Abstract
Gossypium hirsutum L. is the most important fiber crop worldwide and contributes to more than 95% of global cotton production. Marker-assisted selection (MAS) is an effective approach for improving fiber quality, and quantitative trait loci (QTL) mapping of fiber quality traits is important for cotton breeding. In this study, a permanent intra-specific recombinant inbred line (RIL) population containing 137 families was used for fiber quality testing. Based on a previously reported high-density genetic map with an average marker distance of 0.63 cM, 186 additive QTLs were obtained for five fiber quality traits over five consecutive years, including 39 for fiber length (FL), 36 for fiber strength (FS), 50 for fiber uniformity (FU), 33 for micronaire (MC) and 28 for fiber elongation (FE). Three stable QTLs, qMC-A4-1, qMC-D2-3 and qFS-D9-1, were detected in four datasets, and another eight stable QTLs, qMC-A4-2, qMC-D11-2, qFU-A9-1, qFU-A10-4, qFS-D11-1, qFL-D9-2, qFL-D11-1 and qFE-A3-2, were detected in three datasets. The annotated genes in these 11 stable QTLs were collected, and these genes included many transcription factors with functions during fiber development. 33 QTL coincidence regions were found, and these involved nearly half of the total QTLs. Four chromosome regions containing at least 6 QTLs were promising for fine mapping. In addition, 41 pairs of epistatic QTLs (e-QTLs) were screened, including 6 for FL, 30 for FS, 2 for FU and 3 for MC. The identification of stable QTLs adds valuable information for further QTL fine mapping and gene positional cloning for fiber quality genetic detection and provides useful markers for further molecular breeding in enhancing fiber quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cotton is an important cash crop and provides the most important renewable natural resource for the textile industry. Gossypium L., also known as upland cotton, is the most important species and contributes to more than 95% of cotton production worldwide (Chen et al. 2007; Cotton Outlook 2016, see https://www.usda.gov/oce/forum/2016_speeches/Cotton_Outlook_2016.pdf;, Fang et al. 2017). Although cotton cultivars with super fiber quality have long been bred through hybridization (Tang et al. 2015), the development of cultivars with both high yield and super-quality fiber is challenging because these characteristics are negatively correlated (Shen et al. 2007; Wang et al. 2016). As the improvement of fiber quality traits by traditional breeding methods becomes more challenging, breeding for premium-quality fiber is experiencing unprecedented difficulties to meet the increasing demand of spinning technologies (Yu et al. 2013). Due to its superior fiber quality, G. barbadense is 30–50% more expensive than upland cotton and is, therefore, typically used as a favorable allele donor for crossing with G. hirsutum (Ulloa et al. 2005, Si et al. 2017). Nonetheless, inter-species hybridization frequently results in hybrid abortion or degradation, restricting its breeding efficiency (Zhang and Percy 2007; Yu et al. 2013).
Due to clear genetic targets, marker-assisted selection (MAS) is an efficient method for fiber quality improvement (Guo et al. 2003; Kumar et al. 2012; Cao et al. 2014). The first requisite for MAS is a high-density genetic map. Numerous inter- or intra-specific genetic maps based on traditional markers have been constructed since the first genetic map was published in 1994 (Reinisch et al. 1994; Rong et al. 2004; Guo et al. 2007; Yu et al. 2011, 2012; Shi et al. 2015; Khan et al. 2016; Zhang et al. 2016). In addition, more than 1000 inter- or intra-specific QTLs have been reported, and additional studies are under way (Fang et al. 2014; Said et al. 2015; Wang et al. 2015a; Yang et al. 2015; Jamshed et al. 2016). Wang et al. (2015a) published 64 fiber quality-related QTLs based on an intra-specific recombinant inbred line (RIL) population. Jamshed et al. (2016) reported 165 QTLs for fiber quality and noted that four chromosomes, c4(A4), c7(A7), c14(D2) and c25(D6), were more valuable in MAS for improving cotton fiber quality. In addition, Li et al. (2016a, b) detected 47 fiber quality-related QTLs in an upland cotton RIL population based on a single nucleotide polymorphism (SNP)-based genetic map. However, markers in these previously reported QTL regions are limited or not closely linked to target trait(s). Consequently, the direct use of these markers in cotton breeding practices is difficult. In addition, the narrow genetic diversity of upland cotton is another obstacle limiting marker detection or QTL mapping using intra-specific mapping populations. Moreover, with the saturation of traditional markers in genetic maps, particularly for intra-specific populations, new markers or technologies are urgently needed for the construction of super-high-density genetic maps.
SNPs, which are highly abundant and uniformly distributed in plant genomes, are quite appropriate for the construction of high-density genetic maps (Kaur et al. 2014). Additionally, techniques for SNP detection have shown significant progress, providing users with greater marker reliability and lower costs (Cruaud et al. 2014). Reduced-representation sequencing, such as restriction-site associated DNA sequencing (RAD-seq) (Miller et al. 2007), is robust for SNP excavation, and many successful applications have been carried out (Bus et al. 2012; Hegarty et al. 2013; Xu et al. 2014; Wang et al. 2015b). In addition, the release of G. hirsutum genome sequences (Li et al. 2015; Zhang et al. 2015) will facilitate SNP detection and genetic map construction. For example, Wang et al. (2015c) applied RAD-seq for cotton SNP detection and published a genetic map with 4153 loci; based on this map, these authors identified 3 QTLs with high confidence for fiber strength (FS). Zhang et al. (2016) published a high-density genetic map consisting of 5521 SNPs and identified 18 stable QTLs for boll weight. These researches then constructed a genetic map with 2393 loci and a length of 2865.73 cM based on an SNP array and detected 63 additive QTLs for fiber quality (Zhang et al. 2017). Recently, Keerio et al. (2018) applied specific locus amplified fragment sequencing (SLAF-seq) for SNP detection and identified 30 QTLs for fiber quality.
In this study, a permanent RIL mapping population was developed by crossing CCRI36 with G2005 for the detection of stable fiber quality QTLs. CCRI36 is an excellent upland cotton cultivar with a comparatively high yield, whereas G2005 is a G. barbadense introgression line in the background of G. hirsutum that exhibits relatively better fiber quality. Based on the high-density genetic map constructed by RAD-seq (Jia et al. 2016), the aims of this study were (1) to discover more valuable QTLs in an intra-specific upland cotton population and (2) to identify the chromosome region(s) tightly linked to fiber quality, and to (3) perform further fine mapping and candidate gene selection.
Materials and methods
Mapping population and trait evaluation
Detailed information on this population was previously reported by Jia et al. (2016). Briefly, we crossed the G. hirsutum cultivar CCRI36 with an inbred line G2005 in Anyang, Henan Province, in 2006. F1 seeds were planted and self-pollinated in Hainan in the winter of 2006. A total of 137 F2 plants were randomly selected and self-mated in Anyang the next year. From the F2:3 generation, single-seed descent was performed until the F2:9 generation in 2010. All RILs and parents were planted approximately in late April from 2010 to 2015 in a randomized complete block design with three replicates and one row per plot each year. 25 plants were retained in each row, and the rows were 5 m in length and spaced 80 cm apart. The experiment was conducted at the experimental field of the Institute of Cotton Research of CAAS, Anyang, Henan, China, and field management was performed under local practices.
50 naturally opened bolls (2 bolls per plant) were hand-harvested in late September from each row every year from 2011 to 2015 and used for fiber quality assessment. We inspected 5 fiber quality traits, fiber length (FL, mm), FS (cN/tex), fiber uniformity (FU, %), micronaire (MC, unit) and fiber elongation (FE, %), at the Test Center of Cotton Fiber Quality, Institute of Cotton Research of CAAS, Anyang, Henan, China.
Data analysis and QTL detection
Phenotypic data were analyzed by SAS 9.3 (SAS Institute, Inc., Cary, NC, USA). The PROC General Linear Model (GLM) procedure was used to estimate the variance. Analysis of variance (ANOVA) was performed using yearly data and subsequently combined over the 5 years. Broad-sense heritability (HB2) was calculated according to Knapp et al. (1985). A total of 6 datasets were included, namely, 2011, 2012, 2013, 2014, 2015 and combined.
The genetic map used in this study was described previously (Jia et al. 2016). Briefly, the genetic map contains 6,434 loci, including 6,295 SNPs and 139 simple sequence repeat loci. The total length of the genetic map is 4071.98 cM, with an average marker interval of 0.63 cM.
Additive QTLs were analyzed by composite interval mapping using WinQTLCart 2.5. Parameters were set as 5 cM for window size, 1 cM for walk speed and 10 background markers. To detect significant QTLs, a logarithm of odds (LOD) score was determined by 1000-permutation tests at a significance level of P = 0.05. QTLs at the same location for the same trait across different years were regarded as ‘stable’, and QTLs explaining more than 10% of the phenotypic variance (PV) were regarded as ‘major’. In addition, QTLs that overlapped at 2-LOD confidence intervals were considered to be QTL coincidence regions (QCRs). QTLs were named according to McCouch et al. (1997). Epistatic QTLs (e-QTLs) were detected by IciMapping ver. 4.1 software using the multi-environment trials (MET) function and the inclusive composite interval mapping (ICIM) method (Li et al. 2007). And e-QTLs were identified using the pre-adjusted IciMapping parameters of Scan = 5 cM and PIN = 0.0001, and LOD-scores were determined using a 1000-permutation test. Electronic PCR (e-PCR) was performed to verify the mapping results.
Results
Phenotypic evaluation of fiber quality traits
As shown in Table 1, the parents differed in 5 fiber quality traits, and G2005 presented super fiber quality, with the exception of MC. In the RIL population, all 5 fiber quality traits showed transgressive segregation and nearly normal distribution in all environments, except for FL in 2015, indicating that this population is suitable for QTL analysis. In addition, the HB2 values of FL, FS, FU and MC were greater than 70%, suggesting that FL, FS, FU and MC are mainly genetically controlled and that improvement through genetic modification is possible. Consistent with previously reported results (Lacape et al. 2010; Li et al. 2016a, b; Jamshed et al. 2016), the HB2 of FE was low (32.21%). Therefore, FE is relatively less genetically controlled and more easily affected by environmental factors (Table 2).
Table 3 shows the correlation coefficients of the 5 fiber quality traits over 5 years. MC was significantly negatively correlated with FL or FS in most environments (except for FL in 2011 and FS in 2012) but was not significantly correlated with FE. FU was significantly positively correlated with FS. Other trait pairs, such as FU with FE, FU with FL, MC with FU and FL with FS, did not show stable correlations over time, even though they were significantly correlated with each other in a given dataset. For example, FE and FU were significantly negatively correlated in 2011 and 2013 but significantly positively correlated in 2014 and 2015. Therefore, the genes controlling MC and FL might be either the same or tightly linked but have opposite functions. Alternatively, these traits can be controlled by negatively interacting gene networks. The same assumption can be made for MC and FS. MC and FE might be controlled by different genes or by weakly associated gene networks.
Additive QTL analysis
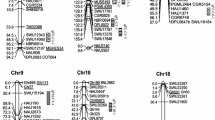
In total, we identified 186 additive QTLs for fiber quality traits, including 32 stable and 37 major ones. Simple sequence repeat (SSR) markers were verified by e-PCR for 106 QTLs (Supplemental file 1). In addition, the number of QTLs for the 5 fiber quality traits varied from 28 to 50, with 39 for FL, 36 for FS, 50 for FU, 33 for MC and 28 for FE (Table 4). The At sub-genome was found to contain 98 QTLs, which is 10 more than the number of QTLs located on the Dt sub-genome (Table 5, Supplemental file 1). The highest number of QTLs was 20 on chromosome (chr.) A10, and no QTL was found on chr. D10. CCRI36 conferred positive additive alleles for 78 QTLs, and G2005 conferred positive additive alleles for 106 QTLs. For the other 2 stable QTLs, different positive additive alleles were inherited from different parents in different datasets. Annotated genes in 11 important QTLs including qFE-A3-2, qFL-D9-2, qFL-D11-1, qFS-D9-1, qFS-D11-1, qFU-A9-1, qFU-A10-4, qMC-A4-1, qMC-A4-2, qMC-D2-3 and qMC-D11-2, which can classify the RIL population into 2 phenotypic groups, were identified (Fig. 1, Supplemental file 2).
FL
For FL, 39 QTLs were detected on 18 chromosomes, including A1, A4, A7, A8, A9, A10, A12, A13, D2, D3, D5, D6, D7, D8, D9, D11, D12 and D13, and 24 of these QTLs were co-located with mapped SSR markers (Supplemental file 1). Among these QTLs, six were detected in at least two datasets, and ten explained more than 10% of the observed PVs. The highest number of QTLs was 6 on chr. D12. Two QTLs (qFL-D9-2 and qFL-D11-1) were detected in 3 datasets, and the first one explained 12.4% of the PV in 2011; qFL-D2-1, qFL-D2-2, qFL-D5-2 and qFL-D5-3 were detected in 2 datasets. CCRI36 conferred the positive additive alleles at qFL-D5-3 and qFL-D9-2, and G2005 conferred the positive additive alleles at the other 4 stable QTLs.
FS
A total of 36 QTLs on 19 chromosomes, including A2, A3, A4, A5, A7, A8, A9, A10, A12, A13, D1, D3, D4, D5, D7, D8, D9, D11 and D12, were found for FS, and 19 of these QTLs fully or partially overlapped with SSR markers (Supplemental file 1). Among these 36 QTLs, five were stably detected in multiple datasets, but only 2 could explain more than 10% of the observed PV. An important QTL, qFS-D9-1, was detected repeatedly in the 2012, 2014, 2015 and combined analyses and explained 12.1% of the PV in 2015, and CCRI36 conferred the positive additive allele. Therefore, this QTL was highly regarded as a candidate region for fine mapping and MAS. Of the other 4 stable QTLs, qFS-D11-1 was detected in the 2012, 2013 and the combined analyses, and the favorable allele was obtained from G2005. And qFS-A10-1, qFS-A10-4 and qFS-D7-1 were detected in 2013 and 2014.
FU
In total, 50 QTLs were detected for FU on 22 chromosomes (excluding chr. A5, D4, D5 and D10), and 27 were verified by e-PCR (Supplemental file 1). Six QTLs (qFU-A8-1, qFU-A9-1, qFU-A10-4, qFU-A11-2, qFU-A11-3 and qFU-D3-1) were detected in at least 2 datasets. The first 3 listed QTLs explained more than 10% of the PV, and CCRI36 conferred their favorable alleles. Among the 6 stable QTLs, qFU-A9-1 and qFU-A10-4 were better; qFU-A9-1 explained 10.1, 12.8 and 10.0% of the observed PVs in the 2013, 2014 and combined analyses, and qFU-A10-4 explained 10.2 and 12.0% of the PVs in 2015 and combined analyses, respectively. Another 10 major QTLs, including qFU-A2-1, qFU-A6-1, qFU-A6-2, qFU-A9-3, qFU-A10-1, qFU-A10-2, qFU-A10-3, qFU-A12-1, qFU-D11-1 and qFU-D11-2, were detected in 1 dataset and could explain 10.1–15.3% of the observed PVs.
MC
For MC, 33 QTLs were detected on 14 chromosomes, including A4, A6, A7, A8, A11, A12, D2, D3, D4, D8, D9, D11, D12 and D13 (Supplemental file 1). Of the 33 QTLs, 22 were reported in previous studies, 10 were stably detected in at least two datasets, and 4 could explain more than 10% of the observed PVs (Supplemental file 1). Two QTLs, qMC-A4-1 and qMC-D2-3, were detected in 4 datasets; qMC-A4-1 was detected from 2011 to 2013 and in the combined analysis and explained 10.5% of the PV in 2013; and qMC-D2-3 was detected from 2013 to 2015 and in the combined analysis, and explained 11.2% of the PV in 2015. These QTLs received favorable alleles from G2005. Two QTLs, qMC-A4-2 and qMC-D11-2, were detected in 3 datasets, and 6 QTLs, namely, qMC-A7-1, qMC-D9-1, qMC-D9-2, qMC-D9-3, qMC-D11-1 and qMC-D11-4, were detected in 2 datasets. The highest number of QTLs was 5 on chr. A8.
FE
For FE, 28 QTLs were detected on 14 chromosomes, including A1, A2, A3, A4, A5, A8, A9, A10, A11, D1, D4, D5, D7 and D11, and 14 of these QTLs were confirmed by SSR markers (Supplemental file 1). Of the total QTLs, 5 were detected in at least 2 datasets that were regarded as stable, and 8 could explain more than 10% of the PV. Four QTLs (qFE-A5-2, qFE-A10-4, qFE-D1-2 and qFE-D4-1) were detected in 2 datasets, and 3 of these (all but qFE-D1-2) were major QTLs. Another QTL (qFE-A3-2) was detected in 2011, 2013 and 2015 and explained 13.0% of the PV in 2015, and the positive additive effect was conferred by G2005. The highest number of QTLs was 5 on chr. A10. Of these QTLs, 2 were major: qFE-A10-4 and qFE-A10-5 explained 11.5% and 11.1% of the PV in 2013, respectively. G2005 conferred the favorable alleles at these 2 loci. The inheritance of different additive alleles for 2 stable QTLs (qFE-A5-2 and qFE-A10-4) in different environments was observed, indicating that these 2 loci were affected more significantly by the environment than the other loci.
Additive QTL coincidence regions
A total of 33 additive QTL coincidence regions (QCRs) were found on 20 chromosomes, and these involved almost half of the QTLs (91/186, 47.85%), including 15 FE-QTLs (53.57%), 15 FL-QTLs (38.46%), 22 FS-QTLs (61.11%), 24 FU-QTLs (48%) and 15 MC-QTLs (45.45%). Moreover, more QTLs detected on a given chromosome corresponded to more QCRs found on that chromosome, and their correlation coefficient reached 0.797 (Tables 4, 5, Supplemental file 2). These regions might explain why these 5 fiber quality traits showed complex correlations. Six FS-QTLs and 8 MC-QTLs overlapped in 5 QCRs with opposite additive effects, and 6 FL-QTLs and 6 MC-QTLs were involved in 5 QCRs with opposite additive effects, which might explain the negative correlations of FS with MC and of FL with MC (Table 6 and Supplemental file 3). 13 FS-QTLs and 12 FU-QTLs overlapped in 11 QCRs with the same additive effects, which was consistent with the positive correlation between FS and FU. Other coinciding QTLs did not show consistent additive effects. For example, FL and FU shared 10 QCRs, but only half of them exhibited the same additive effect direction. Similar phenomena were found between trait pairs such as FE with FS and FE with FU. Therefore, different correlations existed among these fiber quality traits in different environments.
According to the results of the QTL coincidence analysis, we found 4 chromosome regions on A10, D5, D9 and D11 that were strongly related to fiber quality traits (Fig. 2). 13 QTLs were detected in a 40-cM chromosome segment (from 104 to 144 cM) on A10, and these included 3 that were stably detected in two to three datasets and eight that explained more than 10% of the observed PVs. Four fiber quality traits, namely, FL, FS, FU and FE, were found to be involved in these 13 QTLs, and NAU1169 in this region has been reported to be related to fiber quality (Tang et al. 2015). Favorable additive alleles for FE and FL and for FS and FU were obtained from G2005 and CCRI36, respectively. In the region from 135 to 180.7 cM on chr. D5, 10 QTLs were detected for 4 fiber quality traits (FL, FS, FU and FE), and G2005 conferred favorable additive alleles for 8 of these 10 QTLs. Many SSR markers in this region such as CIR024, CIR085 CIR229, BNL0852, BNL3029 and NAU1042, were published previously, strengthening the reliablity of these mapping results (Tang et al. 2015). Six QTLs were detected in the region from 127.8 to 154 cM on chr. D9, and 4 of these QTLs, namely, qFL-D9-2, qFS-D9-1, qMC-D9-2 and qMC-D9-3 were stable. In addition, an e-PCR analysis confirmed that CIR061, CIR383 and BNL3383, which are related to fiber quality, are located in this region (Tang et al. 2015). Another 10 QTLs for FE, MC, FS and FU were found to be clustered in a 32-cM segment (from 20.4 to 52.7 cM) on chr. D11. qFS-D11-1 and qMC-D11-2 were detected in 3 datasets and qMC-D11-1 was detected in two datasets. CCRI36 conferred the additive alleles for FE and MC, whereas, G2005 conferred the additive allele for the other 2 traits. In addition, many SSR markers related to fiber quality, including BL1551, BNL2632, BNL3649, CIR398, JESPR244, NAU2950 and NAU5217, were found to be located in this region (Tang et al. 2015). Accordingly, these 4 chromosome segments might be valuable candidates for fine mapping and MAS. However, a common defect is that these regions span relatively large intervals, and delineating the core region based on the present population is difficult. Therefore, further work is required.
Epistatic QTLs for fiber quality traits
A total of 41 e-QTLs were identified for FL, FS, FU and MC, but none were found for FE (Supplemental file 4). The observed PVs explained by the e-QTLs ranged from 4.42 to 9.25%, indicating that the epistatic effect is an important aspect in controlling cotton fiber quality traits. Six e-QTLs were detected for FL, and 1 locus between Marker4730 and Marker4973 on A4 overlapped with the additive QTL qFL-A4-1. 30 e-QTLs for FS were found, with the marker intervals of Marker16324-Marker16326 on A10 overlapping with qFS-A10-8, BNL3414- Marker18897 on A12 overlapping with qFS-A12-1, and Marker34192-Marker34191 on D8 overlapping with qFS-D8-2, and the marker intervals of Marker34111–Marker34103 and Marker33925–Marker33739 were both on chr. D8. Only 2 and 3 e-QTLs were found for FU and MC, respectively, whereas none was found for FE. Several marker intervals related to more than one trait were found. For example, Marker33513–Marker33511 on D7 had functions for both FL and FS, Marker18984–Marker18977 had functions for FU and FS, Marker35862–Marker35839 had functions for FS and MC. These findings indicate the complexity of epistatic effects on fiber quality.
Discussion
Fiber quality is the most important feature of cotton production and is vital to daily life. However, the quantitative nature of fiber quality has hindered detection of its genetic factors. In this study, two elite upland cotton cultivars/lines, CCRI36 and G2005, were crossed. Although the phenotypic differences between the parents were not as significant as those in inter-specific hybrid combinations (Si et al. 2017), transgressive segregation was observed among the 5 fiber quality traits in the RIL population, and 186 QTLs were detected that showed relatively high mapping efficiency. Both parents conferred favorable alleles for a given trait, indicating the existence of favorable alleles with minor effects. Therefore, QTL mapping studies can be performed based on crossing parents without extreme phenotypic differences, as reported by Tang et al. (2015).
To date, more than 1000 fiber quality QTLs have been published, and these QTLs, QTL clusters or hotspots are distributed throughout the 26 cotton chromosomes, even though the scatter is uneven for a given trait (Said et al. 2015). As almost all published QTL mapping studies regarding fiber quality have been conducted based on traditional markers, few of these QTLs have been applied in breeding practice due to limited marker resolution or genome coverage (Shen et al. 2007; Li et al. 2013). Wang et al. (2015a) reported 64 QTLs for fiber quality based on a genetic map with 3.27 cM between adjacent markers. Tang et al. (2015) published a genetic map of 2842.06 cM in length and detected 62 fiber quality-related QTLs. Shang et al. (2015) detected 20 QTLs for 4 fiber quality traits based on a genetic map with an average marker interval of 6.39 cM. Jamshed et al. (2016) published 165 QTLs for fiber quality based on a genetic map with 5.2 cM between adjacent markers. Li et al. (2016a) reported 47 QTLs for fiber quality based on an SNP genetic map with a total genetic length of 1784.28 cM total genetic length. Recently, Zhang et al. (2017) detected 63 additive QTLs for FS based on a high-density genetic map with relatively less genome coverage (2393 markers and 2865.73 cM in length). Keerio et al. (2018) found 30 QTLs for 5 fiber quality traits based on 3157 SNP markers. Therefore, these newly published QTLs were based on relatively low marker-density genetic maps or maps with a short genetic length, which might result in missing genetic information related to fiber quality. In this study, the QTL analysis was performed based on a high-density genetic map with a total genetic length of 4071.98 cM and an average marker interval of only 0.63 cM, which is superior to other maps used for the detection of fiber quality-related QTLs (Jia et al. 2016). This map contains 6434 loci, including 6295 SNP and 139 SSR loci. Accurate genome position information is available for most of the markers, which will be convenient for further genetic studies or MAS.
A total of 186 additive QTLs were detected on 25 chromosomes (except for chr. D10), with a range of 3 on chr. D6 to 20 on A10. To verify the reliability of our mapping results, e-PCR was conducted with a summary of SSR markers published in studies of QTLs related to fiber quality traits (Said et al. 2015; Tang et al. 2015; Wang et al. 2015a; Jamshed et al. 2016). A total of 106 QTLs detected in this study, including 19 stable QTLs, have published SSR markers in their physical intervals or at distances of less than 2 Mb (Supplemental file 1). Therefore, most of the QTL mapping results reported in this manuscript are reliable, and at least 70 new loci for cotton fiber quality traits are provided. In addition, 11 noteworthy QTLs (qFE-A3-2, qFL-D9-2, qFL-D11-1, qFS-D9-1, qFS-D11-1, qFU-A9-1, qFU-A10-4, qMC-A4-1, qMC-A4-2, qMC-D2-3 and qMC-D11-2) that were detected repeatedly can explain more than 10% of the observed PVs, and the RIL population can be classified into 2 significantly different phenotypic groups based on their homozygous genotypes (Fig. 1). Six of these QTLs were verified by SSR markers, for example, qFS-D9-1, which was previously named qFS-c23-2/qFS-D9-1 and marked by BNL3383, CIR061 and CIR383, and qFS-D11-1, which shares CIR398 and CIR410 with qFS21.2/qFS-c21-2 (Supplemental file 1, Tang et al. 2015). In contrast, verified SSR markers are not available for 5 of the 11 QTLs, namely, qFL-D9-2, qFU-A10-4, qMC-A4-1, qMC-A4-2 and qMC-D2-3. Therefore, these QTLs might be valuable new loci controlling fiber quality. The annotated genes in these regions are listed in Supplemental file 2, and these included many fiber development-related genes included (highlighted), such as the following: the MYB gene family; PPR gene family; WRKY gene family; cellulose synthesis-, pectin synthesis-, tubulin synthesis-, sucrose synthesis-, calmodulin-, auxin-, gibberellin-, ethylene- and brassinosteroid-related genes; and energy metabolism genes (Gou et al. 2007; Lee et al. 2007; Ko et al. 2014; Tang et al. 2014; Hu et al. 2016; Li et al. 2016b; Sun et al. 2017). These findings strengthen the reliability of the mapping results, and some genes could be analyzed in further studies on the molecular mechanism of fiber development.
33 QCRs were found on 20 chromosomes, with chr. A10 showing the most at 6 QCRs. Furthermore, based on the results, more QCRs were observed when more QTLs were detected on a given chromosome, and the correlation coefficient between the QTL number and the QTL coincidence number on one chromosome reached 0.797 (Table 5), which is consistent with the phenomenon reported by Said et al. (2015). With the exception of MC and FE, the fiber quality traits showed significantly complicated correlations. The significant positive correlation between FS and FU can be explained by their co-localized QTLs with the same additive effects (Table 6 and Supplemental file 3). In addition, the significant negative correlation between MC and FS or FL might be explained by their coinciding QTLs with opposite additive effects. Four important chromosome regions on A10, D5, D9 and D11 were strongly related to fiber quality traits and an e-PCR analysis confirmed their reliability (Fig. 2). Among the published fiber quality-related QTLs, at least 30, 62, 52 and 46 have been detected on chr. A10, D5, D9 and D11, respectively (Said et al. 2015; Shang et al. 2015; Tang et al. 2015; Wang et al. 2015a; Jamshed et al. 2016; Li et al. 2016a). Therefore, the four valuable chromosome regions emphasized here are very reliable. In conclusion, these chromosome regions and outstanding QTLs are valuable for further genetic studies and MAS in cotton breeding.
In addition to these additive QTLs, 41 e-QTLs were detected for 4 fiber quality traits, except for FE, and these e-QTLs explained 4.42–9.25% of the observed PVs, which is greater than that reported by Zhang et al. (2017) (Supplemental file 4). Therefore, FL, FS, FU and MC are controlled by both additive effects and epistatic effects. The HB2 value of FE was only 32.21%, which is notably lower than those of FL, FS, FU and MC. This result is consistent with those of other studies (Lacape et al. 2010; Li et al. 2016a, b; Jamshed et al. 2016). This phenomenon suggests that FE might be more easily affected by environmental factors; 2 stable FE-related QTLs, qFE-A5-2 and qFE-A10-4, inherited different additive alleles in different datasets, which might reflect the modifiable genetic effect of FE. In addition, no e-QTLs were found for FE, proving that FE was less genetically controlled.
In this study, 186 additive QTLs were found based on a high-density genetic map, and 11 atable QTLs and 4 important chromosome regions were detected. These regions could be used for QTL fine mapping and candidate gene detection by constructing secondary mapping populations. In addition, the findings obtained in this study could be used as valuable resources for developing reliable fiber quality-related markers and for MAS to accelerate cotton breeding.
Abbreviations
- RAD-seq:
-
Restriction-site associated DNA sequencing
- SNP:
-
Single nucleotide polymorphism
- QTL:
-
Quantitative trait locus
- QCR:
-
QTL coincidence region
- PV:
-
Phenotypic variation
- RIL:
-
Recombinant inbred line
- FE:
-
Fiber elongation
- FL:
-
Fiber length
- FS:
-
Fiber strength
- FU:
-
Fiber uniformity
- MC:
-
Micronaire
- MAS:
-
Marker-assisted selection
- e-PCR:
-
Electronic PCR
References
Bus A, Hecht J, Huettel B, Reinhardt R, Stich B (2012) High-throughput polymorphism detection and genotyping in Brassica napus using next-generation RAD sequencing. BMC Genom 13(1):281
Cao ZB, Wang P, Zhu XF, Chen H, Zhang TZ (2014) SSR marker-assisted improvement of fiber qualities in Gossypium hirsutum using G-barbadense introgression lines. Theor Appl Genet 127(3):587–594
Chen ZJ, Scheffler BE, Dennis E (2007) Toward sequencing cotton (Gossypium) genomes. Plant Physiol 145(4):1303–1310
Cruaud A, Gautier M, Galan M, Foucaud J, Saune L, Genson G, Dubois E, Nidelet S, Deuve T, Rasplus JY (2014) Empirical assessment of RAD sequencing for interspecific phylogeny. Mol Biol Evol 31(5):1272–1274
Fang DD, Jenkins JN, Deng DD, McCarty JC, Li P, Wu JX (2014) Quantitative trait loci analysis of fiber quality traits using a random-mated recombinant inbred population in Upland cotton (Gossypium hirsutum L.). BMC Genom 15(1):397
Fang L, Wang Q, Hu Y, Jia Y, Chen J, Liu B, Zhang Z, Guan X, Chen S, Zhou B, Mei G, Sun J, Pan Z, He S, Xiao S, Shi W, Gong W, Liu J, Ma J, Cai C, Zhu X, Guo W, Du X, Zhang T (2017) Genomic analyses in cotton identify signatures of selection and loci associated with fiber quality and yield traits. Nature Genetics 49(7):1089–1098
Gou JY, Wang LJ, Chen SP, Hu WL, Chen XY (2007) Gene expression and metabolite profiles of cotton fiber during cell elongation and secondary cell wall synthesis. Cell Res 17(5):422–434
Guo WZ, Zhang TZ, Shen XL, Yu JZ, Kohel RJ (2003) Development of SCAR marker linked to a major QTL for high fiber strength and its usage in molecular-marker assisted selection in Upland cotton. Crop Sci 43(6):2252–2256
Guo WZ, Cai CP, Wang CB, Han ZG, Song XL, Wang K, Niu XW, Wang C, Lu KY, Shi B, Zhang TZ (2007) A microsatellite-based, gene-rich linkage map reveals genome structure, function and evolution in Gossypium. Genetics 176(1):527–541
Hegarty M, Yadav R, Lee M, Armstead I, Sanderson R, Scollan N, Powell W, Skot L (2013) Genotyping by RAD sequencing enables mapping of fatty acid composition traits in perennial ryegrass (Lolium perenne (L.)). Plant Biotechnol J 11(5):572–581
Hu HY, He X, Tu LL, Zhu LF, Zhu ST, Ge ZH, Zhang XL (2016) GhJAZ2 negatively regulates cotton fiber initiation by interacting with the R2R3-MYB transcription factor GhMYB25-like. Plant J 88(6):921–935
Jamshed M, Jia F, Gong J, Palanga KK, Shi Y, Li J, Shang H, Liu A, Chen T, Zhang Z, Cai J, Ge Q, Liu Z, Lu Q, Deng X, Tan Y, Or Rashid H, Sarfraz Z, Hassan M, Gong W, Yuan Y (2016) Identification of stable quantitative trait loci (QTLs) for fiber quality traits across multiple environments in Gossypium hirsutum recombinant inbred line population. BMC Genom 17(1):197
Jia X, Pang C, Wei H, Wang H, Ma Q, Yang J, Cheng S, Su J, Fan S, Song M, Wusiman N, Yu S (2016) High-density linkage map construction and QTL analysis for earliness-related traits in Gossypium hirsutum L. BMC Genom 17(1):909
Kaur S, Kimber RBE, Cogan NOI, Materne M, Forster JW, Paull JG (2014) SNP discovery and high-density genetic mapping in faba bean (Vicia faba L.) permits identification of QTLs for ascochyta blight resistance. Plant Sci 217:47–55
Keerio AA, Shen C, Nie Y,C, Ahmed MM, Zhang XL, Lin ZX (2018) QTL mapping for fiber quality and yield traits based on introgression lines derived from Gossypium hirsutum × G. tomentosum. Int J Mol Sci 19(1):243
Khan MKR, Chen HD, Zhou ZL, Ilyas MK, Wang XX, Cai XY, Wang CY, Liu F, Wang KB (2016) Genome wide SSR high density genetic map construction from an interspecific cross of Gossypium hirsutum x Gossypium tomentosum. Front Plant Sci 7:436
Knapp SJ, Stroup WW, Ross WM (1985) Exact confidence intervals for heritability on a progeny mean basis. Crop Sci 25(1):192–194
Ko JH, Jeon HW, Kim WC, Kim JY, Han KH (2014) The MYB46/MYB83-mediated transcriptional regulatory programme is a gatekeeper of secondary wall biosynthesis. Ann Bot 114:1099–1107
Kumar P, Singh R, Lubbers EL, Shen XL, Paterson AH, Campbell BT, Jones DC, Chee PW (2012) Mapping and validation of fiber strength quantitative trait loci on chromosome 24 in Upland cotton. Crop Sci 52(3):1115–1122
Lacape JM, Llewellyn D, Jacobs J, Arioli T, Becker D, Calhoun S, Al-Ghazi Y, Liu SM, Palai O, Georges S, Giband M, de Assuncao H, Augusto P, Barroso V, Claverie M, Gawryziak G, Jean J, Vialle M, Viot C (2010) Meta-analysis of cotton fiber quality QTLs across diverse environments in a Gossypium hirsutum × G. barbadense RIL population. BMC Plant Biol 10(1):132
Lee JJ, Woodward AW, Chen ZJ (2007) Gene expression changes and early events in cotton fibre development. Ann Bot 100(7):1391–1401
Li HH, Ye GY, Wang JK (2007) A modified algorithm for the improvement of composite interval mapping. Genetics 175(1):361–374
Li CQ, Wang XY, Dong N, Zhao HH, Xia Z, Wang R, Converse RL, Wang QL (2013) QTL analysis for early-maturing traits in cotton using two upland cotton (Gossypium hirsutum L.) crosses. Breeding Sci 63(2):154–163
Li F, Fan G, Lu C, Xiao G, Zou C, Kohel RJ, Ma Z, Shang H, Ma X, Wu J, Liang X, Huang G, Percy RG, Liu K, Yang W, Chen W, Du X, Shi C, Yuan Y, Ye W, Liu X, Zhang X, Liu W, Wei H, Wei S, Zhu S, Zhang H, Sun F, Wang X, Liang J, Wang J, He Q, Huang L, Cui J, Song G, Wang K, Xu X, Yu JZ, Zhu Y, Yu S (2015) Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat Biotechnol 33(5):524–530
Li C, Dong YT, Zhao TL, Li L, Li C, Yu E, Mei L, Daud MK, He QL, Chen JH, Zhu SJ (2016a) Genome-wide SNP linkage mapping and QTL analysis for fiber quality and yield traits in the Upland cotton recombinant inbred lines population. Front Plant Sci 7:1356
Li WJ, Shang HH, Ge Q, Zou CS, Cai J, Wang DJ, Fan SM, Zhang Z, Deng XY, Tan YN, Song WW, Li PT, Koffi PK, Jamshed M, Lu QW, Gong WK, Li JW, Shi YZ, Chen TT, Gong JW, Liu AY, Yuan YL (2016b) Genome-wide identification, phylogeny, and expression analysis of pectin methylesterases reveal their major role in cotton fiber development. BMC Genom 17(1):1000
McCouch SR, Chen XL, Panaud O, Temnykh S, Xu YB, Cho YG, Huang N, Ishii T, Blair M (1997) Microsatellite marker development, mapping and applications in rice genetics and breeding. Plant Mol Biol 35:89–99
Miller MR, Dunham JP, Amores A, Cresko WA, Johnson EA (2007) Rapid and cost-effective polymorphism identification and genotyping using restriction site associated DNA (RAD) markers. Genome Res 17(2):240–248
Reinisch AJ, Dong JM, Brubaker CL, Stelly DM, Wendel JF, Paterson AH (1994) A detailed RFLP map of cotton, Gossypium hirsutum x Gossypium barbadense: chromosome organization and evolution in a disomic polyploid genome. Genetics 138(3):829–847
Rong JK, Abbey C, Bowers JE, Brubaker CL, Chang C, Chee PW, Delmonte TA, Ding XL, Garza JJ, Marler BS, Park CH, Pierce GJ, Rainey KM, Rastogi VK, Schulze SR, Trolinder NL, Wendel JF, Wilkins TA, Williams-Coplin TD, Wing RA, Wright RJ, Zhao XP, Zhu LH, Paterson AH (2004) A 3347-locus genetic recombination map of sequence-tagged sites reveals features of genome organization, transmission and evolution of cotton (Gossypium). Genetics 166(1):389–417
Said JI, Song MZ, Wang HT, Lin ZX, Zhang XL, Fang DD, Zhang JF (2015) A comparative meta-analysis of QTL between intraspecific Gossypium hirsutum and interspecific G. hirsutum x G. barbadense populations. Mol Genet Genom 290(3):1003–1025
Shang LG, Liang QZ, Wang YM, Wang XC, Wang KB, Abduweli A, Ma LL, Cai SH, Hua JP (2015) Identification of stable QTLs controlling fiber traits properties in multi-environment using recombinant inbred lines in Upland cotton (Gossypium hirsutum L.). Euphytica 205(3):877–888
Shen XL, Guo WZ, Lu QX, Zhu XF, Yuan YL, Zhang TZ (2007) Genetic mapping of quantitative trait loci for fiber quality and yield trait by RIL approach in Upland cotton. Euphytica 155(3):371–380
Shi YZ, Li WT, Li AG, Ge RH, Zhang BC, Li JZ, Liu GP, Li JW, Liu AY, Shang HH, Gong JW, Gong WK, Yang ZM, Tang FY, Liu Z, Zhu WP, Jiang JX, Yu XN, Wang T, Wang W, Chen TT, Wang KB, Zhang ZS, Yuan YL (2015) Constructing a high-density linkage map for Gossypium hirsutum x Gossypium barbadense and identifying QTLs for lint percentage. J Integ Plant Biol 57(5):450–467
Si ZF, Chen H, Zhu XF, Cao ZB, Zhang TZ (2017) Genetic dissection of lint yield and fiber quality traits of G. hirsutum in G. barbadense background. Mol Breeding 37(1):9
Sun Z, Wang X, Liu Z, Gu Q, Zhang Y, Li Z, Ke H, Yang J, Wu J, Wu L, Zhang G, Zhang C, Ma Z (2017) Genome-wide association study discovered genetic variation and candidate genes of fibre quality traits in Gossypium hirsutum L. Plant Biotechnol J:1–15
Tang WX, Tu LL, Yang XY, Tan JF, Deng FL, Hao J, Guo K, Lindsey K, Zhang XL (2014) The calcium sensor GhCaM7 promotes cotton fiber elongation by modulating reactive oxygen species (ROS) production. New Phytol 202(2):509–520
Tang SY, Teng ZH, Zhai TF, Fang XM, Liu F, Liu DJ, Zhang J, Liu DX, Wang SF, Zhang K, Shao QS, Tan ZY, Paterson AH, Zhang ZS (2015) Construction of genetic map and QTL analysis of fiber quality traits for Upland cotton (Gossypium hirsutum L.). Euphytica 201(2):195–213
Ulloa M, Saha S, Jenkins JN, Meredith WR, McCarty JC, Stelly DM (2005) Chromosomal assignment of RFLP linkage groups harboring important QTLs on an intraspecific cotton (Gossypium hirsutum L.) joinmap. J Hered 96(2):132–144
Wang HT, Huang C, Guo HL, Li XM, Zhao WX, Dai BS, Yan ZH, Lin ZX (2015a) QTL mapping for fiber and yield traits in Upland cotton under multiple environments. PloS One 10(6):e0130742
Wang HT, Jin X, Zhang BB, Shen C, Lin ZX (2015b) Enrichment of an intraspecific genetic map of upland cotton by developing markers using parental RAD sequencing. DNA Res 22(2):147–160
Wang Y, Ning Z, Hu Y, Chen J, Zhao R, Chen H, Ai N, Guo W, Zhang T (2015c) Molecular mapping of restriction-site associated DNA markers in allotetraploid upland cotton. PloS One 10(4):e0124781
Wang HT, Huang C, Zhao WX, Dai BS, Shen C, Zhang BB, Li DG, Lin ZX (2016) Identification of QTL for fiber quality and yield traits using two immortalized backcross populations in Upland Cotton. PloS One 11(12):e0166970
Xu P, Xu SZ, Wu XH, Tao Y, Wang BG, Wang S, Qin DH, Lu ZF, Li GJ (2014) Population genomic analyses from low-coverage RAD-Seq data: a case study on the non-model cucurbit bottle gourd. Plant J 77(3):430–442
Yang XL, Zhou XD, Wang XF, Li ZK, Zhang Y, Liu HW, Wu LQ, Zhang GY, Yan GJ, Ma ZY (2015) Mapping QTL for cotton fiber quality traits using simple sequence repeat markers, conserved intron-scanning primers, and transcript-derived fragments. Euphytica 201(2):215–230
Yu Y, Yuan DJ, Liang SG, Li XM, Wang XQ, Lin ZX, Zhang XL (2011) Genome structure of cotton revealed by a genome-wide SSR genetic map constructed from a BC1 population between Gossypium hirsutum and G. barbadense. BMC Genom 12(1):15
Yu JZ, Kohel RJ, Fang DD, Cho JM, Van Deynze A, Ulloa M, Hoffman SM, Pepper AE, Stelly DM, Jenkins JN, Saha S, Kumpatla SP, Shah MR, Hugie WV, Percy RG (2012) A high-density simple sequence repeat and single nucleotide polymorphism genetic map of the tetraploid cotton genome. G3 Genes Genom Genet 2(1):43–58
Yu JW, Zhang K, Li SY, Yu SX, Zhai HH, Wu M, Li XL, Fan SL, Song MZ, Yang DG, Li YH, Zhang JF (2013) Mapping quantitative trait loci for lint yield and fiber quality across environments in a Gossypium hirsutum × Gossypium barbadense backcross inbred line population. Theor Appl Genet 126(1):275–287
Zhang J, Percy R (2007) Improving Upland cotton by introducing desirable genes from Pima cotton. World Cotton Res Conf 4:10–14
Zhang T, Hu Y, Jiang W, Fang L, Guan X, Chen J, Zhang J, Saski CA, Scheffler BE, Stelly DM, Hulse-Kemp AM, Wan Q, Liu B, Liu C, Wang S, Pan M, Wang Y, Wang D, Ye W, Chang L, Zhang W, Song Q, Kirkbride RC, Chen X, Dennis E, Llewellyn DJ, Peterson DG, Thaxton P, Jones DC, Wang Q, Xu X, Zhang H, Wu H, Zhou L, Mei G, Chen S, Tian Y, Xiang D, Li X, Ding J, Zuo Q, Tao L, Liu Y, Li J, Lin Y, Hui Y, Cao Z, Cai C, Zhu X, Jiang Z, Zhou B, Guo W, Li R, Chen ZJ (2015) Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat Biotechnol 33(5):531–537
Zhang Z, Shang H, Shi Y, Huang L, Li J, Ge Q, Gong J, Liu A, Chen T, Wang D, Wang Y, Palanga KK, Muhammad J, Li W, Lu Q, Deng X, Tan Y, Song W, Cai J, Li P, Rashid HO, Gong W, Yuan Y (2016) Construction of a high-density genetic map by specific locus amplified fragment sequencing (SLAF-seq) and its application to quantitative trait loci (QTL) analysis for boll weight in Upland cotton (Gossypium hirsutum.). BMC Plant Biol 16(1):79
Zhang Z, Ge Q, Liu A, Li J, Gong J, Shang H, Shi Y, Chen T, Wang Y, Palanga KK, Muhammad J, Lu Q, Deng X, Tan Y, Liu R, Zou X, Rashid H, Iqbal MS, Gong W, Yuan Y (2017) Construction of a high-density genetic map and its application to QTL identification for fiber strength in Upland cotton. Crop Sci 57(2):774–788
Acknowledgements
This work was funded by the Major Program of the National Natural Science Foundation of China (No. 31690093) and the Science and Technology Development by Henan province (No. 162102110020). The experiment was performed at the State Key Laboratory of Cotton Biology in the Institute of Cotton Research of the Chinese Academy of Agricultural Sciences.
Author information
Authors and Affiliations
Contributions
Shuxun Yu, Shuli Fan, Meizhen Song and Chaoyou Pang designed the experiment. Xiaoyun Jia and Hantao Wang performed the experiment. Hengling Wei and Xiaoyun Jia analyzed the phenotypic data. Xiaoyun Jia and Hantao Wang performed the QTL analysis. Xiaoyun Jia wrote and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have conflict of interest.
Ethical appoval
This article does not describe any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
438_2018_1424_MOESM1_ESM.xls
Supplemental file 1 Total QTLs of the five fiber quality traits. FL, fiber length; FS, fiber strength; FU, fiber uniformity; MC, micronaire; FE, fiber elongation (XLS 68 KB)
438_2018_1424_MOESM3_ESM.pdf
Supplemental file 3 All QTL coincidence regions on the genetic map. FL, fiber length; FS, fiber strength; FU, fiber uniformity; MC, micronaire; FE, fiber elongation (PDF 308 KB)
438_2018_1424_MOESM4_ESM.xls
Supplemental file 4 Epistatic QTLs of four fiber quality traits. FL, fiber length; FS, fiber strength; FU, fiber uniformity; MC, micronaire (XLS 51 KB)
Rights and permissions
About this article
Cite this article
Jia, X., Wang, H., Pang, C. et al. QTL delineation for five fiber quality traits based on an intra-specific Gossypium hirsutum L. recombinant inbred line population. Mol Genet Genomics 293, 831–843 (2018). https://doi.org/10.1007/s00438-018-1424-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-018-1424-7