue in immature teeth with apical periodontitis using platelet-rich plasma and dental pulp cells. Int Endod J 46:962–970
Author information
Authors and Affiliations
Corresponding author
Additional information
Yong-Jin Chen, Yin-Hua Zhao and Ya-Juan Zhao contributed equally to this work.
Animal experiments were undertaken after being approved by the Animal Experimental Ethical Inspection of FMMU (no. 12011).
This research was supported by the National Natural Science Foundation of China (nos. 81371188, 31170888).
Rights and permissions
About this article
Cite this article
Chen, YJ., Zhao, YH., Zhao, YJ. et al. Potential dental pulp revascularization and odonto-/osteogenic capacity of a novel transplant combined with dental pulp stem cells and platelet-rich fibrin. Cell Tissue Res 361, 439–455 (2015). https://doi.org/10.1007/s00441-015-2125-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2125-8