Abstract
Echinococcosis is a worldwide disease endemic to the western region of China. In 2023, echinococcosis was detected in one of 27 wild boars (Sus scrofa) in Yili Prefecture, Xinjiang, northwestern China. Histopathological staining and full sequence mitochondrial (mt) analysis were used to determine the infection genotype. Echinococcus granulosus was detected in the wild boar liver, and the cystic lesion characteristics indicated the E. granulosus genotype (G1). This case is the first confirmation of wild boar serving as a transmitter for the G1 genotype of E. granulosus within China. These findings suggest that surveillance is needed to assess the risk of E. granulosus sensu lato transmission to humans and wild animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic echinococcosis (CE) is a zoonotic disease caused by the tapeworm Echinococcus granulosus sensu lato (s.l.) (Wen et al. 2019). Echinococcus can be transmitted through domestic, wild, and mixed domestic-wild animal cycles (Carmena and Cardona 2014). CE is characterized by a substantial overall disease burden owing to its global distribution and high regional prevalence (Wen et al. 2019). Many studies have investigated the epidemiology and distribution of Echinococcus spp. worldwide and have increased the identification of endemic areas (Vuitton et al. 2020). The intermediate hosts of E. granulosus s.l. include mainly ungulates and, in Australia, marsupials (Romig and Wassermann 2024). The various genotypes of Echinococcus spp. (G1–G10) are characterized according to the intermediate host, although each genotype may infect many intermediate hosts. According to the “International consensus on terminology to be used in the field of echinococcoses” published by Vuitton et al. in the journal Parasite, the new classifications are E. granulosus s.s. (G1 and G3), E. equinus (G4), E. ortleppi (G5), E. canadensis (G6/7 and G8/10), and E. felidis (Le et al. 2002; Vuitton et al. 2020; Wang et al. 2016).
Echinococcosis caused by E. granulosus s.s. (G1 and G3), E. multilocularis, E. ortleppi (G5), and E. canadensis (G6 and G7) in wild boars (Sus scrofa) has been reported in Portugal, Romania, Spain, France, southern Italy, Bosnia, Herzegovina, Serbia, Ukraine, Germany, and southwestern Iran (Boucher et al. 2005; Paoletti et al. 2019; Romig and Wassermann. 2024; Sarkari et al. 2015; Snábel et al. 2009). In China, echinococcosis is mainly distributed in western regions (Wen et al. 2019). Studies in China have focused on livestock, and there have been no reports of echinococcosis in wild boars. It has been reported that the infection rates of CE in cattle and sheep in Yili Prefecture, Xinjiang, are 4.0% and 3.9%, respectively, and E. granulosus G1 is the predominant genotype, followed by E. granulosus G3 (Guo et al. 2019). Additionally, Yili Prefecture has previously reported many alveolar echinococcosis (AE) cases in humans and small mammals, but CE has not been reported in wild animals. Therefore, we surveyed CE in wild animals in Zhaosu County, Yili Prefecture, Xinjiang.
Materials and methods
Sample collection
From May to August 2023, 27 wild boars were captured in Zhaosu County (Fig. 1). We collected wild boar carcasses or captured and killed live wild boars. We visually inspected the livers and lungs of each wild boar for the presence of cystic lesions and preserved them in a solution containing 4% paraformaldehyde.
Histopathological analysis
Tissues with cystic lesions from the infected samples were embedded in paraffin, sliced into 3-µm sections by a Leica microtome, and dehydrated using a Leica automatic dehydrator. The tissue sections were subjected to hematoxylin and eosin (H&E), Masson, and immunohistochemical (IHC) staining. The characteristic features of E. granulosus s.l. metacestode infections include the presence of an adventitial layer (AL), laminated layer (LL), germinal layer (GL), and host cell infiltration (Li et al. 2019).
DNA extraction
Genomic DNA was extracted from each 4% paraformaldehyde-fixed liver sample using the CW BOI Universal Genomic DNA Kit (CW BOI, Beijing, China) according to the manufacturer’s instructions. DNA was eluted with 2 × 50 µL of RNase-free water by centrifugation of the applied column at 12,000 rpm for 2 min, and the extracted DNA was stored at − 20 °C until use.
Identification of wild boar species
Polymerase chain reaction (PCR) and sequencing were performed to identify the wild boar species according to established methods. The primers used were those described in published papers (Watanobe et al. 1999). Briefly, 2 µL of each extracted DNA sample was added to a PCR tube containing a 25 µL mixture comprising 2 µL of primers and 21 µL of H2O. The reaction conditions included initial denaturation at 94 °C for 3 min; 35 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 1 min; and a final extension at 72 °C for 5 min. The PCR products were subsequently sent to Anhui General Biology (Hefei, China) and processed using the Sanger sequencing method. BioEdit (http://www.mbio.ncsu.edu/bioedit/bioedit.html) was used to compare the obtained sequences with sequences in GenBank.
PCR amplification of E. granulosus s.l.
We used DNA extracted from cystic tissue from infected wild boars to amplify and determine the full mitochondrial (mt) sequences of E. granulosus s.l. A total of 11 primer sets were designed with overlap (Table 1) (Yang et al. 2005). The extracted DNA was stored at − 20 °C for PCR amplification, following a protocol consisting of an initial denaturation step at 94 °C for 5 min; 35 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 2 min; and a final extension step at 72 °C for 5 min. The PCR mixture was composed of a total volume of Master Mix (GoTaq Green Master Mix, Promega, Madison, WI, USA) containing Taq polymerase (1 U), dNTPs (400 µM), MgCl2 (3 mM), and each primer (0.3 µM). The DNA template volume was 2 µL, and nuclease-free water was added to a 50 µL total volume of the PCR mixture. Sequencing was performed as described above.
Sequence analysis of E. granulosus s.l.
The 11 amplified segments were assembled sequentially by DNAMAN version 8.0 software, and the mt ring map was drawn by SnapGene 4.2.4 software. The full mt sequences were aligned using Clustal X2 and BioEdit 5.0 and compared with available full mt sequences from Japanese wild boar (Sus scrofa leucomystax) and Ryukyu wild boar (Sus scrofa riukiuanus) from the GenBank database through BLAST analysis (http://www.ncbi.nlm.nih.gov/Blast.cgi, accessed on May 5, 2020). Phylogenetic analysis was performed using MEGA 11.0. A phylogenetic tree was constructed using the neighbor-joining (NJ), maximum parsimony (MP), and maximum likelihood (ML) methods and the Kimura 2-parameter model. Bootstrapping with 1000 replicates was conducted to test the robustness of the phylogenetic tree.
Results and discussion
Numbers and types of wild boars
During the survey, 27 wild boars were captured, one of which was infected by E. granulosus. PCR amplification of mt cytochrome b (cob) confirmed a sequence similar to that of the published sequences of wild boar in the GenBank database. Among the 27 wild boars, 14, 6, 2, and 1 sequences were identical to the wild boar sequences AB015070, AB015067, AB015068, and AB015066, respectively, published in Japan, and 4 were identical to the wild boar sequence AB015070 published in Ryukyu.
Histopathological morphology
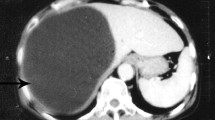
We found a solitary cyst measuring 10 cm × 10 cm on the liver (Fig. 2A). No protoscoleces (PSCs) were observed under a light microscope, and the cyst was not fertile according to H&E staining; however, characteristic features of the cystic lesions included the presence of an AL, LL, and GL, as well as host cell infiltration (Fig. 2B, C, D). Masson staining revealed that liver infection caused a severe inflammatory response and liver fibrosis (Fig. 2C). IHC analysis revealed significant expression of alpha-smooth muscle actin (α-SMA) around the lesions (Fig. 2D) (Abcam, UK). These findings were consistent with those reported by Li et al. (2019). Cystic lesion formation followed the typical pattern of AL, LL, and GL in a gradually increasing number of lesions. At the lesion periphery, numerous fibroblasts and inflammatory cells were present. Granulomas (inflammatory cells with fibrosis) were observed, indicating a typical E. granulosus s.l. infection pattern. Therefore, we determined that the wild boar was infected with E. granulosus s.l. (Li et al. 2019; Vuitton et al. 2020).
Metacestode pathology of Echinococcus granulosus in wild boar liver. A E. granulosus cystic lesions in wild boar liver. B Hematoxylin and eosin (H&E) staining of boar liver (× 200). C Liver fibrosis as determined by Masson’s trichrome staining (× 200). D IHC staining of alpha-smooth muscle actin (α-SMA) in wild boar liver (× 200). Note: the black arrow indicates a cystic lesion, the red arrows indicate the adventitial layer (AL), the yellow arrows indicate the laminated layer (LL), and the green arrows indicate the germinal layer (GL)
Granulomatous inflammatory responses in the infected liver were the most prevalent histopathological changes, which is consistent with previous reports (Díaz et al. 2000). Among the factors that could determine both a distinct type of stimulation of various immune responses and differences at the microenvironmental level, the composition of the LL in E. granulosus s.l. plays a crucial role (Conchedda et al. 2008). The presence of a GL in wild boar was observed through H&E staining, which revealed the specific location where the parasite produces protoscoleces and stem cells (Brehm and Koziol 2017). This observation demonstrated the ability of E. granulosus to adapt to wild boars.
Genotype and sequence analysis
The annotated sequence of E. granulosus G1, with a length of 13,705 base pairs (bp), was deposited in the GenBank database under accession number OR730806. BLAST analysis (http://www.ncbi.nlm.nih.gov/blast) revealed that the annotated sequences obtained from the wild boar exhibited 99.83% similarity to the E. granulosus sequence (accession number: AF297617) available in the GenBank database and 76.41–99.47% identity with the other tapeworm sequences available in the GenBank database (Table 2) (Yang et al. 2005). The near-complete mt genome encoded 12 protein-coding genes (PCGs), 22 transfer RNA (tRNA) genes, and two ribosomal RNA (rRNA) genes (Fig. 3a). The mt ring sequence is shown in Supplementary Table 1.
Map of the near-complete mitochondrial (mt) sequences and phylogenetic tree of the full mt genomes of Echinococcus granulosus genotype 1 and previously published mt sequences of similar species. a Map of the nearly complete E. granulosus mt genome, which included 12 protein-coding genes (PCGs), 22 transfer RNA (tRNA) genes, and two ribosomal RNA (rRNA) genes. b Phylogenetic tree of the full mt genomes of E. granulosus genotype 1 and previously published mt sequences of similar species. The scale bar represents the estimated number of substitutions per site. The red font indicates the wild boar infection genotype reported in this paper
The detection of Echinococcus spp. infection in wild boars across Europe, ranging from France and Romania to Turkey and Iran, has implications for elucidating the transmission dynamics of echinococcosis among wild animals in China. For example, it has been reported that E. canadensis G6/7 occurs in wild boars, and one of four infected individuals harbored fertile cysts (Snábel et al. 2009). This finding highlights the potential role of this wild species as an intermediate host in Spain, France, southern Italy, and southwestern Iran (Boucher et al. 2005; Paoletti et al. 2019; Sarkari et al. 2015). In addition, both the G1 and G7 genotypes were identified in Romania, while G5 was found in wild boars from Portugal (Onac et al. 2013; Mateus et al. 2021). Moreover, E. multilocularis infections have been documented in France, Turkey, and Nordic countries (Boucher et al. 2005).
Phylogenetic analysis
We studied the evolutionary relationship of the G1 genotype derived from the wild boar and other published tapeworm sequences. Two major clades were identified: the Echinococcus spp. clade and the Taenia spp. clade (Fig. 3b). In this study, E. granulosus s.s. (G1, G3) and G1 formed a distinct subclade, while other members of E. granulosus s.l. were grouped into two separate subclades. Based on the topology obtained in our phylogenetic analysis, G1 from this study appears to be closely related to genotypes G1 and G3, forming a small bifurcation. In contrast, G1 in this study showed a more distant relationship to the genotypes of E. equinus and E. felidis, and it had the most distant relationship with genotypes G5–G10 (except for G9) (Fig. 3b). The phylogenetic analysis suggested that G1 from this study has a close evolutionary relationship with G1 and G3 from GenBank but is distantly related to E. vogeli, E. shiquicus, E. multilocularis, and Taenia spp.
This study identified a wild boar infected with E. granulosus G1 in Zhaosu, Yili Prefecture, Xinjiang. These data confirm the transmission of E. granulosus G1 among wild animals and contribute to our understanding of its sporadic appearance in this new area. While many studies have used mt genes for typing E. granulosus s.l. genotypes, they typically use only short or partial segments (Ohiolei et al. 2019), and previous research on wild boar infection did not amplify full-length sequences. Our study revealed the prevalence of echinococcosis among wild boars and reported the amplification of full-length mt genes. The resulting data provide insight into the local E. granulosus s.l. genotype, pathogenicity assessment, and epidemiological analysis.
Conclusions
This study revealed E. granulosus G1 infection in wild boars in Xinjiang, China, suggesting that this pathogen is maintained through circulation between intermediate and definitive hosts. This finding is epidemiologically significant and suggests a transmission route through wild animals. Compared with other Echinococcus species, E. granulosus s.s. is the most pathogenic and virulent species, and wild boar may serve as a suitable transmitter of the parasite in the wildlife cycle, in addition to the dominant domestic cycle that has been described for G1.
Data availability
The study material has been deposited in a curated museum collection, and the data will be made available upon reasonable request.
References
Boucher JM, Hanosset R, Augot D, Bart JM, Morand M, Piarroux R, Pozet-Bouhier F, Losson B, Cliquet F (2005) Detection of Echinococcus multilocularis in wild boars in France using PCR techniques against larval form. Vet Parasitol 129(3–4):259–266
Brehm K, Koziol U (2017) Echinococcus–host interactions at cellular and molecular levels. Adv Parasitol 95:147–212
Carmena D, Cardona GA (2014) Echinococcosis in wild carnivorous species: epidemiology, genotypic diversity, and implications for veterinary public health. Vet Parasitol 202(3–4):69–94
Conchedda M, Gabriele F, Bortoletti G (2008) Morphological study of anomalous “laminated” brood capsules in cystic echinococcosis in humans and sheep. Acta Trop 105(3):215–221
Díaz A, Willis AC, Sim RB (2000) Expression of the proteinase specialized in bone resorption, cathepsin K, in granulomatous inflammation. Mol Med 6(8):648–659
Guo B, Zhang Z, Zheng X, Guo Y, Guo G, Zhao L, Cai R, Wang B, Yang M, Shou X, Zhang W, Jia B (2019) Prevalence and molecular characterization of Echinococcus granulosus sensu stricto in northern Xinjiang. China Korean J Parasito 57(2):153–159
Le TH, Pearson MS, Blair D, Dai N, Zhang LH, McManus DP (2002) Complete mitochondrial genomes confirm the distinctiveness of the horse-dog and sheep-dog strains of Echinococcus granulosus. Parasitology 124(Pt 1):97–112
Li Z, Zhang C, Li L, Bi X, Li L, Yang S, Zhang N, Wang H, Yang N, Abulizi A, Aini A, Lin R, Vuitton DA, Wen H (2019) The local immune response during Echinococcus granulosus growth in a quantitative hepatic experimental model. Sci Rep 9(1):19612
Mateus TL, Gargaté MJ, Vilares A, Ferreira I, Rodrigues M, Coelho C, Vieira-Pinto M (2021) First report of Echinococcus ortleppi in free-living wild boar (Sus scrofa) from Portugal. Microorganisms 9(6):1256
Ohiolei JA, Xia CY, Li L, Liu JZ, Tang WQ, Wu YT, Danqulamu-Danqulamu, ZhuGQ-Zhu GQ, Shi B, Fu BQ, Yin H, Yan HB, Jia WZ (2019) Genetic variation of Echinococcus spp. in yaks and sheep in the Tibet Autonomous Region of China based on mitochondrial DNA. Parasit Vectors 12(1):608
Onac D, Győrke A, Oltean M, Gavrea R, Cozma V (2013) First detection of Echinococcus granulosus G1 and G7 in wild boars (Sus scrofa) and red deer (Cervus elaphus) in Romania using PCR and PCR-RFLP techniques. Vet Parasitol 193(1–3):289–291
Paoletti B, Della Salda L, Di Cesare A, Iorio R, Vergara A, Fava C, Olivastri A, Dessì G, Scala A, Varcasia A (2019) Epidemiological survey on cystic echinococcosis in wild boar from Central Italy. Parasitol Res 118(1):43–46
Romig T, Wassermann M (2024) Echinococcus species in wildlife. Int J Parasitol Parasites Wildl 23:100913
Sarkari B, Mansouri M, Khabisi SA, Mowlavi G (2015) Molecular characterization and seroprevalence of Echinococcus granulosus in wild boars (Sus scrofa) in south-western Iran. Ann Parasitol 61(4):269–273
Snábel V, Altintas N, D’Amelio S, Nakao M, Romig T, Yolasigmaz A, Gunes K, Turk M, Busi M, Hüttner M, Sevcová D, Ito A, Altintas N, Dubinský P (2009) Cystic echinococcosis in Turkey: genetic variability and first record of the pig strain (G7) in the country. Parasitol Res 105(1):145–154
Vuitton DA, McManus DP, Rogan MT, Romig T, Gottstein B, Naidich A, Tuxun T, Wen H, Menezes da Silva A (2020) International consensus on terminology to be used in the field of echinococcoses. Parasite 27:41
Wang N, Xie Y, Liu T, Zhong X, Wang J, Hu D, Wang S, Gu X, Peng X, Yang G (2016) The complete mitochondrial genome of G3 genotype of Echinococcus granulosus (Cestoda: Taeniidae). Mitochondrial DNA A DNA Mapp Seq Anal 27(3):1701–1702
Watanobe T, Okumura N, Ishiguro N, Nakano M, Matsui A, Sahara M, Komatsu M (1999) Genetic relationship and distribution of the Japanese wild boar (Sus scrofa leucomystax) and Ryukyu wild boar (Sus scrofa riukiuanus) analysed by mitochondrial DNA. Mol Ecol 8(9):1509–1512
Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP (2019) Echinococcosis: advances in the 21st century. Clin Microbiol Rev 32(2):e00075-e118
Yang YR, Rosenzvit MC, Zhang LH, Zhang JZ, McManus DP (2005) Molecular study of Echinococcus in west-central China. Parasitology 131(Pt 4):547–555
Acknowledgements
We thank the primary-level workers for their help in the capture and necropsy of the wild boars.
Funding
This study was funded by the Key Research and Development Task Special of Xinjiang Uygur Region (2022B03013-3, 2022B03013-1), the Xinjiang Key Laboratory of Animal Infectious Diseases (Institute of Veterinary Medicine, Xinjiang Academy of Animal Science) (2023KLB002), the Central Government Guides Local Science and Technology Development Fund Projects (ZYYD2023A01), and the State Key Laboratory of Pathogenesis, Prevention and Treatment of Central Asian High Incidence Diseases Fund (SKL-HIDCA-2022-BC4).
Author information
Authors and Affiliations
Contributions
BPG, JYW, RSM, and HW designed the study. BPG, LZ, KA, WQT, CCW, and YM conducted the experiments. BPG conducted the data analysis. BPG, JYW, RSM, and HW wrote the paper. All authors revised the manuscript critically for important intellectual content, read, and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This field study of wild boars was approved by the Ethics Committee of The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China (approval number 20230408–15).
Consent to participate
Not applicable.
Consent for publication
All authors agree to the publication of this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Julia Walochnik
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, B., Cairen, Zhao, L. et al. First report of Echinococcus granulosus genotype 1 in a wild boar (Sus scrofa) from China. Parasitol Res 123, 236 (2024). https://doi.org/10.1007/s00436-024-08249-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-024-08249-3