Abstract
Understanding the evolution of the neurosensory system of man, able to reflect on its own origin, is one of the major goals of comparative neurobiology. Details of the origin of neurosensory cells, their aggregation into central nervous systems and associated sensory organs and their localized patterning leading to remarkably different cell types aggregated into variably sized parts of the central nervous system have begun to emerge. Insights at the cellular and molecular level have begun to shed some light on the evolution of neurosensory cells, partially covered in this review. Molecular evidence suggests that high mobility group (HMG) proteins of pre-metazoans evolved into the definitive Sox [SRY (sex determining region Y)-box] genes used for neurosensory precursor specification in metazoans. Likewise, pre-metazoan basic helix-loop-helix (bHLH) genes evolved in metazoans into the group A bHLH genes dedicated to neurosensory differentiation in bilaterians. Available evidence suggests that the Sox and bHLH genes evolved a cross-regulatory network able to synchronize expansion of precursor populations and their subsequent differentiation into novel parts of the brain or sensory organs. Molecular evidence suggests metazoans evolved patterning gene networks early, which were not dedicated to neuronal development. Only later in evolution were these patterning gene networks tied into the increasing complexity of diffusible factors, many of which were already present in pre-metazoans, to drive local patterning events. It appears that the evolving molecular basis of neurosensory cell development may have led, in interaction with differentially expressed patterning genes, to local network modifications guiding unique specializations of neurosensory cells into sensory organs and various areas of the central nervous system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human brain consists in over 80 billion neurons and similar numbers of glia cells (Herculano-Houzel 2010), engaged in trillions of synapses to process the information gathered by several sensory organs to respond appropriately to environmental stimuli. While the human brain is now recognized as an organ of extraordinary relative and absolute size and packing density of neurons (Herculano-Houzel 2012), its origin as a vertebrate adaptation for information processing has led to various ideas - mostly revolving around single or multiple origins of the central nervous system (CNS) among metazoans (Nieuwenhuys 2002; Northcutt 2012) - and subsequent increase and modification of all or parts of the CNS (Nieuwenhuys et al. 1998; Striedter 2005). In parallel to this traditional interpretation of macroscopic neuronal evolution, the last 20 years have witnessed the generation of another set of data and associated ideas concentrating on molecular and cellular aspects of neuronal evolution as a variation on the theme of ectodermal cellular diversification (Beccari et al. 2013; Fritzsch and Glover 2006; Pani et al. 2012), integrating neurosensory cellular evolution with the evolution of the major sensory systems, eyes and ears (Fritzsch and Straka 2014; Lamb 2013; Patthey et al. 2014; Schlosser et al. 2014). More recently, the molecular origin of evolutionary innovations (Wagner 2011), such as those leading to formation of neurosensory cells and their aggregation into a brain and associated sensory organs, have begun to direct the deluge of genomic data into a theoretical framework of Darwinian evolution of neurosensory systems (Newman 2014). Given that only 14 years have passed since the human genome was published, it is clear that a complete understanding of the different levels of neurosensory evolution from genes, to developmental gene regulatory networks, to neurosensory cell and sensory organ development and CNS evolution is beyond the reach of our current understanding as depicted in this short overview. Admittedly, despite tremendous gains, our understanding of gene regulatory networks and their evolution to govern complex macroscopic phenotype changes is only in its infancy (Sauka-Spengler and Bronner-Fraser 2008; Streit et al. 2013). As of this writing, our ability to translate mutations at the DNA level into altered phenotypes is not yet deep enough to relate single nucleotide changes causing, for example, a different folding of proteins to macroscopic changes comparable to the morphological alterations of hemoglobin in sickle cell anemia. Despite all the gain in detailed information, we are unable to mechanistically explain how limited sequence differences between protein isoforms can cause major diseases (Forrest et al. 2014) or how differences in protein coding genes of humans and chimpanzees of around 2 % can lead to the profound macroscopic differences characterizing these two species.
With this caveat in mind, this overview aims to define some major steps relevant for the evolution of neurosensory systems by casting data on both macroscopic and molecular evolution into a framework of developmental gene regulatory network evolution acting at the cellular level. This review adopts the perspective of Wagner (2011) that an intermediate level of abstraction is paramount for human understanding of otherwise hopelessly entangled innovations across all molecules and levels of analysis. The level of abstraction attempted here will provide a ‘neurocentric’ view of the evolution and development of neurosensory systems of bilaterians. To achieve this, we will progress from the evolution of general cell fate decision-making networks to special cases of limited complexity of lineage-related differential cell fate decisions (inner ear neurons and hair cells) to overall patterning with and without mesodermal induction. Another layer of complexity, related to the gene expression regulation (Davidson 2010) and differential splicing in neurosensory cells (Nakano et al. 2012; Raj et al. 2014), is beyond the scope of this review.
Evolving single cells to multicellular organisms with dedicated neurosensory cells
The origin and molecular repertoire of single and multicellular organisms has been worked out over the last 10 years (Fig. 1). It is commonly agreed that all multicellular animals, including their brains, have evolved from single-celled ancestors s (Fairclough et al. 2013; King 2004) and other related single-celled organisms (Sebé-Pedrós et al. 2013; Suga et al. 2013). These single-celled organisms were able to form transient aggregates with complex life-cycle changes (Levin et al. 2014; Sebé-Pedrós et al. 2013) and already contained many genes previously speculated to be unique to metazoans. While single cells, by definition, do not have specialized neurosensory cells, it seems plausible that some gene regulatory network present only in unicellular organisms leading to metazoans but not those leading to plants or fungi, should have evolved into the gene regulatory network driving the neurosensory cell development of metazoans. In short, we speculate here that the cellular basis of brain and sensory organ evolution lies in the multiplication, diversification and specialization of gene networks (Wagner 2011) that evolved in single-celled precursors of metazoans to guide temporal changes in a transient multicellular organization, most likely associated with reproduction-related life cycles. In essence, metazoans may have evolved, through incompletely understood innovations of the single-celled metazoan ancestors, the molecular repertoire to develop sophisticated developmental fate decision processes (Lai et al. 2013; Stergachis et al. 2013) that generate specific cells capable of collecting and processing information. Whatever the details of this process will eventually turn out to be, it turned the single-celled metazoan ancestors, fully capable of sensing their environment and responding to it in an appropriate way to ensure survival of the species (Dayel et al. 2011; Fairclough et al. 2013; Sebé-Pedrós et al. 2013; Suga et al. 2013), into an aggregate of cells with a segregation of the sensory and information processing aspect to morphologically distinct neurosensory cell types. Ultimately, such neurosensory cells were grouped into the CNS and associated sensory organs (eyes, ears, nose). The evolution of gene networks past single-celled ancestors now governs the transformation of the single fertilized egg at the start of each metazoans life cycle into multiple distinct neurosensory cell types through the topological and temporal precise expression of gene regulatory networks that govern neuronal (Beccari et al. 2013; Puelles et al. 2013) and sensory organ evolution (Fortunato et al. 2014; Fritzsch and Straka 2014).
The relationship of single-celled and multicellular organisms and some critical events concerning the evolution of neurons and sensory systems. 1 Complicated life cycle with transient multicellularity; 2 most cellular communication signals are present, Sox-like and bHLH genes are present; 3 class A bHLH genes and SoxB genes that can induce neurons are present; 4 epithelial nerve nets and sensory organs evolve; 5 miR-124 specific for neurons and miR-183 specific for sensory cells appear; 6 ventral central nervous system (CNS) evolves; 7 ‘skin brains’ with chordate-like patterning evolve; 8 neurons are concentrated in a dorsal neural tube and composite, organ-like sensory cell groups appear; 9 vertebrate sensory organs and the nervous system appear. # note that the position of Ctenophora is disputed. *Acoela are sometimes combined with Xenoturbella, indicating perhaps limited molecular distinction between basic bilaterians and basic deuterostomes. (Compiled after Gyoja 2014; Osigus et al. 2013; Peterson et al. 2013; Schnitzler et al. 2014; Suga et al. 2013; Swalla and Smith 2008)
Integrated into the topological expression regulation is the sequential gene activation that governs coordinated transitions between different stages of cell fate commitment, a process dominated by two closely interacting partners, the SRY (sex determining region Y) - box (Sox) genes (Guth and Wegner 2008) and the basic helix-loop-helix (bHLH) genes (Degnan et al. 2009; Simionato et al. 2007). Metazoan Sox and bHLH genes associated with neurosensory development seem to have no precursors in single-celled metazoan ancestors (Guth and Wegner 2008; Neriec and Desplan 2014). However, both Sox gene-related and bHLH gene-related precursors with similarities in DNA binding sites are well known in single cell organisms (Albert et al. 2013; Gordan et al. 2013) and are also found in plants (Ikeda et al. 2012; Xia et al. 2014). In fact, single bHLH genes such as Tcf25 (Nulp1) are extremely conserved http://www.ensembl.org/Mus_musculus/Gene/Compara_Tree?collapse=6638243%2C6638340%2C6638209%2C6638328%2C6638179%2C6638173%2C6638249%2C6638233%2C6638296;db=core;g=ENSMUSG00000001472;r=8:123373824–123404173 and play a significant role in sensory function (Wolber et al. 2014). Combined, these data suggest that indeed transcription factors may have signaled in single-celled organisms and evolved into a gene regulatory network dedicated to metazoan neurosensory cell development. We propose that gene regulatory networks of metazoan neurosensory development could have originated in DNA binding transcription factors that may already form an interactive network in single cells to regulate specific vegetative or generative states of a cell.
Evolving bHLH and Sox gene networks to specify neurosensory cell types
Single-celled organisms have the molecular machinery to transit through vegetative and generative stages of their life cycle, using molecular cascades to progress into or forgo mitosis, or change cellular communication to engage in multicellularity with or without sexual reproduction. Such intracellular molecular interactions, once evolved in single-celled organisms to define temporal progression of specific stages in the life of one cell, may have evolved to form the basis for the equally fundamental decision of any given cell during development of multicellular organism: to proliferate or to differentiate. It appears that evolution had already picked bHLH and Sox-related transcription factors to translate such cellular decision processes into action in single-celled ancestors (Sebé-Pedrós et al. 2013). Consistent with this assumption is that, during evolution, bHLH and Sox proteins and their DNA binding sites have been mostly multiplied and diversified (Degnan et al. 2009; Guth and Wegner 2008; Pan et al. 2012) and, in particular, bHLH genes govern in extant metazoans the progression of individual cells toward their differentiation (Fig. 2). This ensures that multiple cell types can be differentiated through division of labor between cell types (Arendt et al. 2009), such as stimuli acquisition and information conductance and processing in sensory organs (Pan et al. 2012). Modifying such cell fate-determining genes through topologically restricted transcription factors may have enabled differentiation of specific cell types in specific areas, ultimately evolving into cellular assemblies serving a specific function. Understanding cellular diversification requires an understanding of the regulation of cell fate decision-making proteins in specific cell types (Lai et al. 2013; Stergachis et al. 2013) that interact to develop the cells needed for the function of a specific cellular assembly.
Available data on basic helix-loop-helix transcription factors (bHLH TFs) relevant for the evolution of human hair cells and sensory neurons are shown. Note that Monosiga has 11 bHLH TFs, none of which are orthologous to Metazoans. Eumetazoans have sensory cells with axons and display an asymmetric distribution of microvilli (yellow) and kinocilium (gray). In mammals, the three bHLH TFs are partially overlapping to drive neuronal (Neurog1, Neurod1) and hair cell (Atoh1, Neurod1) development. A superficially similar arrangement of sensory cells and sensory neurons is found in some mollusks but which bHLH genes are expressed in these cells is unknown. Given the distribution in protostomes and deuterostomes, mollusk and vertebrates evolution of sensory cell without an axon likely indicates functional convergence. (Modified after Pan et al. 2012)
The importance of the evolution of this cellular decision making network of group A bHLH genes (Fig. 2) in interaction with the Sox genes for eyes, ears and the CNS cannot be underestimated. For example, only four bHLH genes (Ascl1, Atoh1, Neurog1 and Neurod1) are necessary for the development of ~90 % of the neurons in the mammalian brain (cerebellum, cochlear nuclei, large areas of the cortex and sensory cells and neurons of the eye and ear; Bermingham et al. 2001; Pan et al. 2009) and all sensory neurons bringing information into the brain. Indeed, most of the group A bHLH genes are associated with neurosensory development (Chen et al. 2011; Imayoshi and Kageyama 2014) and seem to be linked to the evolution of neurons (Fig. 2). It seems that the evolution of the Ascl-like family and Atoh-like family are among the earliest group A bHLH genes that had already evolved in sponges out of bHLH genes present in unicellular organisms such as choanoflagellates (Fig. 2). Indeed, recent data suggest that group A bHLH transcription factors, relevant for neuronal development, exist in metazoans that have no trace of a nervous system, raising the issue of function of such neuronally-associated group A bHLH genes in sponges and placozoans (Gyoja 2014). Understanding the evolution of the bHLH gene network and how it became associated with neurosensory development could provide insight into the cellular decision making that stably transforms metazoan ectoderm into neurosensory cells. In fact, bHLH genes cloned from sponges and injected into developing frog embryos can induce formation of neurons in ectoderm (Richards et al. 2008) comparable to bHLH genes of vertebrates (Lee et al. 1995). Recent data (Gyoja 2014) suggest that the molecular evolution of group A bHLH genes, needed for neuronal differentiation, predate the cellular evolution of neurosensory cells (genes to differentiate neurons evolved before neurons). Alternatively, assuming that ctenophores are the sister group of all metazoans (Martindale 2013; Martindale and Lee 2013), the presence of these transcription factors in sponges and placozoans may indicate that the regulatory gene network of these metazoans is either secondarily reduced or a parallel evolution to that found in ctenophores (Ryan 2014).
Consistent with the data on group A bHLH genes (Gyoja 2014), most recent work has already identified true Sox gene members of the high-mobility group-box (HMG) group of transcription factors in early metazoans (Schnitzler et al. 2014). Experimental work in various bilaterians has shown that members of the SoxB, SoxC, SoxD and SoxF families are essential to specify and maintain neurosensory precursors through the clonal expansion of neurosensory precursor cells (NSP cells) to generate the cellular basis of neurosensory development (Guth and Wegner 2008). In particular, SoxB genes are essential to establish neuroectodermal precursor cell fate (Reiprich and Wegner 2014), possibly by preparing cells to respond to bHLH genes with neurosensory differentiation (Bylund et al. 2003). Closer examination shows a regulatory network interaction between Sox genes and bHLH genes such that Sox genes are not only essential to set the stage for bHLH genes to initiate differentiation. In many instances, Sox genes counteract bHLH gene actions by maintaining progenitor status and enhance proliferation. In essence, SoxB genes and bHLH genes act like ‘frenemies’: SoxB genes are needed to set the stage for actions of bHLH genes, play some yet not fully determined role in their expression but ultimately have to be inhibited by bHLH genes to allow neurosensory differentiation (Dabdoub et al. 2008; Reiprich and Wegner 2014). Both bHLH genes and Sox genes are essential for neurosensory development and their interactions within each transcription factor family (Imayoshi and Kageyama 2014; Reiprich and Wegner 2014) and between these transcription factors is essential for neuronal and neurosensory development of vertebrates (Fig. 3). The transition from Sox to bHLH gene expression may be regulated itself by antagonistic actions such as Gdf11 and other factors (Gokoffski et al. 2011). Unfortunately, our insights into the evolution of neurosensory systems have not yet gone beyond identifying relevant orthologs of important transcription factors but not when these feedback loops evolved. Specifically, we need to show experimentally how the intricate gene regulatory network of feed-forward and feed-back loops evolved to regulate differentiation of the three principal cell types of the vertebrate nervous system: neurosensory cells, astrocytes and oligodendrocytes (Fig. 3).
The interactions of Sox and bHLH genes in neurosensory differentiation of a mouse. Experimental data in mice have shown a complex interaction of Sox genes and bHLH genes in the progression of neurosensory cell fate commitment and differentiation. a Sox2 and Sox9 are essential genes in neurosensory precursor cells that ensure self-renewal of precursors but also commitment to the neurosensory lineage. This appears to be in interaction with several bHLH genes that are later found in astrocytes (Hes, Hey, Id). Virtually all of these neurosensory precursor genes are turned off in the neurosensory lineage (arrow with – on transition) and mostly bHLH genes are activated, including Sox21 that antagonizes Sox2. Oligodendrocyte precursors also shut off neurosensory precursor genes but are characterized by a different set of bHLH genes (Olig1/2) and Sox10 and Sox2 for terminal differentiation. b bHLH transcription factors can form complex interactions in a given cell that can undergo periodic changes (oscillates) and their signal can undergo context dependent variation between gene expression and suppression. Data in mice and flies suggest that all proneural transcription factors compete for the E-proteins (Tcf3, 4, 12) to form heterodimers for proper binding. Thus, the level of all proneuronal bHLH TFs (here Atoh1 and Neurod1) and available E-proteins as well as their binding preference will determine how much signaling of heterodimers will occur. Importantly, E-proteins can also interact with Hes/Hey factors and the inhibitors of DNA binding (Ids), limiting availability of E-proteins for proneuronal protein heterodimerization, proportionally to the affinity and concentration of all these interactive partners. In essence, the binding properties and frequency of the binding partners will determine whether a cell is differentiating as a neuron/hair cell, a supporting/glial cell, or is continuing proliferation as a prosensory precursor. HC hair cell, SC supporting cell. (Modified after Forrest et al. 2014; Imayoshi and Kageyama 2014; Pan et al. 2012; Reiprich and Wegner 2014)
Embedded into this emerging concept of molecular evolution of expanding Sox and bHLH gene networks and their cross-regulation to drive neurosensory development is the need of micro-RNA for the development of neurons and sensory cells in bilaterians. Specific micro-RNAs (miR-124) are evolutionarily conserved and evolved first with bilaterians (Campo-Paysaa et al. 2011; Peterson et al. 2009). miR-124 has been shown to be essential for neuronal development through the regulation of chromatin remodeling (Yoo and Crabtree 2009; Yoo et al. 2009) and the downregulation of Sox9 (Cheng et al. 2009). Experimental work in mammals has demonstrated that the absence of miR-124 (and other micro-RNA species) causes rapid degeneration of developing neurons (Kersigo et al. 2011; Rosengauer et al. 2012). Almost as conserved as miR-124 is miR-183 (Peterson et al. 2009), which is associated with sensory systems (Pierce et al. 2008) and is crucial for their development (Soukup et al. 2009). The regulatory potential of miR’s is obvious: expression of just one micro-RNA can transform fibroblasts into neurons and such a transformation is greatly enhanced by Neurod1 (Yoo et al. 2009). This indicates that micro-RNA may have evolved to cooperate with certain bHLH genes apparently to facilitate the action of those bHLH genes by suppressing specific Sox genes (Reiprich and Wegner 2014). More data are needed to understand how many more miR’s can play such a role. It is possible that the rapid expansion of bHLH and Sox gene families required additional gene regulation to fine tune the required detailed cell fate decision. Obviously, the ability of micro-RNA to regulate large sets of transcription factor translation could be a prerequisite for the evolution of a more complex neuronal and sensory system of bilaterians (Fritzsch et al. 2007; Peterson et al. 2009).
In summary, the molecular data on Sox and bHLH transcription factors as well as micro-RNAs suggest the evolution of unique sets of genes within these families of transcription and translation regulating factors. What remains unclear is how that complex gene regulatory network evolved to be associated with the transformation of ectoderm to neurosensory precursor cells, to regulate the clonal expansion of such precursor cells and to guide the differentiation into the principal cell types of the neurosensory system of vertebrates. It is possible that the increasing complexity of these interactions through multiple feed-back and feed-forward loops was enabled with a novel regulatory layer, the micro-RNAs. More experimental data on the functional interaction of Sox and bHLH genes in diploblastic metazoans are needed to verify how genomic evolution relates to evolution of functional interactions as revealed experimentally in vertebrates and flies. In particular, miRs’ hypothetical function in the nervous system evolution of bilaterians requires experimental verification to establish the possibly essential function of these bilaterian specific regulatory elements for neurosensory development and evolution, possibly through misexpression of these miRs in diploblasts.
Evolving intercellular communication mechanisms through regulated release of synaptic and dense core vesicle
The preceding paragraph highlighted basic aspects of molecular evolution of certain transcription factors and their interactions, including interactions with micro-RNA. However, while the essential function of these genes is clear for normal development and differentiation of stem cells, it remains mostly unclear how these embryonic transcription factors regulate the adult neurosensory phenotype. For example, one essential aspect of neurons and sensory cells is the cellular communication via the release of synaptic transmitters to elicit a response in the target cell. The seemingly neurosensory specific aspect of vesicular release evolved out of general vesicular release mechanisms already present in single-celled organisms. In essence, basic molecular aspects needed for synaptic interactions have been identified in the genome of single-celled organisms (Sebé-Pedrós et al. 2013), sponges and placozoans (Cheng et al. 2009; Srivastava et al. 2010), all of which are lacking neurons and synapses. Current data suggest that the basic molecular machinery needed for exocytosis in single-celled organisms was supplemented in neurosensory cells with additional molecules for fast vesicular release necessary for neuronal communication. As with bHLH gene function, such as Myc that evolved perhaps to govern cell-cycle transitions of single cells, such classical synaptic release molecules as SNARE’s (soluble NSF-attachment protein receptor) are already found in yeast and therefore before synaptic communication evolved. Thus, a major aspect of neuronal communication, vesicle release at presynaptic terminals, evolved out of a general exocytosis process through interactions of SNARE proteins with SM (Sec1/Munc18-like) proteins (Südhof and Rizo 2011). The differences in interactions of SM proteins and SNARE complexes to catalyze membrane fusion has been modeled in an evolutionary context to define essential features of this process that can be adjusted through additions of other proteins to allow the fast neuronal transmitter release needed at synapses (Xia et al. 2012). These modifications in neurons consist in chaperons such as Hsc70 and synucleins and their dysfunction can result in neurodegeneration. Synaptotagmins and synaptic “active zone” proteins such as Munc13 and RIMs are central components that adopt the generalized vesicular release mechanism of single-celled organisms such as yeast to the needs of rapid synaptic vesicle release in neurons (Südhof 2012). Recent work has identified for the first time the quantitative ratios of 60 proteins needed to release and recycle synaptic vesicles (Wilhelm et al. 2014). It was also shown that many components critical for synaptic functions are present in diploblastic animals that may or may not have neurons (Ryan et al. 2013; Srivastava et al. 2010). With this detailed, stoichiometric molecular understanding of the most basic process of neuronal communication at hand, one can now start to sort out the evolution of all partner proteins and their diversification to generate the synaptic proteins needed for vesicular release. Ultimately it needs to be established how transmitter choice and synaptic specializations such as ribbon or non-ribbon synapses of vertebrates and insects (Matkovic et al. 2013; Weiler et al. 2014) tie into the emerging network of cell fate decision-making transcription factors and their downstream genes.
In summary, the evolution of synaptic release proteins indicates a molecular transformation of a general vesicular release mechanism of pre-metazoans into a specialized synaptic vesicle release mechanism in metazoans that was further modified in neurons. This transformation parallels the evolution of the gene network of transcription factors to regulate neurosensory development. Possible causalities between the developmental transcription factor network to guide neuronal development and the molecular network to govern synaptic communication as an essential aspect of the function of adult neurosensory networks, need to be established experimentally in the future.
Evolving a communication system to stabilize connections: neurotrophin evolution and function to secure lasting neuronal connections.
Tightly interwoven with the evolution of the synaptic release mechanism is the release of peptides in small and large dense core vesicles to provide a different timetable of cellular interactions. Among these proteins is a set of specialized proteins, the neurotrophins (von Bartheld and Fritzsch 2006), which signal through modified receptor tyrosine kinases (Hallbook et al. 2006) to promote the survival of neurons. These trophic interactions evolved out of tyrosine kinase signaling already known for single-celled organisms (Fairclough et al. 2013; Sebé-Pedrós et al. 2013). Neurotrophins and their receptors are essential for the viability of the peripheral nervous system (PNS) and cause extensive loss in vertebrate embryos when they are deleted. Neurotrophins and other neurotrophic factors (Lindahl et al. 2014; Lindholm and Saarma 2010) are indispensable for proliferation and survival and are the molecular mediators of ‘programmed cell death’ that is widespread in the CNS of many developing organisms (Oppenheim 1991) to achieve numerical matching between independently developing sets of neurons. In addition, neurotrophic factors have been implicated to correct aberrant connections through pruning of such connections (Oppenheim 1991). However, experimentally mis-wired peripheral innervations are not corrected (Mao et al. 2014) even in systems that depend 100 % on neurotrophins for their survival (Fritzsch et al. 2004). Thus, while neurotrophins play a central role in regulating cell death, retention of aberrant connections seems to be of little consequence (Taylor et al. 2012). Moreover, even deletion of two neurotrophins has only a limited effect on the CNS (Silos-Santiago et al. 1997), questioning the basic assumptions underlying the neurotrophin theory (Dekkers and Barde 2013). Molecular differences in protostomian and deuterostomian neuron development dependency on neurotrophins may indicate a lineage-specific use of such cell survival regulation factors but also high levels of conservation of some survival factors, including those responsible for the retention of specific subsets of dopaminergic neurons across phyla (Lindstrom et al. 2013). When those neurotrophic support systems evolved and what was the selective advantage of having a system of survival factors is at the moment unclear.
With this background on the molecular evolution of neurosensory cells in mind, this article will explore aspects of neurosensory evolution of metazoans, with a focus on the evolution of neurosensory cells and organs as well as patterning of the deuterostome brain. This review aims to discuss the molecular basis of locally distinct cellular decision-making processes, orchestrated by local patterning events (Srinivasan et al. 2014) into developing specialized parts of the brain or specific organ systems. This review is organized to reflect the currently accepted systematic relationships among metazoans (Fig. 1), in particular deuterostomes (Bourlat et al. 2006; Nosenko et al. 2013; Osigus et al. 2013; Peterson et al. 2013; Satoh 2008; Swalla and Smith 2008).
From single sensory cells and ‘skin-brains’ to sensory organs and a central nervous system: evolving patterning processes and reorganizing them through embryonic transformation
Generating complex sensory organs or a CNS requires that molecular regulations of cellular development happen in the right cells at the right time to achieve the desired outcome in terms of cellular differentiation (Stergachis et al. 2013) and to coalesce specific cell types into unique aggregates able to perform a distinct function, for example assembling into a sensory organ. Such topological regulation and orchestration of intercellular interactions require a multitude of different diffusible signals (Shh, Fgf, Wnt, BMP; Chen and Streit 2013; Streit, et al. 2000) that will result in localized expression of regional selector genes (for example, Pax genes in sensory organ development; Fortunato, et al. 2014) that translate patterning signals into local, cellular action. Modeling the patterning changes that transform a cnidarian-like organism into a bilaterian suggests that skin patterning evolved as a consequence of mesoderm invagination (Meinhardt 2013) to induce the neural plate and surrounding placodes (Schlosser et al. 2014). Consistent with this gene-centric perspective proposed here is the fact that the molecular basis of patterning of the developing sensory organs and CNS seemingly evolved in pre-metazoans (Fairclough et al. 2013; Fortunato et al. 2014; Sebé-Pedrós et al. 2013). Even amoebazoa, such as the slime mold (Fig. 1), have sophisticated patterning processes to differentiate the homogenous population of amoeba into a stalk and a spore cell type (Chattwood et al. 2013); activation of G protein-coupled receptors are essential for directional growth to ensure mating in yeast cells (Martin and Arkowitz 2014) and gene networks regulate sexual cycles in fungi (Ait Benkhali et al. 2013).
As outlined in the previous section, it is conceivable that sophisticated molecular networks that enable multicellular interactions of single cells for reproduction purposes became the basis of the cellular interaction in metazoans. However, in metazoans, this molecular machinery for cellular communications evolved to pattern the body and its organs, such as the CNS and sensory organs. Obviously, patterning of the body already happened at the level of diploblasts and many molecules found in these patterning processes in bilaterians can be identified in diploblasts that show only 2 (sponges) or 4 (placozoans) distinct cell types. The ideas of a progressive complication of specification of cell types in sponges and placozoans (Osigus et al. 2013) followed by the evolution of gastrulation have recently been blurred with the claims that ctenophores maybe the sister group of all other metazoans (Ryan et al. 2013). In fact, it has been claimed that the ‘gastrulation’ of ctenophores is molecularly and structurally distinct from that of other metazoans (Martindale 2013; Martindale and Lee 2013). Ctenophores also have an unusual development of the apical sensory organ (Schnitzler et al. 2014), a gravity sensing system that uses cilia to hold an otoconia mass so that changes in position affect the beating cilia, the organs of motility in these organisms, to change the course of swimming (Tamm 2014). The nervous system of these animals consists in an assembly of apical neurons and giant fibers that run along the eight strings of combs. Possibly light-sensitive cells are molecularly identified but no response to light has been recorded, indicating that gravity sensing with the single apical sensory organ is the major sensory input for spatial orientation in ctenophores. These unique features as well as a strange double symmetric organization could indicate that ctenophores may indeed be uniquely derived from an unknown metazoan ancestor and have evolved certain features, including a sophisticated multicellular gravity-sensing organ and nervous system in parallel to other metazoans that, nevertheless, share many neuronal patterning genes (bHLH, Sox) and molecules related to synaptic function (Ryan 2014). However, others have argued against this scenario and propose an evolution of metazoan with ctenophores being a highly derived sister group of coelenterates (Nosenko et al. 2013; Osigus et al. 2013), arguments we have adopted here (Fig. 1).
Be this as it may, the evolution of complicated sensory organs dedicated to gravity and light sensing in both ctenophores and cnidarians indicates that sensory organ evolution can be combined with limited development of a nervous system and certainly predates evolution of the sophisticated CNS of bilaterians (Fig. 1). It is possible that the limited motor abilities of diploblastic animals are incompatible with the evolution of a complex nervous system that could integrate sensory stimuli better with a sophisticated motor output provided by mesoderm diversification, requiring only localized ganglion-like concentrations of neurons in an epithelial network (Satterlie 2011). We argue that the ability to generate a refined motor output is a prerequisite to evolve the enhanced computational power of a larger set of interneurons that connect an increasingly sophisticated sensory input to an equally sophisticated motor output (Straka et al. 2014).
For the remainder of this article, we will build on the idea that the molecular ability to form neurosensory cells of complex sensory organs, such as statocysts and eyes for orientation in space (Fritzsch and Straka 2014; Lamb 2013), evolved prior to the bilaterian CNS to process sensory information to guide the limited motor output of animals comparable to ctenophores and cnidarias. Obviously, the evolution of complicated sensory organs is mostly associated with motile diploblastic life forms but they are topographically (opposite the mouth in ctenophores, around the mouth in cnidarians) and structurally so distinct between these two forms of diploblastic animals that they may have independently evolved out of molecular (Gyoja 2014; Schnitzler et al. 2014) and cellular (Fritzsch and Straka 2014) precursors. Experimental gene swapping as conducted in sensory system development of mice and flies (Wang et al. 2002) is needed to verify the function of homologous neuronal transcription factors in diploblasts and bilaterians to further understand the significance of the obvious molecular and anatomical differences in diploblast and bilaterian sensory system development.
‘Splitting hairs’: molecular transformation of single neurosensory cells to neurons and hair cells of the ear
Evolution of the vertebrate CNS with its enormous numbers of distinct neuronal cell types and extensive local and long-range connections (Nieuwenhuys 2002) is beyond the scope of this brief review. However, concentrating on sensory organ evolution in this paragraph will provide a basic insight into general features of gene network evolutions that sort out just two distinct cell types, hair cells and sensory neurons. Evolution of both sensory neurons and hair cells out of the single neurosensory cells of metazoan ancestors (Fig. 2) is a molecularly reasonably understood case of cellular diversification; it follows the predicted multiplication of bHLH genes (Gyoja 2014) combined with changes in cell fate determination (Fritzsch et al. 2010; Pan et al. 2012). Two genes have been associated with neuron and hair cell development through loss of function experiments: respectively, Neurog1 and Atoh1 (Fritzsch et al. 2010). The loss of hair cells in mutants of Neurog1 (Ma et al. 2000) prompted the idea of a lineage relationship between neuronal and hair cell precursors. This idea of a lineage relationship of sensory neurons and hair cells has been accepted among evolutionary biologists (Patthey et al. 2014) but controversies exist among developmental biologists regarding the details of the lineage relationships among all neurosensory cells of the ear (Fritzsch et al. 2006; Raft et al. 2007). Thus, while the severe loss of hair cells as a consequence of neuronal loss (Matei et al. 2005) has been experimentally verified to be due to lineage relationships for the vestibular neurosensory cells of the ear, the relationship of neurons to hair cells is not yet experimentally clarified for the mammalian cochlea (Raft et al. 2007).
Recent data have complicated the picture of molecular interactions to sort different cell fate in lineage-related cells further (Fig. 3) by showing that several other bHLH genes also play a role in ear development (Kruger et al. 2006), in part acting redundantly to other bHLH genes. For example, the bHLH gene Neurod1 is expressed in both neurons and hair cells and seems to suppress Atoh1 expression entirely (neurons) or limit its expression (hair cells; Fig. 3). As a consequence of loss of Neurod1, Atoh1 is more profoundly expressed in neurons and converts neurons into hair cells (Jahan et al. 2010). In contrast to the astonishing fate reversal in neurons to hair cells in the absence of Neurod1, the effect of loss of Neurod1 on hair cells is more subtle and results in alteration of hair cell types but not in hair cell conversion. In the absence of Neurod1, inner hair cell-like cells that express inner hair cell molecular signature genes such as Fgf8, appear among outer hair cells (Jahan et al. 2013).
These data suggest a more complex interaction of various bHLH genes (Fig. 3) to define the fate of neurosensory precursors (Forrest et al. 2014; Jahan et al. 2013). Most pertinent for this effect of Neurod1 is the undisclosed interaction of multiple bHLH genes, including the Hes and Hey genes that are expressed following Delta/Notch upregulation (Raft and Groves 2014). Essentially, the level and number of co-expressed bHLH genes will determine the future fate of a given cell (Fig. 3) in conjunction with the level of expression of other genes that maintain proliferative neurosensory precursors such as Sox2 (Dabdoub et al. 2008). Evolution of Group A bHLH genes in diploblasts and their multiplication in bilaterians (Gyoja 2014) may have generated the molecular basis to evolve the different cell types found in the vertebrate ear that form a complex network of related transcription factors engaged in a stepwise transformation of proliferating neurosensory precursors into the two distinct neurosensory types of the ear, the neurons and hair cells.
Evolving a dedicated precursor population to increase a localized ectodermal transformation
While it is possible that the evolutionary origin of the neurosensory cells of the ear predates the evolution of the vertebrate ear out of placodes (Fritzsch and Straka 2014), the molecular evolution of placodes can now be partially traced in chordates (Schlosser et al. 2014). Chief among ectodermal patterning genes identifying the otic placode are genes also known experimentally to be essential for proper regulation of bHLH gene expression. For example, Eya1/Six1 affects ear development in a dose-dependent fashion (Zou et al. 2008) and regulates neurosensory gene expression through binding to the enhancer elements of bHLH genes (Ahmed et al. 2012a, b). One of the earliest markers of the otic placode, Pax2/8, belongs to an ancient network of selector genes (Fortunato et al. 2014). Pax2/8 is essential for neurosensory development of the ear that is severely disrupted in Pax2/8 double null mutants (Bouchard et al. 2010). Another factor known for gene expression regulation in placodal precursors, Gata3 (Schlosser et al. 2014), is essential for ear neurosensory development, in particular of the newly evolved (Fritzsch et al. 2013) mammalian organ of Corti (Duncan and Fritzsch 2013). Many other factors have been identified as being early markers of the otic placode and have been tested for their significance through genetic manipulation but the basic problem is this: multiple genes need to be coordinately expressed in the placodal region to ensure the transformation of ectoderm into neurosensory cells of the ear.
Combined, these data suggest a scenario that progressively transforms the capacity of ectoderm to develop neurosensory cells through the assembly of a network of neurosensory gene regulating transcription factors (Chen and Streit 2013). This network regulates the localized expression of neurosensory development-mediating Sox and bHLH genes, apparently starting with the Sox2 and Neurog1 as the earliest expressed Sox and bHLH gene in the developing mammalian ear (Ma et al. 1998, 2000; Mak et al. 2009; Puligilla et al. 2010). Several important transcription factors of ear placode development are part of an ancient network predating sensory organ evolution (Bouchard et al. 2010; Fortunato et al. 2014) and are associated with choanocytes, the potential ancestor of hair cells (Fritzsch and Straka 2014). Hair cells can induce surrounding cells to form vesicles and this ability may have been at the basis of ear formation. For example, hair cells induced in Neurod1 mutants in the developing ganglion form mini-vesicles that organize other cells of the ganglion to develop a continuous epithelium (Jahan et al. 2010, 2013) and hair cells can in vitro organize vesicles around them (Koehler et al. 2013). In a way, the otic placode can be viewed as an embryonic adaptation that aggregates sensory cell precursors into a single region through the localized Sox and bHLH expression driven by multiple ancient transcription factors (Fortunato et al. 2014) that in turn are regulated by Fgfs (Chen and Streit 2013; Fritzsch et al. 2006). Understanding the evolution of the otic placode to generate an ear vesicle will require unraveling the molecular basis of the ability of hair cells to induce vesicle formation and its heterochronic shift from hair cells to placodal cells in vertebrates.
Switching gears: the importance of multiple bHLH genes for smooth transitions of fate
Ectodermal transformation to form either single sensory cells, as in insects, or multiple sensory cells and neurons, as in vertebrates, ultimately requires the expression of Sox and bHLH genes to change the fate of ectodermal cells into neurosensory cells (Imayoshi and Kageyama 2014; Reiprich and Wegner 2014). While this general function in particular of bHLH genes has long been established through experimental induction of neurons after bHLH gene mRNA injection into developing Xenopus (Lee et al. 1995), further analysis has shown a puzzling co-expression of several bHLH genes in the developing ear (Jahan et al. 2010), not all of which result in loss of a specific cell type in mutants. The expression of these multiple bHLH genes to achieve transformation of ectodermal cells into neurosensory cells follows an increasingly sophisticated patterning process of the ectoderm (Schlosser et al. 2014; Streit et al. 2013) that readies these cells to respond with differentiation to the upregulation of bHLH genes as a final step to consolidate this decision-making process. Work over the last few years has transformed the simple one gene–one cell type idea generated by early knockout studies that eliminated in Atoh1 null mice all hair cells (Bermingham et al. 1999) and in Neurog1 null mice all neurons (Ma et al. 1998) into a more complicated perspective of an interactive gene regulatory network (Rue and Garcia-Ojalvo 2013). In particular, work on Neurog1 and Neurod1 mutants suggests a sophisticated cross-regulation of multiple bHLH transcription factors (Jahan et al. 2010, 2013; Ma et al. 2000). Understanding these interactions requires a quantitative assessment of binding to the various enhancer regions through interactions with the ubiquitous E-proteins (Forrest et al. 2014), as well as maintaining a proliferative precursor status through interactions with the Sox and Id proteins (Fig. 3). This complicated intracellular gene network is apparently accompanied by an equally sophisticated intercellular network of Delta/Notch interactions that replaces the past simple lateral inhibition model (Sprinzak et al. 2011).
While this complexity of bHLH gene expression has long been noticed, it is now becoming clear that this expression is more than noise generated by stochastic gene expression (Johnston and Desplan 2014; Stergachis et al. 2013). More specifically, it appears that the co-expression of several bHLH genes allows for coordinated transition of cellular states toward diversification from a single precursor (Fig. 3), as has been described as a general principle of neuronal differentiation through coordinated expression level variation (Imayoshi and Kageyama 2014; Roybon et al. 2009). The differential interaction of bHLH genes also results in the differential down-regulation of Sox genes (Bylund et al. 2003), possibly enhanced through positively regulating miRs that set the stage for normal hair cell differentiation (Kersigo et al. 2011).
Reversing decisions: the molecular basis of stability and flexibility of the cellular decision making process in the ear
Consistent with the insight that cell fate decision making is a process and not a single step is the reversal of such a decision at various stages of commitment (Fig. 3). Such decision reversals are particularly interesting to evaluate experimentally the level of fixation of such decision-making processes at an individual cellular level. For example, the hypothesis of lineage relationship of neurons, hair cells and supporting cells (Fritzsch et al. 2006; Ma et al. 2000) implied that either or both can be converted up to a certain level of differentiation into the other neurosensory cell type of the ear. Such evidence was ultimately provided by showing that simple elimination of Neurod1 can convert differentiating neurons into hair cells (Jahan et al. 2010). Likewise, the distinction between supporting cells and hair cells can be reversed at even later stages of development through misexpression of Atoh1 in supporting cells (Cox et al. 2014; Liu et al. 2014). In contrast, a recent attempt to transform differentiating hair cells into neurons through misexpression of Neurog1 did not result in any transdifferentiation (Jahan et al. 2012). However, this negative result could relate, among many things, to the inability of Neurog1 to bind to the Atoh1 enhancer to increase the expression enough to be meaningful for any differentiation.
Other data on ear development already indicate rather dramatic differences in cell fate decision making prior to bHLH gene expression, likely regulated by Sox genes (Reiprich and Wegner 2014). On the one extreme are hair cell precursors that are already committed prior to Atoh1 expression, perhaps through Sox2 (Kiernan et al. 2005), possibly at the time they exit the cell cycle (Kopecky et al. 2013), to differentiate as hair cells so that they cannot be reverted into a different fate even if powerful neurogenic factors are expressed (Jahan et al. 2012). On the other hand are neurons and supporting cells that even in a late stage in their decision-making process can be transdifferentiated into hair cells (Jahan et al. 2010; Mizutari et al. 2013) . Simply speaking, all otocyst-derived cells have the potential, possibly as a default state, to differentiate into hair cells if provided with Atoh1, even at a late stage. In contrast, future hair cells are already committed to hair cell fate prior to Atoh1 expression under the guidance of yet to be specified transcription factor(s). This distinction between cell fate commitment, as supported by cell cycle exit and cell fate execution, as shown by Atoh1 expression, is particularly obvious in the apex of the cochlea. In the apex, a delay of several days exists between cell cycle exit and onset of Atoh1 expression (Fritzsch et al. 2005; Jahan et al. 2013; Matei et al. 2005).
When exactly such fate reversals are irreversible in the ear neurosensory precursors remains to be experimentally evaluated. If properly understood, it has the potential to reverse existing decisions and convert any cell type in the organ of Corti into hair cells, as recently suggested (Mizutari et al. 2013). It is important for this fate reversal to understand the molecular basis of such decision making to enhance the frequency of the outcome and the stability of the induced transdifferentiation. In this context, level of expression of differentiation-inducing bHLH genes, as mediated by early transcription factors in the otic placode (Ahmed et al. 2012a, b), ensures quantitative correct expression of interacting transcription factors (Fig. 3) to differentiate the right type of cell at the right place (Jahan et al. 2013). Translation of this transdifferentiation ability into the clinic will require a more detailed understanding to ensure the reconstitution of a functional organ of Corti, possibly the best strategy to restore some hearing in profoundly deaf people (Zine et al. 2014).
Uncoupling specification from morphogenic transformation: the case of Mauthner cells specification and exo-gastrulae gene expression
Diploblasts have limited aggregation of neurons in combination with an epithelial nerve net (Satterlie 2011) but may have sophisticated sensory organs rivaling in complexity those of vertebrates with a much more complex nervous system (Fritzsch and Straka 2014). It is possible that the bilaterian ancestor had a partially aggregated nervous system like that of Xenoturbella (Raikova et al. 2000) or acorn worms (Bullock 1965) with little to no metameric organization. It appears less likely that a metameric organization around a well-developed CNS of ancient metazoans devolved to form the partially centralized epithelial nerve nets found in diploblasts (Matus et al. 2007b; Satterlie 2011) and the simple CNS found in some basal bilaterians (Brown et al. 2008; Fritzsch and Glover 2006; Harzsch and Muller 2007; Raikova et al. 2000). A non-metamerically organized nervous system with no specialized sensory organs seems to be the most parsimonious assumption for diploblasts and, by logical extension, bilaterian ancestors (Bourlat et al. 2006; Budd 2001; Satoh 2008; Swalla and Xavier-Neto 2008). The different patterns of the nervous system of extant deuterostomes are considered here to be independently derived from such bilaterian ancestors, reflecting either independent formation of a CNS (acorn worms, cephalochordates, urochordates, vertebrates) or show a transformation into a pentameric nerve net (echinoderms). If correct, this hypothesis implies that CNS formation comes about by aggregating epidermal nerve cells of diploblasts into a brain, possibly paralleling the condensation of distributed epithelial sensory cells into sensory organs. A byproduct of this hypothesis of condensation of the CNS and sensory organs would be the de novo appearance of embryonic adaptations (Arendt et al. 2004; Fritzsch et al. 2007; Northcutt and Gans 1983; Sauka-Spengler and Bronner-Fraser 2008) to lead to brain and sensory organ formation such as neural plate, the placodes and the neural crest (Schlosser et al. 2014; Steventon et al. 2014), all of which are embryonic adaptations with poorly-defined cellular and molecular precursors in deuterostomes (Fritzsch and Northcutt 1993; Sauka-Spengler and Bronner-Fraser 2008).
Among deuterostomes, only vertebrates achieved not only the aggregation of all neurons into a CNS but in addition aggregated nearly all peripheral sensory cells into discrete sensory organs and developed a unique set of innervations via neural crest-derived sensory neurons (Fritzsch and Northcutt 1993; Gans and Northcutt 1983; Sauka-Spengler and Bronner-Fraser 2008) and placode-derived sensory neurons (Chen and Streit 2013; Fritzsch et al. 2007). The developmental mechanism to achieve this aggregation of sensory cells in vertebrates is the formation of special embryonic tissue, the neural crest and neurogenic placodes (Begbie et al. 1999; Northcutt and Gans 1983; Streit 2007). While much work has concentrated on the specific gene networks that promote embryonic formation of placodes or neural crest (O’Neill et al. 2012; Ohyama et al. 2007; Streit 2007), much less work has been dedicated toward the molecular mechanism of suppression of neuronal fate determination in the remaining ectoderm. It is now clear that BMP upregulation, combined with limited to no expression of Fgfs and FgfRs will maintain a non-neuronal fate in the ectoderm of chordates (Bertrand et al. 2003; Delaune et al. 2005; Fritzsch et al. 2006) but not in hemichordates (Lowe et al. 2006). That the ectoderm default state of chordates is indeed neurogenic has been demonstrated by overexpressing proneuronal bHLH genes in the ectoderm, revealing a transformation into neurons (Lee et al. 1995; Ma et al. 1996) that even holds for bHLH genes not associated with neuronal development such as bHLH genes isolated from sponges (Richards et al. 2008). It is important to realize that this transformation is limited to ectoderm and does not expand to mesoderm, indicating perhaps the unique ability of ectoderm to respond to pro-neuronal bHLH genes with differentiation as much as mesoderm can respond to the bHLH gene MyoD.
While neuronal patterning, including the formation of placodes, correlates with the inductive interactions of the underling mesoderm, it needs to be stressed that patterning of the neural plate precedes and can be partially independent of mesoderm induction, possibly reflecting ancient patterning mechanisms predating bilaterians. For example, Hox genes and Krox20 expression develops in exogastrulae in which the ectoderm is not in contact with mesoderm (Doniach et al. 1992) and patterning of the neural plate in chicken predates gastrulation (Streit et al. 2000). Even more difficult to reconcile with the ‘traditional’ vertical induction model through endomesoderm involution is the fact that the Mauthner cells, a pair of giant neurons in rhombomere 4 of many aquatic vertebrates, exit the cell cycle at Nieuwkoop and Faber (NF) stage 12, during gastrulation and prior to neurulation in frogs (Lamborghini 1980). Moreover, explants of the future hindbrain during neurulation may generate Mauthner cells (Stefanelli 1950), suggesting that cells are specified at the level of the embryonic equivalent of the ancestral adult ‘skin brain’ found in acorn worms. Logically, Mauthner cells develop normally when all mitosis is arrested after they have become postmitotic (Harris and Hartenstein 1991). Giant fibers, such as Mauthner cell fibers, are known in acorn worms where they form a fast response system also known for many other non-vertebrates (Bullock 1965) and such giant fibers already exist in diploblasts (Satterlie 2011). Moreover, Mauthner cells are in rhombomere 4 that also gives rise to the facial branchial motoneurons, possibly the most conserved motoneurons of the brainstem (Dufour et al. 2006; Elliott et al. 2013).
These developmental experimental data strongly support the notion that ectodermal patterning, including specification of neurons, predates the development and evolution of the deuterostome CNS. It is possible that such giant cells and their fast motor output to induce escape responses were already specified in an epithelial neuronal network of ‘skin brains’ to induce escape through the stimulation by rudimentary sensory organs. Clearly, identifying the molecular basis of this pre-neuronal plate patterning event that may predate mesoderm/ectoderm interaction mediated by gastrulation could shed light on how much ectodermal patterning existed in animals without gastrulation. After all, Sox2 expression increases prior to gastrulation (Yanai et al. 2011) and is already highly expressed in neuroectoderm of NF stage 11 embryos (Sasai et al. 2008). Such early expression in areas of future neuronal development is consistent with the idea developed in mutant mice that Sox2 is an early marker for neuronal fate determination. The alternative, that such data reflect novel developmental reorganizations of amphibians and chickens without any evolutionary significance is possible, but would conflict with significant evidence for an hourglass model of vertebrate development and evolution (Akhshabi et al. 2013; Stergachis et al. 2013), most likely also relevant for vertebrate hindbrain development and evolution.
Concentrating and involuting the nervous system: molecular parallelisms to placodes
While the previous section explored the possible importance of ectodermal patterning events prior to gastrulation, most metazoans gastrulate and use this process of vertical interaction of the involuting endo- or mesoderm to pattern the ectoderm. Patterning the body and the nervous system (Paulin 2014) resulted in regional concentrations of neurons in the nerve net out of patterning the ectoderm of diploblastic ancestors (Meinhardt 2004, 2013). These ‘skin brains’ have ectoderm that consists in many neurons between simple skin cells (Fritzsch and Glover 2006; Pani et al. 2012) with molecular patterning gene expression broadly comparable to the vertebrate brain (Fig. 4). This seems to indicate that the vertebrate brain is essentially a transformed epidermis of the diploblastic ancestor, as predicted in mathematical models (Meinhardt 2013). Simply speaking, molecular evolution of cell type specifying mechanisms evolved in single-celled organisms. However, such mechanisms were through further evolution of cell–cell interactions integrated into regional specific cell type development. In addition, general topological information derived from patterns set up by the diffusion of morphogens was apparently translated into local neurosensory cell type specification. Importantly, the molecular basis of specific neurosensory cell types (Fritzsch and Straka 2014) and the molecular basis allowing the local specialization evolved prior to the evolution of brains in skin-brains. Morphogenetic and inductive events may have led to the formation of CNS in bilaterians through the transformation of an already patterned epidermal nerve cell network into a network with localized specialization, possibly multiple times (Meinhardt 2004; Northcutt 2012). A ‘deep molecular homology’ has been recognized in the development of various organs (Shubin et al. 2009). In the CNS, this may come about through an ancestral patterning network that evolved prior to sensory organ and CNS evolution. Topological information later evolved to guide convergent evolution (Stern 2013) of rather different brains and sensory organs in different phyla that may nevertheless share multiple conserved genes guiding cellular differentiation. A case in point is the different use of hedgehog/sonic hedgehog in flies and vertebrates: whereas Hh of flies is needed to define segment boundaries via short range signaling (Stern 2013), Shh is used in vertebrates to specify dorso-ventral patterning of the CNS via long-range signaling (Echelard, et al. 1993; Ingham and McMahon 2001) instead of adding to the formation of somites (Dias et al. 2014; Newman 2014). Somites are treated by some as indicative of arthropod ‘segments’ despite the fact that in many vertebrates these tissue blocks are at different locations on the left and the right side of the body (Fritzsch and Northcutt 1993).
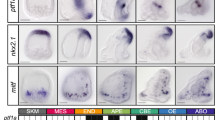
The evolution of gene expression at the midbrain–hindbrain boundary (MHB) is shown for deuterostomes. The MHB of vertebrates shows abutting Otx2 and Gbx2 expression (d–g). This stabilizes the expression of Fgf8 (g), which in turn stabilizes the expression of Wnt1 and engrailed (En1). Mutation of Otx2, Gbx2, Fgf8, or Wnt1 eliminates the MHB. Pax2/5/8 are also expressed at the MHB, whereas the expression of Dmbx occurs immediately rostral to the MHB in the midbrain to later expand into the hindbrain and spinal cord (d). Note the partial overlap of Pax2/5/8 with the caudal expression of Otx2 and the rostral expression of Gbx2 (d). Hemichordates (a) have overlapping expression of Gbx, Otx, Irx and En in the rostral trunk. Pax6 abuts Gbx2 whereas Pax2/5/8 overlaps with the caudal expression of Gbx2. Outgroup data suggest that coelenterates have a Dmbx ortholog, thus raising the possibility that hemichordates (a) also have a Dmbx gene. Cephalochordates (b) have no Dmbx expression in the ‘brain’. Otx abuts with Gbx, like in vertebrates. However, Gbx overlaps with Pax2/5/8 and most of Irx3. Urochordates (c) have no Gbx gene but have a Pax2/5/8 and Pax6 configuration comparable to vertebrates. Dmbx overlaps with the caudal end of the Irx3 expression whereas Dmbx expression is rostral to Irx3 in vertebrates. Together, these data show that certain gene expression domains are topographically conserved (Foxg1, Hox, Otx), whereas others show varying degrees of overlap. It is conceivable that the evolution of nested expression domains of transcription factors is causally related to the evolution of specific neuronal features such as the evolution of oculomotor and trochlear motoneurons (d, e) around the MHB. Experimental work has demonstrated that the development of these motor centers depends on the formation of the MHB. (Adopted from Beccari et al. 2013; Fritzsch and Glover 2006; Pani et al. 2012)
Consistent with this theoretical consideration outlined in the previous paragraph are data (Lowe 2008; Lowe et al. 2003, 2006; Pani et al. 2012) demonstrating that many patterning genes, previously considered to be associated with CNS neuromeres of craniates, are expressed in the skin nerve plexus of acorn worms and play a distinct role in developmental patterning (Fig. 4). The basic organization of repetitive neuronal elements as well as the evolution of neuromeric boundaries can be molecularly dissected as discrete steps in deuterostome and chordate evolution that progresses from a more basic, non-neuromeric organization (Lowe et al. 2003) to a fully developed craniate organization with a clear association with compartmental boundaries (Beccari et al. 2013; Fritzsch and Glover 2006; Murakami et al. 2005). This is most obvious in the hindbrain organization of lampreys, vertebrates that have a different organization of neural elements with respect to rhombomere boundaries (Fritzsch 1998; Murakami et al. 2004, 2005). These overall similarities in expression domains and neuronal organization among bilaterians have long been understood and seemed to indicate a possible homology at the molecular and structural level (Arendt 2005; Arendt et al. 2008; Reichert 2009; Reichert and Simeone 1999). More recent data indicate that the limited molecular toolbox may have biased our understanding and may have falsely identified molecular homology among anatomically analogous organs (Lowe et al. 2006; Newman and Bhat 2009). Clearly, basic protostomes and many basic bilaterians have no metamerically organized ‘nerves’ radiating out in a specific pattern from the epithelial nerve plexus (Bullock 1965; Fritzsch and Glover 2006; Harzsch and Muller 2007). Indeed, the asymmetric but metameric organization of nerves in cephalochordates and basic chordates (Fritzsch and Northcutt 1993) may reflect a peripheral nerve reorganization as much as the molecular basis of boundary formation in the CNS reflects a step-wise evolution of neuromeric boundaries and their underlying molecular basis (Murakami et al. 2005). Rhombomeres of vertebrates, by some considered to be equivalent to arthropod segments because of their comparable Hox-code, may reflect different anatomic solutions to compartment formation while maintaining ectodermal pre-patterning and associated genes in an independently evolved CNS. It should be stressed that through most vertebrate evolution, the majority of neurons of the CNS were concentrated in the spinal cord and this proportion only changed in a few vertebrate radiations with a dramatic increase in brain size, notably in mammals (Nieuwenhuys et al. 1998).
Consistent with this interpretation are the molecularly well-known early steps in CNS formation that reflect the transformation of ectoderm into neuroectoderm. A key player across bilaterians is the Dpp/BMP pathway (Reichert 2009). However, in addition to downregulation of BMP, chordates require the action of Fgfs (Bertrand et al. 2003; Fritzsch et al. 2006; Fritzsch and Glover 2006) to induce neuroectoderm. Fgfs play no role in neuronal specification in arthropods (Urbach and Technau 2004) and hemichordates (Lowe et al. 2006). It remains unclear if this dependence on or absence of Fgfs is linked to the apparent dorso-ventral patterning difference in nervous system induction that has been so long a source of general body plan reversal ideas (Arendt 2005; Arendt et al. 2008; Lowe et al. 2006) but has been questioned by other lines of evidence (Brown et al. 2008; Satoh 2008). In the absence of understanding the full complement of all genes involved in these processes and their interactions, it appears most parsimonious to assume that the known differences are likely to indicate a non-homologous origin of a CNS based on partially homologous transcription factor actions (Newman and Bhat 2009) out of an epidermal nerve plexus, as initially proposed for the acorn worm (Lowe et al. 2003, 2006). Other known similarities, such as the use of different and similar sets of bHLH genes, the ubiquitous use of the Delta-Notch system and certain molecular aspects of proliferation regulation (Arendt 2005; Reichert 2009; Urbach and Technau 2004), could reflect their similar functions in the common diploblast ancestor (Magie et al. 2007; Martindale et al. 2004; Matus et al. 2007a, b; Mazza et al. 2007; Putnam et al. 2007; Seipel et al. 2004; Yamada et al. 2007). What needs to be clarified now is how the aggregation of a nerve net of the diploblast ancestor, most likely also present in the common bilaterian ancestor (Fig. 1), has been tied into the emerging and different general embryonic patterning mechanisms (Meinhardt 2004; Newman and Bhat 2009; Salazar-Ciudad et al. 2003) to elicit a local upregulation of specific neural inducers to form a dorsal or ventral CNS in deuterostomes and protostomes, respectively.
Conclusion
Understanding the evolution of the generalized developmental cell fate specification through topographically restricted gene expression cascades to initiate and regulate cellular differentiation of neurosensory cells out of ectoderm is a key step in sensory organ or CNS evolution. Cellular fate switching evolved as an essential step in early metazoan evolution to transform an assembly of identical cells into a progressively different set of up to over 200 identifiable cell types found in mammals. To transform the cellular evolution into organ differentiation, molecular mechanisms evolved that regulate proliferation of precursor populations in integration with cell fate commitment. Within this process of establishing cellular diversity, Sox and bHLH genes played a major role to consolidate cell fate commitment into cellular differentiation, possibly as an extension of the temporal aggregation of single-celled organisms for vegetative and sexual reproduction. Sox and bHLH genes are essential regulators of neuronal and neurosensory induction and differentiation in the ectoderm and are embedded in an increasingly complex decision-making process that ensures temporal- and intensity-specific expression of interacting intracellular and intercellular networks of transcription factors to drive topologic cell type- and subtype-specific differentiation. Understanding the nodes of such networks will allow inducing transdifferentiation in vivo, once gene expression regulation through manipulation of enhancers is understood.
References
Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX (2012a) Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell 22:377–390
Ahmed M, Xu J, Xu PX (2012b) EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development 139:1965–1977
Ait Benkhali J, Coppin E, Brun S, Peraza-Reyes L, Martin T, Dixelius C, Lazar N, van Tilbeurgh H, Debuchy R (2013) A network of HMG-box transcription factors regulates sexual cycle in the fungus Podospora anserina. PLoS Genet 9:e1003642
Akhshabi S, Sarda S, Dovrolis C, Yi S (2013) An explanatory evo-devo model for the developmental hourglass. arXiv preprint arXiv:13094722
Albert B, Colleran C, Leger-Silvestre I, Berger AB, Dez C, Normand C, Perez-Fernandez J, McStay B, Gadal O (2013) Structure-function analysis of Hmo1 unveils an ancestral organization of HMG-Box factors involved in ribosomal DNA transcription from yeast to human. Nucleic Acids Res 41:10135–10149
Arendt D (2005) Genes and homology in nervous system evolution: comparing gene functions, expression patterns, and cell type molecular fingerprints. Theory Biosci 124:185–197
Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J (2004) Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 306:869–871
Arendt D, Denes AS, Jekely G, Tessmar-Raible K (2008) The evolution of nervous system centralization. Philos Trans R Soc Lond B 363:1523–1528
Arendt D, Hausen H, Purschke G (2009) The ‘division of labour’ model of eye evolution. Philos Trans R Soc Lond B 364:2809–2817
Beccari L, Marco-Ferreres R, Bovolenta P (2013) The logic of gene regulatory networks in early vertebrate forebrain patterning. Mech Dev 130:95–111
Begbie J, Brunet JF, Rubenstein JL, Graham A (1999) Induction of the epibranchial placodes. Development 126:895–902
Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY (1999) Math1: an essential gene for the generation of inner ear hair cells. Science 284:1837–1841
Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY (2001) Proprioceptor pathway development is dependent on Math1. Neuron 30:411–422
Bertrand V, Hudson C, Caillol D, Popovici C, Lemaire P (2003) Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell 115:615–627
Bouchard M, de Caprona D, Busslinger M, Xu P, Fritzsch B (2010) Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev Biol 10:89
Bourlat SJ, Juliusdottir T, Lowe CJ, Freeman R, Aronowicz J, Kirschner M, Lander ES, Thorndyke M, Nakano H, Kohn AB, Heyland A, Moroz LL, Copley RR, Telford MJ (2006) Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature 444:85–88
Brown FD, Prendergast A, Swalla BJ (2008) Man is but a worm: chordate origins. Genesis 46:605–613
Budd GE (2001) Why are arthropods segmented? Evol Dev 3:332–342
Bullock T (1965) The nervous system of hemichordates. In: Bullock T, Horridge G (eds) Structure and function in the nervous systems of invertebrates. Freeman, San Francisco, pp 1567–1157
Bylund M, Andersson E, Novitch BG, Muhr J (2003) Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci 6:1162–1168
Campo-Paysaa F, Semon M, Cameron RA, Peterson KJ, Schubert M (2011) microRNA complements in deuterostomes: origin and evolution of microRNAs. Evol Dev 13:15–27
Chattwood A, Nagayama K, Bolourani P, Harkin L, Kamjoo M, Weeks G, Thompson CR (2013) Developmental lineage priming in Dictyostelium by heterogeneous Ras activation. eLife 2:e01067
Chen J, Streit A (2013) Induction of the inner ear: stepwise specification of otic fate from multipotent progenitors. Hear Res 297:3–12
Chen J, Dai F, Balakrishnan-Renuka A, Leese F, Schempp W, Schaller F, Hoffmann MM, Morosan-Puopolo G, Yusuf F, Bisschoff IJ, Chankiewitz V, Xue J, Chen J, Ying K, Brand-Saberi B (2011) Diversification and molecular evolution of ATOH8, a gene encoding a bHLH transcription factor. PLoS ONE 6:e23005
Cheng LC, Pastrana E, Tavazoie M, Doetsch F (2009) miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci 12:399–408
Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen DH, Chalasani K, Steigelman KA, Fang J, Cheng AG, Zuo J (2014) Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 141:816–829
Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW (2008) Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A 105:18396–18401
Davidson EH (2010) The regulatory genome: gene regulatory networks in development and evolution. Academic, Amsterdam
Dayel MJ, Alegado RA, Fairclough SR, Levin TC, Nichols SA, McDonald K, King N (2011) Cell differentiation and morphogenesis in the colony-forming choanoflagellate Salpingoeca rosetta. Dev Biol 357:73–82
Degnan BM, Vervoort M, Larroux C, Richards GS (2009) Early evolution of metazoan transcription factors. Curr Opin Genet Dev 19:591–599
Dekkers MP, Barde YA (2013) Developmental biology. Programmed cell death in neuronal development. Science 340:39–41
Delaune E, Lemaire P, Kodjabachian L (2005) Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development 132:299–310
Dias AS, de Almeida I, Belmonte JM, Glazier JA, Stern CD (2014) Somites without a clock. Science 343:791–795
Doniach T, Phillips CR, Gerhart JC (1992) Planar induction of anteroposterior pattern in the developing central nervous system of Xenopus laevis. Science 257:542–545
Dufour HD, Chettouh Z, Deyts C, de Rosa R, Goridis C, Joly J-S, Brunet J-F (2006) Precraniate origin of cranial motoneurons. Proc Natl Acad Sci USA 103:8727–8732
Duncan JS, Fritzsch B (2013) Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS ONE 8:e62046
Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP (1993) Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75:1417–1430
Elliott KL, Houston DW, Fritzsch B (2013) Transplantation of Xenopus laevis tissues to determine the ability of motor neurons to acquire a novel target. PLoS ONE 8:e55541
Fairclough SR, Chen Z, Kramer E, Zeng Q, Young S, Robertson HM, Begovic E, Richter DJ, Russ C, Westbrook MJ, Manning G, Lang BF, Haas B, Nusbaum C, King N (2013) Premetazoan genome evolution and the regulation of cell differentiation in the choanoflagellate Salpingoeca rosetta. Genome Biol 14:R15
Forrest MP, Hill MJ, Quantock AJ, Martin-Rendon E, Blake DJ (2014) The emerging roles of TCF4 in disease and development. Trends Mol Med 20: 322-331
Fortunato SA, Leininger S, Adamska M (2014) Evolution of the Pax-Six-Eya-Dach network: the calcisponge case study. EvoDevo 5:23
Fritzsch B (1998) Of mice and genes: evolution of vertebrate brain development. Brain Behav Evol 52:207–217
Fritzsch B, Glover JC (2006) Evolution of the deuterostome central nervous system: an intercalation of developmental patterning processes with cellular specification processes. In: Kaas JH (ed) Evolution of the Nervous System, vol 2. Academic, Oxford, pp 1–24
Fritzsch B, Northcutt RG (1993) Cranial and spinal nerve organization in amphioxus and lampreys: evidence for an ancestral craniate pattern. Acta Anat (Basel) 148:96–109
Fritzsch B, Straka H (2014) Evolution of vertebrate mechanosensory hair cells and inner ears: toward identifying stimuli that select mutation driven altered morphologies. J Comp Physiol A 200:5–18
Fritzsch B, Tessarollo L, Coppola E, Reichardt LF (2004) Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res 146:265–278
Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY (2005) Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn 233:570–583
Fritzsch B, Beisel KW, Hansen LA (2006) The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? Bioessays 28:1181–1193
Fritzsch B, Beisel KW, Pauley S, Soukup G (2007) Molecular evolution of the vertebrate mechanosensory cell and ear. Int J Dev Biol 51:663–678
Fritzsch B, Eberl DF, Beisel KW (2010) The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cell Mol Life Sci 67:3089–3099
Fritzsch B, Pan N, Jahan I, Duncan JS, Kopecky BJ, Elliott KL, Kersigo J, Yang T (2013) Evolution and development of the tetrapod auditory system: an organ of Corti-centric perspective. Evol Dev 15:63–79
Gans C, Northcutt RG (1983) Neural Crest and the Origin of Vertebrates: A New Head. Science 220:268–273
Gokoffski KK, Wu HH, Beites CL, Kim J, Kim EJ, Matzuk MM, Johnson JE, Lander AD, Calof AL (2011) Activin and GDF11 collaborate in feedback control of neuroepithelial stem cell proliferation and fate. Development 138:4131–4142
Gordan R, Shen N, Dror I, Zhou T, Horton J, Rohs R, Bulyk ML (2013) Genomic regions flanking E-box binding sites influence DNA binding specificity of bHLH transcription factors through DNA shape. Cell Rep 3:1093–1104
Guth SI, Wegner M (2008) Having it both ways: Sox protein function between conservation and innovation. Cell Mol Life Sci 65:3000–3018
Gyoja F (2014) A genome-wide survey of bHLH transcription factors in the Placozoan Trichoplax adhaerens reveals the ancient repertoire of this gene family in metazoan. Gene 542:29–37
Hallbook F, Wilson K, Thorndyke M, Olinski RP (2006) Formation and evolution of the chordate neurotrophin and Trk receptor genes. Brain Behav Evol 68:133–144
Harris WA, Hartenstein V (1991) Neuronal determination without cell division in Xenopus embryos. Neuron 6:499–515
Harzsch S, Muller CH (2007) A new look at the ventral nerve centre of Sagitta: implications for the phylogenetic position of Chaetognatha (arrow worms) and the evolution of the bilaterian nervous system. Front Zool 4:14
Herculano-Houzel S (2010) Coordinated scaling of cortical and cerebellar numbers of neurons. Front Neuroanat 4
Herculano-Houzel S (2012) The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci USA 109:10661–10668
Ikeda M, Fujiwara S, Mitsuda N, Ohme-Takagi M (2012) A triantagonistic basic helix-loop-helix system regulates cell elongation in Arabidopsis. Plant Cell 24:4483–4497
Imayoshi I, Kageyama R (2014) bHLH factors in self-renewal, multipotency, and fate choice of neural progenitor cells. Neuron 82:9–23
Ingham PW, McMahon AP (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15:3059–3087
Jahan I, Pan N, Kersigo J, Fritzsch B (2010) Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS ONE 5:e11661
Jahan I, Pan N, Kersigo J, Calisto LE, Morris KA, Kopecky B, Duncan JS, Beisel KW, Fritzsch B (2012) Expression of Neurog1 instead of Atoh1 can partially rescue organ of Corti cell survival. PLoS ONE 7:e30853
Jahan I, Pan N, Kersigo J, Fritzsch B (2013) Beyond generalized hair cells: molecular cues for hair cell types. Hear Res 297:30–41
Johnston RJ Jr, Desplan C (2014) Interchromosomal communication coordinates intrinsically stochastic expression between alleles. Science 343:661–665
Kersigo J, D’Angelo A, Gray BD, Soukup GA, Fritzsch B (2011) The role of sensory organs and the forebrain for the development of the craniofacial shape as revealed by Foxg1-cre-mediated microRNA loss. Genesis 49:326–341
Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS (2005) Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434:1031–1035
King N (2004) The unicellular ancestry of animal development. Dev Cell 7:313–325
Koehler KR, Mikosz AM, Molosh AI, Patel D, Hashino E (2013) Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500:217–221
Kopecky BJ, Jahan I, Fritzsch B (2013) Correct timing of proliferation and differentiation is necessary for normal inner ear development and auditory hair cell viability. Dev Dyn 242:132–147
Kruger M, Schmid T, Kruger S, Bober E, Braun T (2006) Functional redundancy of NSCL-1 and NeuroD during development of the petrosal and vestibulocochlear ganglia. Eur J Neurosci 24:1581–1590
Lai H, Meredith D, Johnson E (2013) bHLH Factors in Neurogenesis and Neuronal Subtype Specification. Patterning and Cell Type Specification in the Developing CNS and PNS. Compr Dev Neurosci 1:333
Lamb TD (2013) Evolution of phototransduction, vertebrate photoreceptors and retina. Prog Retin Eye Res 36:52–119
Lamborghini JE (1980) Rohon–beard cells and other large neurons in Xenopus embryos originate during gastrulation. J Comp Neurol 189:323–333
Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H (1995) Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 268:836–844
Levin TC, Greaney AJ, Wetzel L, King N, Sánchez Alvarado A (2014) The rosetteless gene controls development in the choanoflagellate S. rosetta. eLife 3
Lindahl M, Danilova T, Palm E, Lindholm P, Voikar V, Hakonen E, Ustinov J, Andressoo JO, Harvey BK, Otonkoski T, Rossi J, Saarma M (2014) MANF is indispensable for the proliferation and survival of pancreatic beta cells. Cell Rep 7:366–375
Lindholm P, Saarma M (2010) Novel CDNF/MANF family of neurotrophic factors. Dev Neurobiol 70:360–371
Lindstrom R, Lindholm P, Kallijarvi J, Yu LY, Piepponen TP, Arumae U, Saarma M, Heino TI (2013) Characterization of the structural and functional determinants of MANF/CDNF in Drosophila in vivo model. PLoS ONE 8:e73928
Liu Z, Fang J, Dearman J, Zhang L, Zuo J (2014) In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic atoh1 expression. PLoS ONE 9:e89377
Lowe CJ (2008) Molecular genetic insights into deuterostome evolution from the direct-developing hemichordate Saccoglossus kowalevskii. Philos Trans R Soc Lond B 363:1569–1578
Lowe CJ, Wu M, Salic A, Evans L, Lander E, Stange-Thomann N, Gruber CE, Gerhart J, Kirschner M (2003) Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell 113:853–865
Lowe CJ, Terasaki M, Wu M, Freeman RM Jr, Runft L, Kwan K, Haigo S, Aronowicz J, Lander E, Gruber C, Smith M, Kirschner M, Gerhart J (2006) Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol 4:e291
Ma Q, Kintner C, Anderson DJ (1996) Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87:43–52
Ma Q, Chen Z, del Barco BI, de la Pompa JL, Anderson DJ (1998) neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 20:469–482
Ma Q, Anderson DJ, Fritzsch B (2000) Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol 1:129–143
Magie CR, Daly M, Martindale MQ (2007) Gastrulation in the cnidarian Nematostella vectensis occurs via invagination not ingression. Dev Biol 305:483–497
Mak AC, Szeto IY, Fritzsch B, Cheah KS (2009) Differential and overlapping expression pattern of SOX2 and SOX9 in inner ear development. Gene Expr Patterns 9:444–453
Mao Y, Reiprich S, Wegner M, Fritzsch B (2014) Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PLoS ONE 9:e94580
Martin SG, Arkowitz RA (2014) Cell polarization in budding and fission yeasts. FEMS Microbiol Rev 38:228-253
Martindale MQ (2013) Evolution of development: the details are in the entrails. Curr Biol 23:R25–R28
Martindale MQ, Lee PN (2013) The development of form: Causes and consequences of developmental reprogramming associated with rapid body plan evolution in the bilaterian radiation. Biol Theory 8:253–264
Martindale MQ, Pang K, Finnerty JR (2004) Investigating the origins of triploblasty: ‘mesodermal’ gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa). Development 131:2463–2474
Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B (2005) Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn 234:633–650
Matkovic T, Siebert M, Knoche E, Depner H, Mertel S, Owald D, Schmidt M, Thomas U, Sickmann A, Kamin D (2013) The Bruchpilot cytomatrix determines the size of the readily releasable pool of synaptic vesicles. J Cell Biol 202:667–683
Matus DQ, Pang K, Daly M, Martindale MQ (2007a) Expression of Pax gene family members in the anthozoan cnidarian, Nematostella vectensis. Evol Dev 9:25–38
Matus DQ, Thomsen GH, Martindale MQ (2007b) FGF signaling in gastrulation and neural development in Nematostella vectensis, an anthozoan cnidarian. Dev Genes Evol 217:137–148
Mazza ME, Pang K, Martindale MQ, Finnerty JR (2007) Genomic organization, gene structure, and developmental expression of three clustered otx genes in the sea anemone Nematostella vectensis. J Exp Zool B 308:494–506
Meinhardt H (2004) Different strategies for midline formation in bilaterians. Nat Rev Neurosci 5:502–510
Meinhardt H (2013) From Hydra to Vertebrates: Models for the Transition from Radial-to Bilateral-Symmetric Body Plans. Pattern Formation in Morphogenesis. Springer, Berlin, pp 207–224
Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS (2013) Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77:58–69
Murakami Y, Pasqualetti M, Takio Y, Hirano S, Rijli FM, Kuratani S (2004) Segmental development of reticulospinal and branchiomotor neurons in lamprey: insights into the evolution of the vertebrate hindbrain. Development 131:983–995
Murakami Y, Uchida K, Rijli FM, Kuratani S (2005) Evolution of the brain developmental plan: Insights from agnathans. Dev Biol 280:249–259
Nakano Y, Jahan I, Bonde G, Sun X, Hildebrand MS, Engelhardt JF, Smith RJ, Cornell RA, Fritzsch B, Bánfi B (2012) A mutation in the Srrm4 gene causes alternative splicing defects and deafness in the Bronx waltzer mouse. PLoS Genet 8:e1002966
Neriec N, Desplan C (2014) Different ways to make neurons: parallel evolution in the SoxB family. Genome Biol 15:116
Newman SA (2014) Form and function remixed: developmental physiology in the evolution of vertebrate body plans. J Physiol 592:2403–2412
Newman SA, Bhat R (2009) Dynamical patterning modules: a “pattern language” for development and evolution of multicellular form. Int J Dev Biol 53:693–705
Nieuwenhuys R (2002) Deuterostome brains: synopsis and commentary. Brain Res Bull 57:257–270
Nieuwenhuys R, Donkelaar HJ, Nicholson C, Smeets W, Wicht H (1998) The central nervous system of vertebrates. Springer, Berlin
Northcutt RG (2012) Evolution of centralized nervous systems: two schools of evolutionary thought. Proc Natl Acad Sci USA 109:10626–10633
Northcutt RG, Gans C (1983) The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev Biol 58:1–28
Nosenko T, Schreiber F, Adamska M, Adamski M, Eitel M, Hammel J, Maldonado M, Muller WE, Nickel M, Schierwater B, Vacelet J, Wiens M, Worheide G (2013) Deep metazoan phylogeny: when different genes tell different stories. Mol Phylogenet Evol 67:223–233
Ohyama T, Groves AK, Martin K (2007) The first steps towards hearing: mechanisms of otic placode induction. Int J Dev Biol 51:463–472
O’Neill P, Mak S-S, Fritzsch B, Ladher RK, Baker CV (2012) The amniote paratympanic organ develops from a previously undiscovered sensory placode. Nat Commun 3:1041
Oppenheim R (1991) Cell death during development of the nervous system. Annu Rev Neurosci 14:453–501
Osigus HJ, Eitel M, Schierwater B (2013) Chasing the urmetazoon: striking a blow for quality data? Mol Phylogenet Evol 66:551–557
Pan N, Jahan I, Lee JE, Fritzsch B (2009) Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg(Atoh1-cre) recombination and granule cell requirements for Neurod1 for differentiation. Cell Tissue Res 337:407–428
Pan N, Kopecky B, Jahan I, Fritzsch B (2012) Understanding the evolution and development of neurosensory transcription factors of the ear to enhance therapeutic translation. Cell Tissue Res 349:415–432
Pani AM, Mullarkey EE, Aronowicz J, Assimacopoulos S, Grove EA, Lowe CJ (2012) Ancient deuterostome origins of vertebrate brain signalling centres. Nature 483:289–294
Patthey C, Schlosser G, Shimeld SM (2014) The evolutionary history of vertebrate cranial placodes - I: Cell type evolution. Dev Biol 389:82-97
Paulin MG (2014) Pattern Formation and Animal Morphogenesis. Springer Handbook of Bio-/Neuroinformatics. Springer, Berlin, pp 73–92
Peterson KJ, Dietrich MR, McPeek MA (2009) MicroRNAs and metazoan macroevolution: insights into canalization, complexity, and the Cambrian explosion. Bioessays 31:736–747
Peterson KJ, Su YH, Arnone MI, Swalla B, King BL (2013) MicroRNAs support the monophyly of enteropneust hemichordates. J Exp Zool B 320:368–374
Pierce ML, Weston MD, Fritzsch B, Gabel HW, Ruvkun G, Soukup GA (2008) MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evol Dev 10:106–113
Puelles L, Harrison M, Paxinos G, Watson C (2013) A developmental ontology for the mammalian brain based on the prosomeric model. Trends Neurosci 36:570–578
Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW (2010) Sox2 induces neuronal formation in the developing mammalian cochlea. J Neurosci 30:714–722
Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS (2007) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317:86–94
Raft S, Groves AK (2014) Segregating neural and mechanosensory fates in the developing ear: patterning, signaling, and transcriptional control. Cell Tissue Res (in press)
Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK (2007) Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development 134:4405–4415
Raikova OI, Reuter M, Jondelius U, Gustafsson MKS (2000) An immunocytochemical and utrastructural study of the nervous and muscular system of Xenoturbella westbladi (Bilateria inc. sed.). Zoomorphology 120:107–118
Raj B, Irimia M, Braunschweig U, Sterne-Weiler T, O’Hanlon D, Lin Z-Y, Chen GI, Easton LE, Ule J, Gingras A-C (2014) A Global Regulatory Mechanism for Activating an Exon Network Required for Neurogenesis. Mol Cell 56:90-103
Reichert H (2009) Evolutionary conservation of mechanisms for neural regionalization, proliferation and interconnection in brain development. Biol Lett 5:112–116
Reichert H, Simeone A (1999) Conserved usage of gap and homeotic genes in patterning the CNS. Curr Opin Neurobiol 9:589–595
Reiprich S, Wegner M (2014) From CNS stem cells to neurons and glia: Sox for everyone. Cell Tissue Res (in press)
Richards GS, Simionato E, Perron M, Adamska M, Vervoort M, Degnan BM (2008) Sponge genes provide new insight into the evolutionary origin of the neurogenic circuit. Curr Biol 18:1156–1161
Rosengauer E, Hartwich H, Hartmann AM, Rudnicki A, Satheesh SV, Avraham KB, Nothwang HG (2012) Egr2: cre mediated conditional ablation of dicer disrupts histogenesis of mammalian central auditory nuclei. PLoS ONE 7:e49503
Roybon L, Hjalt T, Stott S, Guillemot F, Li JY, Brundin P (2009) Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS ONE 4:e4779
Rue P, Garcia-Ojalvo J (2013) Modeling gene expression in time and space. Annu Rev Biophys 42:605–627
Ryan JF (2014) Did the ctenophore nervous system evolve independently? Zoology 117:225–226
Ryan JF, Pang K, Schnitzler CE, Nguyen A-D, Moreland RT, Simmons DK, Koch BJ, Francis WR, Havlak P, Smith SA (2013) The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science 342:1242592
Salazar-Ciudad I, Jernvall J, Newman SA (2003) Mechanisms of pattern formation in development and evolution. Development 130:2027–2037
Sasai N, Yakura R, Kamiya D, Nakazawa Y, Sasai Y (2008) Ectodermal factor restricts mesoderm differentiation by inhibiting p53. Cell 133:878–890
Satoh N (2008) An aboral-dorsalization hypothesis for chordate origin. Genesis 46:614–622
Satterlie RA (2011) Do jellyfish have central nervous systems? J Exp Biol 214:1215–1223
Sauka-Spengler T, Bronner-Fraser M (2008) Evolution of the neural crest viewed from a gene regulatory perspective. Genesis 46:673–682
Schlosser G, Patthey C, Shimeld SM (2014) The evolutionary history of vertebrate cranial placodes II. Evolution of ectodermal patterning. Dev Biol 389:98-119
Schnitzler CE, Simmons DK, Pang K, Martindale MQ, Baxevanis AD (2014) Expression of multiple Sox genes through embryonic development in the ctenophore Mnemiopsis leidyi is spatially restricted to zones of cell proliferation. EvoDevo 5:15
Sebé-Pedrós A, Irimia M, Del Campo J, Parra-Acero H, Russ C, Nusbaum C, Blencowe BJ, Ruiz-Trillo I (2013) Regulated aggregative multicellularity in a close unicellular relative of metazoa. eLife 2:e01287
Seipel K, Yanze N, Schmid V (2004) Developmental and evolutionary aspects of the basic helix-loop-helix transcription factors Atonal-like 1 and Achaete-scute homolog 2 in the jellyfish. Dev Biol 269:331–345
Shubin N, Tabin C, Carroll S (2009) Deep homology and the origins of evolutionary novelty. Nature 457:818–823
Silos-Santiago I, Fagan AM, Garber M, Fritzsch B, Barbacid M (1997) Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors. Eur J Neurosci 9:2045–2056
Simionato E, Ledent V, Richards G, Thomas-Chollier M, Kerner P, Coornaert D, Degnan BM, Vervoort M (2007) Origin and diversification of the basic helix-loop-helix gene family in metazoans: insights from comparative genomics. BMC Evol Biol 7:33
Soukup GA, Fritzsch B, Pierce ML, Weston MD, Jahan I, McManus MT, Harfe BD (2009) Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice. Dev Biol 328:328–341
Sprinzak D, Lakhanpal A, LeBon L, Garcia-Ojalvo J, Elowitz MB (2011) Mutual inactivation of Notch receptors and ligands facilitates developmental patterning. PLoS Comput Biol 7:e1002069
Srinivasan S, Hu JS, Currle DS, Fung ES, Hayes WB, Lander AD, Monuki ES (2014) A BMP-FGF Morphogen Toggle Switch Drives the Ultrasensitive Expression of Multiple Genes in the Developing Forebrain. PLoS Comput Biol 10:e1003463
Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, Richards GS, Conaco C, Dacre M, Hellsten U (2010) The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 466:720–726
Stefanelli A (1950) Studies on the development of Mauthner’s cell. Genet Neurol 161–165
Stergachis AB, Neph S, Reynolds A, Humbert R, Miller B, Paige SL, Vernot B, Cheng JB, Thurman RE, Sandstrom R, Haugen E, Heimfeld S, Murry CE, Akey JM, Stamatoyannopoulos JA (2013) Developmental fate and cellular maturity encoded in human regulatory DNA landscapes. Cell 154:888–903
Stern DL (2013) The genetic causes of convergent evolution. Nat Rev Genet 14:751–764
Steventon B, Mayor R, Streit A (2014) Neural crest and placode interaction during the development of the cranial sensory system. Dev Biol 389:28-38
Straka H, Fritzsch B, Glover JC (2014) Connecting ears to eye muscles: evolution of a ‘simple’ reflex arc. Brain Behav Evol 83:162–175
Streit A (2007) The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol 51:447–461
Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD (2000) Initiation of neural induction by FGF signalling before gastrulation. Nature 406:74–78
Streit A, Tambalo M, Chen J, Grocott T, Anwar M, Sosinsky A, Stern CD (2013) Experimental approaches for gene regulatory network construction: the chick as a model system. Genesis 51:296–310
Striedter GF (2005) Principles of brain evolution. Sinauer, Sunderland
Südhof TC (2012) The presynaptic active zone. Neuron 75:11–25
Südhof TC, Rizo J (2011) Synaptic vesicle exocytosis. Cold Spring Harbor Perspect Biol 3:a005637
Suga H, Chen Z, de Mendoza A, Sebé-Pedrós A, Brown MW, Kramer E, Carr M, Kerner P, Vervoort M, Sánchez-Pons N (2013) The Capsaspora genome reveals a complex unicellular prehistory of animals. Nat Commun 4:2325
Swalla BJ, Smith AB (2008) Deciphering deuterostome phylogeny: molecular, morphological and palaeontological perspectives. Philos Trans R Soc Lond B 363:1557–1568
Swalla BJ, Xavier-Neto J (2008) Chordate origins and evolution. Genesis 46:575–579
Tamm SL (2014) Cilia and the life of ctenophores. Invertebr Biol 133:1-46
Taylor AR, Gifondorwa DJ, Robinson MB, Strupe JL, Prevette D, Johnson JE, Hempstead B, Oppenheim RW, Milligan CE (2012) Motoneuron programmed cell death in response to proBDNF. Dev Neurobiol 72:699–712
Urbach R, Technau GM (2004) Neuroblast formation and patterning during early brain development in Drosophila. Bioessays 26:739–751
von Bartheld CS, Fritzsch B (2006) Comparative analysis of neurotrophin receptors and ligands in vertebrate neurons: tools for evolutionary stability or changes in neural circuits? Brain Behav Evol 68:157–172
Wagner A (2011) The molecular origins of evolutionary innovations. Trends Genet 27:397–410
Wang VY, Hassan BA, Bellen HJ, Zoghbi HY (2002) Drosophila atonal fully rescues the phenotype of Math1 null mice: new functions evolve in new cellular contexts. Curr Biol 12:1611–1616
Weiler S, Krinner S, Wong AB, Moser T, Pangrsic T (2014) ATP Hydrolysis Is Critically Required for Function of CaV1.3 Channels in Cochlear Inner Hair Cells via Fueling Ca2+ Clearance. J Neurosci 34:6843–6848
Wilhelm BG, Mandad S, Truckenbrodt S, Krohnert K, Schafer C, Rammner B, Koo SJ, Classen GA, Krauss M, Haucke V, Urlaub H, Rizzoli SO (2014) Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344:1023–1028
Wolber LE, Steves CJ, Tsai P-C, Deloukas P, Spector TD, Bell JT, Williams FM (2014) Epigenome-Wide DNA Methylation in Hearing Ability: New Mechanisms for an Old Problem. PLoS ONE 9:e105729
Xia T, Tong J, Rathore SS, Gu X, Dickerson JA (2012) Network motif comparison rationalizes Sec1/Munc18-SNARE regulation mechanism in exocytosis. BMC Syst Biol 6:19
Xia C, Wang YJ, Liang Y, Niu QK, Tan XY, Chu LC, Chen LQ, Zhang XQ, Ye D (2014) The ARID-HMG DNA-binding protein AtHMGB15 is required for pollen tube growth in Arabidopsis thaliana. Plant J Cell Mol Biol 79:741–756
Yamada A, Pang K, Martindale MQ, Tochinai S (2007) Surprisingly complex T-box gene complement in diploblastic metazoans. Evol Dev 9:220–230
Yanai I, Peshkin L, Jorgensen P, Kirschner MW (2011) Mapping gene expression in two Xenopus species: evolutionary constraints and developmental flexibility. Dev Cell 20:483–496
Yoo AS, Crabtree GR (2009) ATP-dependent chromatin remodeling in neural development. Curr Opin Neurobiol 19:120–126
Yoo AS, Staahl BT, Chen L, Crabtree GR (2009) MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 460:642–646
Zine A, Löwenheim H, Fritzsch B (2014) Toward Translating Molecular Ear Development to Generate Hair Cells from Stem Cells. Adult Stem Cells. Springer, New York, pp 111–161
Zou D, Erickson C, Kim EH, Jin D, Fritzsch B, Xu PX (2008) Eya1 gene dosage critically affects the development of sensory epithelia in the mammalian inner ear. Hum Mol Genet 17:3340–3356
Acknowledgments
This work was supported by NIH (P30 DC 010362, R03 DC013655), NASA Base Program and the OVPR, University of Iowa. We thank Drs. S. Reiprich and U. Ernsberger as well as two unknown reviewers for helpful comments and suggestions to improve this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fritzsch, B., Jahan, I., Pan, N. et al. Evolving gene regulatory networks into cellular networks guiding adaptive behavior: an outline how single cells could have evolved into a centralized neurosensory system. Cell Tissue Res 359, 295–313 (2015). https://doi.org/10.1007/s00441-014-2043-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-2043-1