Abstract
For genetic approaches for controlling insect pests such as the sterile insect technique (SIT), it is advantageous to release only males as females are ineffective as control agents and they consume about 50% of the diet. Here we developed tetracycline-repressible Lucilia cuprina transgenic strains in which adult females were fully fertile and viable on a diet that lacked tetracycline and all of their female offspring died at the embryo stage. The transgenic strains are an improvement over the strains we developed previously, which had the disadvantage that adult females on diet without tetracycline were sterile and died prematurely. This was possibly due to the low level expression of the effector gene in ovaries. In the strains developed in this study, the early promoters from L. cuprina nullo or Cochliomyia macellaria CG14427 genes were used to drive the tetracycline transactivator (tTA) expression in the early embryo. In the absence of tetracycline, tTA activates expression of the proapoptotic gene Lshid which contains a female-specific intron. Consequently, only females produce active HID protein and die at the embryo stage. Crossing the tTA-expressing driver lines with an RFPex reporter line confirmed that there was no expression of the effector gene in the ovary. These new embryonic L. cuprina transgenic sexing strains hold great promise for genetic control programs and the system reported here might also be transferable to other major calliphorid livestock pests such as the New World screwworm, Cochliomyia hominivorax.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic manipulation using transposons has been a valuable tool to develop insect strains with practical applications (Handler and Atkinson 2006). Using piggyBac-mediated germline transformation, transgenic sexing strains (TSSs) of the Australian sheep blow fly Lucilia cuprina, which is a major pest of sheep in Australia and New Zealand (Sandeman et al. 2014), were previously developed to achieve female-specific lethality at embryo or early larval stages (Yan and Scott 2015; Yan et al. 2017). TSSs can be potentially used in sterile insect technique (SIT) programs, in which insects are mass reared, sterilized and released to target a pest population (Knipling 1955). For such control programs, females are ineffective as they mate with sterile males that are co-released and possibly lead to unwanted damage in the field for some pests (Knipling 1959; Papathanos et al. 2009). Further, for some species such as the New World screwworm Cochliomyia hominivorax, high doses of radiation must be used to ensure 100% sterilization of released females (Crystal 1979). Field tests with the Mediterranean fruit fly Ceratitis capitata showed that male-only releases are 3–5 times more effective than bi-sex releases (Rendon et al. 2004; Franz 2005). Additionally, eliminating females at early developmental stages would contribute to significant cost saving, as they consume about 50% of the larval diet (Yan and Scott 2015; Concha et al. 2016). Consequently, the aim of this study was to improve early lethal TSSs to enhance control efficiency and reduce the running cost of SIT programs, thus offering great potential for area-wide pest management (Schetelig and Handler 2012b; Scott et al. 2017).
The previous early lethal TSSs of L. cuprina carried a female-specific conditional lethal genetic system (Yan and Scott 2015; Yan et al. 2017). Specifically, a “driver” cassette that contains the tetracycline transactivator (tTA) gene driven by an early active promoter and an “effector” cassette that contains a cell death gene, L. sericata hid (Lshid), driven by tetO21-hsp70 enhancer promoter. Further, the sex-specifically spliced intron from the Cochliomyia hominivorax transformer gene (Chtra) (Li et al. 2013) was inserted immediately downstream of the ATG translation start codon of Lshid. Only the female transcript encoded functional LsHID protein as the male transcripts have in-frame stop codons shortly after the start codon. When the flies containing both driver and effector transgene were raised on standard diet without tetracycline, binding of tTA to the tetO21 enhancer activated Lshid expression and led to female death at an early stage. Adding tetracycline to diet interrupted the binding between tTA and tetO, so no HID protein was produced and both sexes survived. We found that the stage of female lethality was largely decided by the promoter used for tTA regulation, as all females died at the embryo stage with the cellularization gene bottleneck (Lsbnk) promoter (Yan and Scott 2015), whereas all females were eliminated at the second instar stage with the constitutive spitting image (Lsspt) promoter that has lower activity in embryos than the Lsbnk promoter (Yan et al. 2017).
The promoter from the cellularization gene serendipity alpha (sry-α) has been used in a similar way to develop embryonic TSSs in the tephritid fruit flies Anastrepha suspensa and Ceratitis capitata (Schetelig and Handler 2012a; Ogaugwu et al. 2013). In these strains, females were fertile when raised on diet without tetracycline and their female offspring died at the embryo stage. However when the sry-α promoter was used to build TSSs of Anastrepha ludens, a species that is closely related to A. suspensa, females developed nonvitellogenic oocytes and were sterile, presumably due to expression of tTA in ovaries (Schetelig et al. 2016). Similarly, we found that females from L. cuprina embryonic and early larval TSSs produced very few eggs when fed a diet without tetracycline. We confirmed that tTA was expressed in the ovary in the Lsspt and Lsbnk driver lines (Yan and Scott 2015; Yan et al. 2017), which was likely leading to production of LsHID and induction of apoptosis. The sterility of A. ludens and L. cuprina TSSs could be rescued by adding tetracycline to diet in the first few days following eclosion (Yan and Scott 2015; Schetelig et al. 2016; Yan et al. 2017).
Although L. cuprina and A. ludens TSSs could be used in a control program, they would demand a specific tetracycline feeding scheme which can lead to additional costs in antibiotic treatments and labor. Feeding tetracycline to the adult females also brings the risk that the antibiotic will be passed on to their offspring and prevent activation of the tetO-hid gene. Indeed when TSSs of A. ludens were fed tetracycline for 5 days, some females in the next generation survived to the larval and adult stages (Schetelig et al. 2016). Consequently, TSSs that do not need additional antibiotic treatments would be advantageous in terms of cost saving and ensuring females die at the embryo stage when desired. Assembling such TSSs would require a promoter that is only active in the early embryo and not in later stages of development. In D. melanogaster, the nullo cellularization gene and the closely linked CG14427 gene are strongly expressed in 2–4 h embryos but not expressed in later stages (Graveley et al. 2011). Here we isolated and characterized promoters from two cellularization genes, nullo from L. cuprina and CG14427 from the secondary screwworm Cochliomyia macellaria. Multiple L. cuprina tTA driver lines using these two promoters were generated with tTA highly expressed at the embryo stage in all lines. The newly obtained driver lines were further used to assemble TSSs, from which adult females were fully fertile and viable without any antibiotic treatment, and 100% female lethality was achieved at embryo stage. The TSSs with specific embryonic lethality hold great promise for L. cuprina genetic control programs, and the system reported here can also be transferred to other major calliphorid livestock pests including C. hominivorax, the Old World primary screwworm Chrysomya bezziana and the European green blowfly Lucilia sericata.
Methods
Fly rearing and germline transformation
The LA07 wild-type strain of L. cuprina was maintained and piggyBac-mediated germline transformation performed as previously described (Yan and Scott 2015). In brief, adults were kept in mesh cages at 22 °C and fed a sugar/water/protein biscuit diet. Larvae were raised on 93% ground beef at 27 °C and pupae were kept in a 27 °C incubator until eclosion. The transgenic strains were bred to homozygosity by selecting for brightly fluorescing larvae and confirmed by outcrossing to the wild-type strain.
Promoter isolation and plasmid construction
To assemble the driver constructs, Lcnullo and CmCG14427 gene promoter fragments were amplified from genomic DNA using the primers listed in Table S1 and cloned into pGEM-T (Promega, Madison, WI, USA). Following confirmation of the nucleotide sequences, the promoters were amplified by PCR, digested with NotI and NcoI and ligated to pBS-Lsbnk-tTAopt-p10 (Yan and Scott 2015) that had been cut with the same enzymes. The promoter-tTAopt-SV40 cassettes were excised using unique XhoI and NotI sites and cloned into the unique XhoI and PspOMI sites in the piggyBac transformation vectors pB[Lchsp83-ZsGreen] (Concha et al. 2011) to create the driver constructs DR6 (Lcnullo) and DR7 (CmCG14427). To assemble the tetO-RFPex reporter construct, RFPex fragment was amplified from pBAC[H83-RFPex-pA]attP (Li et al. 2014), digested with NcoI and HindIII and then ligated to pBSFL1 (Li et al. 2014) that had been cut with the same enzymes. The tetO-RFPex cassettes were excised and cloned into pB[Lchsp83-ZsGreen]. The genbank accession numbers for the constructs made in this study are MK509802 for DR6 and MK509803 for DR7.
Female lethality test and embryo-specific lethality assessments
To assess female lethality in a double heterozygous condition, eight newly emerged males from one DR6 or DR7 homozygous driver line and eight newly emerged virgin females from either EF3A or EF1#12 homozygous effector line (Yan and Scott 2015) were put in one bottle and kept on tetracycline-free adult diet for days. Then embryos of 24 h egg lay intervals were reared on tetracycline-free raw ground beef and the number of adult males and females were counted. The specific combinations of driver and effector lines tested are shown in Fig. 3.
To make double homozygous (DH) strains, homozygous virgin females from the effector lines (Yan and Scott 2015) were crossed with homozygous males from the driver lines. The double heterozygous offspring were allowed to interbreed and their progeny screened to select only individuals homozygous for both the driver and effector construct by epifluorescence microscopy based on fluorescence intensity. Adult flies in all crossings were maintained on 100 μg/mL tetracycline water to repress female lethality. For testing the rearing efficiency and female lethality, adults from each DH strain were fed with tetracycline-free or 100 μg/mL tetracycline water, and embryos were collected as previously. Then larvae were reared on tetracycline-free or 100 μg/g tetracycline raw 93% ground beef and the number of third instar larvae, pupae, adult males and females was counted. For staged tests, 1000 embryos from the first egg lay of DH strains were collected and reared on tetracycline-free or 100 μg/mL tetracycline 93% ground beef. Then, the number of first instar, third instar, pupae, adult males and females were counted. All lethality tests were done in triplicate. The adult emergence ratio was calculated as [number of adults emerged/number of pupae] × 100.
Longevity and fertility
For each DH strain, 50 pairs of newly emerged flies were put in a bottle and kept on tetracycline-free adult diet for 30 days. Every day, the dead flies were removed from the bottle, and their sex and number were documented. At 5-day intervals starting at day 10, the tetracycline-free 93% ground beef was put in the bottle for 24 h to induce egg laying, and the number of egg clutches was then counted.
Quantitative real-time and reverse transcriptase polymerase chain reaction
To compare relative gene expression at different developmental stages, total RNA was extracted at different time points using the RNeasy® Mini Kit (QIAGEN) according to the manufacturer’s instructions. Isolated RNA was subsequently treated with RNase-Free DNase Set (Qiagen). 5 µg RNA was used to synthesize cDNA using Superscript III First-Strand Synthesis Supermix (Invitrogen) following the manufacturer’s instructions. A control reaction lacking Superscript III was included for each treatment. qRT-PCR was performed using an Applied Biosystems ABI7900HT Fast Real-time PCR system and Maxima SYBR Green/RoxqPCR Master Mix (Fermentas). All qPCR primer sets were tested with standard PCR, run on a gel to confirm amplification of the desired size products and are listed in Table S1. Samples were assayed in triplicate in a 12.5 µL final volume containing 1 µL of cDNA (less than 100 ng), 1× Maxima SYBR Green/RoxqPCR Master Mix (Fermentas), and 0.3 µM each primer and subjected to the following thermal cycling parameters: initial denaturation for 10 min at 95 °C, 40 cycles of (95 °C for 15 s, 57 °C or 60 °C for 30 s, 72 °C for 30 s), dissociation curve analysis at (72 °C for 30 s). Ct values were called and averaged for each triplicate by SDS software (version 2.4). Samples were normalized to LcGST1 (Bagnall and Kotze 2010) and relative expression levels were calculated using the formula 2−∆∆Ct as described previously (Linger et al. 2015). Graphs were generated using Microsoft Excel.
To determine larval sex, reverse transcription PCR was performed using a Lctra primer pair Table S1) and the following thermal cycling parameters: initial denaturation for 3 min at 94 °C, 34 cycles of (94 °C for 30 s, 54 °C for 30 s, 72 °C for 1 min), final extension for 5 min at 72 °C.
Confocal imaging to assess the ovary-specific expression of tTA
Homozygous males from the driver lines were crossed with homozygous virgin females from the tetO-RFPex reporter line. Homozygous tetO-RFPex females were used as a control. Ovaries were dissected from female offspring at 4 and 8 days following eclosion and kept on ice in 1× PBS for no more than 15 min. Ovaries were fixed by submerging in 4% paraformaldehyde (Biotium) for 15 min with gentle shaking, then washed 4 times with 1× PBS. Ovaries were stored in 1× PBS in 0.5 mL tubes at 4 °C until ready to be imaged. Images were captured on a Zeiss LSM 880 confocal microscope using a 40× lens and the Argon and 561 lasers to capture green and red fluorescence, respectively. Additionally, embryos, first instar larvae, third instar larvae, and adults were screened for red fluorescence using a Leica M205 FA Fluorescent Microscope.
Statistical analysis
To investigate possible changes in tTA expression levels over developmental stages, separate linear mixed effects models (in SAS) were considered for the DR6 and DR7 lines. These models included stage as fixed effects and treated line as random. Since residual plots from this model suggested that variability in expression levels was increasing with the mean, these levels were log transformed, resulting in greater homogeneity of variance across stages. Tukey’s method was used for all pairwise comparisons among the three stage means.
For the analysis of longevity, the PROC LIFETEST (SAS) was used to estimate and plot the survivor functions for the combinations of strain and sex and to obtain test statistics to evaluate the hypothesis of their equality. PROC PHREG (SAS) was used to fit the proportional hazards regression models (“frailty” model when they include a random effect). Two non-parametric tests (Wilcoxon and log rank) of equality across the twelve groups agreed in their indication of significantly different empirical survivor functions. Pairwise comparisons of log hazards estimated using the frailty model were compared with a Tukey–Kramer correction for multiplicity to control for a false positive rate at 0.05. The estimated log-hazard ratios were relative to males from the LA07 strain raised on diet with tetracycline.
Results
Gene identification and characterization
Orthologs of the D. melanogaster nullo and CG14427 genes were identified in the assembled L. cuprina genome (Anstead et al. 2015) and a draft genome of the secondary screwworm Cochliomyia macellaria (CJP, unpublished). Interestingly, the genes are closely linked in both species as in D. melanogaster, house fly and olive fly (Fig. S1). The relative expression of Lcbnk, Lcnullo and LcCG14427 RNAs at different developmental stages was analyzed by quantitative RT-PCR (Fig. 1). Cellularization in L. cuprina embryos occurs 2–3 h after egg laying (Yan and Scott 2015) and the RNA levels of all three genes were highest in these embryos among the tested stages. However, the difference in expression between 2 and 3 h embryos and 2 h female adults was greater than an order of magnitude higher for Lcnullo and LcCG14427 (850 and 2000 times) than Lcbnk (56 times). This in part is because RNA levels of Lcnullo and LcCG14427 in 2–3 h embryos were 3.2 and 5.6 times higher than that of Lcbnk, respectively. Further, RNA levels of Lcnullo and LcCG14427 were lower in adult females, particularly the older 7 d stage (31% and 3% of Lcbnk, respectively). These results confirm Lcnullo and LcCG14427 genes are strongly expressed in early zygotic embryos with little expression in later stages. Thus, the promoters from these genes could have the properties desired for building a TSS with early embryonic female lethality.
Generation and analysis of transgenic L. cuprina
Genomic DNA fragments upstream of and including the translation start codons of the Lcnullo and CmCG14427 genes were isolated and used to build the DR6 and DR7 tTAo driver constructs (Fig. 2a). tTAo is a version of tTA codon optimized for L. cuprina (Yan and Scott 2015). The CG14427 promoter was isolated from C. macellaria for potential future use in C. hominivorax, since a genome assembly for C. hominivorax was not available at the time the constructs were built. Seven DR6 lines were obtained by piggyBac-mediated germline transformation. The Mendelian inheritance patterns of the lines suggested all carried a single autosomal transgene. Twelve DR7 transgenic lines were obtained out of which one line showed mosaic fluorescence, one could not be maintained and two lines were X-linked, which often have poor levels of expression in L. cuprina (Yan and Scott 2015) and consequently were discarded. To identify the strongest expressing lines, RNA was isolated from 2 to 3 h embryos from the remaining DR7 lines and all of DR6 lines. The embryos were collected from crossing of heterozygotes and thus could have zero, one or two copies of the tTAo transgene. However, the average transgene copy number in the embryo collections from different lines should be similar, since they were all collected from heterozygous inbreeding crossings. Based on the tTAo expression level (Fig. S2) and fecundity (data not shown), four DR6 lines (#5, #6, #9 and #11) and four DR7 lines (#2, #4b, #7 and #8) were chosen for breeding to homozygosity. All the chosen lines were homozygous viable and fertile except DR7#4b, which was homozygous lethal.
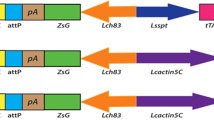
Ovary-specific expression of tTA in the driver lines. a Schematic illustration of the driver and effector constructs. Expression of tTAo is controlled by the Lsbottleneck (Lsbnk) promoter in DR2, the Lsspitting image (Lsspt) promoter in DR3, the Lcnullo promoter in DR6 and the CmCG14427 promoter in DR7. The DsRedexpress2 (RFPex) reporter gene was driven by the tetO21-hsp70 enhancer promoter in the tetO-RFPex construct. All constructs contain a ZsGreen marker driven by the Lchsp83 gene promoter, and contain a phiC31 attP site as well as 5′ and 3′ piggyBac ends. pA: SV40 polyA. b Confocal images of fluorescent ovaries. The images were taken using dissected ovaries from 4-day- (a–c, g–j) and 8-day- (d–f, k–n) old females, which were generated by crossing homozygous males from DR2#6 (a, d), DR3#2 (b, e), DR6#6 (g, k), DR6#11 (h, i), DR7#2 (i, m), DR7#8 (j, n) and tetO-RFPex (c, f, control), with homozygous virgin females from tetO-RFPex. RFPex expression was observed in the early portion of the developing eggs (germarium). The nurse cells showed green fluorescence from the marker
The homozygous viable DR6 and DR7 lines were then analyzed for tTAo expression at embryo cellularization (2–3 h) and in 2 h and 2-day-old adult females. All of the driver lines exhibited significantly higher tTAo expression at the embryo stage than the adult stages (p < 0.01 for DR6 lines and p < 0.01 for DR7 lines), while the difference between the two adult stages was not significant (Fig. S3). However, the difference in tTAo RNA levels between embryos and adults was not as pronounced as for the endogenous Lcnullo and LcCG14427 genes (Fig. 1). In the DR6 and DR7 lines, tTAo RNA was 5–11 and 9–12 times higher in embryos than 2 h adults, respectively (Fig. S3).
Since the DR6 and DR7 lines express significant levels of tTAo RNA in adult females, we next investigated if tTA was present in adult ovaries, as observed previously with the DR2 and DR3 lines, which use the Lsbnk and Lsspt gene promoters to drive tTAo expression respectively (Yan and Scott 2015; Yan et al. 2017). To visualize which cells express tTA, we made a transgenic line that carries the red fluorescent protein gene DsRedexpress2 (RFPex) driven by the tetO21-hsp70 enhancer promoter (Li et al. 2014) (Fig. 2). The tTAo driver lines, DR2#6, DR3#2, DR6#6, DR6#11, DR7#2 and DR7#8 were crossed with the tetO-RFPex reporter line. As expected, red fluorescence was observed in embryos from all six crosses but not in third instar or in the adult whole body. First instar from the crosses with the DR3#2 and both DR6 and DR7 drivers also showed red fluorescence. In contrast, red fluorescence was not observed in first instar from DR2#6 crosses. This could be because tTA RNA levels are higher in embryos from the DR6 and DR7 lines than DR2#6. Upon dissection of adult females, red fluorescence was clearly observed in the germarium region of ovaries from 4- and 8-day-old females from the DR2#6 and DR3#2 crosses (Fig. 2). Under these culture conditions, L. cuprina females lay eggs at 10 days. In contrast, no red fluorescence was observed in the dissected ovaries from females generated by DR6#6, DR6#11, DR7#2 and DR7#8 crosses (Fig. 2). The apparent absence of tTA in ovaries suggests that DR6 and DR7 TSSs adult females may not need to be fed tetracycline to produce eggs, as was needed with the DR2 and DR3 TSSs.
Female-specific lethality of L. cuprina TSSs
DR6 and DR7 homozygous lines were crossed with homozygous effector lines EF1#12 and EF3-A, and the number of adult male and female offspring counted (Fig. 3, Table 1). EF3 contains the wild-type version of proapoptotic gene Lshid under control of the tetO21-Lchsp70 enhancer promoter, whereas EF1 has the more active version that has the two conserved MAPK phosphorylation sites changed to encode alanine (Yan and Scott 2015). Our previous studies showed that EF1#12 is a strong effector line and always achieved 100% dominant lethality when paired with different DR2 lines, while EF3-A was a slightly weaker effector line and gave 96% female lethality when paired with DR2#6 (Yan and Scott 2015). All of the driver lines were effective at reducing female viability, and no female offspring were obtained from any driver/effector combinations (Fig. 3).
Female-specific lethality of double heterozygous lines. Indicated homozygous DR6 (a) and DR7 (b) males were crossed with virgin females from EF3-A or EF1-12 homozygous effector lines. The offspring of the crosses were raised on diet without tetracycline. The number of male and female offspring number from wild-type raised on diet that lacks tetracycline is also shown (b). Error bars show the standard error of the mean (n = 3). Arrow indicates the driver and effector combination for double homozygous (DH) breeding
Since all driver/effector combinations tested were 100% effective (no female offspring) (Fig. 3), any could have been chosen for further inbreeding to produce strains homozygous for both transgenes. We selected driver line DR6#11 as it had the highest embryonic tTAo expression in DR6 homozygous lines (Fig. S3), and paired it with the weaker effector EF3-A to produce the double homozygous (DH) strain DH7. The DR6#6 line, which had lower embryonic tTAo expression (Fig. S3), was paired with the stronger effector EF1#12 to make DH8. For the DR7 drivers, we selected lines #2 and #8, which showed the lowest and highest embryonic tTAo expression, respectively (Fig. S3), to make two additional TSSs. DR7#2 was paired with EF3-A (DH9) and DR7#8 was paired with EF1-12 (DH10). The four DH strains have been stably maintained on tetracycline diet for at least 4 years, confirming the double homozygosity and general fitness of the strains. To test the rearing efficiency, eight pairs of adults from each DH strain were crossed under permissive tetracycline condition, and the number of pupae, adult male and female offspring was counted (Table 1). From the same number of parents, DH7 generated the highest number of adult offspring while DH8 generated the lowest. All DH strains produced an approximate 1:1 ratio of males and females on a diet with tetracycline.
The strains were then tested for female fertility and lethality under a restrictive tetracycline feeding condition, which means tetracycline was only supplied to the parental larvae, but not the parental adults or their offspring. The control wild-type strain produced approximately equal numbers of males and females under these conditions, as also observed when the strain was continuously reared on tetracycline (Fig. 4). For all DH strains, the parental females were fertile unlike DH strains made previously with DR2 and DR3 drivers. Further, no female offspring were obtained under these conditions (Table 1). Notably for DH8 and DH9 offspring, most of pupae emerged as males, suggesting that females died before the pupal stage in these two lines. However, for DH7 and DH10 offspring, approximately half of pupae emerged as males, which suggests some male lethality at the pupal stage. Further, without tetracycline, the DH9 parents produced the highest number of male offspring in both heterozygous and homozygous conditions.
Staged lethality in the wild-type (WT) and double homozygous (DH) strains. WT (a), DH7 (b), DH8 (c), DH9 (d) and DH10 (e) were raised on permissive and restrictive tetracycline conditions. 1000 embryos were collected and the numbers of first instar larvae (L1), L3, pupae, adult males and adult females were recorded. Error bars show the standard error of the mean (n = 3). +W/+M. tetracycline added to adult water and larval diet (meat), −W/−M, adult and larval diet without tetracycline
Females die at the embryo stage if mothers are not fed tetracycline
To identify the stage when females die, the number of flies was scored at all developmental stages except for second instar. Equal numbers of adult males and females were obtained if the strains were raised under the permissive tetracycline condition, while no adult females were obtained from the restrictive tetracycline condition (Fig. 4). For all DH strains, approximately half as many first instar (L1) were obtained from the restrictive tetracycline condition compared to the permissive tetracycline condition. This suggested that the DH females had died in the embryo stage, which was further confirmed using an RT-PCR assay to determine larval sex (Yan and Scott 2015) (Fig. 5), wherein Lctra primers amplify a larger product from male larvae due to the extra male exon (Concha and Scott 2009). At a 1:1 sex ratio, the smaller female PCR product predominates and at a 100:1 male:female sex ratio, the main product is from males but the smaller female product was detectable (Yan and Scott 2015) (Fig. 5, lanes 1, 2). This suggested that if females made up 1% or more of the L1 population, their presence could be identified. Both male and female products were detected in 9–10 h DH8 and DH9 embryos (Fig. 5, lanes 3 and 6). If the mothers were fed a diet with tetracycline, the newly hatched first instar also had both male and female products (Fig. 5, lanes 5 and 8). However, only the male product was detected from L1 samples if mothers were fed a diet without tetracycline (Fig. 5, lanes 4 and 7).
Larval sex identification through detection of Lctra sex-specific splice variants of double homozygous strains DH8 and DH9. RNA was extracted from 100 embryos or L1 larvae and RT-PCR performed using a primer pair that detects the male (636 bp) or female (325 bp) Lctra splice variants. Lane (1) WT♂:♀ = 1; (2) WT♂:♀ = 100; (3) 9–10 h embryos of DH8 without tetracycline; (4) L1 of DH8 without tetracycline; (5) L1 of DH8 with tetracycline; (6) 9–10 h embryos of DH9 without tetracycline; (7) L1 of DH9 without tetracycline; (8) L1 of DH9 with tetracycline; L DNA ladder
Fertility and longevity of L. cuprina TSSs
Expression of tetO-hid in pupae or adults could reduce the longevity of adults. Indeed, for the DH2 strain that contained the Lsbnk-tTA driver, about 80% of females died within 10 days and all females died within 20 days (Yan and Scott 2015). To determine if the new TSSs had higher female survival, we next examined longevity and fertility. As done previously, the DH strains were maintained on 100 μg/mL tetracycline, then tetracycline was added to the larval diet of the offspring but not adult diet. As a control, the parental LA07 strain was maintained on 100 μg/mL tetracycline for a generation, the larval offspring were fed tetracycline but the adults were not. As an additional control, LA07 was raised on standard diet that lacked tetracycline. Mortality and egg production were monitored for 30 days (Fig. 6). With the exception of DH7, the lifespan of DH strain females was significantly shorter than males from the same strain (p < 0.0001) (Table S2). Additionally, the DH7 females continued to lay eggs until 30 days, whereas DH8, DH9 and DH10 females only produced eggs until 20 days. Most of the DH8, DH9 and DH10 females died within 30 days. Nevertheless, the females lived longer than DH2 females, as about half of DH8, DH9 and DH10 females survived to 20 days. For the control strain raised on diet without tetracycline, there was no significant difference in the lifespan of females and males. However, when the control strain was raised under the same tetracycline regimen as the DH strains, females had a significantly shorter lifespan than males. This could in part explain the shorter lifespan of DH8, DH9 and DH10 strain females. However, DH8, DH9 and DH10 females had a significantly shorter lifespan than control LA07 females raised on tetracycline (Table S2). With regard to males, DH7, DH9 and DH10 males had a significantly shorter lifespan than LA07 raised on tetracycline but not LA07 males raised on diet without tetracycline. DH7 males did not live as long as DH8 males, but otherwise there was little difference in longevity of males between the strains.
Longevity and fertility of wild-type (WT) and double homozygous (DH) strains. The WT flies were collected from the wild-type LA07 strain raised without tetracycline (a −Tet), or from LA07 fed with tetracycline (100 μg/mL) for one generation prior to egg collection and fed with tetracycline in the larval diet (b +Tet). The DH pupae were collected from DH7 (c), DH8 (d), DH9 (e) and DH10 (f) that were maintained with tetracycline diet to repress the female lethality. For each DH strain and LA07, 50 pairs of adults were put in one bottle and kept on tetracycline-free diet for 30 days. Each day, dead males and females were counted and removed from the bottle. Error bars show the standard error of the mean (n = 3). On days 10, 15, 20, 25 and 30, ground meat (without tetracycline) was put in the bottle for 24 h and afterwards the number of egg clutches was recorded. “++”indicates more than 10 egg clutches; “+”indicates 2–8 egg clutches; “−”no or very few eggs were laid
Discussion
Promoters from cellularization genes, sry-α and nullo, were first used in a Tet-Off system to achieve embryo lethality in D. melanogaster (Horn and Wimmer 2003). The promoters were selected due to their high but transient activities at the cellular blastoderm stage (Mazumdar and Mazumdar 2002; Graveley et al. 2011). The first experimental identification of such promoters in an agricultural insect pest species was by cDNA subtractions of different embryonic stages of the Medfly Ceratitis capitata (Schetelig et al. 2009). Subsequently, promoters from C. capitata and A. suspensa sry-α genes were successfully applied to develop TSSs for each species (Schetelig and Handler 2012a, b; Ogaugwu et al. 2013). However, TSSs developed for A. ludens that also used the A. suspensa sry-α gene promoter to drive tTA expression were not fertile unless adult females were fed a diet supplemented with tetracycline for several days following eclosion (Schetelig et al. 2016). This suggested that the A. suspensa sry-α gene promoter was active in adult ovaries. Similarly, we found TSSs developed for L. cuprina that used the promoter from the bnk cellularization gene for the driver construct laid few eggs unless adult females were fed a diet with a low dose of tetracycline for a few days after emergence (Yan and Scott 2015). We previously showed by qRT-PCR that this was most likely due to bnk promoter activity in the adult female ovaries (Yan and Scott 2015; Yan et al. 2017). Using a strain that carries a tTA-responsive red fluorescent protein reporter gene, we have confirmed and extended these observations and shown that in the DR2#6 line tTA is produced in the germarium of female ovaries. The DR6 and DR7 drivers, which use promoters from the Lcnullo and CmCG12247 promoters, respectively, did not show any detectable expression in ovaries. This likely explains why TSSs strains assembled using DR6 or DR7 driver lines did not need to be fed a diet supplemented with tetracycline for egg laying. Thus, the sexing strains are an improvement over our previously developed TSSs that required a low dose of tetracycline in the maternal diet for 2 days for egg production.
The endogenous Lcnullo and LcCG14427 genes are expressed at approximately three orders of magnitude higher levels at embryo cellular blastoderm compared to later stages. This appears to be at least in part due to specific activation of the gene promoters in embryos as the DR6 and DR7 lines showed 5–12 times higher tTA expression in embryos compared to 2 h adults. However, it would appear that additional regulatory elements are required to achieve the same high degree of stage specificity as the endogenous genes. One possibility is that the endogenous genes are repressed at later stages by an epigenetic silencing mechanism that spans both genes. The conserved linkage of the genes in Drosophila, blow flies, house fly and olive fly (Fig. S1) suggests that the genes may share distal regulatory elements beyond the promoters used in the DR6 and DR7 constructs. This model could be tested using CRISPR/Cas9 to target a DR6 or DR7 gene construct into the nullo/CG14427 gene region. We have recently used similar technology to make a gene knockin mutation in the L. cuprina no blokes gene (Davis et al. 2018).
We measured the longevity, fecundity and adult emergence ratios of the TSSs on diet that lacked tetracycline. These are some of the fitness parameters that are relevant to mass rearing in a production facility (Concha et al. 2016). While two of the TSSs had an adult emergence ratio similar to the parental wild-type strain (DH8, DH9), the other two TSSs (DH7 and DH10) had a relatively low ratio when raised on diet that lacked tetracycline. We considered two possible explanations for these observations. First, male viability could be reduced because the driver lines used to assemble the DH7 and DH10 showed high levels of expression of tTA. Very high levels of tTA expression are toxic for L. cuprina (Li et al. 2014), which is thought to be due to transcriptional squelching mediated by the VP16 domain of the tTA protein (Gong et al. 2005). However, tTA protein levels would be similar in the DH strains raised on diet with tetracycline but the adult emergence ratio was high for all four DH strains on tetracycline diet. The second possibility we considered is that there is some mis-splicing of the Lshid transcript in males to produce transcripts that encode functional LsHID protein. This could be due to position effects as the local chromatin environment can influence alternative splicing (Allo et al. 2010). However, the same effector lines used in DH7 and DH10 were also used to make DH8 and DH9. Thus, it is unclear why the adult emergence ratio on diet without tetracycline is lower for the DH7 and DH10 strains. With the exception of the DH7 strain, females from the TSSs did not live as long as males from the same strain when raised on diet without tetracycline. This appears to be partly a consequence of raising the TSSs on diet with tetracycline for the generations prior to testing as we found females from the wild-type control strain did not live as long as males when raised under identical conditions. It is unclear why female longevity in the control strain was reduced, but tetracyclines have been reported to induce mitochondrial stress in several model genetic organisms including Drosophila melanogaster (Moullan et al. 2015). Clearly, given that all of the male-only strains have been developed with the Tet-Off system (Gossen and Bujard 1992), further studies are needed to examine the impact (if any) of incorporation of tetracycline in the diet on the fitness of the strains.
The TSSs developed in this study could be used in a future genetic program for control of L. cuprina in Australia or New Zealand where the insect remains a significant pest of sheep. The strains have the advantages that females die at the embryo stage and the parental generation is fertile and viable on diet without tetracycline. Of the four TSSs strains evaluated, DH8 and DH9 appear to be the most promising, as they produce the most males on diet without tetracycline, females were viable and fertile up to 20 days on diet without tetracycline and the adult emergence ratio was comparable to the parental LA07 strain. The genetic systems should be easily transferrable to other blow fly pests such as C. hominivorax. For more distantly related species, promoters from the nullo or CG14427 genes from the species of interest would appear to be ideal for making tTA driver strains.
Data availability
All data are supplied within the main text or as supplementary material.
References
Allo M, Schor IE, Munoz MJ, de la Mata M, Agirre E, Valcarcel J, Eyras E, Kornblihtt AR (2010) Chromatin and alternative splicing. Cold Spring Harb Symp Quant Biol 75:103–111
Anstead CA, Korhonen PK, Young ND, Hall RS, Jex AR, Murali SC, Hughes DS, Lee SF, Perry T, Stroehlein AJ, Ansell BRE, Breugelmans B, Hofmann A, Qu JX, Dugan S, Lee SL, Chao H, Dinh H, Han Y, Doddapaneni HV, Worley KC, Muzny DM, Loannidis P, Waterhouse RM, Zdobnov EM, James PJ, Bagnall NH, Kotze AC, Gibbs RA, Richards S, Batterham P, Gasser RB (2015) Lucilia cuprina genome unlocks parasitic fly biology to underpin future interventions. Nat Commun 6:7344
Bagnall NH, Kotze AC (2010) Evaluation of reference genes for real-time PCR quantification of gene expression in the Australian sheep blowfly, Lucilia cuprina. Med Vet Entomol 24(2):176–181
Concha C, Scott MJ (2009) Sexual development in Lucilia cuprina (Diptera, Calliphoridae) is controlled by the transformer gene. Genetics 182:785–798
Concha C, Belikoff EJ, Carey BL, Li F, Schiemann AH, Scott MJ (2011) Efficient germ-line transformation of the economically important pest species Lucilia cuprina and Lucilia sericata (Diptera, Calliphoridae). Insect Biochem Mol Biol 41:70–75
Concha C, Palavesam A, Sagel A, Li F, Osborne JA, Hernandez Y, Pardo T, Quintero G, Vasquez M, Keller GP, Phillips PL, Welch JB, McMillan WO, Skoda SR, Scott MJ (2016) A transgenic male-only strain of the New World screwworm for an improved control program using the sterile insect technique. BMC Biol 14:72
Crystal MM (1979) Sterilization of screwworm flies (Diptera: Calliphoridae) with gamma rays: restudy after two decades. J Med Entomol 15:103–108
Davis RJ, Belikoff EJ, Scholl EH, Li F, Scott MJ (2018) no blokes is essential for male viability and X chromosome gene expression in the Australian sheep blowfly. Curr Biol 28:1987-1992.e1983
Franz G (2005) Genetic sexing strains in Mediterranean fruit fly, an example for other species amenable to large-scale rearing for the sterile insect technique. Springer, Dordrecht
Gong P, Epton MJ, Fu G, Scaife S, Hiscox A, Condon KC, Condon GC, Morrison NI, Kelly DW, Dafa’alla T, Coleman PG, Alphey L (2005) A dominant lethal genetic system for autocidal control of the Mediterranean fruitfly. Nat Biotechnol 23(4):453–456
Gossen M, Bujard H (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89(12):5547–5551
Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, Brown JB, Cherbas L, Davis CA, Dobin A, Li R, Lin W, Malone JH, Mattiuzzo NR, Miller D, Sturgill D, Tuch BB, Zaleski C, Zhang D, Blanchette M, Dudoit S, Eads B, Green RE, Hammonds A, Jiang L, Kapranov P, Langton L, Perrimon N, Sandler JE, Wan KH, Willingham A, Zhang Y, Zou Y, Andrews J, Bickel PJ, Brenner SE, Brent MR, Cherbas P, Gingeras TR, Hoskins RA, Kaufman TC, Oliver B, Celniker SE (2011) The developmental transcriptome of Drosophila melanogaster. Nature 471(7339):473–479
Handler AM, Atkinson PW (2006) Insect transgenesis: mechanisms, applications, and ecological safety. Biotechnol Genet Eng Rev 23:129–156
Horn C, Wimmer EA (2003) A transgene-based, embryo-specific lethality system for insect pest management. Nat Biotechnol 21(1):64–70
Knipling EF (1955) Possibilities of insect control or eradication through the use of sexually sterile males. J Econ Entomol 48:459–462
Knipling EF (1959) Sterile-male method of population control. Science 130:902–904
Li F, Vensko SPI, Belikoff EJ, Scott MJ (2013) Conservation and sex-specific splicing of the transformer gene in the calliphorids Cochliomyia hominivorax, Cochliomyia macellaria and Lucilia sericata. PLoS One 8:e56303
Li F, Wantuch HA, Linger RJ, Belikoff EJ, Scott MJ (2014) Transgenic sexing system for genetic control of the Australian sheep blow fly Lucilia cuprina. Insect Biochem Mol Biol 51:80–88
Linger RJ, Belikoff EJ, Scott MJ (2015) Dosage compensation of X-linked muller element F genes but not X-linked transgenes in the Australian sheep blowfly. Plos One 10(10):e0141544
Mazumdar A, Mazumdar M (2002) How one becomes many: blastoderm cellularization in Drosophila melanogaster. BioEssays 24(11):1012–1022
Moullan N, Mouchiroud L, Wang X, Ryu D, Williams EG, Mottis A, Jovaisaite V, Frochaux MV, Quiros PM, Deplancke B, Houtkooper RH, Auwerx J (2015) Tetracyclines disturb mitochondrial function across eukaryotic models: a call for caution in biomedical research. Cell Rep 10(10):1681–1691
Ogaugwu CE, Schetelig MF, Wimmer EA (2013) Transgenic sexing system for Ceratitis capitata (Diptera: Tephritidae) based on female-specific embryonic lethality. Insect Biochem Mol Biol 43:1–8
Papathanos PA, Bossin HC, Benedict MQ, Catteruccia F, Malcolm CA, Alphey L, Crisanti A (2009) Sex separation strategies: past experience and new approaches. Malaria J 8(Suppl 2):S5
Rendon P, McInnis D, Lance D, Stewart J (2004) Medfly (Diptera: Tephritidae) genetic sexing: large-scale field comparison of males-only and bisexual sterile fly releases in Guatemala. J Econ Entomol 97(5):1547–1553
Sandeman RM, Levot GW, Heath AC, James PJ, Greeff JC, Scott MJ, Batterham P, Bowles VM (2014) Control of the sheep blowfly in Australia and New Zealand—are we there yet? Int J Parasitol 44:879–891
Schetelig MF, Handler AM (2012a) A transgenic embryonic sexing system for Anastrepha suspensa (Diptera: Tephritidae). Insect Biochem Mol Biol 42:790–795
Schetelig MF, Handler AM (2012b) Strategy for enhanced transgenic strain development for embryonic conditional lethality in Anastrepha suspensa. Proc Natl Acad Sci USA 109(24):9348–9353
Schetelig MF, Caceres C, Zacharopoulou A, Franz G, Wimmer EA (2009) Conditional embryonic lethality to improve the sterile insect technique in Ceratitis capitata (Diptera: Tephritidae). BMC Biol 7:4
Schetelig MF, Targovska A, Meza JS, Bourtzis K, Handler AM (2016) Tetracycline-suppressible female lethality and sterility in the Mexican fruit fly, Anastrepha ludens. Insect Mol Biol 25:500–508
Scott MJ, Concha C, Welch JB, Phillips PL, Skoda SR (2017) Research advances in the screwworm eradication program over the past 25 years. Entomol Exp Appl 164:226–236
Yan Y, Scott MJ (2015) A transgenic embryonic sexing system for the Australian sheep blow fly Lucilia cuprina. Sci Rep 5:16090
Yan Y, Linger RJ, Scott MJ (2017) Building early-larval sexing systems for genetic control of the Australian sheep blow fly Lucilia cuprina using two constitutive promoters. Sci Rep 7:2538
Acknowledgements
We thank Amy Berger and Scott Harrison for assistance with fly rearing and Jason Osborne for statistical analysis. The authors acknowledge the use of the Cellular and Molecular Imaging Facility (CMIF) at North Carolina State University, which is supported by the State of North Carolina and the National Science Foundation. The project benefitted from discussions at International Atomic Energy Agency funded meetings for the Coordinated Research Project: “The Use of Molecular Tools to Improve the Effectiveness of SIT”.
Funding
Funding is gratefully acknowledged from specific cooperative agreements (59-6205-3-001 and 58-3094-7-013) between the USDA-ARS and NCSU, a grant (01-15) from the Panama-United States Commission for the Eradication and Prevention of Screwworm (COPEG) (MJS) and National Institute of Justice Grant 2012-DN-BX-K024 (CJP).
Author information
Authors and Affiliations
Contributions
Y.Y. did qRT-PCR analysis for cellularization genes, carried out the driver plasmid construction, made the transgenic lines, performed the lethality and longevity tests and drafted the manuscript. M.W. made the tetO-RFPex strain and did the confocal microscopy analysis, performed some of the lethality and longevity testing and carried out the RT-PCR analysis for female embryo lethality testing. R.J.L. isolated RNA and performed qRT-PCR analysis for tTA expression of transgenic lines. A.A.A. and C.J.P. assembled and annotated a draft genome of C. macellaria. M.J.S. conceived of the study, participated in its design and coordination and co-wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Stefan Hohmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, Y., Williamson, M.E., Davis, R.J. et al. Improved transgenic sexing strains for genetic control of the Australian sheep blow fly Lucilia cuprina using embryo-specific gene promoters. Mol Genet Genomics 295, 287–298 (2020). https://doi.org/10.1007/s00438-019-01622-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-019-01622-3