Abstract
The host immune response in Oreochromis mossambicus during natural infection with the isopod Cymothoa eremita was investigated. The naturally infected fishes were examined for enzyme profile, viz., respiratory burst activity (RBA), myeloperoxidase activity (MPO), and expression of immune-related genes, viz., toll-like receptor 22 (TLR 22), interleukin-1β (IL-1β), tumor necrosis factor α (TNF-α), complement component (C3), chemokine (CXCa), and β-actin in tissues of various organs (buccal cavity, gills and anterior kidney). Significant reduction (P < 0.05) in RBA and MPO was observed in the parasite-infected fishes when compared to the uninfected control fishes. In the buccal cavity, the expression of the immune-related genes was significantly (P < 0.05) upregulated, whereas all the genes except IL-1β were significantly (P < 0.05) upregulated in the anterior kidney. In the case of gill tissue, the expressed genes showed a varied type of regulation. The immunological responses in O. mossambicus during isopod infection have not been investigated in detail so far, and this is the first study unveiling such insights. Hence, this study will help to improve our molecular understanding of the host-immune response to parasitic isopod infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cymothoids are ectoparasitic, protandrous, and obligate isopods that infect fish in tropical and subtropical regions, leading to detrimental consequences for their hosts (Trilles et al. 2011; Smit et al. 2014). Isopods are found in many commercially important fish species worldwide, and their impact on these fishes includes killing, stunting, or causing damage that results in significant economic losses to fisheries (Ravichandran et al. 2010; Bharadhirajan et al. 2014). Isopod-inflicted mortalities in mariculture facilities are common, causing substantial mortality in cultured fish (Horton and Okamura 2001; Mladineo 2002; Rajkumar et al. 2005). Furthermore, isopod infections have been reported in cultured and wild tilapia, causing mortalities in countries such as the Philippines (Lopez 2001), Thailand (Chinabut 2002) and Egypt (Abdelkhalek et al. 2017).
Cymothoids are prone to infesting the buccal cavity, gill chamber, body surface and fins of fishes (Brusca 1981; Thamban et al. 2015). Host specificity is influenced by the convergence of both parasite and host lineages and refers to the tendency of a parasite to occur on one or a few host species (Poulin 2007). The site specificity of the parasite is determined by its requirements and the impediments applied by the morphology and habits of the host (Morton 1974). These blood-feeding parasites exhibit a variety of pathogenic effects, causing direct harm not only to the host’s skin, gills, and tongue but also to the host’s physiological and reproductive functions (Brusca 1978; Adlard and Lester 1994; Ostlund-Nilsson et al. 2005). Numerous pathogenic effects, such as tissue damage, anemia, a reduction in mean weight and length, growth inhibition, and mortality, have been observed in aquaculture due to cymothoid infection (Marks et al. 1996; Sievers et al. 1996; Leonardos and Trilles 2003).
C. eremita is commonly found attached to the buccal cavity of the host, infecting the basihyal or tongue bone. Distribution has been reported from the Western Indian Ocean to the Western Pacific (Trilles 1994) and known to infect fish of different families, including Aulopidae, Haemulidae, Serranidae, Mugilidae, Siganidae, Sphyraenidae, Psettodidae, Stromateidae, Carangidae, Tetraodontidae (Hadfield et al. 2013), and Lutjanidae (Martin et al. 2016) in different countries such as the Philippines (Trilles 2008), Japan (Saito 2000), South China Sea (Yu and Li 2003), and India (Jayadev Babu and Sanjeeva Raj 1984; Trilles et al. 2011; Rameshkumar et al. 2016; Vigneshwaran et al. 2019; Ray et al. 2022).
Understanding the full range of natural defence mechanisms used by fish to fight off parasites is crucial for effective disease control (Kar et al. 2015). However, the specific defence mechanism during isopod infection through the host immune gene response is unknown apart from hematology and histopathology (Ozdemir et al. 2016; Elgendy et al. 2018). Oreochromis sp. is the third largest aquacultured fish in the world, holding ecological and economic importance (FAO 2022), and it is also a widely studied fish species with well-characterized immune responses, making it a suitable model for investigating immune parameters. While isopods can indeed be host-specific, it is essential to understand the immune response in important host species like Oreochromis mossambicus to gain insights relevant to isopod infections.
This study aimed to investigate the immune response in naturally infected tilapia O. mossambicus which include immune gene expression and non-specific immune parameters during the C. eremita infection. The immune genes such as TLR 22, IL-1β, TNF-α, C3, and CXCa were used for this study based on their known roles and importance in fish immune response. TLRs have a crucial role in fish innate immunity; IL-1β and TNF-α are pro-inflammatory cytokines; C3 is a principal complement factor which is crucial for the generation of the membrane attack complex; and CXCa stimulates immune cell migration to infected regions (Saurabh et al. 2011). We have also postulated eliciting modifications in enzyme activity associated with the immune response in O. mossambicus.
Materials and methods
Samples
Approximately 150 nos of O. mossambicus of various size were collected using a catch net from Pulicat Lake (13.4177° N, 80.3185° E), a brackish water lake situated in the Tiruvallur district, Tamil Nadu, in October 2021 which corresponds to monsoon season. Based on the visual observation, apparently healthy and isopod-infected fishes collected were segregated and maintained in the Pulicat Farm Facility-TNJFU (Tamil Nadu Dr. J. Jayalalithaa Fisheries University) for the study. Both infected and uninfected fish were housed separately in rearing tanks with optimal dissolved oxygen (5 ppm), salinity (27 ppt) and water temperature (24–27 °C) for a duration of 7 days.
The fishes from the infected (n = 15) and control groups (n = 15) from each group were anaesthetized with clove oil at a concentration of 90 mg L−1 The fish skin was thoroughly wiped and cleansed before blood sampling to prevent mucus from contaminating the blood. Blood samples were drawn from each group using a 2-mL syringe from the caudal vein, ensuring no harm to the fish (Kaya and Kocatepe 2014). The samples were processed separately to assess immune parameters, viz., RBA and MPO in triplicates for each sample. Following blood collection from the all the fishes, they were dissected to collect tissues for analysis (buccal cavity, gill, and anterior kidney), which were stored in RNAiso plus (Takara, Japan) at − 20 °C for total RNA extraction. Each target tissue from every five fishes was pooled separately, resulting in three replicates of each organ for gene expression studies. Parasites were carefully removed from the fish and stored in 70% ethanol for PCR-based molecular characterization and in 5% formalin for classical identification.
Molecular characterization and identification of parasites
Parasitological examination was carried out for the identification of isopod parasites attaching to the buccal cavity following the descriptions of Hadfield et al. (2013). For molecular characterization, total DNA was extracted from the parasites using the Qiagen DNA easy Blood and Tissue Kit, following the manufacturer’s instructions. Partial 28S rDNA was amplified using universal primers (Table 1) and the protocol outlined by Roy et al. (2015). The amplified PCR products were resolved in a 1.5% agarose gel containing 0.5 μg/mL ethidium bromide. Visualization and documentation of DNA bands were done using a gel documentation system (Bio-Rad, Germany). Nucleotide sequencing of the amplified products was carried out (Eurofins, Bangalore, India) and sequence similarity was assessed using the BLAST (Basic Local Alignment Search Tool) program of NCBI (National Centre for Biotechnology Information).
Real-time PCR assay
To assess gene expression via real-time PCR (qRT PCR), total RNA was extracted using RNA iso-plus (Takara Bio) following the manufacturer’s protocol from buccal cavity, gill, and anterior kidney tissues. The quality of the extracted RNA was evaluated using a Nanodrop spectrophotometer (Thermo Fisher), and the absorbance ratio at 260 nm and 280 nm indicated a value of approximately 2.0, suggesting a good RNA quality. Residual genomic DNA was removed using RNAse-free DNAse I (Fermentas, USA). RNA extracted from samples was transcribed to cDNA using a first-strand cDNA synthesis kit (Qiagen, Germany, Thermo Scientific) and stored at − 80 °C until use. The real-time PCR (qRT PCR) assay was done in a total volume of 25 μL with 2 μL of cDNA template, 1 μL of each primer (forward and reverse), 12 μL SYBR Green PCR master mix kit (Takara Bio) and 9 μL of RNAs free nuclease water. Gene expression profiles in the tissues of healthy and parasite-infected fish were analyzed using real-time PCR equipment (1000 Touch thermal cycler–CFX96, Bio-Rad, Germany) following a thermal profile of initial denaturation at 95 °C for 3 min, denaturing at 95 °C for 3 s for 40 cycles, and annealing at 56–60 °C (depending upon the targeted gene) for 30 s, with extension at 72 °C for 30 s, finally ending with a dissolution curve. The primers and program used for relative gene expression studies are mentioned in Table 1.
The qRT-PCR was conducted in triplicates, and the relative expression level of the specific gene was presented as 2−ΔΔCt (Livak and Schmittgen 2001) by measuring the amplified threshold cycle (Ct) values. The housekeeping gene β-actin expression was used as an internal control to normalize gene expression, considering its uniform expression across all eukaryotic cell types to compare the relative expression levels of the genes. This choice was influenced by prior research in the field, where β-actin has been successfully used for normalization involving parasitic infection (Zhi et al. 2018; Kar et al. 2015).
Respiratory burst activity (RBA)
RBA was assessed by the reduction in nitroblue tetrazolium (NBT) to formazan (Anderson and Siwicki 1995). Briefly, blood was mixed with 0.2% NBT in equal proportion (1:1), followed by incubation for 30 min at 25 °C using glass tubes. One milliliter of dimethyl formamide (SRL, Mumbai, India) was added to 50 μL of this reaction mixture to solubilize the reduced formazan product, and the mixture was centrifuged at 2000 g for 5 min. The extent of NBT reduction was measured by taking the optical density of the supernatant at 540 nm, using dimethyl formamide as the blank.
Myeloperoxidase activity (MPO)
The total MPO content present in serum was measured according to Quade and Roth (1997) and a partially modified technique of Sahoo et al. (2005). About 10 μL of serum was diluted with 90 μL of Hank’s balanced salt solution without Ca2+ or Mg2+ in transparent U-bottomed 96-well microtitre plates. Then, 35 μL of freshly prepared 20 mM 3,30-,5,50-tetramethyl benzidine hydrochloride and 5 mM of H2O2 were added. The change in color during the reaction was stopped after 2 min by adding 35 μL of 4 Molar sulfuric acid, and at 450 nm, the OD was assessed using an ELISA reader (BioTek, USA).
Statistical analysis
All data were analyzed in triplicates using IBM SPSS 26.0 software for Windows (SPSS Inc.). Normality was assessed using the Shapiro-Wilk test, and as the data were normally distributed (P > 0.05), they were presented as mean ± standard deviation (SD) (descriptive statistics). One-way ANOVA followed by Tukey’s post hoc test for multiple comparisons of means at a significance level of 0.05 was used to determine the variation in parameters (dependent variable) among test groups (independent variable).
Result
Classical identification of the isopods infecting O. mossambicus based on the dorsal and ventral structures confirmed that their identity as C. eremita (Fig. 1) as the observed isopods closely resembled the holotype illustrations of Hadfield et al. (2013) and Martin et al. (2016). Dorsal surfaces appeared glossy, with pereonite 5 being the broadest and pereonite 1 the narrowest. Anterolateral borders of pereonite 1 reached about half the length of the cephalon; Coxae 2 and 3 had subtruncate posteroventral edge angles, while 4–7 was rounded. Pereonites 1–5 increased in length and width, becoming narrower and more rounded posteriorly exhibiting an ivory-white color.
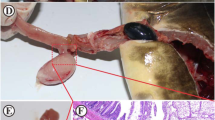
Our surveillance indicated that C. eremita infection tends to be more prevalent during the monsoon season in this region. Both ovigerous females and male isopods were observed in the buccal cavity of the fishes, confirming the site specificity nature of the parasite (Fig. 2). In some cases, we also observed the combined infection where female and male isopod harboring the mouth and buccal cavity of the fishes. Among the 150 fishes collected, 47 specimens were found to be parasitized by C. eremita. Female isopods were present in 41 fishes, male isopods in 3 fishes, and both male and female isopods coexisted in only 3 fishes which resulted in an overall prevalence rate of 31.3% and parasite abundance of 0.33. Notably, the infection exhibited higher prevalence in fishes with length more than 500 mm TL, while smaller fishes displayed a lower susceptibility to this parasitic infection.
PCR amplification of isopod DNA using a universal primer derived from the consensus sequence of 28S rDNA resulted in a 443 bp product. Nucleotide sequencing and the BLAST analysis reveal that no reported sequences targeting 28S rDNA were available for this isopod, and so, the identity was confirmed by the morphometric methods. The sequences were submitted to the Genbank database as C. eremita and the accession number was acquired (Accession No. ON561786).
RBA was significantly lower (P < 0.05) in parasite-infected fishes compared to uninfected ones. Additionally, MPO was also significantly reduced (P < 0.05) in parasite-infected fishes compared to healthy ones (Fig. 3). Real-time expression of immune-related genes relative to the β-actin transcript in the buccal cavity, gills, and anterior kidney is shown in Fig. 4. TLR 22 and TNF expression in the buccal cavity and anterior kidney were up-regulated in infected fishes compared to healthy ones. In contrast, the expression of TLR 22 and TNF in gills was significantly (P < 0.05) down-regulated in infected fishes compared to uninfected controls.
Heat map of relative expression of different immune-related genes in the organs of O. mossambicus infected with C. eremita and uninfected fishes. The color codes on the right represent the expression level of different organs to the genes, where a represents the highest value and b represents the lower value contributing the significant difference between the infected and uninfected organs. CT, control uninfected; IF, isopod infected. The arrow with asterisk (***) was actually provided to indicate the presence of strong significant difference (P < 0.001) between each control and infected organs. One-way ANOVA was followed by Tukey’s post hoc test for multiple comparison of means for individual genes between each control and infected organs
IL-1β gene expression was relatively higher in the buccal cavity and gills of infected fishes compared to controls. However, no significant difference (P < 0.05) in IL-1β gene expression in the anterior kidney was observed between the infected and control groups. The infected fishes exhibited significant (P < 0.05) upregulation in C3 gene expression in the buccal cavity, anterior kidney and gills compared to healthy fish. For the CXCA gene, infected fishes showed significantly (P < 0.05) upregulated expression in the buccal cavity and anterior kidney compared to healthy controls. However, no significant (P < 0.05) alterations in expression were observed in gills between infected and uninfected fish.
Discussion
According to the data, O. mossambicus is predominantly parasitized by female isopod C. eremita, with exceptions noted when both male and female isopods coexist within the same host. C. eremita has been reported to infect the buccal cavity of Parastromateus niger in east coast of India (Rameshkumar et al. 2012; Vigneshwaran et al. 2019) and Lutjanus johnii (Ray et al. 2022). But in this study, it has been recovered from the buccal cavity of O. mossambicus collected from brackish water, which makes it a new host for this species. This research highlights the presence of C. eremita in O. mossambicus during the monsoon season in Pulicat Lake, India. In contrast, Rameshkumar et al. (2012) reported C. eremita infection in P. niger during the summer season along Parangipettai coast of India. The correlation in size between male parasites was less pronounced compared to females, highlighting a defining trait of the Cymothoidae family where males are generally smaller than females, especially in genera that attach to the branchial and buccal regions (Smit et al. 2014). In this study, the isopod infection rate was higher in the fishes with length more than 500 mm TL as observed in P. niger, where the fishes ranging more than 300 mm TL were more susceptible to infection (Vigneshwaran et al. 2019). The study delves into specific organs, buccal cavity, gills, and anterior kidney to capture a holistic picture of the host’s immune reactions encompassing, both local and systemic responses in fishes infected with female isopod as they were more prevalent in this study. Additionally, comparing multiple organs provides a comprehensive view of the host-parasite interaction.
Innate immune responses in the fish hosts get activated during pathogen infection to maintain homeostasis. Transcriptional analysis of immune-related genes provides a true picture of the host’s immunological condition (Dash et al. 2014). Expression of immune-regulatory genes in fish with several different parasite infections and their role in particular disease processes have been documented in various fish species (Zhi et al. 2018; Li et al. 2022; Zeng et al. 2023). A non-specific immune response serves as the first line of defence during a pathogen infection (Dash et al. 2014). The respiratory burst is an important host response that produces reactive oxygen species (ROS) to evade invading pathogens. The myeloperoxidase is a chief oxidative enzyme in fish generated by neutrophils that works against infections by producing reactive oxygen molecules. It is known to have microbicidal action when discharged into phagolysosomes after the phagosome and lysosome junction is used to assess superoxide generation (Siwicki and Anderson 1993).
In this study, both RBA and MPO activity were found to be significantly lower (P < 0.05) in the C. eremita infected O. mossambicus when compared to the healthy fishes. This result suggests that the C. eremita-infected O. mossambicus exerts very low oxidative components towards the invaded ectoparasite; as a result, the host defense could not be eliminated leaving them to develop upon feeding the host tissues and blood. Supporting this, a similar reduction in RBA was observed in the bogue fish (Boops boops) infected with Ceratothoa oestroides (Ozdemir et al. 2016). However, increased RBA has been observed in the Scophthalmus maximus infected with Enteromyxum scophthalmi and Labeo rohita infected with Dactylogyrus catlaius (Sitja-Bobadilla et al. 2006; Dash et al. 2014) which might be due to their histozoic nature and smaller size respectively facilitating the host defense. Similarly, an increased MPA was observed in Diplodus puntazzo infected with Enteromyxum leei (Munoz et al. 2007; Ozdemir et al. 2016). However, no significant changes in MPA were observed in the D. catlaius-infected Labeo rohita (Dash et al. 2014) suggesting that the parasite that feeds on host circulatory fluids might not able to be eliminated by the host oxidative defense.
Toll-like receptors (TLRs) are the best characterized among the functionally different groups of Pattern Recognition Receptors (PRRs), which stimulate innate immune functions such as cytokine generation, cell differentiation, the formation of reactive nitrogen and oxidative radicals, and cell differentiation and also trigger intracellular signal transduction in inflammation, antiviral responses and dendritic cell maturation (Alvarez-Pellitero 2008; Aoki and Hirono 2006). TLR22 is engaged mainly in viral nucleic acid recognition, in addition to bacterial and parasite ligands regulating the gene’s mRNA expression levels, thereby proving their multiple roles (Su et al. 2012). In the present study, significantly upregulated (P < 0.05) expressions of TLR 22 were observed in the buccal cavity and the anterior kidney of O. mossambicus infected with C. eremita. Saurabh et al. (2011) and Kar et al. (2015) observed downregulated expression of TLR 22 in the anterior kidney in L. rohita infected with Argulus siamensis, whereas significant down-regulation of TLR 22 expression was also observed in the gills of O. mossambicus infected with C. eremita as observed by Dash et al. (2014) in the gills of L. rohita parasitized with D. catlaius. The differences in the transcription of immune genes in the buccal cavity in this study might be due to parasite-induced inflammatory reactions in the tissue with the attraction of leucocytes in situ, whereas the expression of immune genes in the anterior kidney could be modulated as an immune response due to the loss of blood caused by the blood-sucking isopod parasite.
IL-1β is a well-known cytokine that plays a crucial role in immune responses, infection and inflammation in cells. IL-1β is a vital participant in the immune system’s defense against microorganism invasion and tissue damage, and it may activate NK cells, macrophages, and lymphocytes by activating lymphocytes or increasing the release of other cytokines (Low et al. 2003). Induced expression of IL-1β was reported in the skin of Oncorhynchus mykiss parasitized by Gyrodactylus derjavini (Neary et al. 2012); spleen and gills of Dicentrarchus labrax infected with Diplectanum aequans (Faliex et al. 2008). Zhi et al. (2018) observed up-regulated expressions of IL-1β in both the skin and gills of Oreochromis niloticus after G. cichlidarum, and Cichlidogyrus sclerosus infections, due to their local inflammatory reactions. Our study indicated a significant upregulation (P < 0.05) of the IL-1β gene in the infected buccal cavity and gill samples when compared to control groups. Conversely, no significant difference in IL-1β gene expression was observed in the kidney sample. It seems that the inflammatory responses due to the attachment of the isopod to the buccal cavity and the gills being in closer proximity may have altered the expression level at the site of infection and not at the site of hemopoiesis.
TNF-α is a vital antibacterial and inflammatory regulatory cytokine generated by macrophages, monocytes, natural killer cells and T cells (Whyte et al. 2013; Grayfer et al. 2008). The expression of TNF-α in the O. mossambicus infected with C. eremita also showed significant upregulation in the buccal cavity and the anterior kidney, with a significant downregulation in the gill of the infected fishes when compared to the control fishes, which is consistent with the trend observed in the TLR gene expression in our study. This result correlates with the study by Sigh et al. (2004) where upregulated expression of TNF-α in the kidney of rainbow trout infected with Ichthyophthirius multifiliis at day 26 conversely downregulated expression of TNF-α which was also observed in the kidney and was also observed in L. rohita and Sparus aurata infected by A. siamensis and Enteromyxum leei (Saurabh et al. 2011; Cuesta et al. 2006). Expressions of TNFα were upregulated in the impact region (buccal cavity) of the O. mossambicus infected with C. eremita. Similarly, it has been observed in various studies where TNFα were upregulated in the impact regions of the parasitic infections, viz., the skin of rainbow trout after infection with Gyrodactylus derjavini (Lindenstrom et al. 2004) in the gills of goldfish and O. niloticus infected with Dactylogyrus intermedius and C. sclerosus (Lu et al. 2013; Zhi et al. 2018), respectively. The head kidney is rich in monocytes and macrophages that are engaged in phagocytosis of invading pathogens and secretion of cytokines such as the pro-inflammatory cytokines interleukin-1β and TNF-α, which are crucial in the initiation of the immunological response in fish (Kumar et al. 2016; Sahoo 2006). In this study, higher expression of TNF-α in the kidney tissue of infected fishes indicates that the effects of the isopod infection can induce a systemic inflammatory reaction in the kidney, which in turn elevates the expression level in the most affected buccal cavity region.
The chemokines CXCa belong to the chemotactic cytokine’s superfamily, which is important in promoting neutrophilic granulocyte migration to infection sites (Huising et al. 2003). The immunological response to bacteria (Baoprasertkul et al. 2004), viral (Li et al. 2012), and parasitic (Huising et al. 2003) infections alters the immune response leading to significant increase in CXC-chemokine transcripts and proteins. They are regarded as crucial immune defence regulators, acting as the link between adaptive and innate immune responses (Alejo and Tafalla 2011). In this study, significant upregulation (P < 0.05) of CXCa was noted in the buccal cavity and anterior kidney in the infected fishes when compared to healthy fishes. A similar increased expression of CXCa has been documented in the head kidney of L. rohita infected with A. siamensis (Kar et al. 2015) and in the skin of the common carp infected with Argulus japonicas and I. multifiliis, respectively (Forlenza et al. 2008; Gonzalez et al. 2007). The upregulated expression of CXCa in the infected site (buccal cavity and anterior kidney) is altered by the leucocyte chemotaxis that attracts different immune cells to sites of infection, resulting in upregulated expression during the isopod infection.
Complementary element C3 is the basis of three stimulation pathways and is crucial for the development of the membrane attack complex. Complement’s functions include the generation of opsonin molecules, anaphylatoxin, direct pathogen killing and maintaining homeostasis (Nikoskelainen et al. 2002). Upregulated expression (P < 0.05) of C3 gene was recorded in all the samples, such as buccal cavity, anterior kidney and gills of infected fish, which was not the case for the genes TLR, IL-1β, TNF-α, and CXCa examined in our study. This may indicate the specific activity of C3 against C. eremita infection and its potential to be utilized as a marker for immune response when infected with such an isopod. Upregulated expression of C3 in the skin and lymphoid organs of rainbow trout infected with I. multifiliis, a parasitic ciliate (Sigh et al. 2004), in the liver of grass carp infected with Sinergasilus major (Chang et al. 2005), and in the head kidney, liver, and spleen of common carp infected with Trypanoplasma borreli (Saeij et al. 2003) were documented earlier. Hence, the findings of this study provide a comprehensive understanding of the immune responses in O. mossambicus during C. eremita isopod infection.
Conclusion
In conclusion, the non-specific immune response and the modulation of specific gene expression in tilapia during isopod C. eremita infection provide valuable molecular insights into the host’s immune response, contributing to a better understanding of the host’s reaction to the isopod infection. As there is no data available on the gene expression in fish during an isopod infection, this appears to be the first report that looks into the changes in the immunity in fish in response to isopod infection. More importantly, it contributes to a broader understanding of the intricate dynamics between isopods and their hosts in aquatic ecosystems.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abdelkhalek NK, El-Adl M, Salama M, Al-Araby MA (2017) Mass mortalities associated with Isopoda infestation as a result of uncontrolled biosecurity measures in Lake Qarun. World Aquaculture Society, Egypt https://www.was.org/MeetingAbstracts/ShowAbstract/47509
Adlard RD, Lester R (1994) Dynamics of the interaction between the parasitic isopod, Anilocra pomacentri, and the coral reef fis, Chromis nitida. Parasitology 109(3):311–324
Alejo A, Tafalla C (2011) Chemokines in teleost fish species. Dev Comp Immunol 35(12):1215–1222
Alvarez-Pellitero P (2008) Fish immunity and parasite infections: from innate immunity to immunoprophylactic prospects. Vet Immunol Immunopathol 126:171–198
Anderson DP, Siwicki AK (1995) Basic haematology and serology for fish health programs. In: Shariff M, Arthur JR, Subasinghe JP (eds) Diseases in Asian aquaculture II. Manila, Philippines, Fish Health Section, Asian Fisheries society, pp 185–202
Aoki T, Hirono I (2006) Immune relevant genes of Japanese flounder, Paralichthys olivaceus. Comp Biochem Physiol Part D Genom Proteo 1(1):115–121. https://doi.org/10.1016/j.cbd.2005.07.002
Baoprasertkul P, Peatman E, Chen LQ, He CB, Kucuktas H, Li P, Simmons M, Liu ZJ (2004) Sequence analysis and expression of a CXC chemokine in resistant and susceptible catfish after infection of Edwardsiella ictaluri. Dev Comp Immunol 28(7-8):769–780. https://doi.org/10.1016/j.dci.2003.12.002
Bharadhirajan P, Murugan S, Sakthivel A, Selvakumar P (2014) Isopods parasites infection on commercial fishes of Parangipettai waters, southeast coast of India. Asian Pacific J Trop Dis 4:S268–S272. https://doi.org/10.1016/S2222-1808(14)60453-9
Brusca RC (1978) Studies on the cymothoid fish symbionts of the eastern Pacific (Isopoda, Cymothoidae) I. Biol Nerocila californica Crustaceana 34:141–154. https://doi.org/10.1163/156854078X00718
Brusca RC (1981) A monograph on the Isopoda Cymothoidae (Crustacea) of the eastern Pacific. Zool JLinn Soc 73:117–199. https://doi.org/10.1111/j.1096-3642.1981.tb01592.x
Chang MX, Nie P, Liu GY, Song Y, Gao Q (2005) Identification of immune genes in grass carp Ctenopharyngodon idella in response to infection of the parasitic copepod Sinergasilus major. Parasitol Res 96:224–229. https://doi.org/10.1007/s00436-005-1340-8
Chinabut S (2002) A case study of isopod infestation in tilapia cage culture in Thailand. FAO Fish Tech Paper 406:201–202
Cuesta A, Munoz P, Rodriguez A, Salinas I, Sitja-Bobadilla A, Alvarez-pellitero P, Estaben MA, Mesequer J (2006) Gilthead Sea bream (Sparus aurata L.) innate defence against the parasite Enteromyxum leei (Myxozoa). Parasitology 132(1):95–104. https://doi.org/10.1017/S0031182005008759
Dash P, Kar B, Mishra A, Sahoo PK (2014) Effect of Dactylogyrus catlaius (Jain 1961) infection in Labeo rohita (Hamilton 1822): innate immune responses and expression profile of some immune related genes. Indian J Exp Biol 52:267–280 https://www. ncbi.nlm.nih.gov/pubmed/24669670
Elgendy MY, Hassan AM, Zaher MFA, Abbas HH, Soliman WS, Bayoumy EM (2018) Nerocila bivittata massive infestations in Tilapia zillii with emphasis on hematological and histopathological changes. Asian J Sci 11(1):134–144. https://doi.org/10.3923/ajsr.2018.134.144
Faliex E, Da Silva C, Simon G, Sasal P (2008) Dynamic expression of immune response genes in the sea bass, Dicentrarchus labrax, experimentally infected with the monogenean Diplectanum aequans. Fish Shellfish Immunol 24(6):759–767. https://doi.org/10.1016/j.fsi.2008.02.011
FAO (2022) The State of World Fisheries and Aquaculture 2022. In: Towards blue transformation. The state of world fisheries and aquaculture (SOFIA). https://doi.org/10.4060/cc0461en
Forlenza M, Walker PD, De Vries BJ, Bonga SEW, Wiegertjes GF (2008) Transcriptional analysis of the common carp (Cyprinus carpio L.) immune response to the fish louse Argulus japonicus Thiele (Crustacea: Branchiura). Fish Shellfish Immunol 25(1-2):76–83. https://doi.org/10.1016/j.fsi.2007.12.013
Gonzalez SF, Buchmann K, Nielsen ME (2007) Real-time gene expression analysis in carp (Cyprinus carpio L.) skin: inflammatory responses caused by the ectoparasite Ichthyophthirius multifiliis. Fish Shellfish Immunol 22(6):641–650. https://doi.org/10.1016/j.fsi.2006.08.011
Grayfer L, Walsh JG, Belosevic M (2008) Characterization and functional analysis of goldfish (Carassius auratus L.) tumor necrosis factor-alpha. Dev Comp Immunol 32(5):532–543. https://doi.org/10.1016/j.dci.2007.09.009
Hadfield KA, Bruce NL, Smit NJ (2013) Review of the fish-parasitic genus Cymothoa Fabricius, 1793 (Isopoda, Cymothoidae, Crustacea) from the southwestern Indian Ocean, including a new species from South Africa. Zootaxa 3640(2):152–176. https://doi.org/10.11646/zootaxa.3640.2.2
Horton T, Okamura B (2001) Cymothoid isopod parasites in aquaculture: a review and case study of a Turkish sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus) farm. Dis Aquat Organ 46(3):181–188. https://doi.org/10.3354/dao046181
Huising MO, Stolte E, Flik G, Savelkoul HF, Verburg-van Kemenade BML (2003) CXC chemokines and leukocyte chemotaxis in common carp (Cyprinus carpio L.). Dev Comp Immunol 27(10):875–888. https://doi.org/10.1016/S0145-305X(03)00082-X
Huttenhuis HB, Grou CP, Taverne-Thiele AJ, Taverne N, Rombout JH (2006) Carp (Cyprinus carpio L.) innate immune factors are present before hatching. Fish Shellfish Immunol 20(4):586–596. https://doi.org/10.1016/j.fsi.2005.07.008
Jayadev Babu S, Sanjeeva Raj PJ (1984) Isopod parasites of fish of Pulicat Lake. In: Proceedings of the Symposium on Coastal Aquaculture (Fin Fish), vol 3, pp 818–823
Kar B, Mohanty J, Hemaprasanth KP, Sahoo PK (2015) The immune response in rohu, Labeo rohita (Actinopterygii: Cyprinidae) to Argulus siamensis (Branchiura: Argulidae) infection: kinetics of immune gene expression and innate immune response. Aquacult Res 46(6):1292–1308. https://doi.org/10.1111/are.12279
Kaya Y, Kocatepe D (2014) Chemical composition and nutritional quality of Scorpion fish (Scorpaena porcus, Linnaeus 1758) muscle. Indian J Anim Res 48(1):83–87. https://doi.org/10.5958/j.0976-0555.48.1.018
Kole S, Anand D, Sharma R, Tripathi G, Makesh M, Rajendran KV, Bedekar MK (2017) Tissue specific expression profile of some immune related genes in Labeo rohita to Edwardsiella tarda infection. Fish Shellfish Immunol 66:575–582. https://doi.org/10.1016/j.fsi.2017.05.047
Kumar R, Joy KP, Singh SM (2016) Morpho-histology of head kidney of female catfish Heteropneustes fossilis: seasonal variations in melano-macrophage centers, melanin contents and effects of lipopolysaccharide and dexamethasone on melanins. Fish Physiol Biochem 42:1287–1306. https://doi.org/10.1007/s10695-016-0218-2
Leonardos I, Trilles JP (2003) Host-parasite relationships: occurrence and effect of the parasitic isopod Mothocya epimerica on sand smelt Atherina boyeri in the Mesolongi and Etolikon Lagoons (W. Greece). Dis Aquat Organ 54(3):243–251. https://doi.org/10.3354/dao054243
Li Y, Luo X, Dan X, Qiao W, Huang X, Li A (2012) Molecular cloning of orange-spotted grouper (Epinephelus coioides) TLR21 and expression analysis post Cryptocaryon irritans infection. Fish Shellfish Immunol 32(3):476–481. https://doi.org/10.1016/j.fsi.2011.11.021
Li Z, Jiang B, Zhong Z, Cao J, Li H, Wang C, Li A (2022) Skin transcriptomic analysis and immune-related gene expression of golden pompano (Trachinotus ovatus) after Amyloodinium ocellatum infection. Fish Shellfish Immunol 128:188–195. https://doi.org/10.1016/j.fsi.2022.07.052
Lindenstrom T, Secombes CJ, Buchmann K (2004) Expression of immune response genes in rainbow 430 trout skin induced by Gyrodactylus derjavini infections. Vet Immunol Immunopathol 97(3-4):137–148. https://doi.org/10.1016/j.vetimm.2003.08.016
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lopez NC (2001) Parasitic crustaceans in fishes from some Philippine lakes. In: Santiago CB, Cuvin-Aralar M, Basiao ZU (eds) Conservation and ecological management of Philippine lakes in relation to fisheries and aquaculture. Aquaculture Department, Southeast Asian Fisheries Development Center, pp 75–79
Low C, Wadsworth S, Burrells C, Secombes CJ (2003) Expression of immune genes in turbot (Scophthalmus maximus) fed a nucleotide-supplemented diet. Aquaculture 221(1-4):23–40. https://doi.org/10.1016/S0044-8486(03)00022-X
Lu C, Ling F, Ji J, Kang YJ, Wang GX (2013) Expression of immune-related genes in goldfish gills induced by Dactylogyrus intermedius infections. Fish Shellfish Immunol 34(1):372. https://doi.org/10.1016/j.fsi.2012.11.004
Marks RE, Juanes F, Hare JA, Conover DO (1996) Occurrence and effect of the parasitic isopod, Lironeca ovalis (Isopoda: Cyymothoidae), on young-of-the-year bluefish, Pomatomus saltatrix (Pisces: Pomatomidae). Can J Fish Aquat Sci 53(9):2052–2057. https://doi.org/10.1139/f95-155
Martin MB, Bruce NL, Nowak BF (2016) Review of the fish-parasitic genus Cymothoa Fabricius, 1793 (Crustacea: Isopoda: Cymothoidae) from Australia. Zootaxa 4119(1):1–72. https://doi.org/10.11646/ZOOTAXA.4119.1.1
Mladineo I (2002) Prevalance of Ceratothoa oestroides (Risso, 1826), a cymothoid isopode parasite, in cultured sea bass Dicentrarchus labrax L. on two farms in middle Adriatic Sea. Acta Adriat 43(1):97–102
Morton B (1974) Host specificity and position on the host in Nerocila phaeopleura Bleeker (Isopoda, Cymothoidae). Crustaceana 26(2):143–148 http://www.jstor.org/stable/20102075
Munoz P, Cuesta A, Athanassopoulou F, Golomazou H, Crespo S, Padrós F, Sitjà-Bobadilla A, Albiñana G, Esteban, M.A, Alvarez-Pellitero P, Meseguer J (2007) Sharpsnout sea bream (Diplodus puntazzo) humoral immune response against the parasite Enteromyxum leei (Myxozoa). Fish Shellfish Immunol 23(3):636-645. https://doi.org/10.1016/j.fsi.2007.01.014
Neary ET, Develi N, Özgül G (2012) Occurrence of Dactylogyrus species (platyhelminths, monogenean) on cyprinids in Almus Dam Lake, Turkey. Turkish J Fish Aquat Sci 12(1) https://dergipark.org.tr/en/pub/trjfas-ayrildi/issue/13270/160272
Nikoskelainen S, Lehtinen J, Lilius EM (2002) Bacteriolytic activity of rainbow trout (Oncorhynchus mykiss) complement. Dev Comp Immunol 26(9):797–804. https://doi.org/10.1016/S0145-305X(02)00032-0
Ostlund-Nilsson S, Curtis L, Nilsson GE, Grutter AS (2005) Parasitic isopod Anilocra apogonae, a drag for the cardinal fish Cheilodipterus quinquelineatus. Mar Ecol Prog Ser 287:209–216. https://doi.org/10.3354/meps287209
Ozdemir G, Celik ES, Yılmaz S, Gurkan M, Kaya H (2016) Histopathology and blood parameters of bogue fish (Boops boops, Linnaeus 1758) parasitized by Ceratothoa oestroides (Isopoda: Cymothoidae). Turkish J Fish Aquat Sci 16(3):579–590. https://doi.org/10.4194/1303-2712-v16_3_28
Poulin R (2007) Are there general laws in parasite ecology? Parasitology 134(6):763–776. https://doi.org/10.1017/S0031182006002150
Quade MJ, Roth JA (1997) A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol 58(3-4):239–248. https://doi.org/10.1016/S0165-2427(97)00048-2
Rajkumar M, Perumal P, Trilles JP (2005) Cymothoa indica (Crustacea, Isopoda, Cymothoidae) parasitizes the cultured larvae of the Asian seabass Lates calcarifer under laboratory conditions. Dis Aquat Organ 66(1):87–90. https://doi.org/10.3354/dao066087
Rameshkumar G, Ravichandran S, Ramesh M (2016) Distribution of isopod parasites in Carangid fishes from Parangipettai, Southeast coast of India. J Parasit Dis 40:124–128. https://doi.org/10.1007/s12639-014-0460-4
Rameshkumar G, Ravichandran S, Trilles JP (2012) Observation on an isopod parasitizing the edible fish Parastromateus niger in the Parangipettai coast of India. J Environ Biol 33(2):191–193
Ravichandran S, Rameshkumar G, Balasubramanian T (2010) Infestation of isopod parasites in commercial marine fishes. J Parasit Dis 34:97–98. https://doi.org/10.1007/s12639-010-0014-3
Ray D, Mohapatra P, Ghorai N, Seth JK, Mohapatra A (2022) Infection of the parasitic isopods on commercial fishes of the northern part of the east coast of India. J Parasit Dis 46(2):440–453. https://doi.org/10.1007/s12639-021-01463-1
Roy P, Panda SP, Sethi SN, Rana N, Sethi G, Das BK (2015) Morphometric and molecular identification of Isopod parasite Joryma hilsae in Pellona ditchela (Val 1847). J Aquac Trop 30(1/2):15
Saeij JP, de Vries BJ, Wiegertjes GF (2003) The immune response of carp to Trypanoplasma borreli: kinetics of immune gene expression and polyclonal lymphocyte activation. Dev Comp Immunol 27(10):859–874. https://doi.org/10.1016/S0145-305X(03)00083-1
Sahoo P (2006) Immunocompetent organs in teleosts. In: In Fish and shellfish immunology: an introduction. Narendra Publishing House, New Delhi, pp 1–11
Sahoo PK, Kumari J, Mishra BK (2005) Non-specific immune responses in juveniles of Indian major carps. J Appl Ichthyol 21(2):151–155. https://doi.org/10.1111/j.1439-0426.2004.00606.x
Saito N (2000) A preliminary check list of isopod crustaceans in Japan. Bull Toyama Sci Mus 23:11–107
Saurabh S, Mohanty BR, Sahoo PK (2011) Expression of immune-related genes in rohu Labeo rohita (Hamilton) by experimental freshwater lice Argulus siamensis (Wilson) infection. Vet Parasitol 175(1-2):119–128. https://doi.org/10.1016/j.vetpar.2010.10.001
Sievers G, Lobos C, Inostroza R, Ernst S (1996) The effect of the isopod parasite Ceratothoa gaudichaudii on the body weight of farmed Salmo salar in southern Chile. Aquaculture 143(1):1–6. https://doi.org/10.1016/0044-8486(96)01262-8
Sigh J, Lindenstrøm T, Buchmann K (2004) The parasitic ciliate Ichthyophthirius multifiliis induces expression of immune relevant genes in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 27(7):409–417. https://doi.org/10.1111/j.1365-2761.2004.00558.x
Sitja-Bobadilla A, Redondo MJ, Bermúdez R, Palenzuela O, Ferreiro I, Riaza A, Quiroga I, Nieto JM, Alvarez-Pellitero P (2006) Innate and adaptive immune responses of turbot, Scophthalmus maximus (L.), following experimental infection with Enteromyxum scophthalmi (Myxosporea: Myxozoa). Fish Shellfish Immunol 21(5):485–500. https://doi.org/10.1016/j.fsi.2006.02.004
Siwicki AK, Anderson DP (1993) Immunostimulation in fish: measuring the effects of stimulants by serological and immunological methods. In: Siwicki AK, Anderson DP (eds) The Nordic Symposium On Fish Immunology, 1st edn, Lysekil, Sweden, pp 1–24
Smit NJ, Bruce NL, Hadfield KA (2014) Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae. Int J Parasitol Parasites Wildl 3(2):188–197. https://doi.org/10.1016/j.ijppaw.2014.03.004
Su YL, Guo ZX, Xu LW, Jiang JZ, Wang JY, Feng J (2012) Identification of a cobia (Rachycentron canadum) CC chemokine gene and its involvement in the inflammatory response. Fish Shellfish Immunol 32(1):204–210. https://doi.org/10.1016/j.fsi.2011.10.005
Thamban AP, Kappalli S, Kottarathil HA, Gopinathan A, Paul TJ (2015) Cymothoa frontalis, a cymothoid isopod parasitizing the belonid fish Strongylura strongylura from the Malabar Coast (Kerala, India): redescription, description, prevalence and life cycle. Zool Stud 54(1):1–28. https://doi.org/10.1186/s40555-015-0118-7
Trilles JP (1994) Les Cymothoidae (Crustacea: Isopoda) du Monde. Podrome pour une faune Studia Marina 21(22):1–288
Trilles JP (2008) Some marine isopods from the Senckenberg Research Institute (Frankfurt am Main, Germany) (Crustacea, Isopoda: Cymothoidae, Aegidae, Corallanidae, Cirolanidae). Senckenbergiana Biologica 88:21–28
Trilles JP, Ravichandran S, Rameshkumar G (2011) A checklist of the Cymothoidae (Crustacea, Isopoda) recorded from Indian fishes. Acta Parasitol 56(4):446–459. https://doi.org/10.2478/s11686-011-0077-z
Vigneshwaran P, Ravichandran S, Prema M (2019) Parasitic isopod Cymothoa eremita (Brünnich 1783) (Isopoda: Cymothoidae) affects the growth of black pomfret Parastromateus niger (Bloch 1795) in the southeast coast of India. Thalassas: Int J Mar Sci 35:109–115. https://doi.org/10.1007/s41208-018-0097-7
Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA (2013) Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153(2):307–319. https://doi.org/10.1016/j.cell.2013.03.035
Yu H, Li X (2003) Study on the Cymothoidae from Chinese waters. Stud Mar Sin 45:223–238
Zeng S, Duan Y, Li X, Hu Y, Mo Z, Dan X, Li Y (2023) Effects of Cryptocaryon irritans infection on the histopathology, oxidative stress, immune response, and intestinal microbiota in the orange-spotted grouper Epinephelus coioides. Fish Shellfish Immunol 133:108562. https://doi.org/10.1016/j.fsi.2023.108562
Zhi T, Xu X, Chen J, Zheng Y, Zhang S, Peng J, Brown CL, Yang T (2018) Expression of immune-related genes of Nile tilapia Oreochromis niloticus after Gyrodactylus cichlidarum and Cichlidogyrus sclerosus infections demonstrating immunosupression in coinfection. Fish Shellfish Immunol 80:397–404. https://doi.org/10.1016/j.fsi.2018.05.060
Acknowledgements
The authors acknowledge and thank Tamil Nadu Dr. J. Jayalalithaa Fisheries University, Nagapattinam, Tamil Nadu, India, for extending the research facilities.
Funding
This study was funded by Tamil Nadu State Planning Commission under the category “Tamil Nadu Innovation Initiatives (TANII)” in the project entitled “E-Fish Health surveillance and monitoring to improve the fisheries production in Tamil Nadu” operated at State Referral Laboratory for Aquatic Animal Health, Tamil Nadu Dr. J. Jayalalithaa Fisheries University-Madhavaram campus, Chennai, India.
Author information
Authors and Affiliations
Contributions
AP: sampling, molecular works, analysis of the data, and drafting of manuscript. AU: conceptualization, designing of the study, and manuscript correction.
Corresponding author
Ethics declarations
Ethical approval
In our study, the handling and experimentation of fishes were carried out in accordance with the “CPCSEA guidelines for experimentation on fishes, Government of India.” All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and are aware that with minor exceptions; no changes can be made to authorship once the paper is submitted.
Human and animal ethics
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
The work has been carried out as a part of post graduate research work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ponsrinivasan, A., Uma, A. Unraveling the host-parasite interaction: immune response in Oreochromis mossambicus to Cymothoa eremita (Isopoda, Cymothoidae) infection. Parasitol Res 122, 3233–3242 (2023). https://doi.org/10.1007/s00436-023-08012-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-08012-0