Abstract
Leigh syndrome (LS) is a rare heterogeneous progressive neurodegenerative disorder usually presenting in infancy or early childhood. Clinical presentation is variable and includes psychomotor delay or regression, acute neurological or acidotic episodes, hypotonia, ataxia, spasticity, movement disorders, and corresponding anomalies of the basal ganglia and brain stem on magnetic resonance imaging. To date, 35 genes have been associated with LS, mostly involved in mitochondrial respiratory chain function and encoded in either nuclear or mitochondrial DNA. We used whole-exome sequencing to identify disease-causing variants in four patients with basal ganglia abnormalities and clinical presentations consistent with LS. Compound heterozygote variants in ECHS1, encoding the enzyme enoyl-CoA hydratase were identified. One missense variant (p.Thr180Ala) was common to all four patients and the haplotype surrounding this variant was also shared, suggesting a common ancestor of French-Canadian origin. Rare mutations in ECHS1 as well as in HIBCH, the enzyme downstream in the valine degradation pathway, have been associated with LS or LS-like disorders. A clear clinical overlap is observed between our patients and the reported cases with ECHS1 or HIBCH deficiency. The main clinical features observed in our cohort are T2-hyperintense signal in the globus pallidus and putamen, failure to thrive, developmental delay or regression, and nystagmus. Respiratory chain studies are not strikingly abnormal in our patients: one patient had a mild reduction of complex I and III and another of complex IV. The identification of four additional patients with mutations in ECHS1 highlights the emerging importance of this pathway in LS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leigh syndrome (LS), also known as subacute necrotizing encephalomyelopathy, is a rare progressive neurodegenerative disorder usually presenting in infancy or early childhood (Aulbert et al. 2014; Jellinger and Seitelberger 1970; Shrikhande et al. 2010). Characteristic findings on cerebral magnetic resonance imaging (MRI) include bilateral, symmetric brain lesions that affect the basal ganglia, brain stem and cerebellum (Aulbert et al. 2014; Peters et al. 2014). In addition to these MRI findings, patients present with variable clinical features that frequently include psychomotor delay or regression, hypotonia, ataxia, spasticity, and movement disorders (Peters et al. 2014; Thorburn and Rahman 2003). Atypical or later onset cases have been reported in the literature and are referred to as Leigh-like diseases (McKelvie et al. 2012; Peters et al. 2014). To date, more than 35 genes have been identified as a cause of LS (Ruhoy and Saneto 2014). In most cases, mutations occur in nuclear or mitochondrial DNA genes involved in the assembly or maintenance of the complexes of the mitochondria respiratory chain, or that encode the catalytic subunits themselves. Complex I and IV (cytochrome c oxidase) are frequently affected. However, deficiencies of the pyruvate dehydrogenase complex, ATP synthase subunit 6, SDHA (subunit of complex II), and recent enzymes of the valine degradation pathway have also been shown to cause LS (Ferdinandusse et al. 2013; Peters et al. 2014).
Most of the valine metabolic pathway is mitochondrial, and deficiencies of several of these enzymes are associated with human diseases (Wanders et al. 2012). Among them, deficiency in 3-hydroxyisobutyryl-CoA hydrolase (HIBCH) results in a progressive infantile neurodegenerative phenotype (Ferdinandusse et al. 2013; Loupatty et al. 2007; Reuter et al. 2014) with clinical features similar to LS. More recently, mutations in enoyl-CoA hydratase (ECHS1), the enzyme immediately upstream of HIBCH, have been associated with LS in 13 patients (Haack et al. 2015; Peters et al. 2014; Sakai et al. 2014). ECHS1 catalyzes the reversible hydration of methacrylyl-CoA, a potentially toxic coenzyme A ester, and also catalyzes the hydration of short chain fatty acyl-CoAs in the beta-oxidation pathway (Mitchell et al. 2008). The clinical overlap and the metabolite abnormalities observed in reported patients with HIBCH or ECHS1 deficiency suggest a dysfunction of the valine degradation pathway as a common pathological mechanism (Peters et al. 2014).
The precise diagnosis of genetically heterogeneous diseases, such as LS, remains difficult, and next-generation sequencing technologies have proved to be successful in the identification of disease-causing genes (Bamshad et al. 2011; Boycott et al. 2013). In this paper, we describe the identification of novel mutations in ECHS1 using whole-exome sequencing (WES), in four patients from three families with LS-like disease. A missense mutation was shared by all four of the patients, suggesting a common, likely French-Canadian ancestor. The identification of four additional patients with mutations in ECHS1 highlights the importance of the valine degradation pathway in LS.
Patients and methods
Patients
This study presents findings on four children presenting with neurodegenerative conditions involving the basal ganglia. These four patients were recruited at different times and part of larger cohorts. Patient P1 was part of the Care4Rare Consortium research protocol approved by the Research Ethics boards of the Children’s Hospital of Eastern Ontario. Patients P2, P3 and P4 were part of a Montreal cohort with suspected mitochondrial disease and the research protocol was approved by the Research Ethics Board of CHU-Sainte-Justine. Informed consent was obtained for all participating individuals. DNA was isolated from peripheral blood using standard procedures. The patients were grouped together post-analysis based on shared mutations.
Whole-exome sequencing
Due to clinical and genetic heterogeneity in LS, all four patients were sent for WES with the aim to identify known or novel gene(s) associated with LS. Whole-exome library preparation, exon capture and sequencing were performed at Genome Quebec Innovation Center (Montreal, QC, Canada) as previously described (Fahiminiya et al. 2014). Genomic DNA (3 μg) from each patient was captured using the SureSelect Human 50 Mb All Exon kit v3 (P4), v4 (P2, P3) or v5 (P1) (Agilent Technologies, Santa Clara, CA, USA). Sequencing was performed on an Illumina HiSeq 2000 (San Diego, CA, USA) with paired-end 100-bp reads. A mean coverage of 154X (P1), 131X (P2), 121X (P3), and 63X (P4) was obtained. Read alignment, variant calling, and annotation were done with a pipeline based on BWA, SAMtools, Annovar, and custom annotation scripts. All sequences were aligned to Human genome Hg19. We excluded variants with minor allele frequency greater than 5 % in either the 1000 genomes project (http://browser.1000genomes.org/index.html) or the 6500 NHLBI EVS (http://evs.gs.washington.edu/EVS), and seen in more than 30 samples from our in-house database (containing approximately 2000 samples). The WES data were further filtered to keep protein-damaging variants (nonsense, missense, frameshift, indel and splice variants).
Sanger sequencing
Variants in ECHS1 were confirmed using Sanger sequencing at Genome Quebec Innovation Center (Montreal, QC, Canada). Exons containing variants were amplified by polymerase chain reaction (PCR) and directly sequenced using an Applied Biosystem 3730xl sequencer (Applied Biosystems, Foster City, CA, USA).
Results
Clinical assessment
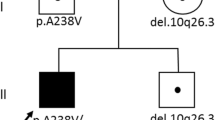
We recruited four affected individuals from three apparently unrelated French-Canadian families with clinical features suggestive of LS. Patients P2 and P3 are siblings. All four affected cases presented with global developmental delay (Table 1). For patients P2, P3 and P4 initial development was considered normal but delay or regression was noted in the first month of life. The first symptoms, hypotonia and failure to thrive, appeared at 2.5 months of age in patient P1 and developmental regression was observed at 4 months. Failure to thrive was noted in all patients, leading to a poor weight gain and subsequent growth retardation. The presence of nystagmus was also observed in all four patients, and three of them (P2, P3 and P4) subsequently developed optic atrophy. Sensorineural deafness was observed in three patients (P2, P3 and P4); hearing impairment was severe in patient P4 requiring hearing aids, but was milder in the other two patients and remained stable over the years. Hypotonia was observed in patient P1 and P4. Microcephaly and dystonia were observed in patients P2 and P4. In the context of viral infections, patient P2 had episodes characterized by ataxia and dystonia involving mainly the lower left limb. These episodes were occurring once or twice a year and were necessitating hospitalization. The ataxia initially receded, whereas the dystonia improved but persisted between episodes. In the last 10 years, these episodes became less frequent and were dominated by fatigability. His brother, P3, also had an episode (7 years of age) characterized by dysarthria, dysmetria and ataxia followed by regression associated with a viral infection. Acute episodes of neurological changes were also observed for patient P4 (from 6 years of age). These episodes were characterized by a loss of contact with environment and lasted 30–60 s and would occur several times a day. There were no tonic/clonic movements. They were no identified provoking factors and no history of regression. The EEG (11 years of age) was normal. The episodes became less frequent with age.
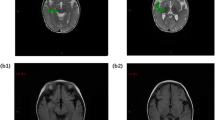
All patients showed abnormalities on brain magnetic resonance imaging (MRI). Patient P1 had an MRI at 6 months of age showing bilaterally symmetric hyperintense T2 and low T1 signals in the medullary pyramids, dorsal brainstem tracts, cerebral peduncles, substantia nigra, putamen, caudate nuclei, globus pallidus, and supratentorial white matter (Fig. 1a–c). The first MRI for patient P2 at 35 months of age showed the presence of hyperintense T2 signals in the globus pallidus bilaterally. A follow-up MRI performed at 5 years and 11 months of age showed a decrease in the volume of the globus pallidi, lacunar lesions in the putamina, and the appearance of new lesions involving the posterior aspect of both putamina and of the caudate nuclei. Most recently, MRI at 11 years of age revealed the presence of T2 hypersignal involving mainly the two putamina and to a lesser extent the globi pallidi and the caudate nuclei. His brother, patient P3, also had multiple MRI scans showing T2 hyperintense signals in the globi pallidi, putamina and caudate nuclei with atrophy of the globi pallidi. Patient P4’s first MRI at 18 months of age showed hyperintense T2-weighted images, with strongest signal in the caudate nuclei, globi pallidi and putamina. A milder signal was also observed in the pons underlying the floor of the fourth ventricle and in the corona radiata, with mild atrophy of the superior vermis. A second MRI at age 12 years showed persistent hyperintensities in T2 and FLAIR particularly in the putamina and the occipital and frontal periventricular white matter with cerebellar atrophy.
T2-weighted MRI images of patient P1. a Dilated ventricles and extensive white matter changes with involvement of dorsal brainstem tracts. b Midbrain signal changes. c Caudate nuclei basically absent and abnormal putamen. d MRS showing a lactate doublet peak (arrow). e Diffusion restricted to affected areas
Biochemical analysis
Plasma lactate levels were elevated initially in patient P1 with associated increase in plasma alanine. Plasma lactate levels were normal in others, except for discrete increases in patients P2 and P3 during acute episodes of motor deterioration (Table 1). Urinary organic acids were normal for patients P2 and P3. For patient P1, urine organic acids measurement performed at 4 months of age revealed mild increase of methylmalonic acid, but was normal at 6 months of age. In P4, the urinary organic acids measurement during an acute decompensation revealed severe ketosis and hyperlactaturia, both of which resolved with intravenous volume replacement and glucose, l-carnitine, thiamine and biotin administration. Repeat urine organic acid analyses were normal. Plasma acylcarnitine profiles were normal in all affected individuals. A lactate doublet on magnetic resonance spectroscopy (MRS) was only observed for patient P1 (Fig. 1d, e). Respiratory chain studies in fibroblasts showed normal activity levels of pyruvate dehydrogenase in patients P2 and P4 but slightly decreased for patient P1. Cytochrome c oxidase (complex IV) and succinate cytochrome c reductase (complexes II and III) activity levels were normal in fibroblasts from all four individuals. For patient P4, a semiquantitative assessment of the respiratory chain complexes in fibroblasts by blue native gel electrophoresis (BN-PAGE) showed a mild reduction of complex IV (Fig. 2). Muscle respiratory chain enzymologies showed a slight reduction in complexes I and III activity for patient P1, which is unlikely to be clinically significant. Muscle assays of respiratory chain complexes revealed normal activity levels for P2, P3 and P4.
Mutation identification
DNA from all four affected individuals was analyzed by WES. Since they were recruited at different times, each family was initially analyzed separately. Given the type of clinical presentation and sib recurrence in one family (Family 2), autosomal recessive inheritance was suspected. For this reason, we searched for rare homozygous or compound heterozygous variants within each family. In family 2, we selected only those candidate variants observed in both affected brothers (P2 and P3). The analysis of exome data for patient P1 uncovered two missense variants in exon 5 of the gene ECHS1 (NM_004092), a gene recently identified as a cause of LS: p.Thr180Ala and p.Gly195Ser. Screening of our in-house database allowed us to identify three additional LS-like patients carrying the p.Thr180Ala variant (Table 1). Re-analysis of the exome data of these four patients by focusing on variants or genes shared by more than one affected individual, allowed the identification of a second variant in the three additional patients. Only ECHS1 was mutated in all four patients (Table Supp1). None of the patients had other compound heterozygous or homozygous variants in nuclear genes associated with mitochondrial diseases including genes previously associated with LS (Chinnery 2000) (online updated version 2014). In addition, we did not identify other samples from our in-house rare disease database (more than 1000 samples) with homozygous or compound heterozygous variants in ECHS1, supporting the likely pathogenicity of these variants. To assess the possibility that the common mutation (p.Thr180Ala) arose from a single event, we looked at genotypes of known SNPs surrounding this shared variant. Polymorphisms were selected if they were reported in dbSNP138, had mapping quality of 60 and coverage higher than 10×. Carriers of this mutation shared a common 7.1 Mb haplotype (rs11245007-rs3737031), suggesting that the p.Thr180Ala variant in patients 1–4 derives from a single ancestral mutation (Table Supp2). This haplotype was not observed in 10 unrelated French-Canadian (20 chromosomes) control samples. In addition to this common variant, siblings P2 and P3 share a missense variant in exon 6 (p.Ala238Val) and P4 carries a missense variant in exon 4 (p.Gln159Arg) (Table 1). The missense variants p.Gly195Ser and p.Gln159Arg were recently reported in patients affected by a mitochondrial encephalopathy with clinical features very similar to our patients (Haack et al. 2015), suggesting the involvement of these variants in the disease. All four ECHS1 variants observed in our patients are extremely rare in control populations. In fact, they are not present in 1000 genomes, the 6500 NHLBI EVS, dbSNP138 or our in-house exome database, with the exception of missense p.Gln159Arg that is reported to be heterozygous in one individual from EVS and a second from our in-house database. Each of these variants are predicted to be pathogenic by at least one-prediction software (Table Supp 3) and observed at a conserved position in vertebrates (Fig. 3d). The missense variants p.Gln159Arg and p.Thr180Ala are not well conserved in C. elegans as arginine and alanine are the observed amino acids. Although valine is required for population growth in C. elegans, high concentration of this essential amino acid is not toxic (Perelman 2000). This difference in metabolism between human and C. elegans suggest that these amino acids changes may result in an accumulation of toxic metabolites in human that are tolerated in C. elegans. It has been previously reported that high damaging variants in human allele can be benign in other species, especially distantly related species such as nematodes, because of compensatory changes in other sites of the same protein or interacting partners (Jordan et al. 2010). All four missense variants are present in the large enoyl-CoA hydratase/isomerase domain, responsible for substrate binding and the catalytic activity of the enzyme, and are predicted to decrease protein stability (MuPro and Auto-Mute). Haack et al. have also reported a decrease ECHS1 protein level and enzyme activity in a patient carrying the p.Gln159Arg mutation, further supporting pathogenicity of the described variants (Haack et al. 2015).
To confirm the WES results, Sanger sequencing of genomic DNA was performed. All four heterozygous variants were confirmed in the corresponding affected patients (Fig. 3a–c). DNA from parents of P2 and P3 was available for segregation analysis and the variants were confirmed to be inherited in trans (Fig. 3a). DNA from the mother of patient P4 was also available and demonstrated that the mother is only carrying the p.T180A variant in a heterozygote state, suggesting an in trans transmission. Unfortunately, parental DNA was not available for patient P1 to assess the segregation of the variants. Since all patients share a common allele and haplotype corresponding to the variant p.Thr180Ala, we can infer that the second variant is likely to be on the other allele, and hence the variants were also transmitted in trans.
Discussion
In this study, we report four patients presenting with clinical features consistent with LS. Because of the high genetic heterogeneity in LS and LS-like disorders, we opted for whole-exome sequencing to uncover the genetic causes of disease in these patients. This allowed the identification of four mutations, including two novel, in the recently identified LS-associated disease gene, ECHS1 (Haack et al. 2015; Peters et al. 2014; Sakai et al. 2014). One particular missense variant (p.Thr180Ala) was observed in all four patients. SNPs analysis demonstrated the presence of a shared haplotype suggesting a common French-Canadian ancestor. To our knowledge, this represents the third ethnic founder effect described in LS, and the second in the French-Canadian population. A founder mutation in the nuclear-encoded gene LRPPRC is most prevalent in French-Canadians who trace their ancestry to the Saguenay-Lac-St-Jean region of Quebec. A mutation in the nuclear-encoded gene SUCLA2 is found in the Faroese population (Ruhoy and Saneto 2014). The missense variant p.Gln159Arg has been observed in one patient from our study as well as in three patients from Haack et al. This variant is the first recurrent mutation describe to date in ECHS1 that is observed in unrelated patients from different ethnic background, suggesting that p.Gln159Arg is a common mutation and more patients carrying this variation will be identified in the future.
ECHS1 is a small protein of 290 amino acids localized in the mitochondrial matrix. The protein is mainly composed of two important domains, a mitochondrial transit peptide (amino acids 1–27) and a large enoyl-CoA hydratase/isomerase domain (amino acids 30–289) (www.rcsb.org). The first domain encodes the signal peptide that targets the protein to the mitochondrial matrix. The enoyl-CoA hydratase/isomerase domain is responsible for the substrate binding and catalytic activity of the enzyme. The missense variants we have identified in our LS patients are located in three consecutive exons (exons 4–6) all part of the enoyl-CoA hydratase/isomerase domain, so it is unlikely that they influence mitochondrial import. In fact, MitoProt predicted similar mitochondrial targeting scores for the wild type (0.996) and mutant (0.995) protein (MitoProt II; http://ihg.gsf.de/ihg/mitoprot.html). Possible impact of the mutations reported here includes dysregulation of enzymatic function by changing the structure of the catalytic or binding pocket, or by destabilizing the protein resulting in degradation.
The patients reported here present with many clinical features associated with LS, including general (failure to thrive), neurologic (hypotonia, dystonia, ataxia, developmental delay, MRI findings), audiologic (sensorineural hearing loss) and ophthalmologic (nystagmus, optic atrophy) features (Ruhoy and Saneto 2014). Our study brings the total number of reported patients with mutations in ECHS1 to 17; four patients from three families reported here and 13 patients from 10 families previously reported (Haack et al. 2015; Peters et al. 2014; Sakai et al. 2014). The common clinical features (Table 1) are onset before 3 years of age, presence of white matter abnormalities (T2-weighted hyperintensities), failure to thrive, hypotonia, and developmental delay or regression. Sensorineural deafness and optic atrophy are observed in three of our cases (P2, P3 and P4) and were reported in three and seven previous ECHS1 patient (Haack et al. 2015; Sakai et al. 2014). The presence of acute episodes of neurological change and/or acidosis was frequently observed in 3 out of 4 patients (P2, P3 and P4). Such acute decompensating episodes have also been described in patient F5 II:3, and was responsible for the death of her brother (Haack et al. 2015). Although a clear clinical overlap is observed among patients with ECHS1 mutations, the metabolic analysis results are variable. Increase in plasma levels is a common feature in reported ECHSI patients (10/13) (Haack et al. 2015; Peters et al. 2014; Sakai et al. 2014). In our patients, plasma lactate was elevated on presentation for patient P1 and a discrete increase was observed for patients P2 and P3 only during acute episodes of motor deterioration. Pyruvate dehydrogenase activity is normal in the majority of the patients described; a decrease was only observed in two patients (Patients 1 and 2 from Peters et al.). Peters et al. has described a specific pattern of urine metabolites (increase methacrylate metabolites and 2-methyl-2,3-dihydroxybutyric), suggesting that the phenotypes associated with ECHS1 or HIBCH deficiency is due to the valine degradation pathway (Peters et al. 2014). Urine organic acids measurement are normal in our patient and in three patients from Haack et al. (F1 II:2, F2 II:1 and F6 II1). The metabolite 2-methyl-2,3-dohydroxybutyric acid) has not been measured in our patients, but was increased in three out of the four patients (F2 II:1, F5 II:3 and F7 II:2) tested by Haack et al. (2015), supporting a defect in the valine degradation pathway.
The mitochondrial respiratory chain does not seem to be severely impacted in ECHS1 patients, at least as assessed in fibroblasts and muscle. Only three reported patients showed a decrease in mitochondrial respiratory chain complexes (Table 1) (Haack et al. 2015; Sakai et al. 2014), patient P1 had a small reduction of complexes I and III and patient P4 had a mild reduction of complex IV on BN-PAGE. A lactate peak on MRS seems to be associated with a more severe outcome it was observed in five reported patients (Haack et al. 2015; Peters et al. 2014) and patient P1. The disease progression in these patients was more severe, leading to a premature death due to respiratory failure. Clinical and biochemical similarities between patients with ECHSI and HIBCH deficiencies have previously been reported (Peters et al. 2014); however, one main difference was the severity of the disease with HIBCH patients surviving beyond 1 year of age. Patients P2, P3 and P4 are more similar to HIBCH patients in that aspect, and are currently living.
ECHS1 catalyzes the reversible hydration of methylacrylyl-CoA to 3-hydroxyisobutyryl-CoA in the mitochondrial matrix. Methylacrylyl-CoA is a potentially toxic intermediate (Mitchell et al. 2008; Wanders et al. 2012) that can react with mitochondrial enzymes containing essential cysteine residues (pyruvate dehydrogenase and respiratory chain enzymes), reducing their activities and causing oxidative damage (Ferdinandusse et al. 2013). Because coenzyme A esters do not cross the mitochondrial membrane, the damage related to the accumulation of methylacrylyl-CoA is expected to be concentrated in mitochondria. Although mitochondria are present in other tissues, the most substantial damage of ECHS1 deficiency is in the brain, as seen by the patient’s severe neurological symptoms and marked changes on cerebral magnetic resonance images. Similar clinical observations, such as basal ganglia abnormalities have been reported in other patients with inborn errors of branched-chain amino acid metabolism (Buhas et al. 2013; Mitchell et al. 2008) consistent with the theory that toxic branched-chain amino acid-related acyl-CoA esters are produced in brain mitochondria and cause local damage there.
In conclusion, a total of 17 patients with impaired valine degradation have been identified to date. The disease severity is variable among the ECHS1 patients. The majority of the previously reported patients present a more severe phenotype. In this group of patients, an increased level of lactate in serum and on magnetic resonance spectrometry, as well as an increase 2-methyl-2,3-dihydroxybutyrate, are common features. However, a considerable number of patients are less severely affected and are still living despite being in their adolescence or adulthood. The diagnosis of these patients is difficult due to the lack of specific clinical features. In fact, reduced ECHS1 protein level and increased 2-methyl-2,3-dihydroxybutyrate seem to correlate with disease severity and might not be detected in less severely affected patients (Table 1; patient F10 II:1) (Haack et al. 2015). The description of four additional patients, among which three are presenting a milder phenotype, will contribute to defining the clinical spectrum and highlights the clinical variability observed in ECHS1 patients. Early diagnosis of ECHS1 deficiency by exome sequencing or targeted panels in a clinical setting will provide an essential tool for optimal genetic counseling and for the design of clinical trials.
References
Aulbert W, Weigt-Usinger K, Thiels C, Kohler C, Vorgerd M, Schreiner A, Hoffjan S, Rothoeft T, Wortmann SB, Heyer CM, Podskarbi T, Lucke T (2014) Long survival in Leigh syndrome: new cases and review of literature. Neuropediatrics 45:346–353. doi:10.1055/s-0034-1383823
Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, Shendure J (2011) Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet 12:745–755. doi:10.1038/nrg3031
Boycott KM, Vanstone MR, Bulman DE, MacKenzie AE (2013) Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat Rev Genet 14:681–691. doi:10.1038/nrg3555
Buhas D, Bernard G, Fukao T, Decarie JC, Chouinard S, Mitchell GA (2013) A treatable new cause of chorea: beta-ketothiolase deficiency. Mov Disord 28:1054–1056. doi:10.1002/mds.25538
Chinnery PF (2000) Mitochondrial disorders overview. In: Pagon RA, Adam MP, Ardinger HH et al (eds) GeneReviews® [Internet]. University of Washington, Seattle, WA, 1993–2015
Fahiminiya S, Majewski J, Al-Jallad H, Moffatt P, Mort J, Glorieux FH, Roschger P, Klaushofer K, Rauch F (2014) Osteoporosis caused by mutations in PLS3: clinical and bone tissue characteristics. J Bone Miner Res 29:1805–1814. doi:10.1002/jbmr.2208
Ferdinandusse S, Waterham HR, Heales SJ, Brown GK, Hargreaves IP, Taanman JW, Gunny R, Abulhoul L, Wanders RJ, Clayton PT, Leonard JV, Rahman S (2013) HIBCH mutations can cause Leigh-like disease with combined deficiency of multiple mitochondrial respiratory chain enzymes and pyruvate dehydrogenase. Orphanet J Rare Dis 8:188. doi:10.1186/1750-1172-8-188
Haack TB, Jackson CB, Murayama K, Kremer LS, Schaller A, Kotzaeridou U, de Vries MC, Schottmann G, Santra S, Buchner B, Wieland T, Graf E, Freisinger P, Eggimann S, Ohtake A, Okazaki Y, Kohda M, Kishita Y, Tokuzawa Y, Sauer S, Memari Y, Kolb-Kokocinski A, Durbin R, Hasselmann O, Cremer K, Albrecht B, Wieczorek D, Engels H, Hahn D, Zink AM, Alston CL, Taylor RW, Rodenburg RJ, Trollmann R, Sperl W, Strom TM, Hoffmann GF, Mayr JA, Meitinger T, Bolognini R, Schuelke M, Nuoffer JM, Kolker S, Prokisch H, Klopstock T (2015) Deficiency of ECHS1 causes mitochondrial encephalopathy with cardiac involvement. Ann Clin Transl Neurol 2:492–509. doi:10.1002/acn3.189
Jellinger K, Seitelberger F (1970) Subacute necrotizing encephalomyelopathy (LEIGH). Ergeb Inn Med Kinderheilkd 29:155–219
Jordan DM, Ramensky VE, Sunyaev SR (2010) Human allelic variation: perspective from protein function, structure, and evolution. Curr Opin Struct Biol 20:342–350. doi:10.1016/j.sbi.2010.03.006
Loupatty FJ, Clayton PT, Ruiter JP, Ofman R, Ijlst L, Brown GK, Thorburn DR, Harris RA, Duran M, Desousa C, Krywawych S, Heales SJ, Wanders RJ (2007) Mutations in the gene encoding 3-hydroxyisobutyryl-CoA hydrolase results in progressive infantile neurodegeneration. Am J Hum Genet 80:195–199. doi:10.1086/510725
McKelvie P, Infeld B, Marotta R, Chin J, Thorburn D, Collins S (2012) Late-adult onset Leigh syndrome. J Clin Neurosci 19:195–202. doi:10.1016/j.jocn.2011.09.009
Mitchell GA, Gauthier N, Lesimple A, Wang SP, Mamer O, Qureshi I (2008) Hereditary and acquired diseases of acyl-coenzyme A metabolism. Mol Genet Metab 94:4–15. doi:10.1016/j.ymgme.2007.12.005
Perelman DLN (2000) Requirements for branched chain amino acids and their interactions in the nematode Caenorhabditis elegans. Nematology 2:6
Peters H, Buck N, Wanders R, Ruiter J, Waterham H, Koster J, Yaplito-Lee J, Ferdinandusse S, Pitt J (2014) ECHS1 mutations in Leigh disease: a new inborn error of metabolism affecting valine metabolism. Brain 137:2903–2908. doi:10.1093/brain/awu216
Reuter MS, Sass JO, Leis T, Kohler J, Mayr JA, Feichtinger RG, Rauh M, Schanze I, Bahr L, Trollmann R, Uebe S, Ekici AB, Reis A (2014) HIBCH deficiency in a patient with phenotypic characteristics of mitochondrial disorders. Am J Med Genet A 164:3162–3169. doi:10.1002/ajmg.a.36766
Ruhoy IS, Saneto RP (2014) The genetics of Leigh syndrome and its implications for clinical practice and risk management. Appl Clin Genet 7:221–234. doi:10.2147/TACG.S46176
Sakai C, Yamaguchi S, Sasaki M, Miyamoto Y, Matsushima Y, Goto YI (2014) ECHS1 mutations cause combined respiratory chain deficiency resulting in Leigh syndrome. Hum Mutat. doi:10.1002/humu.22730
Shrikhande DY, Kalakoti P, Syed MM, Ahya K, Singh G (2010) A rare mitochondrial disorder: Leigh syndrome—a case report. Ital J Pediatr 36:62. doi:10.1186/1824-7288-36-62
Thorburn DR, Rahman S (2003) Mitochondrial DNA-associated Leigh syndrome and NARP. In: Pagon RA, Adam MP, Ardinger HH et al (eds) GeneReviews® [Internet]. University of Washington, Seattle, WA, 1993–2015
Wanders RJ, Duran M, Loupatty FJ (2012) Enzymology of the branched-chain amino acid oxidation disorders: the valine pathway. J Inherit Metab Dis 35:5–12. doi:10.1007/s10545-010-9236-x
Acknowledgments
We would like to thank the families who participated in this study. We would also like to thank Pierre Lepage and his team at the Genome Quebec Innovation Centre for Sanger validation. This work was funded in part by a Grant (MT_15460) from the CIHR to EAS and performed under the Care4Rare Canada Consortium (Enhanced Care for Rare Genetic Diseases in Canada) funded by Genome Canada, the Canadian Institutes of Health Research, the Ontario Genomics Institute, Ontario Research Fund, Genome Quebec, and Children’s Hospital of Eastern Ontario Foundation. The project was selected for analysis by the Care4Rare Consortium Gene Discovery Steering Committee consisting of Kym Boycott (lead; University of Ottawa), Alex MacKenzie (co-lead; University of Ottawa), Jacek Majewski (McGill University), Michael Brudno (University of Toronto), Dennis Bulman (University of Ottawa), David Dyment (University of Ottawa). The authors wish to acknowledge the contribution of the high throughput-sequencing platform of the McGill University and Génome Québec Innovation Centre, Montréal, Canada. MT received a post-doctoral fellowship from the Réseau de Médecine Génique Appliquée (RMGA) and the Fond de Recherche du Québec en Santé. SF received a post-doctoral fellowship from RMGA. URL: MuPro: http://mupro.proteomics.ics.uci.edu, Auto-Mute: http://proteins.gmu.edu.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tetreault, M., Fahiminiya, S., Antonicka, H. et al. Whole-exome sequencing identifies novel ECHS1 mutations in Leigh syndrome. Hum Genet 134, 981–991 (2015). https://doi.org/10.1007/s00439-015-1577-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-015-1577-y