Abstract
Echinococcal disease (hydatid disease (HD) is an endemic parasitosis caused by Echinococcus granulosus in the larval stage, and it is typically due to the production of unilocular cystic lesions, usually involving the liver for the majority of patients and the lungs in 25%, but also any other organs can be potentially involved in developing echinococcal disease. We report a case of extrahepatic, retroperitoneal echinococcal disease, caused by Echinococcus granulosus. The patient underwent a surgical removal of the abdominal mass, revealed by abdominal ultrasound and computerized tomography scanning, and in the founded clinical and radiological suspicion of echinococcal disease, multiple bioptical samples were sent for microbiological analysis and albendazole therapy was started; Echinococcus granulosus protoscolices were found on the bioptical sample, and the diagnosis was successfully confirmed. According to the current parasitology literature on echinococcal disease, extrahepatic localization, although rare, can be found, and it should be considered in the differential diagnosis of an abdominal mass when epidemiological risk factors and anamnestic data are present, regardless of the usual site of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case history
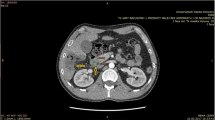
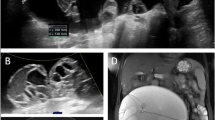
A 61-year-old Romanian female was admitted to our emergency department on February 22, 2023, with a 1-month history of intense abdominal pain and discomfort. No history of fever or weight loss was reported. The patient had immigrated from Romania to Italy in 2008 and used to return frequently (> two times a year) to Romania, in a rural setting where she lived since birth. Previous contact with farm animals was referred by the patient, at the anamnestic investigation, and no other travel history was declared. She had a history of severe chronic kidney disease, with a reported surgical history of left nephrectomy in 2009 because of a suspected cystic renal tumor, and right nephrostomy placement in 2022 in Romania, for worsening of kidney function. In 2011, she also underwent a surgical operation of bilateral iliac stent placement for atherosclerotic aneurism. On an accurate abdominal examination, pain was mainly located in the right flank and mesogastric area. Abdominal ultrasound and computerized tomography scanning were performed and revealed a solid retroperitoneal expansive polylobed heterogeneous mass, of large dimension (axial 15.3 × 12.2 cm, CC 12.5 cm) which incorporated the right iliac stent. Chest X-ray showed no focal parenchymal abnormalities. In the following days, a progressive worsening of clinical condition was observed; an urgent abdominal computerized tomography examination revealed a hemorrhagic leaking localized inside the abdominal mass. An urgent surgical implantation of aorto-bi-iliac stent graft was needed to be performed. In suspicion of a gynecological cancer origin of the abdominal mass, elective surgery of a complete resection was performed on March 3. During an initial laparoscopic approach, multiple subhepatic cystic formations with regular walls were found, and samples were sent for extemporaneous histopathological examination (EHE). Histopathology revealed plurilaminated acellular structure, suggestive of hydatidosis. Therefore, laparotomic conversion was necessary to perform a complete and radical excision of the cystic formations [F]. A careful and radical removal of the mass was assessed, in order to avoid cyst rupture and possible subsequent metastatic spread. The procedure required a multidisciplinary consultation comprehensive of urological and vascular evaluations, for iliac veins and ureteral involvement. In the well-founded suspicion of echinococcal disease, multiple bioptical samples were sent for microbiological analysis and albendazole therapy was started. The biopsy specimen was delivered to the Microbiology and Virology Laboratory in an appropriate swab for transport and storage (eSwab, Copan, Italy). The sample was resuspended by vortex (VELP Scientifica, Italy) for 30 s, and then, a series of unstained wet mount slides were prepared for the observation under a light microscope Olympus BX60 (Olympus, Japan) at 100 × and 400 × magnification [A, B,C]. Echinococcus granulosus protoscolices were found on the bioptical sample, and the diagnosis was successfully confirmed (Fig. 1). A new computerized tomography of the low abdomen and thighs was performed, showing an ulterior localization of the disease, in postero-lateral compartment of the right thigh [D, E]. The patient was subsequently discharged from hospital without any post-surgery complications and with a plan for follow-up care with an infectious disease specialist.
A Invaginated protoscolices (wet mount, 400 × magnification); B evaginated protoscolices (wet mount, 400 × magnification); C free hooklets (wet mount, 400 × magnification); D computerized tomography scan of the abdomen before surgery; E post-surgical computerized tomography scan of right thigh; F intraoperatory view of the abdominal mass
Discussion and results
Hydatid disease (HD) is an extremely widespread parasitosis caused by the larval stage of Echinococcus granulosus; it is characterized by the production of unilocular cystic lesions, usually affecting the liver in the majority of patients and the lungs in 25% of affected individuals, but other organs may also be implicated as an alternative and rare site of infection (Tsaroucha et al. 2005; Ozturk et al. 2014). Extrahepatic localizations are infrequent and reported in a small proportion of patients; primary retroperitoneal hydatid cysts are rare and first described in 1958 by Lockhart and Sapinza (Erdem et al. 2009; Tepetes et al. 2007; Kiresi et al. 2003). Roughly 90% of patients with Echinococcus granulosus infection have only one organ involvement and most of patients present with only one cyst (Sirus et al. 2006). Cystic echinococcosis (CE) has an overall prevalence in pastoral regions due to an increased possibility of zoonotic transmission. It is considered endemic in areas such as the Middle East, the Mediterranean region, Greece and Lebanon, Australia, Argentina, and Africa. The life cycle of Echinococcus includes a definitive host (typically dogs) and an intermediate host (sheep, cattle, cervids, goats and horses). Humans are an incidental host who can acquire the infection through the ingestion of eggs, from the feces of the definitive host. Human to human transmission does not occur (Ammann and Eckert 1996). Oncospheres are released in the intestine, and hydatid cysts can grow in a variety of organs. If cysts rupture, the liberated protoscolices may create secondary cysts in other sites within the body (secondary echinococcosis) like lungs, liver, and other organs (https://www.cdc.gov/dpdx/echinococcosis/index.html). The endocyst has an outer acellular laminated layer and an inner, germinal layer that give rise to protoscolices. In some stages of cyst development, daughter elements of different sizes can be identified (Ammann and Eckert 1996). In degenerating cyst, one might see free floating hooklets [c]. Referring to our patient, she presented the typical risk factor overmentioned, and it is likely that she acquired the infection in Romania, in pastoral areas (Agudelo Higuita et al. 2016). She reported a several-years history of kidney severe disease, with no definite diagnosis, and parasitosis was not considered in differential diagnosis. According to the current literature, it is not clear to understand the growth rate of the echinococcal cyst. Some studies reveal the growth rate of cysts over time, making it difficult to establish the exposure time. The incubation period and subsequent clinical presentation are highly variable between infected individuals: it depends on several cofactors such as the organ involved, the relationship with surrounding structures, and size. The diagnosis of cystic echinococcosis is still primarily based on imaging. The World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) published in 2003 has been dedicated to the clinician to be used in the clinical practice and to implement tailored management for each stage of infection (Anonymous 2003). In our case, cysts were fully active, like in CE1 and CE2 groups. This classification was developed based on analysis of cystic echinococcosis cysts in the liver, but it can also be applied in the management and follow-up of other non-hepatic localization. Immunodiagnostics plays an ancillary role in diagnosis due to limitations in sensitivity and specificity, and the World Health Organization recommends sequential testing with primary screening including immunosorbent assays (ELISA), indirect hemagglutination antibody test (IHAT), and immunoelectrophoresis (IEP) (Eckert et al. n.d.). In our case, ELISA serology test was performed after surgery, and it resulted negative, not excluding the presence of cystic echinococcosis, according to the overmentioned variable sensitivity and specificity of these tests. The overview of the management of this condition is very varied around the world even though the WHO-IWGE published an expert consensus in 2010 (Brunetti et al. 2010). The refinement of surgical techniques and the introduction of minimally invasive treatments (such as (PAIR)) and anthelmintic therapy such as benzimidazoles have really changed the life expectancy of patients with hydatid disease (Nunnari et al. 2012). In our case, treatment with albendazole was administered before the parasite was directly observed in the bioptical samples, due to the high previous clinical suspicious of HD. Cystic echinococcosis remains a diagnostic challenge for physicians and a disease of complex management. Extrahepatic localization is still little discussed. Diagnosis is based on radiological imaging and is then confirmed by direct observation, in histopathology and microbiology, of protoscolices and hooklets in cystic fluids. Total cystectomy, when possible, is the treatment of choice for large extrahepatic echinococcal cysts. The surgical treatment should be combined with a careful use of scolicidal fluids and aspiration of the cyst to avoid contamination and spread, thus minimizing the risk of recurrence. Anthelmintic therapy is still the optimal choice of therapy. Although rare, retroperitoneal localization has been reported in literature, and it should be considered in the differential diagnosis of a suspected abdominal mass when epidemiological risk factors and suggestive anamnestic data are present, regardless of the usual site of disease localization.
Data availability
The datasets for the current study are available from the corresponding author on reasonable request.
References
Agudelo Higuita NI, Brunetti E, McCloskey C (2016) Cystic echinococcosis. J Clin Microbiol 54:518–523
Ammann RW, Eckert J (1996) Cestodes: echinococcus. Gastroenterol Clin 25:655–689
Anonymous. (2003) International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop 85:253–261
Brunetti E, Kern P, Vuitton DA (2010) Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 114:1–16
Eckert J, Gemmell MA, Meslin F-X et al (n.d.) WHO/ OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organization for Animal Health (Office International des Epizooties) Paris, France, and World Health Organization, Geneva, Switzerland
Erdem MR, Akbaş A, Onol FF, Tanidir Y, Onol SY (2009) An unusual retroperitoneal sero-negative hydatid cyst presenting with lower urinary tract symptoms. Turk Parazitol Derg 33:82–84
Kiresi DA, Karabacakoglu A, Odev K et al (2003) Uncommon locations of hydatid cysts. Acta Radiol 44:622–636
Nunnari G, Pinzone MR, Gruttadauria S et al (2012) Hepatic echinococcosis: clinical and therapeutic aspects. World J Gastroenterol 18:1448–1458
Ozturk S, Unver M, Kibar Ozturk B, Kebapci E, Bozbiyik O, Erol V, Zalluhoglu N, Olmez M (2014) Isolated retroperitoneal hydatid cyst invading splenic hilum. Case Rep Surg 2014:303401
Sirus M, Zhianpour M, Golshahi F (2006) Omental and retroperitoneal hydatid cyst: a case report. Iran J Radiol 217–220
Tepetes K, Christodoulidis G, Spryridakis M et al (2007) Large solitary retroperitoneal echinococcal cyst: a rare case report. World J Gastroenterol 13:6101–6103
Tsaroucha AK, Polychronidis AC, Lyrantzopoulos N et al (2005) Hydatid disease of the abdomen and other locations. World J Surg 9:1161–5
Author information
Authors and Affiliations
Contributions
S.D.—designed the study and main revision of text and figures.
A.F.—clinical investigation and diagnosis and data collection.
G.Q.—microbiology investigation and data observation, figures.
V.I.—data collection, wrote main manuscript text and revisions, submission, revision, and re-submission.
R.J.S.—data collection, figures, and wrote manuscript.
G.M.—radiology figures and text revision.
G.F.—microbiology investigation and data observation, figures.
L.M.—microbiology investigation direction and data observation and analysis.
E.T.—clinical investigation and diagnosis and data collection.
A.B.—data collection and main manuscript revisions.
A.N.—clinical investigation and diagnosis and data collection.
G.S.—diagnosis, clinical investigation direction and data collection, text revisions.
Corresponding author
Ethics declarations
Ethical approval
The study was performed in accordance with the 1964 Declaration of Helsinki and later amendments and in conformity with good clinical practice.
Consent to participate
Consent to participate was obtained by the subject of the study.
Consent for publication
Consent to publish was obtained by the subject of the study.
Competing interests
All authors declare no interests of financial nature or personal nature.
Additional information
Handling Editor: Julia Walochnik
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Di Giambenedetto, S., Fagotti, A., Quaranta, G. et al. Reporting a single case of cystic echinococcosis in retroperitoneal mass of uncertain origin. Parasitol Res 123, 40 (2024). https://doi.org/10.1007/s00436-023-08018-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-023-08018-8